Abstract
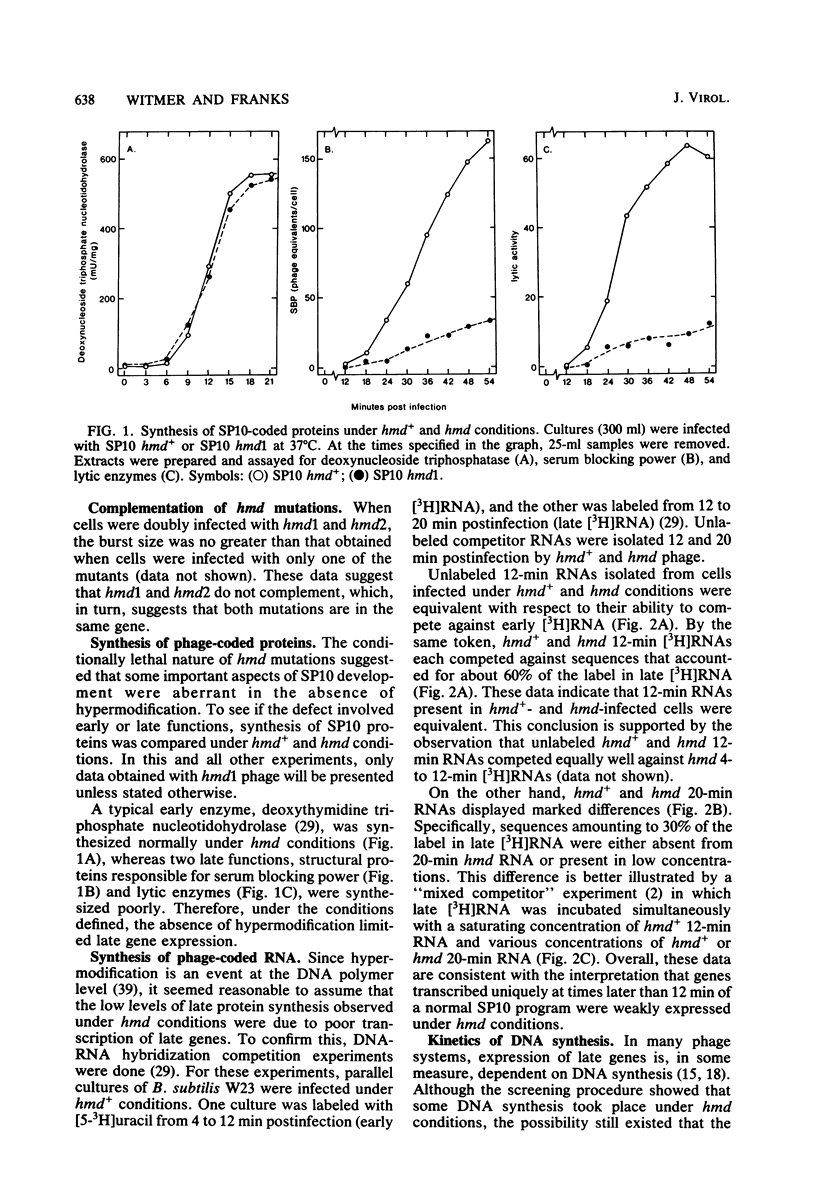
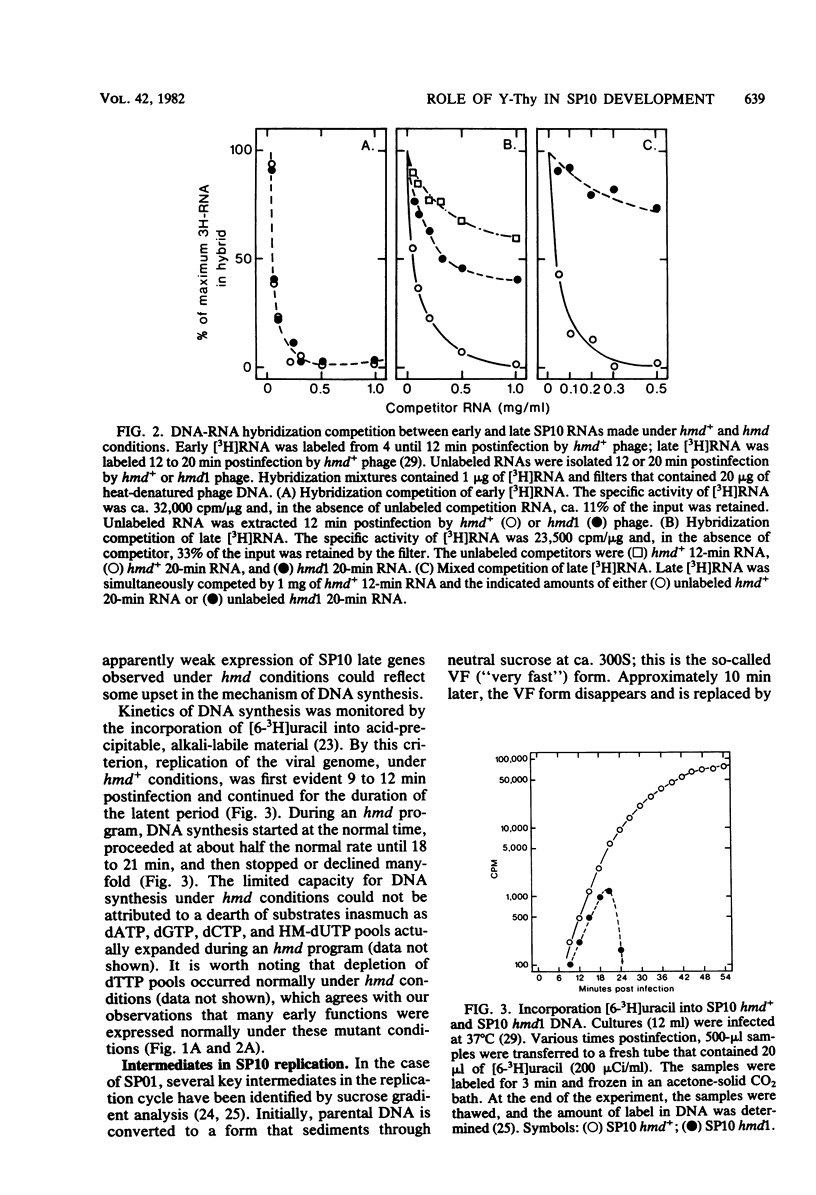
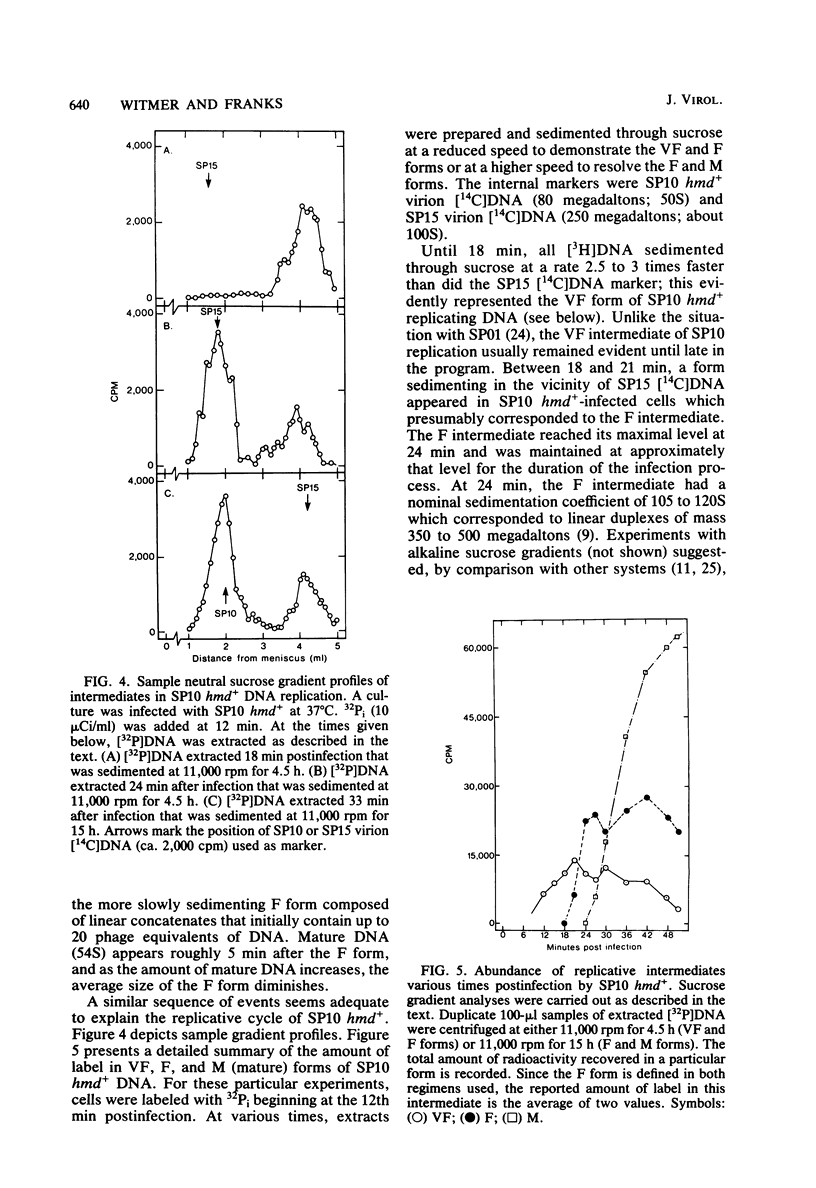
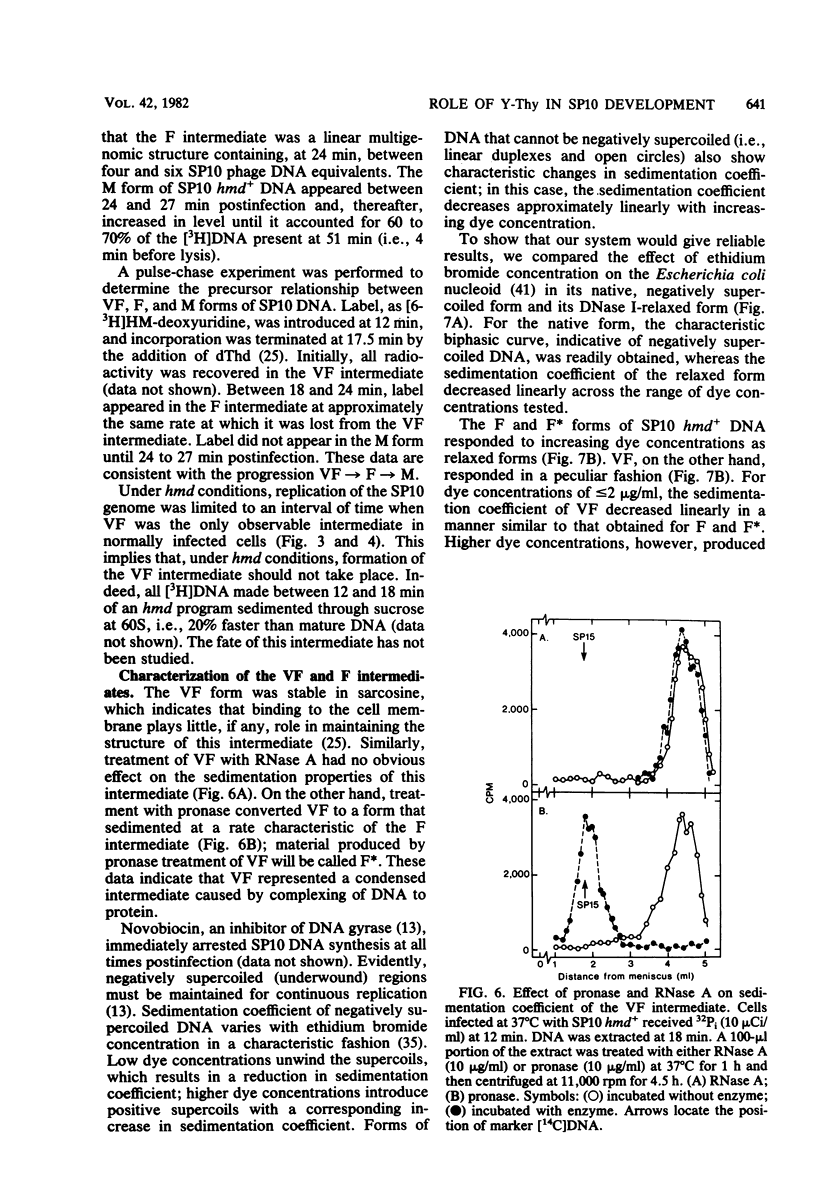
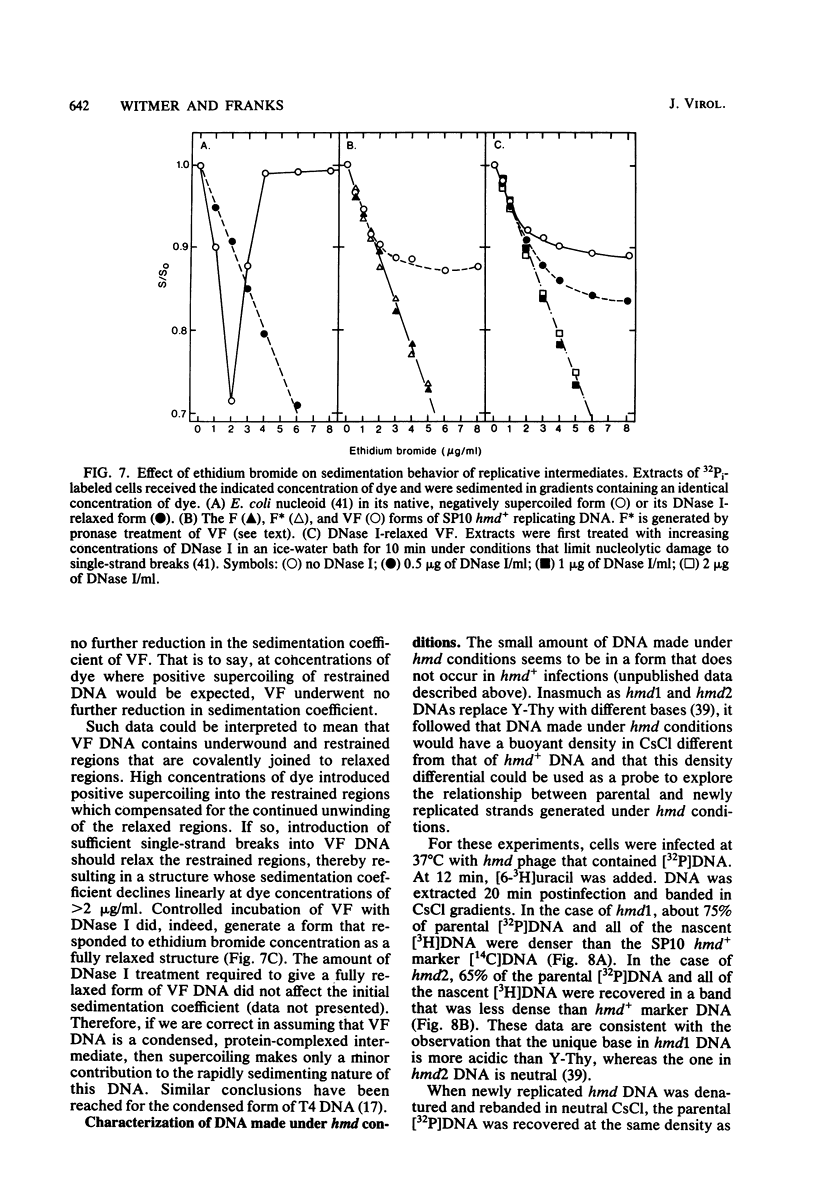
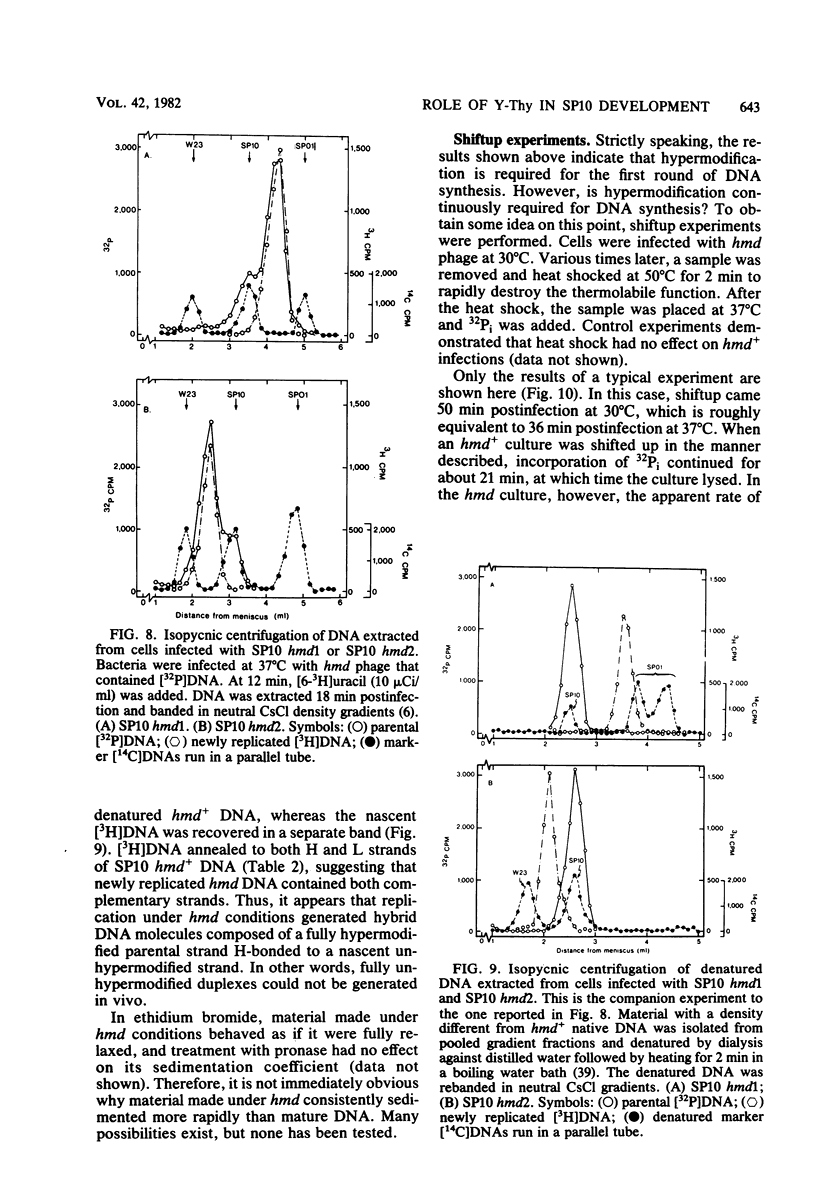
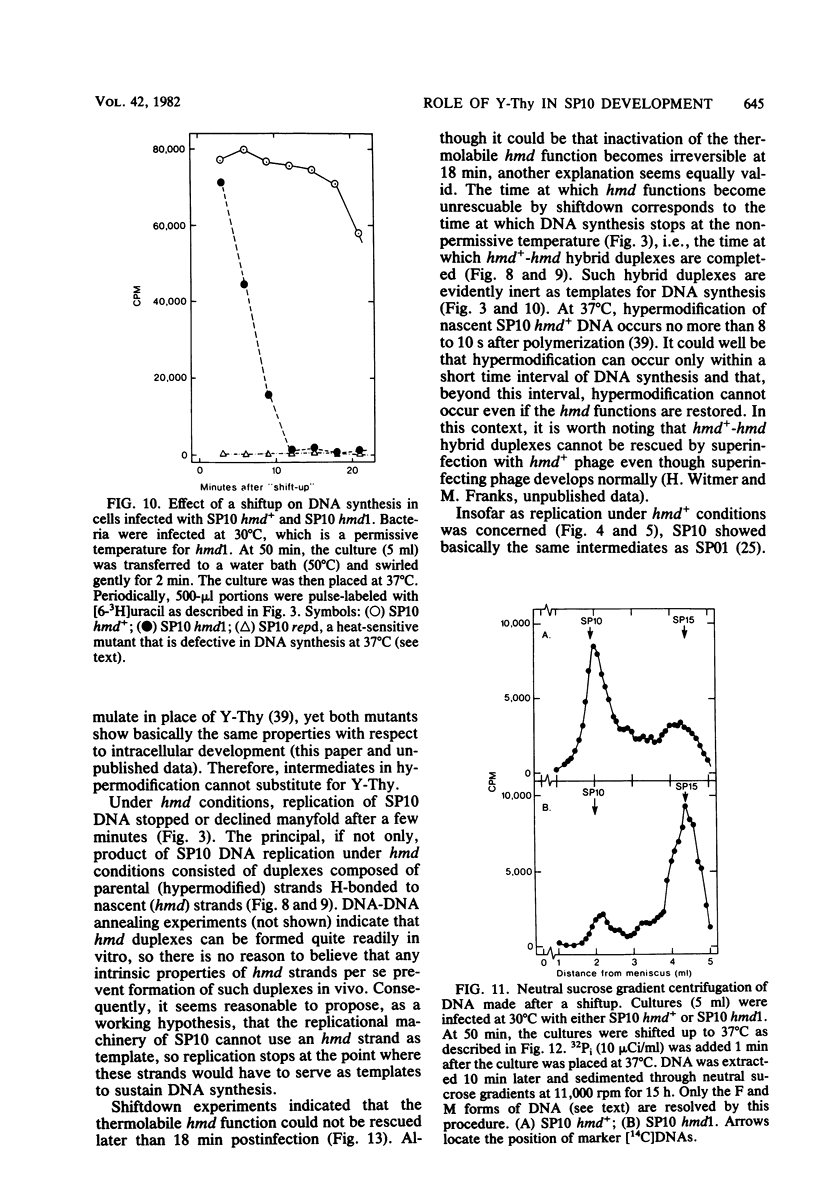
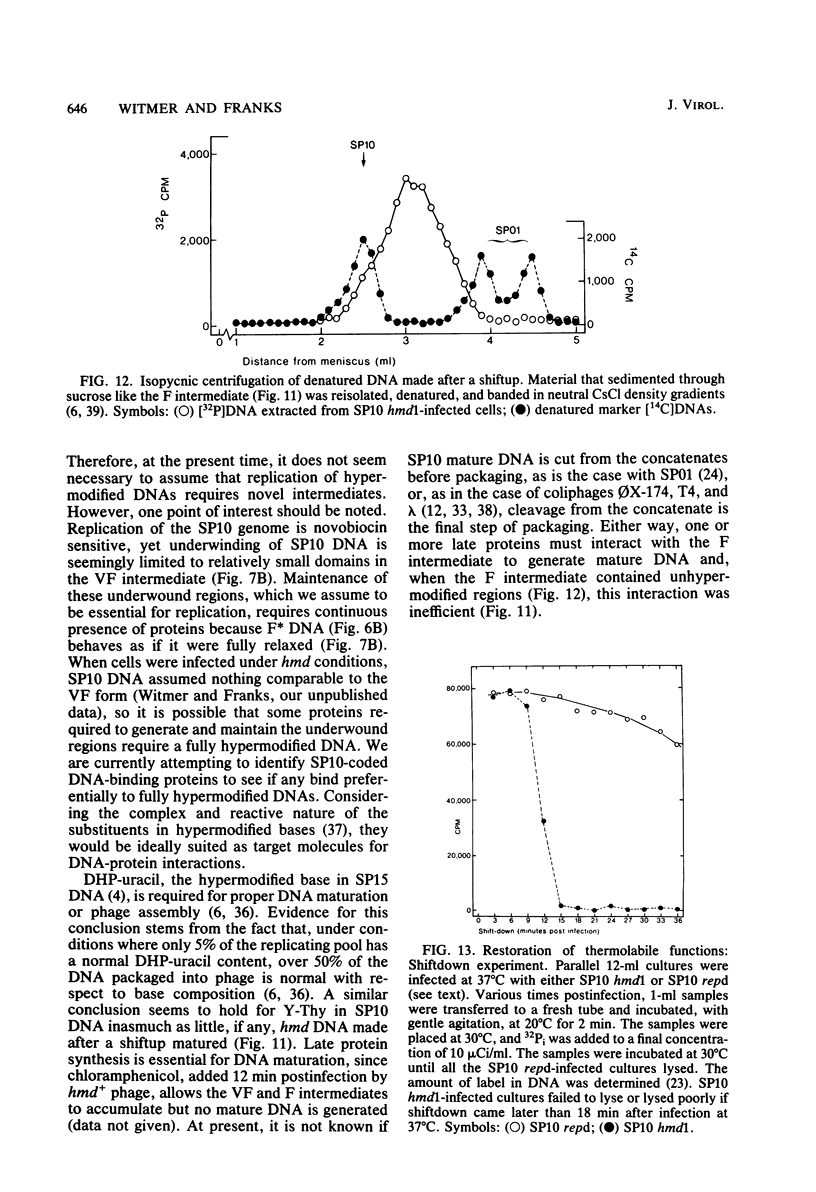
A hypermodified base (Y-Thy) replaces 20% of the thymine (Thy) in mature DNA of Bacillus subtilis phage SP10. Two noncomplementing hypermodification-defective (hmd) mutants are described. At 30°C, hmd phage carried out a normal program, but at temperatures of ≥37°C, the infection process was nonproductive. When cells were infected at 37°C with hmd phage, DNA synthesis started at its usual time (12 min), proceeded at about half the normal rate for 6 to 8 min, and then stopped or declined manyfold. All, or nearly all, of the DNA made under hmd conditions consisted of fully hypermodified parental DNA strands H-bonded to unhypermodified nascent strands. The reduced levels of DNA synthesis observed under hmd conditions were accompanied by weak expression of late genes. A sucrose gradient analysis of SP10 hmd+ replicating DNA intermediates was made. Two intermediates, called VG and F, were identified. VF consisted of condensed DNA complexed to protein; VF also contained negatively supercoiled domains covalently joined to relaxed regions. F was composed of linear concatenates from which mature DNA was cleaved. None of those intermediates was evident in cells infected at 37°C with hmd phage. Shiftup experiments were performed wherein cells infected with hmd phage at 30°C were shifted to 37°C at a time when replication was well under way. DNA synthesis stopped or declined manyfold 10 min after shiftup. The hmd DNA made after shiftup was conserved as a form sedimentationally equivalent to the F intermediate, but little mature DNA was evident. It is proposed that Y-Thy is required for replication and DNA maturation because certain key proteins involved with these processes interact preferentially with hypermodified DNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alegria A. H. Hydroxymethylation of pyrimidine mononucleotides with formaldehyde. Biochim Biophys Acta. 1967 Dec 19;149(2):317–324. doi: 10.1016/0005-2787(67)90159-1. [DOI] [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Brandon C., Gallop P. M., Marmur J., Hayashi H., Nakanishi K. Structure of a new pyrimidine from Bacillus subtilis phage SP-15 nucleic acid. Nat New Biol. 1972 Sep 20;239(90):70–71. doi: 10.1038/newbio239070a0. [DOI] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella E., Markewych O., Dosmar M., Witmer H. Production and expression of dTMP-enriched DNA of bacteriophage SP15. J Virol. 1978 Dec;28(3):753–766. doi: 10.1128/jvi.28.3.753-766.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MARS R. I. The production of phage-related materials when bacteriophage development in interrupted by proflavine. Virology. 1955 May;1(1):83–99. doi: 10.1016/0042-6822(55)90007-6. [DOI] [PubMed] [Google Scholar]
- Donelli G., Dore E., Frontali C., Grandolfo M. E. Structure and physico-chemical properties of bacteriophage G. III. A homogeneous DNA of molecular weight 5 times 10(8). J Mol Biol. 1975 Jun 5;94(4):555–565. doi: 10.1016/0022-2836(75)90321-6. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Glassberg J., Slomiany R. A., Stewart C. R. Selective screening procedure for the isolation of heat- and cold-sensitive, DNA replication-deficient mutants of bacteriophage SPO1 and preliminary characterization of the mutants isolated. J Virol. 1977 Jan;21(1):54–60. doi: 10.1128/jvi.21.1.54-60.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Szybalski W. Fractionation of the complementary strands of coliphage T4 DNA based on the asymmetric distribution of the poly U and poly U,G binding sites. Virology. 1968 Apr;34(4):608–616. doi: 10.1016/0042-6822(68)90082-2. [DOI] [PubMed] [Google Scholar]
- Hamilton S., Pettijohn D. E. Properties of condensed bacteriophage T4 DNA isolated from Escherichia coli infected with bacteriophage T4. J Virol. 1976 Sep;19(3):1012–1027. doi: 10.1128/jvi.19.3.1012-1027.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman R., Goldberg E. B. A genetic assay for mRNA's of phage T4. Proc Natl Acad Sci U S A. 1969 Sep;64(1):198–204. doi: 10.1073/pnas.64.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan E. A genetic study of temperature-sensitive mutants of the subtilis phage SP82. Virology. 1966 Dec;30(4):650–660. doi: 10.1016/0042-6822(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legault-Demare L., Chambliss G. H. Natural messenger ribonucleic acid-directed cell-free protein-synthesizing system of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1300–1307. doi: 10.1128/jb.120.3.1300-1307.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- Levner M. H., Cozzarelli N. R. Replication of viral DNA in SPO1-infected Bacillus subtilis. I. Replicative intermediates. Virology. 1972 May;48(2):402–416. doi: 10.1016/0042-6822(72)90051-7. [DOI] [PubMed] [Google Scholar]
- Levner M. H. Replication of viral DNA in SPO1-infected Bacillus subtilis. II. DNA maturation during abortive infection. Virology. 1972 May;48(2):417–429. doi: 10.1016/0042-6822(72)90052-9. [DOI] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Lewis H. A., Warren R. A. Synthesis of thymine and alpha-putrescinylthymine in bacteriophage phi W-14-infected Pseudomonas acidovorans. J Virol. 1980 May;34(2):354–359. doi: 10.1128/jvi.34.2.354-359.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Newlon M. C. Control of DNA synthesis in Bacillus subtilis by phage phi e. Virology. 1971 Apr;44(1):83–93. doi: 10.1016/0042-6822(71)90155-3. [DOI] [PubMed] [Google Scholar]
- Markewych O., Boghosian A., Dosmar M., Ende D., Witmer H. SP-10 bacteriophage-specific nucleic acid and enzyme synthesis in Bacillus subtilis W23. J Virol. 1977 Jan;21(1):84–95. doi: 10.1128/jvi.21.1.84-95.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Maltman K. L., Warren R. A. Bacteriophage phi W-14-infected Pseudomonas acidovorans synthesizes hydroxymethyldeoxyuridine triphosphate. J Virol. 1980 May;34(2):347–353. doi: 10.1128/jvi.34.2.347-353.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. M., Brown N. C. Inhibition of a discrete bacterial DNA polymerase by 6-(p-hydroxyphenylazo)-uracil and 6-(p-hydroxyphenylazo-)-isocytosine. Nat New Biol. 1972 Nov 15;240(98):80–82. doi: 10.1038/newbio240080a0. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. S., Mandel M. Biosynthesis of 5-(4'5'-dihydroxypentyl) uracil as a nucleoside triphosphate in bacteriophage SP15-infected Bacillus subtilis. J Virol. 1978 Feb;25(2):500–509. doi: 10.1128/jvi.25.2.500-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. A. Modified bases in bacteriophage DNAs. Annu Rev Microbiol. 1980;34:137–158. doi: 10.1146/annurev.mi.34.100180.001033. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Bartl P., Salzman L. A. The structure of replicative lambda DNA--electron microscope studies. Cold Spring Harb Symp Quant Biol. 1968;33:525–531. doi: 10.1101/sqb.1968.033.01.060. [DOI] [PubMed] [Google Scholar]
- Witmer H. Synthesis of deoxythymidylate and the unusual deoxynucleotide in mature DNA of Bacillus subtilis bacteriophage SP10 occurs by postreplicational modification of 5-hydroxymethyldeoxyuridylate. J Virol. 1981 Aug;39(2):536–547. doi: 10.1128/jvi.39.2.536-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Wu R., Geiduschek E. P. The role of replication proteins in the regulation of bacteriophage T4 transcription. I. Gene 45 and hydroxymethyl-C-containing DNA. J Mol Biol. 1975 Aug 25;96(4):513–538. doi: 10.1016/0022-2836(75)90137-0. [DOI] [PubMed] [Google Scholar]


