Abstract
Leptin controls body weight by activating the long form of the leptin receptor (LEPRb). Janus kinase 2 (JAK2) is associated with LEPRb and autophosphorylates in response to leptin. JAK2 also phosphorylates LEPRb, STAT3, and multiple other downstream molecules. Surprisingly, here we show that JAK2 is not required for leptin stimulation of STAT3 phosphorylation. Leptin time- and dose-dependently stimulated tyrosine phosphorylation of STAT3 in both human and mouse JAK2-null cells. Leptin also increased the viability of JAK2-null cells. Overexpression of c-Src or Fyn, two Src family members, promoted STAT3 phosphorylation, whereas inhibition of the endogenous Src family members by either pharmacological inhibitors or dominant negative Src(K298M) decreased the ability of leptin to stimulate the phosphorylation of STAT3 and ERK1/2. Leptin also stimulated tyrosine phosphorylation of kinase-inactive JAK2(K882E) in JAK2-null cells. Overexpression of JAK2(K882E) enhanced the ability of leptin to stimulate STAT3 phosphorylation in JAK2-null cells. Tyr1138 in LEPRb was required for leptin-stimulated phosphorylation of STAT3 but not JAK2(K882E). These data suggest that leptin stimulates non-JAK2 tyrosine kinase(s), including the Src family members, which phosphorylate JAK2, STAT3, and other molecules downstream of LEPRb. JAK2 mediates leptin signaling by both phosphorylating its substrates and forming a signaling complex as a scaffolding/adaptor protein. The non-JAK2 kinase(s) and JAK2 may act coordinately and synergistically to mediate leptin response.
Leptin, an adipocyte-derived hormone, controls energy balance and body weight via its long form receptor LEPRb.2 Genetic deficiency of either leptin or LEPRb results in severe obesity and obesity-associated type 2 diabetes in both rodents and humans (1–7). LEPRb in the brain mediates leptin regulation of energy homeostasis and body weight (8, 9), whereas LEPRb in peripheral tissues (e.g. the liver, skeletal muscle, adipose tissue, pancreatic islets, and immune cells) directly regulates the metabolic activities of these tissues (10–16). Interestingly, obesity is associated with markedly elevated circulating leptin, a hallmark of leptin resistance (17, 18). Defects in LEPRb signaling are primary determents for leptin resistance and obesity (17, 19–21).
LEPRb is a member of the cytokine receptor family (e.g. receptors for interferon-γ, erythropoietin, growth hormone, prolactin, and various interleukins) (13). Cytokine receptors activate the JAK tyrosine kinases (JAK1, JAK2, JAK3, and Tyk2) that subsequently tyrosyl phosphorylate and activate the STAT transcription factors (22). LEPRb is associated with JAK2 (23–25). Leptin stimulates JAK2, which autophosphorylates as well as tyrosyl phosphorylates LEPRb (13). Phosphorylation of Tyr1007/1008 within the activation loop of JAK2 is required for the full activation of JAK2, whereas phosphorylated Tyr813 in JAK2 binds to the SH2 domain of SH2B1 (26). SH2B1 is an endogenous enhancer of leptin signaling, and genetic deletion of SH2B1 results in leptin resistance and obesity (18, 27). Phosphorylated Tyr1138 in LEPRb binds to STAT3, which is required for tyrosine phosphorylation and activation of STAT3 by LEPRb-associated JAK2 (23, 28). Importantly, genetic mutation of Tyr1138 not only abolishes leptin-stimulated phosphorylation of STAT3 but also results in morbid obesity, suggesting that Tyr1138-mediated phosphorylation of STAT3 is required for leptin regulation of energy balance and body weight (28). Consistent with these observations, neuron-specific deletion of STAT3 results in severe obesity and obesity-associated metabolic disease (29, 30). Therefore, the STAT3 pathway appears to be required for LEPRb to regulate body weight and metabolism.
In this study, we unexpectedly found that leptin still stimulated tyrosine phosphorylation of STAT3 and kinase-inactive JAK2(K882E) in JAK2-deficient cells. Our results suggest that leptin stimulates non-JAK2 tyrosine kinase(s), including the Src family tyrosine kinases, which tyrosyl phosphorylate both STAT3 and JAK2, thereby mediating the leptin pathway.
EXPERIMENTAL PROCEDURES
Material—αpSTAT3 (pY705), αSTAT3, and αERK2 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-phospho-MAPK was from Cell Signaling Technology Inc. (Beverly, MA). αJAK2 was from Biosource (Camarillo, CA) and Upstate Biotechnology Inc. (Lake Placid, NY). Anti-phospho-JAK2 (pY1007/1008) and αPY20 were from Upstate Biotechnology Inc. (Lake Placid, NY). SU6656 and PP2 were from Calbiochem Bioscience Inc. (La Jolla, CA). Leptin was from the National Hormone and Peptide Program, NIDDK, National Institutes of Health (Torrance, CA). Growth hormone (GH) and nerve growth factor (NGF) were from Sigma-Aldrich.
Generation of Stable Cell Lines—LEPRb or LEFRbY1138F was stably introduced into γ2A cells, which are genetically deficient of JAK2 (31, 32), via retrovirally or lentivirally mediated gene transfer, to generate γ2ALEPRb or γ2AY1138F cell lines as described previously (33). Wild type JAK2 or kinase-inactive JAK2(K882E) were stably introduced into γ2ALEPRb cells via retrovirally mediated gene transfer to generate γ2ALEPRb/JAK2 or γ2ALEPRb/K882E cell lines, respectively. JAK2-deficient mouse embryonic fibroblast (MEFKO) cells were prepared from JAK2 knock-out embryos (34) and infected with LEPRb lentiviruses to generate MEFKO-LEPRb cell lines, which stably express LEPRb. JAK2 and JAK2(K882E) were stably introduced into MEFKO-LEPRb cells via retrovirally mediated gene transfer to generate MEFKO-LEPRb/JAK2 and MEFKO-LEPRb/K882E cell lines, respectively. Rat adrenal pheochromocytoma (PC12) cells were infected with LEPRb retroviruses to generate PC12LEPRb cell lines, which stably express LEPRb. GH receptor (GHR) cDNA was prepared from mouse livers and stably introduced into γ2A cells via retrovirally mediated gene transfer to generate γ2AGHR cell lines. JAK2 or JAK2(K882E) were stably introduced into γ2AGHR cells via retrovirally mediated gene transfer to generate γ2AGHR/JAK2 or γ2AGHR/K882E cell lines, respectively.
Cell Culture, Neuronal Differentiation, and Transfection—γ2A and MEFKO cells were cultured at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 25 mm glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5% heat-inactivated fetal bovine serum (FBS). PC12LRb cells were grown on collagen-coated dishes in DMEM supplemented with 25 mm glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 10% heat-inactivated horse serum, and 5% FBS. To induce neuronal differentiation, PC12LRb cells were cultured for 6 days in DMEM supplemented with 25 mm glucose, 2% horse serum, 1% FBS, 100 ng/ml NGF, 100 units/ml penicillin, and 100 μg/ml streptomycin. HEK293LEPRb cells, which stably express LEPRb (35), were grown at 37 °C in 5% CO2 in DMEM supplemented with 25 mm glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, and 8% calf serum. HEK293LEPRb cells were transiently transfected with expression plasmids using Lipofectamine™ 2000 reagents (Invitrogen) according to the manufacturer's instructions.
Immunoprecipitation and Immunoblotting—Confluent cells were deprived of serum overnight (∼16 h) in DMEM containing 0.6% bovine serum albumin and treated with leptin. The cells were rinsed two times with ice-cold phosphate-buffered saline, solubilized in lysis buffer (50 mm Tris, pH 7.5, 1% Nonidet P-40, 150 mm NaCl, 2 mm EGTA, 1 mm Na3VO4, 100 mm NaF, 10 mm Na4P2O7, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin), and centrifuged at 14,000 × g for 15 min at 4 °C. Protein concentrations in the supernatant (cell extracts) were measured using a protein assay kit (Bio-Rad). The cell extracts were incubated with the indicated antibody on ice for 2 h. The immune complexes were collected on protein A-agarose during 1 h of incubation at 4 °C. The beads were washed three times with washing buffer (50 mm Tris, pH 7.5, 1% Nonidet P-40, 150 mm NaCl, 2 mm EGTA) and boiled for 5 min in SDS-PAGE sample buffer (50 mm Tris-HCl, pH 6.8, 2% SDS, 2% β-mercaptoethanol, 10% glycerol, 0.005% bromphenol blue). The solubilized proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes (Amersham Biosciences), and detected by immunoblotting with the indicated antibody using the Odyssey detection system or ECL. Some membranes were subsequently incubated at 55 °C for 30 min in stripping buffer (100 mm β-mercaptoethanol, 2% SDS, 62.5 mm Tris-HCl, pH 6.7) to prepare them for a second round of immunoblotting.
In Vitro Kinase Assays—JAK2 was immunoprecipitated with αJAK2 and purified using protein A-agarose beads. Protein A-agarose bead-bound JAK2 was washed extensively with a kinase reaction buffer (50 mm HEPES, pH 7.6, 10 mm MnCl2, 100 mm NaCl, 0.5 mm dithiothreitol, 1 mm Na3VO4) and incubated at room temperature for 30 min in the kinase reaction buffer supplemented with [γ-32P]ATP (10 μCi), 20 μm cold ATP, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Protein A-agarose bead-bound JAK2 was washed with lysis buffer, boiled for 5 min in the SDS-PAGE sample buffer, resolved by SDS-PAGE, and transferred onto nitrocellulose membranes. Phospho-JAK2 was detected by autoradiography. The same blots were immunoblotted with αJAK2.
Cell Viability Assays—Cell viability was measured by the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. MTT is taken up via endocytosis and metabolized by mitochondrial dehydrogenase activity in viable cells to form purple formazan. MEFKO-LEPRb or γA2LEPRb cells were grown in DMEM supplemented with 5% FBS in 24-well plates for 8 h and then in DMEM supplemented without (control) or with 100 ng/ml leptin for an additional 8 h. The cells were treated with H2O2 (600 μm for γA2LEPRb and 100 μm for MEFKO-LEPRb cells) in the absence (control) or presence of 100 ng/ml leptin for various times as indicated. H2O2 and leptin were replaced every 24 h by changing culture medium. The cells were then incubated with 75 μg/ml MTT for 2 h at 37 °C, washed with phosphate-buffered saline, and solubilized in 0.04 n HCl in isopropyl alcohol. Formazan was quantified spectrophotometrically using a microplate reader. The absorbance at 570 nm (A570) of the cells prior to H2O2 treatment was converted to 100% viability, and all other A570 was normalized to these values as a percentage of viability.
RESULTS
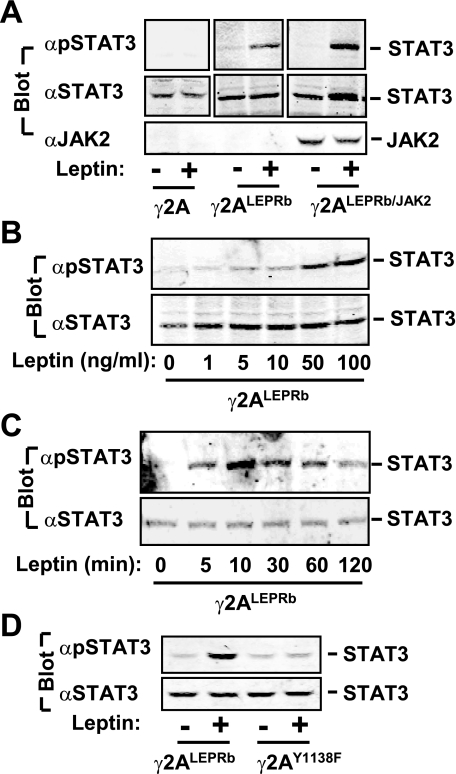
Leptin Stimulates JAK2-independent Tyrosine Phosphorylation of STAT3 in γ2ALEPRb Cells—To determine whether JAK2 is required for leptin signaling, LEPRb was stably expressed in JAK2-deficient γ2A human fibroblast cells (γ2ALEPRb) (33). JAK2 was also stably introduced into γ2ALEPRb cells via retrovirally mediated gene transfer to generate γ2ALEPRb/JAK2 cell lines. The cells were deprived of serum overnight and treated with 100 ng/ml leptin for 10 min. The cell extracts were immunoblotted with anti-phospho-STAT3 (pTyr705) (αpSTAT3) that recognizes phosphorylated and active STAT3. Leptin stimulated phosphorylation of endogenous STAT3 in both γ2ALEPRb/JAK2 and γ2ALEPRb cells (Fig. 1A). In contrast, leptin did not stimulate STAT3 phosphorylation in γ2A cells (Fig. 1A). JAK2 was detected in γ2ALEPRb/JAK2 cells but not in γ2A and γ2ALEPRb cells as revealed by immunoblotting cell extracts with anti-JAK2 antibody (αJAK2) (Fig. 1A). These observations indicate that leptin stimulates tyrosine phosphorylation of STAT3 via LEPRb but independent of JAK2.
FIGURE 1.
JAK2 is not required for leptin stimulation of tyrosine phosphorylation of STAT3 in γ2A cells. A, γ2A, γ2ALEPRb, and γ2ALEPRb/JAK2 cells were treated without or with 100 ng/ml leptin for 10 min. The cell extracts were immunoblotted with anti-phospho-STAT3 (Tyr(P)705)(αpSTAT3), αSTAT3, or αJAK2 as indicated. B, γ2ALEPRb cells were treated with leptin for 10 min at the concentrations of 0, 1, 5, 10, 50, or 100 ng/ml. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3, respectively. C, γ2ALEPRb cells were treated with 100 ng/ml leptin for 0, 5, 10, 30, 60, and 120 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3, respectively. D, γ2ALEPRb and γ2ALEPRb(Y1138F) cells were treated with 100 ng/ml leptin for 10 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3.
Leptin stimulated tyrosine phosphorylation of STAT3 in JAK2-deficient γ2ALEPRb cells in a dose- and time-dependent fashion (Fig. 1, B and C). STAT3 phosphorylation was detected within 5 min of leptin stimulation, reached maximal levels within 10 min, and gradually declined 30 min after leptin stimulation (Fig. 1C).
To determine whether Tyr1138 in LEPRb is required for leptin stimulation of STAT3 phosphorylation, LEPRbY1138F, in which Tyr1138 is replaced with Phe, was stably introduced into γ2A cells via lentivirally mediated gene transfer to generate γ2AY1138F cell lines. Leptin stimulated tyrosine phosphorylation of endogenous STAT3 in γ2ALEPRb but not γ2AY1138F cells (Fig. 1D). These observations suggest that Tyr1138 is phosphorylated by non-JAK2 tyrosine kinase(s) and binds to STAT3 in response to leptin, thereby allowing the non-JAK2 tyrosine kinase(s) to phosphorylate STAT3.
Leptin Stimulates Tyrosine Phosphorylation of Kinase-inactive JAK2(K882E) in γ2ALEPRb/K882E Cells—Kinase-inactive JAK2(K882E) was prepared by replacing Lys882 in the kinase domain, which is required for the binding of ATP to JAK2, with Glu. To determine whether JAK2 serves as a substrate for the non-JAK2 tyrosine kinase(s), kinase-inactive JAK2(K882E) was stably introduced into γ2ALEPRb cells via retrovirally mediated gene transfer to generate γ2ALEPRb/K882E cell lines. The cells were deprived of serum overnight and treated with 100 ng/ml leptin for 10 min. JAK2 and JAK2(K882E) were immunoprecipitated with αJAK2 and immunoblotted with anti-phosphotyrosine antibody (αPY). Leptin stimulated tyrosine phosphorylation of both JAK2 in γ2ALEPRb/JAK2 cells and JAK2(K882E) in γ2ALEPRb/K882E cells (Fig. 2A). To determine whether leptin stimulates phosphorylation of JAK2(K882E) at physiological phosphorylation sites, αJAK2 immunoprecipitates were immunoblotted with anti-phospho-JAK2 (pTyr813)(αp813) that specifically recognizes phosphorylated JAK2 on Tyr813 (26, 33). Leptin stimulated Tyr813 phosphorylation in both JAK2(K882E) and JAK2 (Fig. 2A). In parallel experiments, the cell extracts were immunoblotted with anti-phospho-JAK2 (pTyr1007/1008) (αpY1007/8), which specifically recognizes phosphorylated Tyr1007/1008 in JAK2. Leptin also stimulated Tyr1007/1008 phosphorylation in both JAK2(K882E) and JAK2 (Fig. 2A). As expected, γ2ALEPRb cells did not express endogenous JAK2, whereas γ2ALEPRb/K882E cells expressed recombinant JAK2(K882E) (Fig. 2A). The expression levels of JAK2-(K882E) and JAK2 were comparable between γ2ALEPRb/K882E and γ2ALEPRb/JAK2 cells (Fig. 2A).
FIGURE 2.
Leptin stimulates tyrosine phosphorylation of kinase-inactive JAK2(K882E) in γ2A cells. A, γ2ALEPRb, γ2ALEPRb/K882E, or γ2ALEPRb/JAK2 cells were treated with 100 ng/ml leptin for 10 min. The cell extracts were immunoprecipitated (IP) with anti-JAK2 antibody (αJAK2) and immunoblotted with anti-phosphotyrosine antibody (αPY). The same blots were reprobed with anti-phospho-JAK2 (pY813) (αpY813) or αJAK2. In parallel experiments, cell extracts were immunoblotted with anti-phospho-JAK2 (pY1007/1008) (αpY1007/8) or αJAK2. B, γ2ALEPRb/K882E or γ2ALEPRb/JAK2 cells were treated with 100 ng/ml leptin for 0, 5, 10, 30, and 60 min. The cell extracts were immunoblotted with αpY813, αpY1007/8, or αJAK2 as indicated. C, γ2ALEPRb, γ2ALEPRb/K882E, γ2AY1138F, and γ2AY1138F/K882E cells were treated with 100 ng/ml leptin for 10 min. The cell extracts were immunoblotted with αpY1007/8 or αJAK2. In parallel experiments, the cell extracts were immunoprecipitated with αJAK2, immunoblotted with αPY, and reprobed with αJAK2. D, γ2ALEPRb, γ2ALEPRb/K882E, and γ2ALEPRb/JAK2 cells were treated with leptin for 10 min at the indicated concentrations. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3, respectively.
Leptin stimulated Tyr813/1007/1008 phosphorylation in JAK2(K882E) in a time-dependent manner. Tyr813/1007/1008 phosphorylation in both JAK2(K882E) and JAK2 was maximal with 5 min of leptin stimulation and gradually declined 10 min after leptin stimulation (Fig. 2B). However, leptin stimulated Tyr813/1007/1008 phosphorylation to a greater extent in JAK2 than in JAK2(K882E) (Fig. 2B). Together, these data indicate that leptin stimulates Tyr813/1007/1008 phosphorylation through both JAK2 autophosphorylation and non-JAK2 autophosphorylation.
To determine whether Tyr1138 in LEPRb is involved non-JAK2 kinase-mediated tyrosine phosphorylation of JAK2, γ2ALEPRb, γ2ALEPRb/K882E, γ2AY1138F, and γ2AY1138F/K882E cells were treated with 100 ng/ml leptin for 10 min. γ2ALEPRb and γ2AY1138F cells did not express JAK2, whereas the expression level of JAK2(K882E) was similar between γ2ALEPRb/K882E and γ2AY1138F/K882E cells (Fig. 2C). Leptin similarly stimulated tyrosine phosphorylation of kinase-inactive JAK2(K882E), including Tyr1007/1008 phosphorylation, in both γ2ALEPRb/K882E and γ2AY1138F/K882E cells (Fig. 2C). These results suggest that Tyr1138 in LEPRb is not involved in leptin stimulation of the non-JAK2 tyrosine kinase(s), which subsequently phosphorylate JAK2; however, Tyr1138 is required for the non-JAK2 kinase(s) to phosphorylate STAT3 (Fig. 1D).
To determine whether JAK2-dependent and -independent pathways act coordinately to mediate leptin signaling, γ2ALEPRb, γ2ALEPRb/K882E, and γ2ALEPRb/JAK2 cells were treated with leptin (0, 10, or 50 ng/ml) for 10 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3. Consistent with the previous data, leptin dose-dependently stimulated STAT3 phosphorylation in γ2ALEPRb cells; interestingly, kinase-inactive JAK2(K882E) significantly enhanced leptin-stimulated STAT3 phosphorylation in γ2ALEPRb/K882E cells (Fig. 2D). These data suggest that JAK2 is able to promote leptin signaling independent of its tyrosine kinase activity, presumably by functioning as a scaffolding and/or adaptor protein. However, leptin stimulated STAT3 phosphorylation to a greater extent in γ2ALEPRb/JAK2 cells (presumably by both the JAK2-dependent and -independent mechanisms) than in γ2ALEPRb/K882E cells (Fig. 2D). Taken together, these data suggest that the JAK2-independent pathway regulates the JAK2-dependent pathway upon leptin activation of LEPRb.
Leptin Stimulates Phosphorylation of STAT3, ERK1/2, and JAK2(K882E) in JAK2 Knock-out Mouse Embryonic Fibroblast (MEFKO) Cells—To determine whether JAK2 is dispensable for leptin signaling in other cell types, MEFKO cells were generated from JAK2 knock-out mice (34). LEPRb was stably introduced into MEFKO cells via lentivirally mediated gene transfer to generate MEFKO-LEPRb cell lines. JAK2(K882E) or JAK2 were stably introduced into MEFKO-LEPRb cells via retrovirally mediated gene transfer to generate MEFKO-LEPRb/K882E or MEFKO-LEPRb/JAK2 cell lines, respectively. MEFKO-LEPRb, MEFKO-LEPRb/K882E, and MEFKO-LEPRb/JAK2 cells were deprived of serum overnight and treated with leptin for 10 min. The cell extracts were immunoblotted with αpSTAT3, αSTAT3, anti-phospho-MAPK (pThr202/Tyr204) (αpMAPK), αERK2 that recognizes both ERK1 and ERK2, αpY1007/8, or αJAK2. Leptin stimulated phosphorylation of both STAT3 and ERK1/2 in all three cell lines even though MEFKO-LEPRb cells did not express JAK2 (Fig. 3A). Moreover, leptin stimulated Tyr1007/1008 phosphorylation in both JAK2(K882E) and JAK2 (Fig. 3A). To verify that JAK2(K882E) is kinase-dead, MEFKO-LEPRb/K882E and MEFKO-LEPRb/JAK2 cells were treated with 100 ng/ml leptin for 10 min. JAK2(K882E) or JAK2 was immunoprecipitated with αJAK2 and subjected to in vitro kinase assays. Leptin stimulated autophosphorylation of JAK2 but not JAK2(K882E) under these conditions (Fig. 3B). Together, these data indicate that leptin stimulates the JAK2-independent pathway in multiple cell types, including γ2A and MEFKO cells.
FIGURE 3.
JAK2 is not required for leptin stimulated phosphorylation of STAT3 and ERK1/2 in MEFKO cells. A, MEFKO-LEPRb, MEFKO-LEPRb/K882E, and MEF KO-LEPRb/JAK2 cells were treated with 200 ng/ml leptin for 10 min. The cell extracts were immunoblotted with the indicated antibodies. B, MEFKO-LEPRb/K882E and MEF KO-LEPRb/JAK2 cells were treated with 100 ng/ml leptin for 10 min. The cell extracts were immunoprecipitated with αJAK2. Immunopurified JAK2 was subjected to in vitro kinase assays in the presence of [γ-32P]ATP, resolved by SDS-PAGE, and transferred onto nitrocellulose membranes. JAK2 was visualized by autoradiography and subsequently immunoblotted with αJAK2.
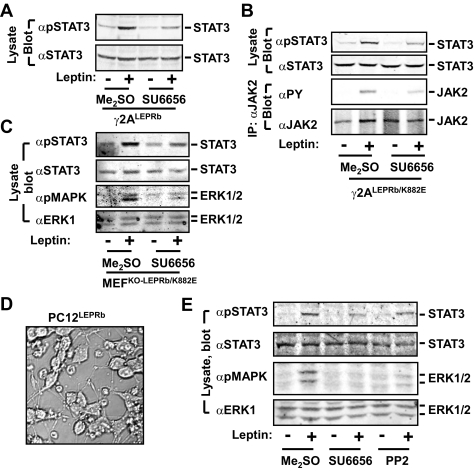
Src Inhibitors Attenuate Leptin Signaling in Multiple Cell Types—Because the Src family tyrosine kinases are expressed in leptin target cells and phosphorylate STAT3, we determined whether they were involved in the JAK2-independent pathway in response to leptin. γ2ALEPRb cells were pretreated with Me2SO (vehicle) or SU6656 (Src inhibitor) for 4 h and stimulated with 100 ng/ml leptin for an additional 10 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3. SU6656 significantly attenuated leptin stimulation of STAT3 phosphorylation in γ2ALEPRb cells (Fig. 4A). SU6656 also inhibited leptin-stimulated phosphorylation of both STAT3 and JAK2(K882E) in γ2ALEPRb/K882E cells (Fig. 4B). To determine whether the Src family tyrosine kinases are also involved in leptin signaling in other cell types, MEFKO-LEPRb/K882E cells were pretreated with SU6656 and stimulated with 100 ng/ml leptin for 10 min. Leptin stimulated phosphorylation of both STAT3 and ERK1/2 in Me2SO-pretreated cells as expected (Fig. 4C). SU6656 significantly inhibited phosphorylation of both STAT3 and ERK1/2 (Fig. 4C).
FIGURE 4.
Src inhibitors inhibit leptin signaling in multiple cell types. A, γ2ALEPRb cells were pretreated with Me2SO or SU6656 (10 μm) for 4 h and treated with 100 ng/ml leptin for 10 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3. B, γ2ALRb/K882E cells were pretreated with Me2SO or SU6656 (10 μm) for 4 h, and treated with 100 ng/ml leptin for 10 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3. In parallel, the cell extracts were immunoprecipitated (IP) with αJAK2 and immunoblotted with αPY. The same blots were reprobed with αJAK2. C, MEFKO-LEPRb/K882E cells were pretreated with Me2SO or SU6656 (10 μm) for 4 h and treated with 100 ng/ml leptin for an additional 10 min. The cell extracts were immunoblotted with the indicated antibodies. D, representative images of differentiated PC12LEPRb cells. PC12LEPRb cells were incubated with 100 ng/ml NGF for 6 days to induce neuronal differentiation. E, PC12LRb cells were differentiated into neurons with 100 ng/ml NGF treatment for 6 days. Differentiated cells were pretreated with dimethyl sulfoxide, (DMSO), SU6656 (10 μm), or PP2 (10 μm) for 4 h, and treated with 100 ng/ml leptin for an additional 10 min. The cell extracts were immunoblotted with the indicated antibodies.
Leptin regulates energy balance and body weight primarily by activating LEPRb in neurons (8, 9, 17). LEPRb was stably introduced into rat adrenal pheochromocytoma (PC12) cells via retrovirally mediated gene transfer to generate PC12LEPRb cell lines that express endogenous JAK2. To induce neuronal differentiation, PC12LEPRb cells were treated with 100 ng/ml NGF for 6 days. NGF stimulated neurite growth and neuronal differentiation of PC12LEPRb cells (Fig. 4D). To determine whether the Src family tyrosine kinases mediate leptin signaling in PC12LEPRb neurons, PC12LEPRb cells were differentiated into neurons with NGF, pretreated with either SU6656 or PP2, two structurally distinct inhibitors of the Src family tyrosine kinases, and stimulated with 100 ng/ml leptin. Leptin stimulated phosphorylation of both STAT3 and ERK1/2 in Me2SO-pretreated PC12LEPRb neurons (Fig. 4E). Both SU6656 and PP2 significantly attenuated leptin-stimulated phosphorylation of both STAT3 and ERK1/2 (Fig. 4E). Therefore, the Src family members may be involved in the activation of the JAK2-independent pathway in response to leptin.
The Src Family Members Tyrosyl Phosphorylate Both STAT3 and JAK2—To determine whether the Src family tyrosine kinases phosphorylate STAT3, c-Src was coexpressed with STAT3 in HEK293LEPRb cells. The cell extracts were immunoblotted with αpSTAT3, αSTAT3, anti-phospho-Src (pTyr416) (αpSrc) that recognizes active c-Src, or αSrc. Overexpression of c-Src markedly increased phosphorylation of STAT3 (Fig. 5A), consistent with previous reports (36). Endogenous Src tyrosine kinases were inactive under basal conditions; in contrast, overexpressed c-Src was tyrosyl-phosphorylated and constitutively active (Fig. 5A). Fyn, another Src family member, also phosphorylated STAT3 in a similar fashion (Fig. 5B).
FIGURE 5.
The Src family members phosphorylate both STAT3 and JAK2. A, c-Src was transiently coexpressed with STAT3 in HEK293LEPRb cells. The cell extracts were immunoblotted with αpSTAT3, αSTAT3, anti-phospho-Src (pTyr416) (αpSrc), or αSrc as indicated. B, FLAG-tagged Fyn was transiently coexpressed with STAT3 in HEK293LEPRb cells. The cell extracts were immunoblotted with αpSTAT3, αSTAT3, or αFLAG, respectively. C, c-Src was transiently coexpressed with kinase inactive-JAK2(K882E) in HEK293LEPRb cells. The cell extracts were immunoprecipitated with αJAK2 and immunoblotted with αPY. The same blots were reprobed with αJAK2. The cell extracts were immunoblotted with αpSrc or αSrc. D, STAT3 expression vectors (0.4 μg) were transiently cotransfected with either c-Src (2 ng) or Fyn expression plasmids (2 ng) into HEK293LEPRb cells. 48 h after transfection, the cells were deprived of serum overnight and treated with leptin for 10 min. The cell extracts were immunoblotted with either αpSTAT3 or αSTAT3, respectively. E, STAT3 expression vectors (0.4 μg) were transiently cotransfected with kinase-inactive Src(K298M) expression plasmids (from 0.4 to 1.6 μg) into HEK293LEPRb cells. The cells were treated with leptin for 10 min, and the cell extracts were immunoblotted with either αpSTAT3 or αSTAT3 as described for D. Con, control.
To determine whether the Src family tyrosine kinases phosphorylate JAK2, c-Src was coexpressed with kinase-inactive JAK2(K882E) in HEK293LEPRb cells. The cell extracts were immunoprecipitated with αJAK2 and immunoblotted with αPY. Overexpression of c-Src dose-dependently increased tyrosine phosphorylation of JAK2(K882E) (Fig. 5C). These data suggest that the Src family tyrosine kinases phosphorylate JAK2, thereby enhancing the activation of the leptin pathways downstream of JAK2.
To determine whether the Src family members promote leptin signaling, c-Src or Fyn were transiently coexpressed with STAT3 in HEK293LEPRb cells. Both c-Src and Fyn enhanced leptin stimulation of STAT3 phosphorylation (Fig. 5D). To further determine whether the endogenous Src family members are involved in leptin stimulation of STAT3 phosphorylation, kinase-inactive Src(K298M) was coexpressed with STAT3 in HEK293LEPRb cells. Src(K298M) dose-dependently inhibited leptin stimulation of STAT3 phosphorylation as a dominant negative mutant (Fig. 5E). Together, these results suggest that the Src family members are candidates for the non-JAK2 kinases that mediate leptin signaling.
The JAK2-independent Pathway Mediates Leptin Stimulation of Cell Survival—To determine whether the JAK2-independent pathway mediates leptin response, we examined the ability of leptin to promote the survival of JAK2-null cells. Cell death was induced by a combination of serum deprivation and H2O2 treatment, and cell viability was measured by MTT assays. H2O2 treatment reduced cell viability in a time-dependent manner; leptin significantly increased the viability of JAK2-deficient γ2ALEPRb cells (Fig. 6A). After 120 h of H2O2 treatment, cell viability was reduced to 16%, and leptin increased the viability of γ2ALEPRb cells by 94%. Leptin also increased the viability of MEFKO-LEPRb cells (Fig. 6B). These results suggest that the JAK2-independent pathway mediates leptin survival signals.
FIGURE 6.
The non-JAK2 pathway(s) promote cell survival. A, γ2ALEPRb cells were pretreated with or without 100 ng/ml leptin for 8 h and then treated with 600 mm H2O2 for 0, 24, 48, 72, 96, or 120 h in the absence or presence of 100 ng/ml leptin. Cell viability was measured by MTT assays. B, MEFKO-LEPRb cells were pretreated with or without 100 ng/ml leptin for 8 h and then treated with 100 mm H2O2 for 0 or 144 h in the absence or presence of 100 ng/ml leptin. Cell viability was measured by MTT assays. *, p < 0.05. Con, control.
JAK2 Is Required for Growth Hormone to Stimulate Tyrosine Phosphorylation of STAT3—To determine whether other cytokines also stimulate JAK2-independent tyrosine phosphorylation of STAT3, GHR cDNA was prepared from mouse livers and stably introduced into γ2A cells via retrovirally mediated gene transfer to generate γ2AGHR cell lines. JAK2 or JAK2(K882E) was stably introduced into γ2AGHR cells via retrovirally mediated gene transfer to generate γ2AGHR/JAK2 or γ2AGHR/K882E cell lines, respectively. γ2AGHR and γ2AGHR/JAK2 cells were stimulated with GH for 10 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3. GH stimulated STAT3 phosphorylation in γ2AGHR/JAK2 but not γ2AGHR cells (Fig. 7A). JAK2 was detected in γ2AGHR/JAK2 but not γ2AGHR cells, and GH stimulated tyrosine phosphorylation of JAK2 in γ2AGHR/JAK2 cells as expected (Fig. 7A). LEPRbY1138F was also unable to mediate STAT3 phosphorylation in response to leptin (Fig. 1D). Both GHR and LEPRbY1138F do not bind to STAT3; therefore, the interaction of STAT3 with the cytokine receptors may be required for cytokine-stimulated STAT3 phosphorylation by the non-JAK2 tyrosine kinase(s).
FIGURE 7.
JAK2 is required for growth hormone-stimulated phosphorylation of STAT3. A, γ2AGHR and γ2AGHR/JAK2 cells were treated with GH (8 × 10–3 unit/ml) for 10 min. The cell extracts were immunoblotted with αpSTAT3 or αSTAT3. In parallel experiments, cell extracts were immunoprecipitated (IP) with αJAK2 and immunoblotted with αPY. The same blots were reprobed with αJAK2. B, γ2AGHR/K882E and γ2AGHR/JAK2 cells were treated with GH (8 × 10–3 unit/ml) for 10 min. The cell extracts were immunoprecipitated with αJAK2 and immunoblotted with αPY. The same blots were immunoblotted with αJAK2.
To determine whether the intrinsic kinase activity is required for GH-stimulated tyrosine phosphorylation of JAK2, γ2AGHR/K882E and γ2AGHR/JAK2 cells were stimulated with GH for 10 min. JAK2 was immunoprecipitated with αJAK2 and immunoblotted with αPY. GH stimulated tyrosine phosphorylation of JAK2 in γ2AGHR/JAK2 cells as well as tyrosine phosphorylation of JAK2(K882E) in γ2AGHR/K882E cells (Fig. 7B). These data suggest that GH also stimulates the non-JAK2 tyrosine kinase(s) that subsequently tyrosyl phosphorylate JAK2 but not STAT3.
DISCUSSION
Leptin controls energy balance and body weight primarily by activating LEPRb in the brain (8, 9, 17). Leptin stimulates LEPRb-associated JAK2. JAK2 phosphorylates LEPRb on multiple tyrosines. Phosphorylated Tyr985 binds to SHP2 or SOCS3 (23, 37, 38). SHP2 mediates leptin stimulation of the MAPK pathway, which is involved in the regulation of body weight (38, 39). In contrast, the binding of SOCS3 to phosphorylated Tyr985 inhibits leptin signaling and action (37, 40). Phosphorylated Tyr1138 binds to STAT3, which is required for leptin stimulation of phosphorylation and activation of STAT3 by JAK2 (23, 28). Surprisingly, we observed that leptin stimulated LEPRb-mediated tyrosine phosphorylation of STAT3 in both human and mouse JAK2-null cells and increased the viability of JAK2-null cells. These findings demonstrate the existence of a new JAK2-independent pathway that mediates leptin response.
The Src family members are a candidate for the non-JAK2 tyrosine kinases that mediate LEPRb signaling. The Src family tyrosine kinases consist of 11 members (Blk, Brk, Fgr, Frk, Fyn, Hck, Lck, Lyn, Src, Srm, and Yes), which are widely expressed in multiple tissues, including leptin targets (e.g. the brain, liver, adipose tissue, and skeletal muscles) (41). Leptin stimulates Hck in isolated human neutrophils and c-Src in rat kidney (42, 43). We demonstrated that overexpressed c-Src was constitutively active and tyrosyl-phosphorylated STAT3. c-Src and Fyn enhanced the ability of leptin to stimulate STAT3 phosphorylation. Conversely, Src-specific inhibitors (both SU6656 and PP2) significantly attenuated leptin-stimulated tyrosine phosphorylation of STAT3 in both fibroblasts and neurons. Dominant negative Src(K298M) inhibited leptin-stimulated phosphorylation of STAT3. However, our data do not exclude the possibility that other non-JAK2 and non-Src tyrosine kinases may also be involved in leptin signaling.
Leptin stimulates JAK2 that autophosphorylates on multiple tyrosines. Phosphorylation of Tyr1007/1008 in the activation loop is required for the full activation of the intrinsic tyrosine kinase activity of JAK2 (44, 45). Phosphorylated Tyr813 binds to SH2B1, an essential endogenous enhancer of leptin sensitivity (18, 27, 33). Surprisingly, leptin also stimulated Tyr813/1007/1008 phosphorylation in kinase-inactive JAK2(K882E) in JAK2-null cells, indicating that JAK2 serves as a substrate for the non-JAK2 tyrosine kinase(s) in response to leptin. The non-JAK2 tyrosine kinase(s) may stimulate JAK2 by phosphorylating JAK2 on Tyr1007/1008 in response to leptin. Interestingly, overexpression of JAK2(K882E) enhanced leptin stimulation of STAT3 phosphorylation in JAK2-null cells, suggesting that JAK2(K882E) forms a signaling complex as a scaffolding/adaptor protein to promote leptin signaling. Therefore, the JAK2-independent pathway may cross-talk with the JAK2-dependent pathway in mediating leptin signaling.
Leptin stimulated LEPRbY1138F-mediated phosphorylation of JAK2(K882E) but not STAT3 in JAK2-null cells, suggesting that the non-JAK2 tyrosine kinase(s) phosphorylate JAK2 and STAT3 by different mechanisms. Consistent with this idea, GH stimulated GHR-mediated tyrosine phosphorylation of JAK2(K882E) but not STAT3 in JAK2-null cells. These observations also raise the possibility that the JAK2-independent pathway may cross-talk with the JAK2-dependent pathway in response to multiple cytokines in addition to leptin.
In summary, we have identified the JAK2-independent pathway that mediates leptin stimulation of the tyrosine phosphorylation of STAT3 and ERK1/2. The Src family members of tyrosine kinases are candidates for the non-JAK2 tyrosine kinase(s) that initiate the JAK2-independent pathway. JAK2 mediates leptin signaling both by phosphorylating its substrates and by forming a signaling complex as a scaffolding/adaptor molecule. The non-JAK2 tyrosine kinase(s) and JAK2 may act coordinately and synergistically to promote leptin signaling.
Acknowledgments
We thank David Morris and Drs. Yingjiang Zhou and Lawrence Argetsinger for helpful discussion. We thank Catherine Fang (Pioneer High School, Ann Arbor, MI) for technical assistance. We thank Dr. George Stark (Cleveland Clinic Foundation, Cleveland, OH) for providing γ2A cells, Dr. James Ihle (Department of Biochemistry, St. Jude Children's Research Hospital, Memphis, TN) for JAK2-deficient MEF cells, Dr. Christin Carter-Su (University of Michigan, Ann Arbor, MI) for αp813, and Dr. Kun-Liang Guan (University of California San Diego, La Jolla, CA) for Src and Src(K298M) plasmids.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 DK 065122, RO1 DK073601, 5P60 DK20572, 5P30 CA46592, P30AG013283, and DK34933. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LEPRb, long isoform of leptin receptor; JAK, janus kinase; STAT, signal transducers and activators of transcription; MEF, mouse embryonic fibroblast; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; NGF, nerve growth factor; GH, growth hormone; GHR, GH receptor; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
References
- 1.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J. M. (1994) Nature 372 425–432 [DOI] [PubMed] [Google Scholar]
- 2.Chen, H., Charlat, O., Tartaglia, L. A., Woolf, E. A., Weng, X., Ellis, S. J., Lakey, N. D., Culpepper, J., Moore, K. J., Breitbart, R. E., Duyk, G. M., Tepper, R. I., and Morgenstern, J. P. (1996) Cell 84 491–495 [DOI] [PubMed] [Google Scholar]
- 3.Chua, S. C., Jr., Chung, W. K., Wu-Peng, X. S., Zhang, Y., Liu, S. M., Tartaglia, L., and Leibel, R. L. (1996) Science 271 994–996 [DOI] [PubMed] [Google Scholar]
- 4.Lee, G. H., Proenca, R., Montez, J. M., Carroll, K. M., Darvishzadeh, J. G., Lee, J. I., and Friedman, J. M. (1996) Nature 379 632–635 [DOI] [PubMed] [Google Scholar]
- 5.Vaisse, C., Halaas, J. L., Horvath, C. M., Darnell, J. E., Jr., Stoffel, M., and Friedman, J. M. (1996) Nat. Genet. 14 95–97 [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia, L. A., Dembski, M., Weng, X., Deng, N., Culpepper, J., Devos, R., Richards, G. J., Campfield, L. A., Clark, F. T., Deeds, J., Muir, C., Sanker, S., Moriarty, A., Moore, K. J., Smutko, J. S., Mays, G. G., Wool, E. A., Monroe, C. A., and Tepper, R. I. (1995) Cell 83 1263–1271 [DOI] [PubMed] [Google Scholar]
- 7.Clement, K., Vaisse, C., Lahlou, N., Cabrol, S., Pelloux, V., Cassuto, D., Gourmelen, M., Dina, C., Chambaz, J., Lacorte, J. M., Basdevant, A., Bougneres, P., Lebouc, Y., Froguel, P., and Guy-Grand, B. (1998) Nature 392 398–401 [DOI] [PubMed] [Google Scholar]
- 8.Cohen, P., Zhao, C., Cai, X., Montez, J. M., Rohani, S. C., Feinstein, P., Mombaerts, P., and Friedman, J. M. (2001) J. Clin. Investig. 108 1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Luca, C., Kowalski, T. J., Zhang, Y., Elmquist, J. K., Lee, C., Kilimann, M. W., Ludwig, T., Liu, S. M., and Chua, S. C. (2005) J. Clin. Investig. 115 3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, P., Yang, G., Yu, X., Soukas, A. A., Wolfish, C. S., Friedman, J. M., and Li, C. (2005) J. Biol. Chem. 280 10034–10039 [DOI] [PubMed] [Google Scholar]
- 11.Minokoshi, Y., Kim, Y. B., Peroni, O. D., Fryer, L. G., Muller, C., Carling, D., and Kahn, B. B. (2002) Nature 415 339–343 [DOI] [PubMed] [Google Scholar]
- 12.Park, B. H., Wang, M. Y., Lee, Y., Yu, X., Ravazzola, M., Orci, L., and Unger, R. H. (2006) J. Biol. Chem. 281 40283–40291 [DOI] [PubMed] [Google Scholar]
- 13.Bjorbaek, C., and Kahn, B. B. (2004) Recent Prog. Horm. Res. 59 305–331 [DOI] [PubMed] [Google Scholar]
- 14.Lord, G. M., Matarese, G., Howard, J. K., Baker, R. J., Bloom, S. R., and Lechler, R. I. (1998) Nature 394 897–901 [DOI] [PubMed] [Google Scholar]
- 15.Anderwald, C., Muller, G., Koca, G., Furnsinn, C., Waldhausl, W., and Roden, M. (2002) Mol. Endocrinol. 16 1612–1628 [DOI] [PubMed] [Google Scholar]
- 16.Morioka, T., Asilmaz, E., Hu, J., Dishinger, J. F., Kurpad, A. J., Elias, C. F., Li, H., Elmquist, J. K., Kennedy, R. T., and Kulkarni, R. N. (2007) J. Clin. Investig. 117 2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz, M. W., and Porte, D., Jr. (2005) Science 307 375–379 [DOI] [PubMed] [Google Scholar]
- 18.Ren, D., Li, M., Duan, C., and Rui, L. (2005) Cell Metabolism 2 95–104 [DOI] [PubMed] [Google Scholar]
- 19.Flier, J. S. (2004) Cell 116 337–350 [DOI] [PubMed] [Google Scholar]
- 20.Friedman, J. M. (2003) Science 299 856–858 [DOI] [PubMed] [Google Scholar]
- 21.Friedman, J. M., and Halaas, J. L. (1998) Nature 395 763–770 [DOI] [PubMed] [Google Scholar]
- 22.Herrington, J., Smit, L. S., Schwartz, J., and Carter-Su, C. (2000) Oncogene 19 2585–2597 [DOI] [PubMed] [Google Scholar]
- 23.Banks, A. S., Davis, S. M., Bates, S. H., and Myers, M. G., Jr. (2000) J. Biol. Chem. 275 14563–14572 [DOI] [PubMed] [Google Scholar]
- 24.Bjorbaek, C., Uotani, S., da Silva, B., and Flier, J. S. (1997) J. Biol. Chem. 272 32686–32695 [DOI] [PubMed] [Google Scholar]
- 25.Bjorbak, C., El-Haschimi, K., Frantz, J. D., and Flier, J. S. (1999) J. Biol. Chem. 274 30059–30065 [DOI] [PubMed] [Google Scholar]
- 26.Kurzer, J. H., Argetsinger, L. S., Zhou, Y. J., Kouadio, J. L., O'Shea, J. J., and Carter-Su, C. (2004) Mol. Cell. Biol. 24 4557–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren, D., Zhou, Y., Morris, D., Li, M., Li, Z., and Rui, L. (2007) J. Clin. Investig. 117 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates, S. H., Stearns, W. H., Dundon, T. A., Schubert, M., Tso, A. W., Wang, Y., Banks, A. S., Lavery, H. J., Haq, A. K., Maratos-Flier, E., Neel, B. G., Schwartz, M. W., and Myers, M. G., Jr. (2003) Nature 421 856–859 [DOI] [PubMed] [Google Scholar]
- 29.Cui, Y., Huang, L., Elefteriou, F., Yang, G., Shelton, J. M., Giles, J. E., Oz, O. K., Pourbahrami, T., Lu, C. Y., Richardson, J. A., Karsenty, G., and Li, C. (2004) Mol. Cell. Biol. 24 258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao, Q., Wolfgang, M. J., Neschen, S., Morino, K., Horvath, T. L., Shulman, G. I., and Fu, X. Y. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burfoot, M. S., Rogers, N. C., Watling, D., Smith, J. M., Pons, S., Paonessaw, G., Pellegrini, S., White, M. F., and Kerr, I. M. (1997) J. Biol. Chem. 272 24183–24190 [DOI] [PubMed] [Google Scholar]
- 32.Kohlhuber, F., Rogers, N. C., Watling, D., Feng, J., Guschin, D., Briscoe, J., Witthuhn, B. A., Kotenko, S. V., Pestka, S., Stark, G. R., Ihle, J. N., and Kerr, I. M. (1997) Mol. Cell. Biol. 17 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Z., Zhou, Y., Carter-Su, C., Myers, M. G., Jr., and Rui, L. (2007) Mol. Endocrinol. 21 2270–2281 [DOI] [PubMed] [Google Scholar]
- 34.Parganas, E., Wang, D., Stravopodis, D., Topham, D. J., Marine, J. C., Teglund, S., Vanin, E. F., Bodner, S., Colamonici, O. R., van Deursen, J. M., Grosveld, G., and Ihle, J. N. (1998) Cell 93 385–395 [DOI] [PubMed] [Google Scholar]
- 35.Duan, C., Li, M., and Rui, L. (2004) J. Biol. Chem. 279 43684–43691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu, C. L., Meyer, D. J., Campbell, G. S., Larner, A. C., Carter-Su, C., Schwartz, J., and Jove, R. (1995) Science 269 81–83 [DOI] [PubMed] [Google Scholar]
- 37.Bjorbak, C., Lavery, H. J., Bates, S. H., Olson, R. K., Davis, S. M., Flier, J. S., and Myers, M. G., Jr. (2000) J. Biol. Chem. 275 40649–40657 [DOI] [PubMed] [Google Scholar]
- 38.Li, C., and Friedman, J. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 9677–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, E. E., Chapeau, E., Hagihara, K., and Feng, G.-S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 16064–16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjornholm, M., Munzberg, H., Leshan, R. L., Villanueva, E. C., Bates, S. H., Louis, G. W., Jones, J. C., Ishida-Takahashi, R., Bjorbaek, C., and Myers, M. G., Jr. (2007) J. Clin. Investig. 117 1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roskoski, R., Jr. (2004) Biochem. Biophys. Res. Commun. 324 1155–1164 [DOI] [PubMed] [Google Scholar]
- 42.Montecucco, F., Bianchi, G., Gnerre, P., Bertolotto, M., Dallegri, F., and Ottonello, L. (2006) Ann. N. Y. Acad. Sci. 1069 463–471 [DOI] [PubMed] [Google Scholar]
- 43.Beltowski, J., Wojcicka, G., Trzeciak, J., and Marciniak, A. (2006) Peptides 27 3234–3244 [DOI] [PubMed] [Google Scholar]
- 44.Feng, J., Witthuhn, B. A., Matsuda, T., Kohlhuber, F., Kerr, I. M., and Ihle, J. N. (1997) Mol. Cell. Biol. 17 2497–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ungureanu, D., Saharinen, P., Junttila, I., Hilton, D. J., and Silvennoinen, O. (2002) Mol. Cell. Biol. 22 3316–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]