Abstract
The most common type of ultrasound contrast agents are encapsulated microbubbles, typically 1–5 microns in diameter. These microbubbles are injected into the bloodstream to provide image enhancement during an ultrasound exam. Due to their compressibility, these microbubbles are inherently sensitive to changes in pressure. For imaging, this is beneficial in that these microbubbles oscillate in an acoustic field and allow imaging systems to detect their response uniquely from tissue. However, this sensitivity also means that microbubbles can be readily destroyed by significant hydrostatic pressure. Injection of these microbubbles through a small-gauge catheter, such as sometimes performed in small animal imaging studies, can result in microbubble destruction. In this manuscript, the effects of microbubble injection through catheters of varying diameter are examined. Our results indicate that the concentration and size distribution of microbubbles can be substantially altered in cases of rapid injection through small needles.
Keywords: microbubble, contrast agent, size distribution, ultrasound, molecular imaging
INTRODUCTION
Ultrasound contrast agents (UCAs) are currently being used in biomedical applications such as radiology and cardiology (Goldberg et al. 2001). These contrast agents are micron-sized gas bubbles which are injected intravascularly during an imaging exam. In response to an acoustic pulse, microbubble contrast agents resonate at a diagnostic ultrasound frequency and improve the contrast between blood and tissue (Villanueva et al. 1995); (Lang et al. 2006), or highlight disease or tissue injury (Lindner 2004); (Dayton et al. 2002). A thin shell of lipid, protein, or polymer is placed around the gas core to stabilize these microbubbles, as unencapsulated gas would diffuse in milliseconds (Dayton et al. 1999). Although first-generation agents utilized an air or nitrogen core, current microbubble agents are typically filled with a high-molecular weight gas such as a perfluorocarbon due to lower gas solubility.
One of the characteristics of a microbubble contrast agent which makes it highly echogenic is the ability to resonate in an acoustic pressure field. This property is directly related to the shell rigidity (Postema et al. 2007). Commercially available agents produce a strong scattered echo either by oscillating with an intact flexible shell (Chomas et al. 2002); (de Jong et al. 2002), or by bursting, releasing a free gas bubble which oscillates before it dissolves (Bouakaz et al. 2005); (Chomas et al. 2001); (Frinking et al. 2001). Since these agents are sensitive to an acoustic pressure field, they are also sensitive to a hydrostatic pressure field. A large change in hydrostatic pressure will burst many of the microbubbles.
Contrast agent microbubbles are being increasingly used in small animal imaging, particularly with the development of new high-resolution pre-clinical imaging systems (Foster et al. 2002) and the advancement of molecular imaging (Lindner 2004). Although some small researchers utilize jugular vein cannulation for contrast administration (Ellegala et al. 2003), this technique is more complicated and difficult for serial studies. For contrast injection into small animals, or occasionally in in-vitro studies, many researchers utilize small gauge catheters/needles with tail vein injection which can result in a large hydrostatic pressure differential during injection. The literature reports examples of 24 gauge needles used for injection into rat tail veins (Miller et al. 2007), 27 gauge needles utilized for tail vein injection into mice (Howard et al. 2006), and 30 gauge needles used for contrast studies of the lymphatic system (Wisner et al. 2002). Often, researchers do not report infusion rate, although some reported rates include 0.5 mL/kg/min into rats (Miller et al. 2007), as well as “bolus” injection into mice and rats (Choi et al. 2007) (Stieger et al. 2006).
In this study, we examine the sensitivity of lipid-shelled microbubbles to injection through various gauge needles to determine thresholds for contrast injection with minimal bubble disruption. We examine flow rates from 0.6 ml/minute to 0.5 ml/second to simulate ranges from fast infusion to rapid bolus injection. In addition to catheter size the injection rates, we consider microbubble concentration, as it is known that microbubble contrast agents are more stable in a high concentration. Dilutions of lipid shelled microbubble contrast agents utilized for small animal imaging reported in the literature cover a wide range, including ~1:10 to ~1:50 (Miller et al. 2007, Stieger et al. 2006).
MATERIALS AND METHODS
Lipid-encapsulated microbubble contrast agents were prepared as previously described (Borden et al. 2002). Briefly, 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1, 2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Methoxy(Polyethylene glycol)-2000] (DSPE-PEG2000) (Avanti Polar Lipids; Alabaster, AL) were combined at a molar ratio of 9:1, and suspended in a 0.1 M Tris buffer (Sigma-Aldrich Corp.; Louis, MO). The solution was placed into sonic bath for 45 min and then aliquoted into gas tight vials to which decafluorobutane gas was added (SynQuest Labs; Alachua, FL). Microbubbles were formed by shaking method using a CAPMIX machine (ESPE; Seefeld, Germany) for 45 s.
Microbubble concentrations of approximately 1 · 109, 5 · 108 and 1 · 108 microbubble/ml (equivalent to ~1:10 to ~1:100 for some lipid-encapsulated perfluorocarbon agents (http://www.definityimaging.com/pdf/prescribinginfo.pdf) were used. 23, 27 and 30 gauge needles were used with an addition of 6” polyethylene tubing with diameters matching the needle gauge. 5 different flow rates (0.01, 0.03, 0.1, 0.3, and 0.5 ml/sec) were provided by a high precision Harvard Apparatus (Holliston, MA) syringe pump. The microbubble concentration and size distribution before and after the injection of the microbubbles were measured with an Accusizer (Particle Sizing Systems Inc.; Santa Barbara, CA). All experiments were repeated three times.
RESULTS
The size distribution of microbubbles before injection was approximately 0.95 µm ± 0.75 µm for all concentrations. Results are separated according to injection needle size.
23-gauge needle
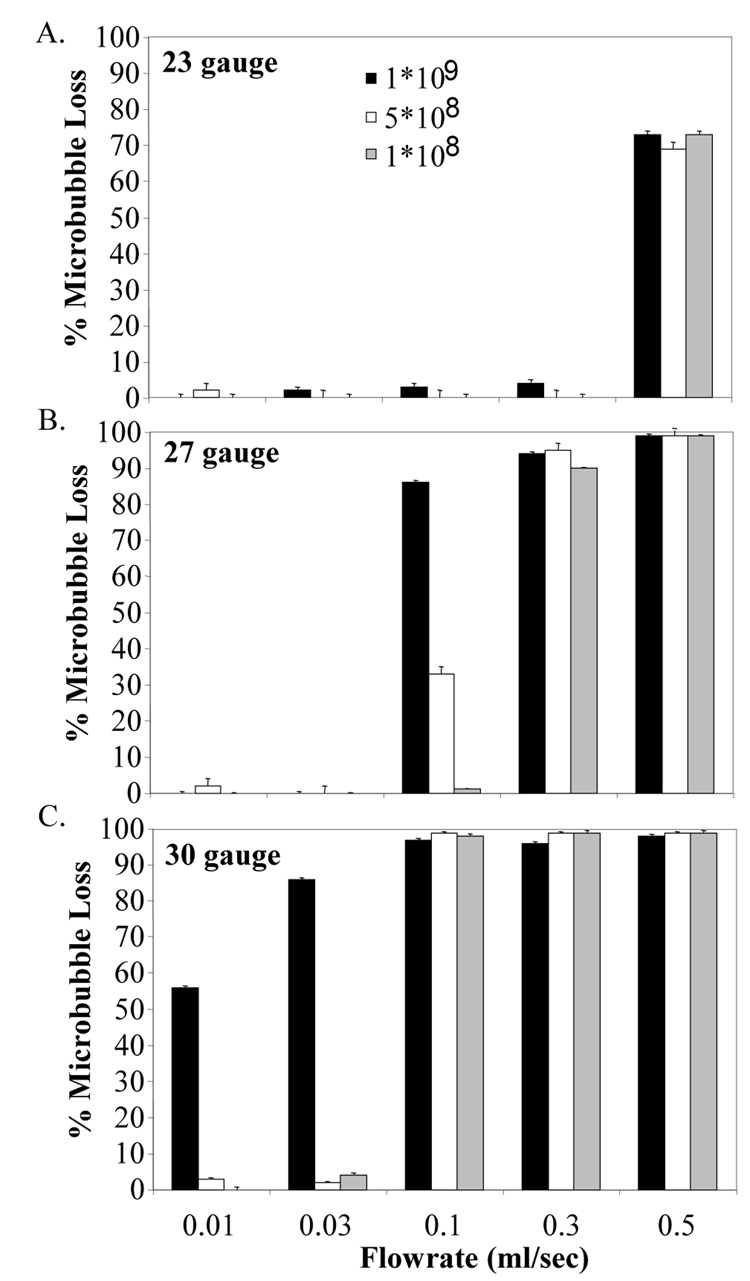
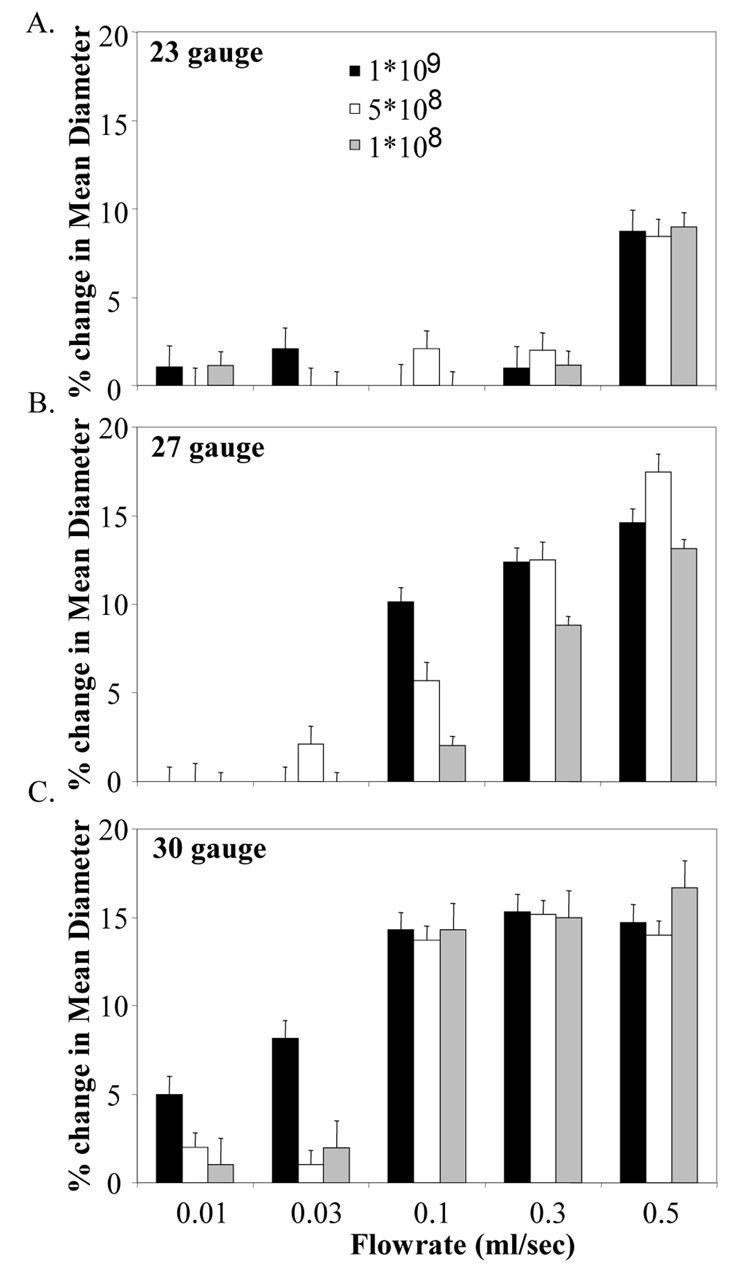
Injection through the 23-gauge needle did not affect either the microbubble concentration or the size distribution except for the highest flowrate tested. At a flowrate of 0.5 ml/sec, the percentage of microbubbles destroyed during injection was 70 ± 2 % (Figure 1A) and the mean diameter of the size distribution was reduced by 10 ± 1 % (Figure 2A) for all concentrations.
Figure 1.
% Microbubble destruction during infusion through a catheter at as a function of flow rate, concentration, and needle gauge A) 23-gauge, B) 27-gauge, C) 30-gauge.
Figure 2.
% change in mean diameter of microbubble population due to injection as a function of flow rate, concentration, and needle gauge A) 23-gauge, B) 27-gauge, C) 30-gauge.
27-gauge needle
When 27-gauge needle was used at a 1 · 108 microbubble/ml concentration, more than 85 ± 2 % of the microbubbles were destroyed for flowrates higher than 0.1 ml/sec. At a microbubble concentration of 5 · 108 microbubble/ml, only 30 ± 3 % microbubble loss was observed for 0.1 ml/sec; however, further increase in the flowrate above 0.3 ml/sec resulted in more than 95 % microbubble loss. At a microbubble concentration of 1 · 108 microbubble/ml, no destruction was observed up to 0.1 ml/sec, but 82 ± 3 % and 98 ± 2 % microbubble destruction was observed for 0.3 and 0.5 ml/sec, respectively (Figure 1B).
The mean diameter of the size distribution dropped more than 10 % with the use of 27-gauge needle at all microbubble concentrations when the flowrate was more than 0.3 ml/sec. At a flowrate of 0.1 ml/sec, decrease in mean diameter was observed to be 2, 6 and 10 % at 1 · 109, 5 · 108 and 1 · 108 microbubble/ml, respectively (Figure 2B).
30-gauge needle
The 30-gauge needle was observed to be destructive to microbubbles at all flow rates tested at 1 · 108 microbubble/ml concentration. More than 55 % of the microbubbles were destroyed at 0.01 ml/sec, and 96 % of the microbubbles were lost for flowrates 0.1 ml/sec and above. With the use of the 30-gauge needle at both 1 · 109 and 5 · 108 microbubble/ml concentration, 99 % of the microbubbles were destroyed at flowrates of more 0.1 ml/sec (Figure 1C).
Approximately 15 % reduction in size distribution was observed at all microbubble concentrations at flowrates above 0.1 ml/sec (Figure 2C). However, at these flow rates – there were very few contrast agents remaining after being pumped through the catheter.
DISCUSSION
Our results indicate that injection of contrast through a 23-gauge needle was relatively non-destructive to microbubble contrast agents for all concentrations tested at flow rates up to 300 microliters/sec, which would be a very rapid bolus injection. In contrast, we observed that injection through a 30-gauge needle was very destructive (>50% loss in concentration) for contrast agents of 1 · 108 bubbles/ml concentration at all flow rates tested, and very destructive for 5 · 108 and 1 · 109 bubbles/ml at bolus injection rates > 30 microliters per second.
In addition to destruction of microbubbles, a shift in the mean diameter of the population towards a smaller diameter was also observed for cases where microbubble loss during injection was observed. This likely indicated either that large bubbles were destroyed preferentially, or the population diameter was reduced, possibly by forced compression and resulting gas loss. Further investigations will elucidate specific mechanisms for these changes.
This study was largely limited by the fact that only one type of lipid-shelled contrast agent was tested. However, we anticipate similar trends for the stability of other microbubble type contrast agents in response to injection parameters.
Maintaining consistency in microbubble population is important in contrast enhanced ultrasound, particularly in applications where contrast is used for quantitative measurement. This is complicated further by the fact that the contrast intensity is not always a linear function of the microbubble concentration (Porter et al. 2003). One application where experimental consistency might be particularly sensitive to administered concentration is ultrasonic molecular imaging, where intensity of contrast agents retained at a target site provides information as to the expression of a specific molecular marker.
Additionally, microbubble concentration has also been found to play an important role in therapeutic applications, where microbubble concentration has been shown to be related to efficiency of gene transfection (Zhao et al. 2007) and localized heating (Razansky et al. 2006).
Microbubble concentration is also related to the likelihood of bioeffects, where higher concentrations are related to increased cavitation (Tu et al. 2006), and differences in the amount of cavitation based effects may be statistically significant at concentration differences as low as 1% (Sassaroli and Hynynen 2007). Part of this mechanism may be due to the formation of larger bubbles as single smaller microbubbles aggregate and then fuse due to secondary radiation force, which is dependent on concentration (Caskey et al. 2007).
Additionally, microbubble size distribution effects contrast agent behavior. Almost all properties of a microbubble contrast agent are related to diameter, including echogenicity and resonant frequency (de Jong et al. 2002, Morgan et al. 2000), susceptibility to radiation force (Dayton et al. 2002), and potential biodistribution (Talu et al 2008). Thus, size distribution should be maintained in order to preserve experimental or diagnostic consistency.
CONCLUSIONS
Lipid-coated and perfluorocarbon-filled microbubbles are known to be stable ultrasound contrast agents. We have shown that the stability of these microbubbles during a contrast injection depends on needle size, contrast agent concentration, and injection rate. We have illustrated several combinations of parameters which are non-destructive for contrast injection, and parameters which will destroy a large majority of the injected dose. Our results indicate that researchers using contrast injection through a needle or catheter should pay specific attention to the injection conditions in an imaging study to avoid deleterious affects on the microbubble contrast agents and to maintain experimental consistency.
Acknowledgements
This work is funded by the National Institutes of Health through the NIH Roadmap for Medical Research (Grant R21 EB005325).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Borden MA, Longo ML. Dissolution behavior of lipid monolayer-coated, air-filled microbubbles: effect of lipid hydrophobic chain length. Langmuir. 2002;18:9225–9233. [Google Scholar]
- Bouakaz A, Versluis M, de Jong N. High-speed optical observations of contrast agent destruction. Ultrasound Med Biol. 2005;31:391–399. doi: 10.1016/j.ultrasmedbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med Biol. 2007;33:95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Chomas J, Dayton P, May D, Ferrara K. Nondestructive subharmonic imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:883–892. doi: 10.1109/tuffc.2002.1020158. [DOI] [PubMed] [Google Scholar]
- Chomas JE, Dayton P, May D, Ferrara K. Threshold of fragmentation for ultrasonic contrast agents. J Biomed Opt. 2001;6:141–150. doi: 10.1117/1.1352752. [DOI] [PubMed] [Google Scholar]
- Dayton PA, Ferrara KW. Targeted imaging using ultrasound. J Magn Reson Imaging. 2002;16:362–377. doi: 10.1002/jmri.10173. [DOI] [PubMed] [Google Scholar]
- Dayton PA, Morgan KE, Klibanov AL, Brandenburger GH, Ferrara KW. Optical and acoustical observations of the effects of ultrasound on contrast agents. Ieee T Ultrason Ferr. 1999;46:220–232. doi: 10.1109/58.741536. [DOI] [PubMed] [Google Scholar]
- Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. Journal of the Acoustical Society of America. 2002;112:2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- de Jong N, Bouakaz A, Frinking P. Basic acoustic properties of microbubbles. Echocardiography. 2002;19:229–240. doi: 10.1046/j.1540-8175.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- Ellegala DB, Leong-Poi H, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME, Sklenar J, Lindner JR. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108:336–341. doi: 10.1161/01.CIR.0000080326.15367.0C. [DOI] [PubMed] [Google Scholar]
- Foster FS, Zhang MY, Zhou YQ, Liu G, Mehi J, Harasiewicz KA, Starkoski GB, Zan L, Knapik DA, Adamson SL. A new Ultrasound Instrument for in vivo microimaging of mice. Ultrasound Med Biol. 2002;28:1165–1172. doi: 10.1016/s0301-5629(02)00567-7. [DOI] [PubMed] [Google Scholar]
- Frinking PJ, Cespedes EI, Kirkhorn J, Torp HG, de Jong N. A new ultrasound contrast imaging approach based on the combination of multiple imaging pulses and a separate release burst. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48:643–651. doi: 10.1109/58.920687. [DOI] [PubMed] [Google Scholar]
- Howard CM, Forsberg F, Minimo C, Liu J-B, Merton Daniel A, Claudio PP. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol. 2006;209:413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- http://www.definityimaging.com/pdf/prescribinginfo.pdf. Definity package insert.
- Lang RM, Mor-Avi V. Clinical utility of contrast-enhanced echocardiography. Clin Cardiol FIELD Full Journal Title:Clinical cardiology. 2006;29:I15–I25. doi: 10.1002/clc.4960291304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004;11:215–221. doi: 10.1016/j.nuclcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Miller DL, Dou C, Wiggins RC, Wharram BL, Goyal M, Williams AR. An in vivo rat model simulating imaging of human kidney by diagnostic ultrasound with gas body contrast agent. Ultrasound Med Biol. 2007;33:129–135. doi: 10.1016/j.ultrasmedbio.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibanov AL, Ferrara KW. Experimental and theoretical evaluation of microbubble behavior: effect of transmitted phase and bubble size. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47:1494–1509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- Porter TR, Oberdorfer J, Rafter P, Lof J, Xie F. Microbubble responses to a similar mechanical index with different real-time perfusion imaging techniques. Ultrasound Med Biol. 2003;29:1187–1192. doi: 10.1016/s0301-5629(03)00900-1. [DOI] [PubMed] [Google Scholar]
- Postema M, Schmitz G. Ultrasonic bubbles in medicine: Influence of the shell. Ultrasonics Sonochemistry. 2007;14:438–444. doi: 10.1016/j.ultsonch.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Razansky D, Einziger PD, Adam DR. Enhanced heat deposition using ultrasound contrast agent-modeling and experimental observations. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53:137–147. doi: 10.1109/tuffc.2006.1588399. [DOI] [PubMed] [Google Scholar]
- Sassaroli E, Hynynen K. Cavitation threshold of microbubbles in gel tunnels by focused ultrasound. Ultrasound Med Biol. 2007;33:1551–1560. doi: 10.1016/j.ultrasmedbio.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger SM, Bloch SH, Foreman O, Wisner ER, Ferrara KW, Dayton PA. Ultrasound assessment of angiogenesis in a matrigel model in rats. Ultrasound Med Biol. 2006;32:673–681. doi: 10.1016/j.ultrasmedbio.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talu E, Hettiarachchi K, Zhao S, Powell R, Lee AP, Longo ML, Dayton PA. Tailoring the size distribution of ultrasound contrast agents: A possible method for improving sensitivity in molecular imaging. Molecular Imaging. 2008 In press. [PMC free article] [PubMed] [Google Scholar]
- Tu J, Matula TJ, Brayman AA, Crum LA. Inertial cavitation dose produced in ex vivo rabbit ear arteries with Optison by 1 MHz pulsed ultrasound. Ultrasound Med Biol. 2006;32:281–288. doi: 10.1016/j.ultrasmedbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Villanueva FS, Kaul S. Assessment of myocardial perfusion in coronary artery disease using myocardial contrast echocardiography. Coron Artery Dis. 1995;6:18–28. doi: 10.1097/00019501-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Wisner ER, Ferrara K, Gabe JD, Patel D, Nyland TG, Short RE, Ottoboni TB. Contrast enhanced intermittent power Doppler ultrasound with sub-micron bubbles for sentinel node detection. Acad Radiol. 2002;9 Suppl 2:S389–S391. doi: 10.1016/s1076-6332(03)80241-6. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Luo Y, Liang H, Mei X, Tang J, Lu C, Zhang Y, Lin Q. Comparing transfection efficiency and safety for antisense oligodeoxyribonucleotide between phospholipids-based microbubbles and liposomes. J Drug Targeting. 2007;14:687–693. doi: 10.1080/10611860600965849. [DOI] [PubMed] [Google Scholar]