Abstract
The contribution of triacylglycerol to energy provision in the hypertrophied heart, mediated through lipoprotein lipase (LPL) is largely unknown and the contribution of very-low-density lipoprotein (VLDL) receptor to control of LPL presentation at the endothelium is unclear. For isolated perfused rat hearts, cold acclimation (CA) induced volume-overload hypertrophy, with decreased developed pressure (P < 0.01), increased end-diastolic volume of the left ventricle (P < 0.001) and a loss of contractile reserve in response to dobutamine challenge (P < 0.01). Oleate utilisation by perfused hearts was unchanged by CA, however uptake of intralipid emulsion increased 3-fold (P < 0.01). CA increased the proportion of lipid deposited in tissue lipids from 10% in euthermic controls to 40% (P < 0.01) although the overall contribution of individual lipid classes was unaffected. Cold acclimation significantly increased heparin-releasable LPL (P < 0.05) and tissue residual LPL (P < 0.01). Western blot analysis indicated preserved expression of proteins coding for SERCA2, muscle-CPT1 and VLDL-receptor following CA, while AMPKα2 and phospho-AMPKα2 were unaffected. These observations indicate that for physiological hypertrophy AMPK phosphorylation does not mediate the enhanced translocation of LPL to cardiac endothelium.
Keywords: Cardiac hypertrophy, Very-low-density lipoprotein receptor, AMPK, Triacylglycerol
1. Introduction
The heart has a high and unremitting demand for energy and relies on metabolic flexibility to derive this energy from the prevailing substrates present in plasma [1]. In the fed (postprandial) state the majority of energy is derived from lipids in the diet. These are principally transported in the form of chylomicrons and must be assimilated into the myocardium through lipoprotein lipase, an endothelium-expressed enzyme that controls the transport of triacylglycerol-derived fatty acids (FA) into the cardiomyocyte across the endothelium. Chylomicrons may provide the majority of FA-derived energy as measured for the in vitro perfused myocardium [2] and in the whole animal [3]. Moreover, given that mammals are predominantly in the ‘postprandial state’ chylomicrons may be the primary fuel for the heart. Interestingly, LPL is not synthesised in the endothelium but is derived from the cardiomyocyte itself [4] and must be translocated across the interstitial space to be re-expressed on the luminal surface of the capillary endothelium [5].
LPL is intricately coupled to the very-low-density lipoprotein receptor (VLDL-receptor) and investigations have demonstrated the potency of the VLDL-receptor to bind lipoproteins and act as an anchor [6], facilitating the lipolysis of lipoprotein particles to release NEFA and thus generating a high local concentration at the endothelium. The VLDL-receptor may also facilitate uptake of core lipids from lipoproteins [7]. Further studies have also demonstrated the ability of VLDL-receptor to bind LPL directly, and elegant experiments have proposed the exploitation of this mechanism for the translocation and subsequent re-expression of LPL from the cardiomyocyte, across the endothelium to the luminal surface of capillaries [8]. More recent investigations noted the expression of VLDL-receptor protein may also be coupled to cellular energy status through activation of AMPK [9].
While the control of cardiac expression of LPL remains controversial, the involvement of the VLDL-receptor in trafficking implies a degree of control that is critically located at the capillary endothelium. This provides a mechanism regulated by prevailing plasma substrate concentration which may be important during hypertriacylglycerolemia. Indeed, given the location of LPL-synthesis within the cardiomyocyte control of LPL expression at the mRNA level may appear somewhat distant from the site of LPL action, namely the capillary endothelium. An important recent observation suggests that activation of adenosine monophosphate-activated protein kinase (AMPK) in the cardiomyocyte by chemical activators (AICAR) led to increased endothelial expression of LPL [10]. However, this appears at odds with the importance of AMPK in the signalling of low energy status in tissues, typically seen after ischaemia-reperfusion injury [11]. Under metabolic conditions where oxygen is limiting for the production of ATP through oxidative phosphorylation the teleological rationale for increasing FA uptake into tissue that cannot utilise this substrate efficiently is unclear, particularly given the adverse consequences of myocardial TAG accumulation [12]. Clearly, this has implications for pathological cardiac hypertrophy, characterised by a decrease in metabolic flexibility and increased reliance on glucose metabolism and glycolysis for ATP synthesis in an oxygen-sparing manner. However, no estimate of the involvement of VLDL-receptor and AMPK is available for physiological hypertrophy.
We exploited the cold-acclimated rat as a model for physiological cardiac hypertrophy to investigate for the first time the influence of hypertrophy on the expression of LPL and VLDL-receptor proteins and quantify the metabolic contribution of triacylglycerol to energy metabolism. In addition, the presentation of LPL at the endothelial surface was correlated with levels of VLDL-receptor to investigate whether control of LPL translocation may reside in the VLDL-receptor.
2. Materials and methods
2.1. Materials
3H-[9,10]-oleic acid and 3H-[9,10]-triolein were purchased from Amersham Biosciences (Chalfont, UK). Intralipid lipid emulsion (10%w/v triacylglycerol) was obtained from Fresenius Kabi Ltd, (Runcorn, UK). Dobutamine was obtained from Boehringer Ingelheim, (Bracknell, UK). Fatty acid-free bovine albumin and all buffer salts were purchased from Sigma (Poole, UK). Antibodies to AMPKα2 and threonine 172 phospho-AMPKα2 were obtained from Kinasource Ltd. (Dundee, Scotland). Ventricular balloons were constructed ‘in house’ using Saran Wrap polythene film.
2.2. Methods
2.2.1. Animal maintenance
Animals were maintained in accordance with the UK Home Office, Animal Scientific Procedures Act (1986) and housed at 22 °C 12 h light/12 h dark with ad libitum access to food and water. Male Wistar rats (60 g starting body mass) were cold acclimated as previously described [13,14]. Briefly, rats were housed in an environmental chamber and exposed to 22 °C 12 h light/12 h dark. Over the subsequent 4 week period the temperature and light period duration were gradually decreased until at 4 weeks the rats were exposed to 1 h light/23 h dark and environmental temperature 4 °C. Throughout this period animals had ad libitum access to both food and water. Control animals were purchased at the appropriate experimental body mass (250–300 g).
2.2.2. Radio-isotope tracer preparation
Oleic acid (final concentration 0.4 mM) was pre-bound to fatty acid-free bovine albumin as previously described [2]. Briefly, oleic acid sodium salt was dissolved in pre-warmed saline and added to bovine albumin (2.0 g, to give a final concentration in perfusate 2.0%w/v). 3H-[9,10]-oleic acid (9.0 MBq/100 ml final concentration) was saponified (0.1 M KOH) and added to the oleic acid. Intralipid was pre-labelled with 3H-[9,10]-triolein (9.0 MBq/12 mg intralipid triacylglycerol). The intralipid mixture was homogenised (ultraturrax blade homogeniser, full power 15 s, 4-cycles, over ice) and added to fatty acid-free bovine albumin (final concentration 2.0%w/v in perfusate) to give the final triacylglycerol working concentration (0.4 mM).
2.2.3. Tissue isolation and heart perfusion
Animals were prepared surgically as outlined previously [2]. Briefly, anaesthesia was induced with pentobarbital (60 mg/kg ip in saline) and following thoracotomy hearts excised with lungs and thymus in situ and immersed in ice-cold Krebs–Hensleit medium. Excess tissue was dissected away, the thymus was divided to reveal the aortic arch and the aorta was trimmed at the level of the carotid artery branches and cannulated (16G cannula). Hearts were perfused in retrograde fashion as outlined previously [15,16]. Flow through the heart was established and extra tissue was dissected away. An incision was made in the right ventricle and the left atrial appendage was removed. A small flexible non-elastic balloon was inserted into the left atrium through the mitral valve and into the left ventricle. This fluid-filled balloon was attached to a fine plastic catheter and connected to a pressure transducer (MEMSCAP, Skoppum, Norway) and a graduated syringe (0–1000 μl: Hamilton, Nevada, USA). Hearts were maintained at 37 °C and perfused at a constant pressure (100 cm H2O) with a Krebs–Hensleit crystalloid medium supplemented with glucose (10 mM) and CaCl2 (1.3 mM) gassed with oxygen/CO2 (95:5). Developed pressure was measured following isovolumic contraction of the fluid-filled balloon and recorded to computer using a digital interface (AD Instruments, Chalgrove, Oxford, UK).
2.2.4. Ventricular performance
The initial balloon volume was adjusted until the diastolic pressure recorded measured 0 mmHg and the developed pressure (difference between systolic and diastolic pressures) was < 10 mmHg. Balloon volume was increased from this baseline, in incremental steps (50 μl) and developed pressure was recorded in real time. Pressures were allowed to stabilise until diastolic pressure remained constant before initiating further increases in balloon volume. Incremental increases in balloon volume were performed until the peak systolic pressure developed exceeded 200 mmHg. The balloon was then deflated and the process repeated. For selected experiments, the sympathomimetic inotrope dobutamine was added (final concentration 300 nM) and the preparation was allowed to stabilise for 10 min before new measurements of developed pressure, heart rate and coronary flow measured. For metabolism experiments a single balloon volume was used, corresponding to a fixed end-diastolic pressure (EDP). Balloon volume was increased until diastolic pressure reached 20 mmHg which was designated the ‘working pressure’ of the myocardium. Coronary flow was estimated from timed collections of a known volume of perfusate and expressed as volume/unit mass of cardiac tissue.
Ventricular performance was calculated off-line following the experiment using computer analysis software (Chart Version 5.0, AD Instruments, Chalgrove, Oxford, UK). Heart rate, systolic pressure, diastolic pressure and hence developed pressure were measured. Rate of change of pressure (+ dP/dt) was calculated from the maxima of first order derivative of pressure trace. Rate-pressure product (RPP) was calculated at each balloon volume as the product of heart rate (bpm) × developed pressure (mmHg). End-diastolic volume was estimated from the linear regression of diastolic performance curve for values greater than zero at the point the regression line bisected the balloon volume at diastolic pressure = zero.
2.2.5. Quantitation of plasma tritiated water
Metabolism of oleic acid and intralipid was estimated from quantitation of tritiated water as previously described [2]. Briefly, aliquots of perfusate (1.0 ml) were extracted with chloroform:methanol (2:1) (20 ml). Following addition of water (4.0 ml), tritiated water was estimated in the aqueous fraction by scintillation counting. Metabolism was calculated with reference to the specific activity at the start of the experiment.
2.2.6. Total lipid extraction
Total cholesterol and triglycerides were also extracted from the hearts as described previously [14]. Briefly, aliquots (100 mg) of heart powder were extracted with methanol:chloroform (1:2). Extracts were evaporated to dryness and resuspended in absolute ethanol. Cardiac TAG and cholesterol were measured using commercial kits. For selected extracts, lipids were separated into phospholipids, diacylglycerol, fatty acid, triacylglycerol and cholesterol ester as outlined previously [2]. Briefly, ethanol extracts of tissue (100 mg) were separated on thin layer chromatography (TLC) plates (Silica Gel 60, 250 μm) and separated using the solvent system hexane:diethyl ether:acetic acid (70/30/1.6). Lipids were visualised using rhodamine 6G and UV light. Lipids were mechanically recovered from the TLC plate and quantified by liquid scintillation counting.
2.2.7. Lipoprotein lipase activity
Lipoprotein lipase (LPL) activity was measured as previously described [17,2]. Briefly, separate groups of hearts from control and cold-acclimated rats were perfused with Krebs–Hensleit medium containing glucose (10 mM) and CaCl2 (1.3 mM) as outlined above. Perfusion was maintained initially in non-recirculating mode to wash out erythrocytes. Recirculating perfusion was established and maintained for 5 min, after which heparin (10 U/ml final concentration) was added and recirculated for a further 2 min. Samples of perfusate were isolated and frozen in liquid nitrogen. Cardiac tissue was then snap-frozen in liquid nitrogen and cardiac mass was noted. Aliquots of post-heparin perfusate and acetone-dried heart powders (10 mg) were reacted with triacylglycerol emulsion (final concentration 5.6 mM) pre-labelled with 3H-[9,10]-triolein supplemented with human plasma (ratio plasma to final reaction volume 1:6) as a source of Apolipoprotein CII. Reactions were carried out in Tris–HCl buffer (0.1 M, pH = 8.0) supplemented with fatty acid-free bovine albumin (final concentration 2.0%w/v). Incubations were carried out at 37 °C and activities were expressed per unit mass of cardiac tissue. Total cardiac LPL activity was estimated as the sum of tissue residual LPL and heparin-releasable LPL activity.
2.2.8. Immunoblotting for proteins
Standard Western immunoblotting techniques were used for the detection and estimation of relative amounts of sarcoplasmic–endoplasmic reticulum Ca2+-ATPase (SERCA2), VLDL-receptor, muscle-carnitine palmitoyl transferase-1 (CPT1), AMPKα2, phospho-AMPKα2 and tubulin proteins. Briefly, cardiac tissue (50 mg) was powdered in liquid nitrogen and extracted with RIPA buffer containing protease inhibitors, followed by centrifugation (10,000 rpm for 10 min) and recovery of the supernatant. The membranes were probed with antibodies specific for SERCA2, VLDL-receptor, muscle-CPT1 (all Santa Cruz — initial dilution 1:1000) AMPKα2, Threonine-172 phospho-AMPKα2 (both Kinasource Ltd, Dundee, Scotland — initial dilution 1:1500) and mouse monoclonal anti-Tubulin (Sigma — initial dilution 1:2500). Differing sample protein loadings were used for different antibodies (SERCA2, Threonine-172 phospho-AMPKα2 20 μg; VLDL-receptor, muscle-CPT1, AMPKα2 10–15 μg; Tubulin 10 μg). Densitometry of Western blots was estimated using ImageJ software. Protein expression was corrected for the expression of an internal control (Tubulin).
2.2.9. In vitro rates of β-oxidation
Rates of β-oxidation were estimated as outlined previously [18,14]. Peroxisomal oxidation of oleate was estimated following inhibition of oxidative phosphorylation using rotenone, Antimycin A and potassium cyanide in the reaction buffer and repeating the assay. Mitochondrial β-oxidation activity was quantified following subtraction of the peroxisomal from total β-oxidation. Activity was normalised against tissue protein determined by the BCA protein assay kit (Sigma, Poole, UK).
2.2.10. Statistical analysis
Statistical analysis was carried out using Single Factor ANOVA analysis with Bonferroni correction for multiple comparisons where appropriate. Data represents mean ± standard deviation.
3. Results
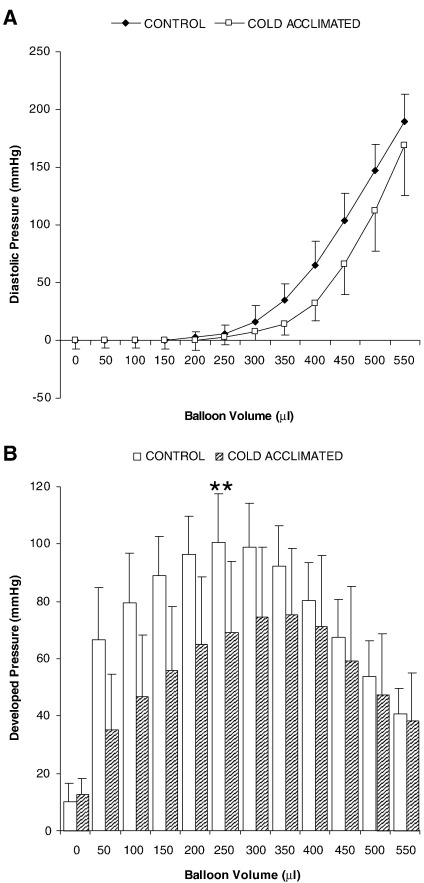
During cold acclimation all rats gained weight normally and appeared to tolerate the cold exposure. Under anaesthesia, mean arterial pressure was significantly increased in CA-rats (P < 0.01), whereas heart rate was unaffected by CA. At post-mortem comparison of body mass for control and CA-rats revealed no difference in growth despite the progressive cold stress (body mass 262 ± 6 g control vs 276 ± 35 g cold acclimated: n = 6; NS). However for CA-rats heart mass was significantly increased 20% (P < 0.01: Table 1). Estimates of end-diastolic volume indicate a statistically significant 20% increase in volume (P < 0.001: Table 1). Estimation of developed pressure throughout the range of balloon volumes indicated that for CA-rats, at peak developed pressure, measured pressures were decreased 40% (P < 0.01; Fig. 1).
Table 1.
Cardiac performance in vivo for control and cold-acclimated hearts and the effects of dobutamine addition (300 nM) on cardiac performance of perfused control and cold-acclimated hearts
| Control |
Cold acclimated |
|||
|---|---|---|---|---|
| Untreated | + Dobutamine | Untreated | + Dobutamine | |
| Heart rate (in vivo — bpm) | 430 ± 37 | 418 ± 45 | ||
| Mean arterial pressure (mmHg) | 106 ± 12 | 134 ± 9++ | ||
| RPP (mmHg/min) | 30962 ± 5685 | 29305 ± 3760 | ||
| Cardiac mass (wet mass — g) | 1.51 ± 0.13 | 1.82 ± 0.24++ | ||
| Estimated EDV (μl) | 313 ± 24 | 377 ± 15++ | ||
| Cardiac TAG (μmol/g) | 4.82 ± 1.75 | 4.65 ± 1.60 | ||
| Heart rate (bpm) | 292.4 ± 39.7 | 298.9 ± 51.7 | 247.0 ± 25.5 | 279.2 ± 25.9 |
| Peak systolic pressure (mmHg) | 106.8 ± 12.9 | 127.8 ± 15.2⁎ | 71.8 ± 13.1++ | 88.2 ± 16.0++ |
| Developed pressure (mmHg) | 90.5 ± 11.4 | 113.7 ± 12.6⁎⁎ | 58.3 ± 16.1++ | 75.8 ± 19.8++ |
| + dP/dt (mmHg/s) | 1682.5 ± 130.5 | 2409.8 ± 139.1⁎⁎⁎ | 1419.6 ± 522.6 | 1723.8 ± 454.1++ |
| − dP/dt (mmHg/s) | − 1288.8 ± 94.1 | − 1875.0 ± 71.2⁎⁎⁎ | − 1133.6 ± 415.1 | − 1434.8 ± 484.7 |
Data represents mean ± SD (n = 6). Statistical significance is measured as ANOVA with correction for multiple comparisons and represented as: The effects of cold acclimation; ++P < 0.01: the effects of dobutamine; ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001.
Fig. 1.
Diastolic performance (A) and developed pressure (B) for Langendorff-perfused rat hearts from control or CA-rats. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance indicated as: Effect of cold acclimation ⁎⁎P < 0.01.
3.1. Cardiac performance
Cold acclimation led to a 30% decrease in peak systolic pressure (P < 0.01) and a 35% decrease in developed pressure for a fixed end-diastolic pressure (P < 0.01: Table 1) compared with euthermic control rats. Addition of dobutamine to control heart perfusions increased peak systolic pressure 20% (P < 0.05) and developed pressure 25% (P < 0.01: Table 1). In addition, dobutamine also induced a 40% increase in the rate of change of pressure (measured as + dP/dt) for control hearts (P < 0.001). For cold-acclimated (CA) rats dobutamine was without effect on peak systolic pressure, developed pressure and + dP/dt (NS: Table 1).
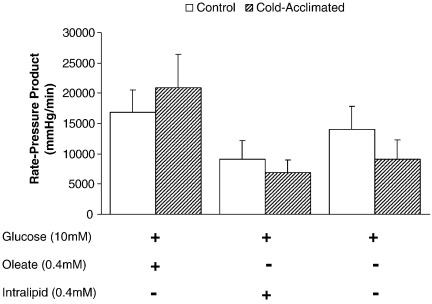
3.2. Rate-pressure product
Comparing RPP for control and CA-rat hearts perfused with glucose alone, or in combination with oleate or intralipid the differing substrates were without effect on cardiac performance when perfused at fixed end-diastolic pressure (Fig. 2).
Fig. 2.
Influence of substrate on rate-pressure product for control and cold-acclimated rat hearts. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance set at P < 0.05.
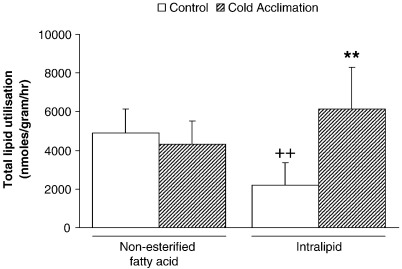
3.3. Total lipid utilisation
Total utilisation was estimated as the sum of tissue lipids remaining and β-oxidation at 60 min. Total utilisation was preserved in CA-rat hearts perfused with oleate-containing buffer (NS: Fig. 3). Intralipid perfusion for control rat hearts led to a 55% decrease in total utilisation compared with control oleate-perfused hearts (P < 0.01: Fig. 3). However, when compared with CA-rats, intralipid utilisation increased 3-fold following CA (P < 0.01: Fig. 3).
Fig. 3.
Total lipid utilisation for control and cold-acclimated hearts perfused with either oleate or intralipid. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance indicated as: Effect of perfusion with intralipid ++P < 0.01; Effect of cold acclimation ⁎⁎P < 0.01.
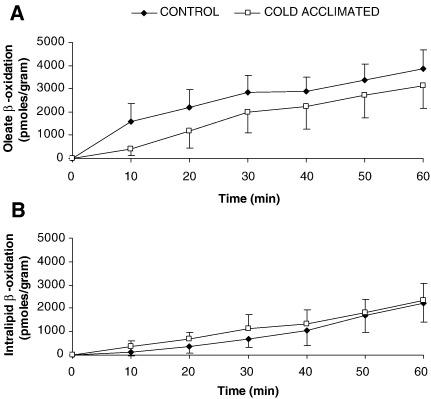
3.4. β-oxidation
Oxidation of both oleate and intralipid remained linear throughout the period of perfusion. Cold acclimation was without effect on oleate oxidation by perfused hearts (Fig. 4). For intralipid-perfused rat hearts rate of β-oxidation was preserved in CA hearts (Fig. 4).
Fig. 4.
Estimation of fatty acid β-oxidation from oleate and intralipid with time for control or cold-acclimated rat hearts. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance set at P < 0.05.
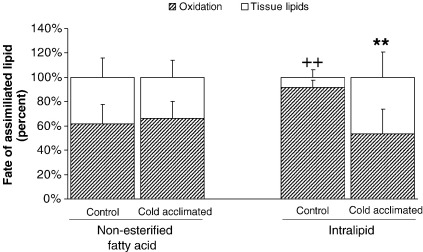
3.5. Lipid fate
Fate was estimated as the proportion of total uptake represented by tissue lipid or β-oxidation. For control hearts perfused with oleate, β-oxidation represented 60% of total uptake, and tissue lipids represented the remaining 40%. This proportion was preserved in CA-rat hearts perfused with oleate (NS: Fig. 5). For intralipid-perfused control hearts the proportion undergoing β-oxidation increased to 91.6 ± 6.1% (P < 0.01: Fig. 5). For CA hearts perfused with intralipid β-oxidation represented 53% of total utilisation and was significantly different from intralipid-perfused controls (P < 0.01: Fig. 5).
Fig. 5.
Fate of assimilated lipid taken up by perfused hearts after 60 min perfusion. Control and cold-acclimated hearts were perfused with either oleate or intralipid. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance indicated as: Effect of perfusion with intralipid ++P < 0.01; Effect of cold acclimation ⁎⁎P < 0.01.
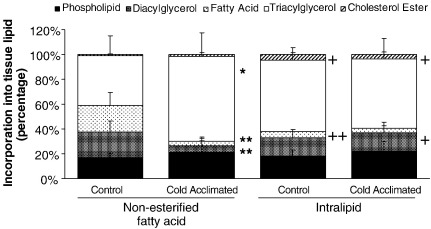
3.6. Tissue lipids
Comparison of the tissue components of labelled lipids revealed that CA significantly reduced the proportion of oleate deposited as diacylglycerol (20.7 ± 8.8% control vs 4.9 ± 4.1% CA; P < 0.01: Fig. 6) and non-esterified fatty acid (21.3 ± 10.5% control vs 3.8 ± 2.3% CA; P < 0.01: Fig. 6). This was accompanied by a significant, 70% greater accumulation of oleate as triacylglycerol in CA-rat hearts (P < 0.05: Fig. 6). Perfusion of hearts with labelled intralipid resulted in a 3-fold increase in labelled lipids deposited as cholesterol ester, comparing oleate and intralipid-perfused hearts (P < 0.05: Fig. 6). This was preserved for both control and CA-rat hearts (P < 0.05: Fig. 6). Estimation of cardiac tissue unlabelled TAG concentration revealed that CA had no effect on tissue TAG content (Table 1).
Fig. 6.
Distribution of tissue lipid between different lipid classes following perfusion with either oleate or intralipid. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance indicated as: Effect of perfusion with intralipid +P < 0.05, ++P < 0.01; Effect of cold acclimation ⁎P < 0.05, ⁎⁎P < 0.01.
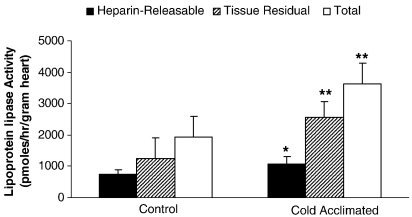
3.7. Lipoprotein lipase activity
For both control- and CA-hearts, tissue residual LPL activity was approximately double that found for heparin-releasable LPL activity (Fig. 7). Cold acclimation led to a 45% greater heparin-releasable LPL activity (733 ± 146 pmol/g/h control rat vs 1068 ± 236 pmol/g/h CA-rat; P < 0.05; Fig. 7). Tissue residual LPL for CA-rat hearts was 2-fold greater than controls (P < 0.01: Fig. 7). By calculation, total cardiac LPL activity was increased 90% for CA-rat hearts compared with corresponding euthermic controls (P < 0.01: Fig. 7).
Fig. 7.
Cardiac lipoprotein lipase activity for heparin-releasable, tissue residual or total LPL. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance indicated as: Effect of cold acclimation ⁎P < 0.05, ⁎⁎P < 0.01.
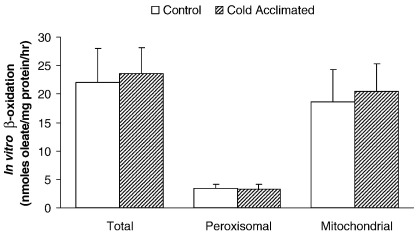
3.8. In vitro β-oxidation
Total β-oxidation was unaffected following CA of rats (22.1 ± 5.9 nmol/mg protein/h control vs 23.6 ± 4.5 nmol/mg protein/h CA: Fig. 8). Following inhibition of oxidative phosphorylation, estimation of peroxisomal β-oxidation also remained unchanged following CA (3.48 ± 0.58 nmol/mg protein/h control vs 3.22 ± 0.96 nmol/mg protein/h CA: Fig. 8). Therefore by calculation, mitochondrial β-oxidation was unchanged following CA in the heart.
Fig. 8.
Estimation of in vitro β-oxidation of oleate by homogenates of cardiac tissue following cold acclimation. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance set at P < 0.05.
3.9. Western blot analysis
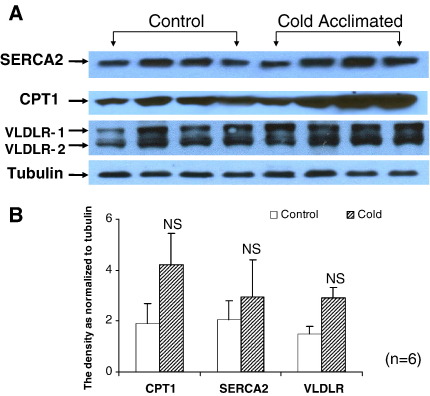
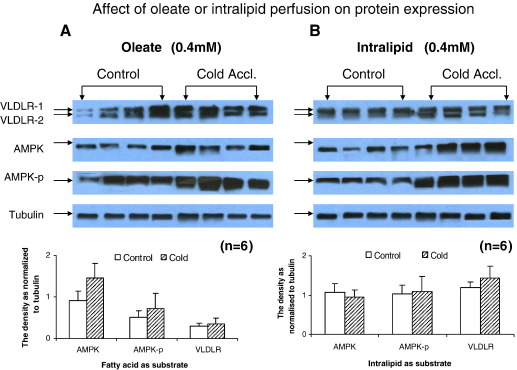
Densitometric analysis of Western blots from control and CA-hearts perfused with oleate revealed that CPT1, SERCA2 and VLDL-receptor protein expression was unaffected by CA (Fig. 9). Investigation of the effect of perfusion substrate (oleate or intralipid) on protein expression revealed that neither oleate (Fig. 10A) nor intralipid (Fig. 10B) affected the expression of AMPKα2 protein or phospho-AMPKα2 protein during the course of the perfusion in either control or CA-rat hearts.
Fig. 9.
Analysis of the effect of cold acclimation on protein expression by Western blot for SERCA2, CPT1 and VLDL-receptor proteins. (A) Representative western blots from cardiac tissue homogenates. (B) Densitometric analysis of Western blots for estimation of expression of different proteins. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance set at P < 0.05.
Fig. 10.
Analysis of the effect of perfusion substrate on protein expression by Western blot for VLDL-receptor, AMPKα2 and threonine 172 phospho-AMPKα2 proteins. (A) Representative western blots from cardiac tissue perfused with oleate. (B) Representative western blots from cardiac tissue perfused with intralipid. Densitometric analysis of Western blots carried out for estimation of expression of different proteins. Results represent mean ± SD (n = 6 hearts in all groups). Statistical significance set at P < 0.05.
4. Discussion
We document for the first time that increases in LPL activity associated with physiological hypertrophy are not connected with changes to VLDL-receptor, one proposed mechanism for the translocation of LPL to the cardiac endothelium. Cold acclimation (CA) produced a model of volume-overload hypertrophy characterised by an enlarged heart, increased end-diastolic volume and preserved expression of both SERCA2a and muscle-CPT1 (m-CPT1). This is analogous to other forms of physiological hypertrophy including pregnancy [19] and exercise [20]. Moreover, previous investigations of cold acclimation also describe volume-overload hypertrophy with elevated systolic blood pressure [21], a preserved capillary density [22] and oxygen delivery [23]. CA was chosen over other forms of physiological hypertrophy to overcome the variability in different levels of exercise stress and the degree of hypertrophy induced [24] or the influence of phases of pregnancy on myocardial LPL activity [25]. We postulate that the acclimation phase delivers a steadily increasing challenge to energy demands in the rat. Rats defend core temperature and achieve this through increased activity, thermogenesis and hyperphagia accompanied with changes to blood flow distribution [26]. We demonstrate relatively preserved absolute myocardial performance, yet when corrected for tissue mass cardiac performance appears poor. Dobutamine challenge reveals a loss of contractile reserve in CA hearts. It is unclear whether this reflects an absolute loss of β-adrenoceptors, hence desensitisation, or a loss of the ability to increase contractility [27,28]. Previous studies with cold acclimation noted enhanced catecholamine release [29] coupled with increased diurnal activity, and raised heart rate [30,31] suggesting similarities with low intensity exercise. It may thus be anticipated the loss of contractile reserve reflects desensitisation and down-regulation of β-adrenoceptors following catecholamine exposure.
Our experiment documents an increased tissue LPL activity following CA as both increased heparin-releasable LPL (functional LPL) and tissue residual LPL. Similar observations have been noted previously for cardiac muscle following CA [32,33]. By contrast, exercise was without effect on myocardial LPL protein content [34] and pregnancy led to decreased myocardial LPL activity [35]. What is not clear is the stimulus for increased LPL translocation to the endothelial surface following CA. Our hypothesis detailing enhanced VLDL-receptor expression necessary for the improved translocation of LPL to the endothelium is not proven. Indeed it is conceivable that if VLDL-receptor represents a ‘transport mechanism’ for LPL then it may not necessarily be functional at maximum capacity, however this study was not designed to test such redundancy. Other models have shown a direct correlation between VLDL-receptor expression and heparin-releasable LPL for muscle and adipose tissue as a result of glomerulosclerosis [36], showing a negative correlation between these two proteins and plasma triacylglycerol concentration. By contrast, sepsis led to decreased cardiomyocyte VLDL-receptor expression [37] yet earlier experiments document increased cardiac LPL resulting from sepsis [38]. Clearly, the coupling of VLDL-receptor with LPL is neither direct nor straightforward. It was also noted that the rate of β-oxidation for intralipid is linear for the duration of the experiment, suggestive for LPL retention at the endothelium [39].
Our data reveals that control of LPL activity is probably multifactorial. Indeed, we confirm that cold acclimation increased cardiac LPL activity, but this was not mediated through changes to AMPK/p-AMPK. Oxidative phosphorylation was thus sufficient to meet the metabolic demands of the heart for both normal and hypertrophied hearts suggesting that under these perfusion conditions AMPK does not control translocation of LPL to the heparin-releasable compartment. Few studies describe physiological changes to AMPK activity, indeed neither chronic calorie restriction nor overnight fasting altered cardiac AMPK activity in mice [40], conditions previously noted to increase cardiac heparin-releasable LPL [41]. Previous investigations exploiting pressure-overload hypertrophy document increased p-AMPK levels for hearts perfused with palmitate, while supplementation with octanoate restored levels to normal, improving mechanical performance [42]. Our experiments were of sufficient duration to observe changes to the translocation rate of LPL to the endothelium, should perfusion conditions have had an acute effect on cardiac LPL activity [43].
Of interest is the observation that for both control and CA hearts β-oxidation of FA derived from oleate or intralipid remains constant, this was further supported by the in vitro estimate of β-oxidation capacity and the analysis of m-CPT1 protein expression. This is crucial to our interpretations, as previous experiments exploiting more severe models of volume-overload hypertrophy document changes to m-CPT1 activity accompanied by preserved mitochondrial function [44]. We are therefore confident that for these experimental conditions we provide adequate lipids to fuel oxidation. The estimates for uptake and β-oxidation of lipid substrates documented here are in accordance with data collected previously for perfused working hearts [2,45]. Interestingly, the enhanced uptake of intralipid as a result of increased heparin-releasable LPL for CA hearts appears ‘channelled’ towards storage, supporting the importance for fuelling muscle contraction first whereas biosynthetic processes are of secondary importance [7]. The increase in the proportion of intralipid β-oxidised in control hearts, compared with oleate most likely reflects the slow rate of lipolysis and then transfer of intralipid-derived FA across the endothelium. This may result from the heterogeneity in intralipid emulsion particle sizes or the absence of apolipoprotein-assisted binding. The increased heparin-releasable LPL activity described for CA-hearts restored the relative proportions of intralipid oxidised and deposited as tissue lipids to levels similar to oleate implying that uptake (and hence delivery to the cardiomyocyte) is the rate-limiting step and that oxidative phosphorylation the primary sink for lipids. The increased lipid deposition into all lipid classes following CA supports the predominance of acyl-CoA synthetase 1 (ACS1) in cardiac tissue [46] but may also indicate the increased activity of glycerol phosphate acyl transferase (GPAT) enzyme in the mitochondria, a control point dividing lipid between energy provision and storage of glycerolipids [47] and increased activity of GPAT was previously described for the rat following CA [48]. This lipid accumulation may also be a protective mechanism to limit fatty acid-induced apoptosis – lipotoxicity – in the myocardium [49]. We cannot comment directly on the utilisation of endogenous sources of triacylglycerol during this experiment, however we have previously shown that myocardial deposits of triacylglycerol are small [14] and we note that CA did not alter myocardial unlabelled TAG content. Previous experiments detail a rapidly cycling small intracellular pool of triacylglycerol for cardiomyocytes that FAs are diverted into before partition between β-oxidation and tissue lipids [50].
The use of intralipid emulsions rather than chylomicrons may appear controversial due to the heterogeneity of lipid particle size and the absence of apolipoproteins E and CII (Apo E and ApoCII). However, previous experiments have documented the clearance of protein-free lipid emulsions from the plasma of rats [51]. Moreover, this was inhibited by Triton WR1339 and protamine sulphate — both treatments documented to decrease the activity of LPL [52]. In addition, particle number rather than particle size was more important for the clearance of intralipid in vivo [53]. More recent experiments describe the efficient clearance of intralipid from plasma from mice (T1/2 = 2.5 min); chemical inhibition of LPL decreased intralipid clearance by perfused hearts 82% [3]. Perfusion of mouse hearts in the absence of ApoE and ApoCII yielded LPL-dependent uptake of intralipid emulsions [54]. Intralipid increased cardiac lipid content in a dose-dependent manner and increased LPL translocation across the cardiac endothelium [39]. Therefore we are confident that for the investigation of LPL activity in situ undertaken here the use of intralipid emulsions is valid.
The choice of Langendorff perfusion over the working heart preparation was made in order to normalise the workloads placed on the perfused hearts. Given that hypertrophy of myocardium results from increased wall stress in the ventricle and is a compensatory mechanism to decrease this [55], we were unable to make accurate predictions of the afterload (‘work’) with which to challenge the hearts. The assumption was therefore made to use a fixed measured end-diastolic pressure and hence normalise the wall tension in control and CA hearts.
LPL activity was measured in separate groups of tissues to those used for metabolic investigations as we have previously shown that the composition of the perfusion medium will alter the presentation of LPL at the endothelial surface. Indeed, perfusion with triacylglycerol increased heparin-releasable LPL activity [2]. A potential shortcoming for our protocol may involve the measurement of ‘active’ LPL enzyme only. Early investigations outline a post-translational regulation for LPL activity in the hearts following fasting and refeeding [56]. Neither levels of mRNA nor protein coding for LPL were altered following fasting. The recent observation that over-expression of angiopoietin-like protein 4 (Angptl4) inhibited cardiac LPL and decreased intralipid utilisation by perfused hearts [57], coupled with the conversion of active LPL dimers into inactive monomers by Angptl4 in adipose tissue [58], demonstrates a further level of complexity for LPL regulation. The characterisation of a transcription-dependent mechanism for modulating LPL activity in the heart through conversion of active dimeric LPL to inactive monomers [59] suggests the involvement of an Angplt4-like protein. Our protocol only measures the active component of LPL secreted, therefore translocation of LPL may be continuous and the acute control of LPL activity may reside in an Angplt4-like mechanism. The experimental protocol remains valid, however, as the critical component is lipid substrate delivered to the myocardium rather than estimates of total enzyme released.
5. Concluding remarks
Cold acclimation results in a physiological hypertrophy that is characterised by preserved energetics and normal phosphorylation status for AMPK. Abundant chylomicrons that result from hyperphagia will provide an increasing contribution to substrate utilisation for the myocardium via enhanced LPL protein levels but this was not mediated through anticipated changes to either AMPK or the VLDL-receptor. It is unclear whether the activation of AMPK that results from pathological forms of hypertrophy [42] will trigger enhanced LPL translocation to the capillary endothelium [10]. In addition, enhanced VLDL-receptor expression following AMPK phosphorylation [8] may also contribute to increased lipid uptake, increasing lipid accumulation into tissue less able to metabolise FA leading to lipotoxicity [60].
Acknowledgements
The authors are grateful to the British Heart Foundation (Project Grant PG/06/007) for their financial support of this work.
References
- 1.Lopaschuk G.D., Belke D.D., Gamble J., Itoi T., Schonekess B.O. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim. Biophys. Acta. 1994;1213:263–267. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 2.Hauton D., Bennett M.J., Evans R.D. Utilisation of triacylglycerol and non-esterified fatty acid by the working rat heart: myocardial lipid substrate preference. Biochim. Biophys. Acta. 2001;1533:99–109. doi: 10.1016/s1388-1981(01)00146-9. [DOI] [PubMed] [Google Scholar]
- 3.Augustus A.S., Kako Y., Yagyu H., Goldberg I.J. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am. J. Physiol. 2003;284:E331–E339. doi: 10.1152/ajpendo.00298.2002. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien K.D., Ferguson M., Gordon D., Deeb S.S., Chait A. Lipoprotein lipase is produced by cardiac myocytes rather than interstitial cells in human myocardium. Arterioscler. Thromb. 1994;14:1445–1451. doi: 10.1161/01.atv.14.9.1445. [DOI] [PubMed] [Google Scholar]
- 5.Sambandam N., Abrahani M.A., St Pierre E., Al-Atar O., Cam M.C., Rodrigues B. Localisation of lipoprotein lipase in the diabetic heart: regulation by acute changes in insulin. Arterioscler. Thromb. Vasc. Biol. 1999;19:1526–1534. doi: 10.1161/01.atv.19.6.1526. [DOI] [PubMed] [Google Scholar]
- 6.Goudriaan J.R., Espirito-Santo S.M., Voshol P.J., Teusink B., van Dijk K.W., van Vlijmen B.J., Romijn J.A., Havekes L.M., Rensen P.C. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J. Lip. Res. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Niu Y.G., Hauton D., Evans R.D. Utilisation of triacylglycerol-rich lipoproteins by the working rat heart: routes of uptake and metabolic fates. J. Physiol. 2004;558:225–237. doi: 10.1113/jphysiol.2004.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obunike J.C., Lutz E.P., Li Z., Paka L., Katopodis T., Strickalnd D.K., Kozarsky K.F., Pillarisetti S., Goldberg I.J. Transcytosis of lipoprotein lipase across cultured endothelial cells requires both heparan sulphate proteoglycans and the very low density lipoprotein receptor. J. Biol. Chem. 2001;276:8934–8941. doi: 10.1074/jbc.M008813200. [DOI] [PubMed] [Google Scholar]
- 9.Zenimaru Y., Takahashi S., Takahashi M., Yamada K., Iwasaki T., Hattori H., Imagawa M., Ueno M., Suzuki J., Miyamori I. Glucose deprivation accelerates VLDL receptor-mediated TG-rich lipoprotein uptake by AMPK activation in skeletal muscle cells. Biochem. Biophys. Res. Comm. 2008;368:716–722. doi: 10.1016/j.bbrc.2008.01.154. [DOI] [PubMed] [Google Scholar]
- 10.An D., Kewalramani G., Qi D., Pulinilkunnil T., Ghosh S., Abrahani A., Wambolt R., Allard M., Innis S.M., Rodrigues B. B-agonist stimulation produces changes in cardiac AMPK and coronary lumen LPL only during increased workload. Am J. Physiol. 2005;288:E246–E253. doi: 10.1152/ajpendo.00588.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kudo N., Gillespie J.G., Kung L., Witters L.A., Schulz R., Clanachan A.S., Lopaschuk G.D. Characterisation of %'AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim. Biophys. Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson L.L., Kozak R., Kelly S.E., Onay-Besikci A., Russell J.C., Lopaschuk G.D. Potential mechanisms and consequences of cardiac triacylglycerol accumulation in insulin-resistant rats. Am. J. Physiol. 2003;284:E923–E930. doi: 10.1152/ajpendo.00360.2002. [DOI] [PubMed] [Google Scholar]
- 13.Deveci D., Egginton S. Differing mechanisms of cold-induced changes in capillary supply in m. tibialis anterior of rats and hamsters. J. Exp. Biol. 2002;205:829–840. doi: 10.1242/jeb.205.6.829. [DOI] [PubMed] [Google Scholar]
- 14.Hauton D., Richards S.B., Egginton S. The role of the liver in lipid metabolism during cold acclimation in non-hibernator rodents. Comp. Physiol. Biochem. Part B. 2006;144:372–381. doi: 10.1016/j.cbpb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Li G., Xiao Y., Estrella J.L., Ducsay C.A., Gilbert R.D., Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in adult rat. J. Soc. Gynecol. Invest. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Williams S.J., O'Brien D., Davidge S.T. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodelling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:E536–E545. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson-Ehle P., Schotz M.C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J. Lip. Res. 1976;17:536–541. [PubMed] [Google Scholar]
- 18.Herpin P., Vincent A., Fillaut M., Bonito B.P., Hocquette J.F. Mitochondrial and peroxisomal fatty acid oxidation capacities increase in the skeletal muscles of young pigs during early postnatal development but are not affected by cold stress. Reprod. Nutr. Dev. 2003;93:155–166. doi: 10.1051/rnd:2003013. [DOI] [PubMed] [Google Scholar]
- 19.Eghbali M., Deva R., Alioua A., Minosyan T.Y., Ruan H., Wang Y., Toro L., Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ. Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 20.Naylor L.H., George K., O'Driscoll G., Green D.J. The athlete’s heart: a contemporary appraisal of the ‘Morganroth hypothesis’. Sports Med. 2008;38:69–90. doi: 10.2165/00007256-200838010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Roukoyatkina N.I., Shefer S.I., Rifkind J., Ajmani R., Talan M.I. Cold acclimation-induced increase of systolic blood pressure in rats is associated with volume expansion. Am. J. Hypertens. 1999;12:54–62. doi: 10.1016/s0895-7061(98)00213-1. [DOI] [PubMed] [Google Scholar]
- 22.Heroux O., St Pierre J. Effect of cold acclimation on vascularisation of ears, heart, liver and muscles of white rats. Am. J. Physiology. 1956;188:163–168. doi: 10.1152/ajplegacy.1956.188.1.163. [DOI] [PubMed] [Google Scholar]
- 23.Kayar S.R., Banchero N. Volume overload hypertrophy elicited by cold and its effects on myocardial capillarity. Respir. Physiol. 1985;59:1–14. doi: 10.1016/0034-5687(85)90013-1. [DOI] [PubMed] [Google Scholar]
- 24.Konhilas J.P., Widegren U., Allen D.L., Paul A.C., Cleary A., Leinwand L.A. Loaded wheel running and muscle adaptation in the mouse. Am. J. Physiol. 2005;289:H455–H465. doi: 10.1152/ajpheart.00085.2005. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Luna P., Olea J., Herrera E. Effect of starvation on lipoprotein lipase activity in different tissues during gestation in the rat. Biochim. Biophys. Acta. 1994;1215:275–279. doi: 10.1016/0005-2760(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 26.Foster D.O., Frydman M.L. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 27.Tan L.B., Benjamin I.J., Clark W.A. Beta adrenergic desensitisation may serve a cardioprotective role. Cardiovasc. Res. 1992;26:608–614. doi: 10.1093/cvr/26.6.608. [DOI] [PubMed] [Google Scholar]
- 28.Matthews J.M., Falckh P.H., Molenaar P., Summers R.J. Chronic (−)-isoprenaline infusion down-regulates beta 1- and beta 2-adrenoceptors but does not transregulate muscarinic cholinoceptors in rat heart. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353:213–225. doi: 10.1007/BF00168760. [DOI] [PubMed] [Google Scholar]
- 29.Depocas F., Behrens W.A. Levels of noradrenaline in plasma during thermogenesis induced by cold-exposure or by noradrenaline infusion in warm- and cold-acclimated rats. Experientia Suppl. 1978;32:135–146. doi: 10.1007/978-3-0348-5559-4_15. [DOI] [PubMed] [Google Scholar]
- 30.Ishii K., Kuwahara M., Tsubone H., Sugano S. The telemetric monitoring of heart rate, locomotor activity and body temperature in mice and voles (Microtus arvalis) during ambient temperature changes. Lab. Animal. 1996;30:7–12. doi: 10.1258/002367796780744992. [DOI] [PubMed] [Google Scholar]
- 31.Chambers J.B., Williams T.D., Nakamura A., Henderson R.P., Overton J.M., Rashotte M.E. Cardiovascular and metabolic responses to hypertensive and normotensive rats to one week of cold exposure. Am. J. Physiol. 2000;279:1486–1494. doi: 10.1152/ajpregu.2000.279.4.R1486. [DOI] [PubMed] [Google Scholar]
- 32.Begin-Heick N., Heick H.M. Increased lipoprotein lipase activity of skeletal muscle in cold-acclimated rats. Can. J. Biochem. 1977;55:1241–1243. doi: 10.1139/o77-186. [DOI] [PubMed] [Google Scholar]
- 33.Bertin R., Goubern M., Portet R. Effects of diets and cold acclimation on lipoprotein lipase activity and cyclic nucleotide levels in some tissues of rats. Experientia Suppl. 1978;32:185–190. doi: 10.1007/978-3-0348-5559-4_20. [DOI] [PubMed] [Google Scholar]
- 34.Ong J.M., Simsolo R.B., Saghizadeh M., Goers J.W., Kern P.A. Effects of exercise training and feeding on lipoprotein lipase gene expression in adipose tissue, heart, and skeletal muscle of the rat. Metabolism. 1995;44:1596–1605. doi: 10.1016/0026-0495(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Luna P., Olea J., Herrera E. Effect of starvation on lipoprotein lipase activity in different tissues during gestation in the rat. Biochim. Biophys. Acta. 1994;1215:275–279. doi: 10.1016/0005-2760(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 36.Sato T., Liang K., Vaziri N.D. Down-regulation of lipoprotein lipase and VLDL receptor in rats with focal glomerulosclerosis. Kid. Int. 2002;67:157–162. doi: 10.1046/j.1523-1755.2002.00104.x. [DOI] [PubMed] [Google Scholar]
- 37.Jia L., Takahashi M., Morimoto H., Takahashi S., Izawa A., Ise H., Iwasaki T., Hattori H., Wu K.J., Ikeda U. Changes in cardiac lipid metabolism during sepsis: the essential role of very-low-density lipoprotein receptors. Cardiovasc. Res. 2006;69:545–555. doi: 10.1016/j.cardiores.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Bennett M.J., Hauton D., Hole D.G., Evans R.D. Utilisation of very low density lipoprotein by the rat heart: the effect of endotoxin. Am. J. Physiol. 2000;278:E802–E810. doi: 10.1152/ajpendo.2000.278.5.E802. [DOI] [PubMed] [Google Scholar]
- 39.Qi D., Kuo K.H., Abrahani A., An D., Qi Y., Heung J., Kewalramani G., Pulinilkunnil T., Ghosh S., Innis S.M., Rodrigues B. Acute intralipid infusion reduces cardiac luminal lipoprotein lipase but recruits additional enzyme from cardiomyocytes. Cardiovasc. Res. 2006;72:124–133. doi: 10.1016/j.cardiores.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez A.A., Kumar R., Mulligan J.D., Davis A.J., Weindruch R., Saupe K.W. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle and liver do not include changes to AMPK activity. Am. J. Physiol. 2004;287:E1032–E1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- 41.Ruge T., Bergo M., Hultin M., Olivecrona G., Olivecrona T. Nutritional regulation of binding sites for lipoprotein lipase. Am. J. Physiol. 2000;278:E211–E218. doi: 10.1152/ajpendo.2000.278.2.E211. [DOI] [PubMed] [Google Scholar]
- 42.Allard M., Parsons H.L., Saeedi R., Wambolt R.B., Brownsey R. AMPK and metabolic adaptation by the heart to pressure-overload. Am. J. Physiol. 2007;292:140–148. doi: 10.1152/ajpheart.00424.2006. [DOI] [PubMed] [Google Scholar]
- 43.Liu G.Q., Olivecrona T. Pulse-chase study on lipoprotein lipase in perfused guinea pig heart. Am. J. Physiol. 1991;261:H2044–H2050. doi: 10.1152/ajpheart.1991.261.6.H2044. [DOI] [PubMed] [Google Scholar]
- 44.el Alaoui-Talibi Z., Landormy S., Loireau A., Moravec J. Fatty acid oxidation and mechanical performance of volume-overloaded rat hearts. A. J. Physiol. 1992;262:H1068–H1074. doi: 10.1152/ajpheart.1992.262.4.H1068. [DOI] [PubMed] [Google Scholar]
- 45.Saeedi R., Parsons H.L., Wambolt R.B., Paulson K., Sharma V., Dyck J.R.B., Brownsey R.W., Allard M.F. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am. J. Physiol. 2008 doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- 46.de Jong H., Neal A.C., Coleman R.A., Lewin T.A. Ontogeny of mRNA expression and activity of long-chain acyl-CoA synthetase (ASCL) isoforms in Mus muscles heart. Biochim. Biophys. Acta. 2007;1771:75–82. doi: 10.1016/j.bbalip.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammond L.E., Gallagher P.A., Wang S., Hiller S., Kluckman K.D., Posey-Marcos E.L., Maeda N., Coleman R.A. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol. Cell. Biol. 2002;22:8204–8214. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darnley A.C., Carpenter C.A., Saggerson E.D. Changes in activities of some enzymes of glycerolipid synthesis in brown adipose tissue of cold-acclimated rats. Biochem. J. 1988;253:351–355. doi: 10.1042/bj2530351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Listenberger L.L., Han X., Lewis S.E., Cases S., Farese R.V., Jr., Ory D.S., Schaffer J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saddik M., Lopaschuk G.D. Myocardial triglyceride turnover and contribution to energy utilisation in isolated working rat hearts. J. Biol. Chem. 1991;266:8162–8170. [PubMed] [Google Scholar]
- 51.Oliveira H.C., Hirata M.H., Redgrave T.G., Maranhao R.C. Competition between chylomicrons and their remnants for plasma removal: a study with artificial emulsions models of chylomicrons. Biochim. Biophys. Acta. 1998;958:211–217. doi: 10.1016/0005-2760(88)90179-8. [DOI] [PubMed] [Google Scholar]
- 52.Hirata H.M., Oliveira H.C., Quintao E.C., Redgrave T.G., Maranhao R.C. The effects of Triton WR-1339, protamine sulphate and heparin on the plasma removal of emulsion models of chylomicrons and remnants in rats. Biochim. Biophys. Acta. 1987;917:344–346. doi: 10.1016/0005-2760(87)90141-x. [DOI] [PubMed] [Google Scholar]
- 53.Martins I.J., Mortimer B.C., Miller J., Redgrave T.G. Effects of particle size and number on the plasma clearance of chylomicrons and remnants. J. Lipid Res. 1996;37:2696–2705. [PubMed] [Google Scholar]
- 54.Pillutla P., Hwang Y.C., Augustus A., Yokoyama M., Yagyu H., Johnston T.P., Kaneko M., Ramasamy R., Goldberg I.J. Perfusion of hearts with triglyceride-rich particles reproduces the metabolic abnormalities in lipotoxic cardiomyopathy. Am. J. Physiol. 2005;288:E1229–E1235. doi: 10.1152/ajpendo.00273.2004. [DOI] [PubMed] [Google Scholar]
- 55.Yamakawa H., Imamura T., Matsuo T., Onitsuka H., Tsumori Y., Kato J., Kitamura K., Koiwaya Y., Eto T. Diastolic wall stress and ANGII in cardiac hypertrophy and gene expression induced by volume overload. Am. J. Physiol. 2000;279:H2939–H2946. doi: 10.1152/ajpheart.2000.279.6.H2939. [DOI] [PubMed] [Google Scholar]
- 56.Doolittle M.H., Ben-Zeev O., Elovson J., Martin D., Kirchgessner T.G. The response of lipoprotein lipase to feeding and fasting. Evidence for posttranslational regulation. J. Biol. Chem. 1990;265:4570–4577. [PubMed] [Google Scholar]
- 57.Yu X., Bugess S.C., Ge H., Wong K.K., Nassem R.H., Garry D.J., Sherry A.D., Mallory C.R., Berger J.P., Li C. Inhibition of cardiac lipoprotein utilisation by transgenic overexpression of Angptl4 in the heart. Proc. Nat. Acad. Sci. 2005;102:1767–1772. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sukonina V., Lookene A., Olivecrona T., Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Nat. Acad. Sci. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.G. Wu, L. Zhang, J. Gupta, G. Olivecrona, T. Olivecrona. A transcription-dependent mechanism, akin to that in adipose tissue, modulates lipoprotein lipase activity in rat heart. Am. J. Physiol. 293 (207) E908–E925. [DOI] [PubMed]
- 60.Sharma S., Adrogue J.V., Golfman L., Uray I., Lemm J., Youker K., Noon G.P., Frazier O.H., Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]