Abstract
Anaplerosis, the synthesis of citric acid cycle intermediates, by pancreatic beta cell mitochondria has been proposed to be as important for insulin secretion as mitochondrial energy production. However, studies designed to lower the rate of anaplerosis in the beta cell have been inconclusive. To test the hypothesis that anaplerosis is important for insulin secretion, we lowered the activity of pyruvate carboxylase (PC), the major enzyme of anaplerosis in the beta cell. Stable transfection of short hairpin RNA was used to generate a number of INS-1 832/13-derived cell lines with various levels of PC enzyme activity that retained normal levels of control enzymes, insulin content, and glucose oxidation. Glucose-induced insulin release was decreased in proportion to the decrease in PC activity. Insulin release in response to pyruvate alone, 2-aminobicyclo[2,2,1]heptane-2-carboxylic acid (BCH) plus glutamine, or methyl succinate plus β-hydroxybutyrate was also decreased in the PC knockdown cells. Consistent with a block at PC, the most PC-deficient cells showed a metabolic crossover point at PC with increased basal and/or glucose-stimulated pyruvate plus lactate and decreased malate and citrate. In addition, in BCH plus glutamine-stimulated PC knockdown cells, pyruvate plus lactate was increased, whereas citrate was severely decreased, and malate and aspartate were slightly decreased. The incorporation of 14C into lipid from [U-14C]glucose was decreased in the PC knockdown cells. The results confirm the central importance of PC and anaplerosis to generate metabolites from glucose that support insulin secretion and even suggest PC is important for insulin secretion stimulated by noncarbohydrate insulin secretagogues.
Glucose is the most potent physiological insulin secretagogue in the pancreatic beta cell, and it stimulates insulin secretion via its metabolism by aerobic glycolysis. Pyruvate, the terminal product of glycolysis, is metabolized in mitochondria to make ATP to power intracellular processes. However, somewhat surprisingly, in the beta cell, a large amount of glucose-derived pyruvate, equal to one-half the total pyruvate entering mitochondrial metabolism, is carboxylated in the pyruvate carboxylase reaction to form oxaloacetate (1–5). The oxaloacetate can combine with pyruvate-derived acetyl-CoA to form citrate enabling the beta cell mitochondrion to increase the rate of synthesis of any citric acid cycle intermediate (anaplerosis) (6–9). The rate of pyruvate carboxylation correlates with the glucose concentration applied to islets and is thus correlated with the rate of insulin secretion (2). The level of pyruvate carboxylase in the pancreatic islet beta cell is higher than in most body tissues (4, 10–13) and is equal to the levels in gluconeogenic tissues, such as liver and kidney (4), which possess very high levels of the enzyme. However, the beta cell does not possess the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (11, 14, 15) or fructose-1,6-bisphosphatase (8), and this explains why it is incapable of gluconeogenesis (11). Although the reason for the high level of pyruvate carboxylase and the apparent high rate of anaplerosis in the beta cell is the subject of much study (1–5, 7, 9, 16–24) and conjecture, there is still doubt about the necessity of anaplerosis for insulin secretion, since convincing specific roles for products of anaplerosis have not been identified. Unlike mutations in the genes encoding the ATP-sensitive potassium channel, the sulfonylurea receptor and glucokinase, mutations in the pyruvate carboxylase gene do not cause a clinically detectable alteration in insulin secretion.
In addition, there are only a few studies of insulin secretion in which the amount of pyruvate carboxylase was specifically lowered, and the results of these studies were inconclusive. In a study designed to validate a process to scan chromosomal regions in order to identify diabetes susceptibility loci, Antinozzi et al. (25) transfected two separate small interfering RNA (siRNAs)3 targeted against the pyruvate carboxylase mRNA into INS-1 cells and observed about a 60% inhibition of hormone secretion. However, they did not report whether the level of the mRNA encoding pyruvate carboxylase or the activity of the enzyme was lowered by their treatment. A study by Jensen et al. (26) focused specifically on pyruvate carboxylase in which the pyruvate carboxylase protein in INS-1 cells and rat pancreatic islets was decreased 40–56% with siRNA delivered by a virus. This did not inhibit insulin release, and the lack of inhibition was attributed to a compensatory increase in the level of acetyl carnitine that could act as a substitute for acetyl-CoA, which is an essential activator of pyruvate carboxylase. Although phenylacetate, an inhibitor of pyruvate carboxylase, has been used in studies of insulin release, there are reasons to doubt its specificity. It is a weak inhibitor of the enzyme, and high concentrations of phenylacetate (5–10 mm) are necessary to achieve even partial inhibition of the enzyme and insulin release (17, 19). In addition, phenylacetate has been shown to inhibit many cellular processes not related to pyruvate carboxylase (27, 28) and aromatic compounds at millimolar concentrations uncouple mitochondrial oxidative phosphorylation.
In order to test the hypothesis that lowering pyruvate carboxylase could decrease secretagogue-induced insulin release, we generated cell lines from INS-1 832/13 cells that expressed shRNA targeted to various sites of the pyruvate carboxylase mRNA. This gave cell lines with various reductions in pyruvate carboxylase enzyme activity ranging up to a 90% reduction compared with the control cell lines. Decreases in insulin release induced by glucose and other secretagogues were correlated with the level of the enzyme. Malate, citrate, pyruvate, and lactate were measured in secretagogue-stimulated pyruvate carboxylase-deficient cells to discern whether a block at the pyruvate carboxylase reaction was present. In addition, the incorporation of 14C from [U-14C]glucose into lipid products was measured to determine whether lowering of the enzyme influenced the rate of formation of anaplerotic products.
EXPERIMENTAL PROCEDURES
Materials—Chemicals were from Sigma in the highest purity available. [U-14C]glucose was from PerkinElmer Life Sciences. The parental INS-1 832/13 cell line was from Chris Newgard (29).
Generation Of Pyruvate Carboxylase Knockdown Cell Lines—The stably transfected control cell lines CHS and U6 were generated by transfection of INS-1 832/13 cells with pSilencer2.1-U6/Hygro negative control vector (Ambion) and pRNA-U6.1/Hygro (GenScript), respectively, and both cell lines were selected for hygromycin resistance. The first vector contains a 62-bp DNA fragment cloned into the BamHI/HindIII sites of pSilencer2.1-U6/Hygro GATCCACTACCGTTGTTATAGGTGTTCAAGAGACACCTATAACAACGGTAGTTTTTTGGAAA. The two 19-bp inverted repeats (underlined) express siRNA with limited homology to any known sequence in the human, mouse, and rat genomes. The vectors used for the expression of shRNAs were derived from pRNA-U6.1/Hygro, except in two cases the pSilencer2.1-U6/Hygro vector was used (cell lines PCX1 and PCX3). The pRNA-U6.1/Hygro vector contains a 12-bp sequence between the BamHI and the HindIII sites. This sequence was replaced with the 64- or 65-bp DNA inserts that express the various shRNAs. The DNA inserts were cloned into the BamHI and the HindIII sites downstream of the U6 promoter for stable expression of shRNAs that are processed in vitro to generate siRNA. The design of target sequences was performed using Web-based siRNA design engines (Table 1). The transfection of INS-1 832/13 cells was performed using Lipofectamine 2000 reagent (Invitrogen) in 6-well tissue culture plates. Following transfection, the cells were incubated overnight without selection. Subsequently, the cells were grown in the presence of 150 μg/ml hygromycin in INS-1 tissue culture medium (RPMI 1640 medium (contains 11.1 mm glucose) supplemented with 10% fetal calf serum, 1 mm sodium pyruvate, 50 μm β-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin in 10 mm sodium Hepes buffer, pH 7.3 (23, 24, 30). Pools of cells stably transfected with each shRNA vector directed against one pyruvate carboxylase mRNA target (Table 1) were used to study pyruvate carboxylase activity and insulin release. In an attempt to reduce pyruvate carboxylase activity further, two regions of the pyruvate carboxylase mRNA were targeted. Hygromycin-resistant cell lines that stably expressed shRNA against a single pyruvate carboxylase mRNA target were transfected with a pSilencer2.1-U6/Puro vector that expressed siRNA targeted against a second region of pyruvate carboxylase mRNA and would render the cells puromycin-resistant. The hygromycin-resistant cell lines PC118 and PC3064 were transfected with the pyruvate carboxylase gene target constructs containing the puromycin gene, and the cell lines were selected using 150 μg/ml hygromycin and 250 ng/ml puromycin. Cell lines were designated such that the first number refers to the pyruvate carboxylase mRNA target on the hygromycin-resistant vector and the second number refers to the pyruvate carboxylase mRNA target on the puromycin-resistant vector.
TABLE 1.
Nucleotide sequences used to target the pyruvate carboxylase gene mRNA
The nucleotide positions are shown according to the numbering sequence in the rat pyruvate carboxylase mRNA (accession number NM_012744).
| Cell line | Nucleotide positions | Target sequence |
|---|---|---|
| PC118 | 184-202 | GTAATGGTGGCCAACAGAG |
| PCX1 | 296-314 | GGCAGAAAGCTGATGAAGC |
| PC352 | 384-402 | CAAGGAGAATGGTGTAGAT |
| PC169 | 387-405 | GGAGAATGGTGTAGATGCT |
| PC701 | 767-785 | TTGTGGAGAAATTCATTGA |
| PC1422 | 1454-1472 | AGAATGT(G/T) CTCAACAACCAC |
| PC1971 | 2037-2055 | CGTGGTCTTCAAGTTCTGT |
| PC1973 | 2038-2057 | GTGGTCTTCAAGTTCTGTGA |
| PC2887 | 2953-2971 | GTTCCGTTCTAAGGTGCTAA |
| PC3064 | 3130-3148 | GACTTCACGGCTACCTTTG |
| PCX3 | 3291-3309 | GGTGTTCTTTGAACTCAAT |
Real Time PCR mRNA Quantification—Cells grown on 100-mm plates as described above were harvested at 60–80% confluence and washed twice with 10 ml of phosphate-buffered saline. The cells were then lysed on the plates using the RNeasy® minikit (Qiagen), and total RNA was prepared as recommended by the manufacturer. Equal amounts of RNA were reverse-transcribed from each cell line, using the RETRO-script™ kit (Ambion) and oligo(dT) primers. Primers used for the PCR were as follows. For the pyruvate carboxylase mRNA (NM_012744), a 123-bp PCR product was amplified using the primers 5′-GCACAACCATTGATTCGGATG-3′ and 5′-GCACAACCATTGATTCGGATG-3′; for ATP citrate lyase mRNA (NM_016987), a 169-bp PCR product was amplified using the primers 5′-ACCCAGAGGAAGCCTACATTGC-3′ and 5′-TTCGCCAGTTCGTTGACACC-3′; and for glutamate dehydrogenase mRNA (NM_012570), a 154-bp PCR product was amplified using the primers 5′-CGAGAAGCAGTTGACCAAATCC-3′ and 5′-CACTCCTCCAGCATTCAGGTAGAG-3′. All quantitative reverse transcription-PCRs were performed using the following amplification conditions: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 58 °C for 20 s, and extension at 72 °C for 30 s. Amplification and real time analysis were performed using the Syber Green-based MyiQ™ single-color real-time PCR detection system (Bio-Rad). A standard curve prepared from dilutions of INS-1 832/13 cDNA was included with each run, and results for pyruvate carboxylase mRNA were expressed relative to expression of ATP citrate lyase mRNA or glutamate dehydrogenase mRNA.
Insulin Release Studies—Insulin release from the cell lines derived from INS-1 832/13 cells and expressing shRNA targeted against the pyruvate carboxylase mRNA was measured as previously described (23). Cells were maintained in the INS-1 medium (23, 24, 30) described above. Insulin release was performed in 24-well tissue culture plates. One day before an insulin release experiment was to be performed, the glucose concentration in the tissue culture medium was reduced to 5 mm. Two hours before the experiment, the medium was replaced with Krebs-Ringer bicarbonate buffer, pH 7.3 (modified to contain 15 mm Hepes and 15 mm NaHCO3 with the NaCl concentration adjusted to maintain osmolarity at 310) containing 3 mm glucose and 0.5% bovine serum albumin (BSA) (23, 24, 29). Cells were washed once with the Krebs-Ringer Hepes BSA solution, and insulin release was studied in 1 ml of this same solution in the presence or absence of secretagogues. After 1 h at 37 °C, samples of incubation solution were collected and centrifuged to sediment any cells floating in the incubation solution. An aliquot of the supernatant fraction was removed and saved for insulin measurements by radioimmunoassay. The plates were then washed once with Krebs-Ringer solution containing no BSA, water was added to the plates, and the mixture containing the cells was removed and saved for estimation of total protein by the Bradford method using a dye reagent from Bio-Rad.
Insulin Content of Cells—Cells from individual wells of a 24-well plate were suspended in 1 ml of 1 m acetic acid containing 0.1% BSA and sonicated for 10 s (29). The mixture was diluted appropriately to estimate the insulin concentration by radioimmunoassay.
Enzyme Activities—Pyruvate carboxylase and propionyl carboxylase activities were measured in a whole cell homogenate, and the activities of other enzymes were measured in the cytosol of cells prepared as previously described (4, 24). Briefly, cells were washed once with phosphate-buffered saline and treated with 0.05% trypsin, 0.5 mm EDTA in Hepes-buffered saline solution, followed by RPMI 1640 tissue culture medium containing 10% fetal bovine serum. Cells were washed three times with phosphate-buffered saline and suspended in KMSH solution (220 mm mannitol, 70 mm sucrose, and 5 mm potassium Hepes buffer, pH 7.5) containing protease inhibitor mixture (Pierce). The cell homogenate was obtained by two cycles of freezing at –80 °C and thawing with vigorous vortexing and pipetting the sample up and down. The cytosol was the supernatant fraction obtained from centrifuging the whole cell homogenate at 20,000 × g for 10 min. Pyruvate carboxylase enzyme activity was measured by a CO2 fixation assay in a reaction mixture containing 20 mm NaH14CO3, 8 mm pyruvate, 2 mm Na3ATP, 1.6 mm acetyl-CoA, 10 mm MgCl2, 100 mm KCl, 0.1% Triton X-100, and 100 mm Tris-chloride buffer, pH 7.85, and 10 μl of whole cell homogenate in a final volume of 50 μl incubated at 37 °C for 30 min. The reaction was stopped by adding 50 μl of 10% trichloroacetic acid, and after 1 h, 80 μ l of the mixture was removed and placed in a scintillation vial that was left open overnight to eliminate the unincorporated CO2. The next day, 10 ml of Biosafe scintillation mixture was added to the vial, and the carbon fixed was measured. Values from blank test tubes containing no pyruvate were subtracted from test tubes containing the complete reaction mixture to give the rate attributed to pyruvate carboxylase (31, 32). The activity of propionyl-CoA carboxylase was measured the same as for pyruvate carboxylase, except 2 mm propionyl-CoA was present instead of pyruvate and there was no acetyl-CoA in the reaction mixture (31, 32). Activities of ATP citrate lyase (24), malic enzyme (1, 33, 34), NADP isocitrate dehydrogenase (34, 35), glucose-6-phosphate dehydrogenase (34), and aspartate aminotransferase (36) were measured as previously described.
Metabolite Measurements—Alkali-enhanced fluorescence was used to measure malate, citrate, aspartate, alanine, pyruvate, and lactate in the cell lines as previously described in detail (37–39). Briefly, cells were maintained as monolayers on 100-mm plates in the presence of 5 mm glucose in INS-1 medium (23, 24, 30) for 24 h before an experiment was performed as described above. (A single exception to this was when pyruvate was omitted from the INS-1 medium when metabolites were to be measured after stimulation with BCH plus glutamine, because part of the purpose of the experiment was to test whether BCH plus glutamine would stimulate pyruvate formation. We have shown that omitting pyruvate from the INS-1 tissue culture medium containing 5 mm glucose for the final 24 h before an experiment does not alter insulin release.4) The plates were washed and incubated with or without glucose or BCH plus glutamine in the Hepes-fortified Krebs-Ringer bicarbonate buffer for 30 min. Metabolism was stopped with perchloric acid, and metabolites were measured in the KOH-neutralized extract, as previously described (23). NAD(P)(H) levels were measured with slight modifications to the procedure of Queval and Noctor (40). Briefly, 0.5 ml of 0.5 m KOH in ethanol was added to each 100-mm plate of cells to extract NADH and NADPH, and 0.5 ml of 6% perchloric acid was added to a companion plate to extract NAD and NADP (41). Plates were placed at –80 °C for 5–10 min, the cells were then thawed and scraped into a Microfuge test tube, and plates were washed with an additional 0.5 ml of extraction solution. Test tubes were centrifuged at 12,000 × g for 5 min, and the supernatant fractions were removed and neutralized to pH ∼7. Approximately 0.8 ml of the alkaline extract was neutralized with 0.6 ml of 0.5 m triethanolamine plus 0.5 m potassium phosphate, pH 6, and 0.9 ml of the perchloric acid extract was neutralized with about 0.15 ml of 30% KOH. Pyridine nucleotides were measured by enzymatic cycling in a reaction mixture containing 1 mm phenazine methosulfate, 0.14 mm dichloroindophenol, 1 mm EDTA, and 30 μl of extract in 50 mm sodium Hepes buffer, pH 7.5, in a final volume of 200 μl with 0.5 mm glucose 6-phosphate and 10 units/ml glucose-6-phosphate dehydrogenase for NADP and NADPH and 7.5% ethanol and 1250 units/ml yeast alcohol dehydrogenase for NAD and NADH. Enzyme rates were monitored at 600 nm every 30 s beginning immediately after samples were added to the wells of a 96-well plate maintained at room temperature in a Molecular Devices SpectraMax M2 microplate reader. The average slopes from triplicate wells for each sample read between ∼1.5 and ∼6 min were used to calculate the amount of nucleotide.
[14C]Glucose Metabolism—The incorporation by the cell lines of 14C from [U-14C]glucose into acid-precipitable material was measured after incubating cells in suspension in the Krebs-Ringer BSA solution and precipitating cellular material with 20 volumes of 10% trichloroacetic acid, followed by washing the pellet 6–9 times with 10% trichloroacetic acid until the radioactivity in the supernatant fraction of the final wash equaled background levels. Incorporation of 14C into acid-precipitable protein is assumed to be negligible during this short time period as protein synthesis inhibitors, cycloheximide and puromycin, added together to the incubation medium of INS-1 832/13 cells, do not change the amount of 14C incorporated into the analyzed fractions. An aqueous suspension of the washed pellet was then extracted with 2.5 volumes of chloroform/methanol (3:1) to extract lipids. Radioactivity in CO2, the acid precipitate, and the organic fraction was measured by liquid scintillation spectrometry as previously described (1–4, 42, 43).
Data Analysis—Statistical significance was confirmed with Student's t test or the nonparametric Wilcoxon rank sum test.
RESULTS
Reduction Of Pyruvate Carboxylase and Insulin Release—Pyruvate carboxylase mRNA (Fig. 1) and enzyme activities (Figs. 1 and 2) in several cell lines that integrated the shRNA vectors targeted against the pyruvate carboxylase mRNA were significantly reduced as compared with the parent INS-1 832/13 cell line or the cell lines U6 and CHS that integrated the control vectors. The greatest reduction in glucose-induced insulin release was observed in the cell lines PCX3, PC1971, PC1973, PC3064, and PC118 (Fig. 2). Less reduction was observed with other cell lines. Although pyruvate carboxylase activity was 12% lower in the U6 cell line and 20% lower in the CHS cell line than in the INS-1 832/13 cell line, the insulin release from the U6 cell line was about the same as that of the parent INS-1 832/13 cell line, and the insulin release from the CHS cell line was 28% higher than that of the INS-1 832/13 cell line. The decreases in insulin release were proportional to the decreases in mRNA and pyruvate carboxylase enzyme activities. However, in general within individual cell lines, the decrease in mRNA exceeded the decrease in enzyme activity, and the decrease in enzyme activity exceeded the decrease in insulin release (cf. Figs. 1 and 2). The enzyme activity usually needed to be lower than 40–50% of the control value to cause a significant reduction in insulin release. The results obtained with our cell lines PC1422, PC169, PCX1, and PC352 are very similar to the results that Jensen et al. (26) obtained with Ad-siPC2. These cell lines showed 20–45% decreases in pyruvate carboxylase enzyme activity with little or no effect on glucose-stimulated insulin release (Fig. 2). The single exception was with the PC2887 cell line, where a 45% reduction in pyruvate carboxylase enzyme activity was associated with an equal (and significant) lowering of insulin release. It is noteworthy that the PC169 cell line possesses shRNA identical to siPC2 used by Jensen et al. (26). In addition, the targets of PC169 and PC352 (Table 1) and those of Jensen, Ad-siPC1, and Ad-siPC2, have overlapping nucleotide sequences (siPC1 (nucleotides 378–396), siPC2 (nucleotides 387–405), PC169 (nucleotides 387–405), and PC352 (nucleotides 384–402)).
FIGURE 1.
Quantitative reverse transcription-PCR shows decreases in pyruvate carboxylase mRNA correlate with decreases in pyruvate carboxylase enzyme activity in INS-1 cell lines. Results for quantitative reverse transcription-PCR are expressed as the ratio of pyruvate carboxylase mRNA to either ATP citrate lyase mRNA (top) or glutamate dehydrogenase mRNA (bottom) of the same cell line and expressed as a percentage of the same ratios for the INS-1 832/13 parent cell line equal to 100%. Pyruvate carboxylase enzyme activity is expressed as a percentage of pyruvate carboxylase enzyme activity of the 832/13 cell line equal to 100%. (The absolute enzyme activity is mentioned in the legend of Fig. 2.) Values of mRNA are the average of duplicate measurements, and those of pyruvate carboxylase activities are the mean ± S.E. of measurements on 4–9 cell preparations. The ACL 940-12 cell line is 88% deficient in ATP citrate lyase enzyme activity (24) and is shown as a control (bottom).
FIGURE 2.
Decreases in glucose-stimulated insulin release correlate with reductions in pyruvate carboxylase in INS-1 cell lines. Pyruvate carboxylase enzyme activity was measured in pyruvate carboxylase-deficient cell lines generated with shRNA targeting one region of the pyruvate carboxylase gene in INS-1 832/13 cells. Enzyme activity and insulin release are expressed as a percentage of those of the parent INS-1 832/13 control parent cell line equal to 100%. The specific pyruvate carboxylase activity (mean ± S.E.) in the untransfected INS-1 832/13 cell line was 56 ± 3.5 (n = 21) nmol of oxaloacetate formed/min/mg of cell protein. Unstimulated insulin release was 0.7 ± 0.02 (n = 94) milliunits of insulin/mg of cell protein, and glucose (11.1 mm)-stimulated insulin release was 13.8 ± 0.8 (n = 105) milliunits of insulin/mg of cell protein (means ± S.E.) in the untransfected INS-1 832/13 parent control cell line during a 1-h incubation. For the pyruvate carboxylase-targeted cell lines, enzyme activity data are from the mean ± S.E. of 4–9 preparations, and insulin release data are from 16 or more replicate incubations. Insulin release of any control cell line incubated without a secretagogue was always 5% or less of the glucose-stimulated value of the same cell line. Enzyme activity of 40% of the control value or lower and insulin release of 65% of the control value or lower were significantly different from those of any of the three controls (U6, CHS, or 832/13) with p ≤ 0.001 by Student's t test.
In an attempt to cause a further reduction in pyruvate carboxylase activity, cell lines were transfected with a second vector that also targeted the pyruvate carboxylase mRNA. This produced drastic decreases in pyruvate carboxylase activity in some instances. In other instances, the presence of the two shRNAs attenuated the decreases in enzyme activity, probably because competition between two siRNAs targeted against the same mRNA can lessen their efficiency (44, 45). In both types of instance, the degrees of decreases in pyruvate carboxylase were well correlated with the degrees of decreases in insulin release and again a lowering of enzyme activity of more than 50% was required for a decrease in insulin release (Fig. 3). The greatest reductions in pyruvate carboxylase activity and insulin release were seen in cell lines PC118/1971, PC118/3064, and PC118/1973.
FIGURE 3.
Decreases in glucose-stimulated insulin release correlate with reductions in pyruvate carboxylase in INS-1 cell lines with shRNAs targeted against two regions of the pyruvate carboxylase mRNA. These experiments were performed identically to those in Fig. 2, except they were performed with cell lines generated with two shRNAs targeted against separate regions of the pyruvate carboxylase gene. Enzyme data are from 4–12 preparations of cells, and insulin release data are from 9–32 replicate incubations of the pyruvate carboxylase cell lines. Enzyme activity of 40% of the control value or lower and insulin release of 65% of the control value or lower were significantly different from those of any of the three control cell lines (U6, CHS, or 832/13) with p ≤ 0.001 by Student's t test.
Pyruvate by itself is a potent stimulant of insulin release in INS-1 cells (23, 25, 46, 47), and pyruvate-induced insulin release was also decreased in the pyruvate carboxylase-deficient cells (Fig. 4). BCH plus glutamine (10 mm each) is a potent stimulus of insulin release in INS-1 832/13 cells (Fig. 5) (48) and methyl succinate plus β-hydroxybutyrate is fairly potent (47). Insulin release in response to BCH plus glutamine (Fig. 5) as well as to methyl succinate plus β-hydroxybutyrate (Fig. 6) were also decreased in proportion to reductions in pyruvate carboxylase.
FIGURE 4.
Decreased pyruvate-stimulated insulin release in pyruvate carboxylase-deficient cell lines. Results are the mean ± S.E. of ≥16 replicate incubations in each case (PC118/1971, p < 0.001 versus 832/13 parent control and CHS vector control; PC1973, p = 0.025 versus 832/13 and p < 0.005 versus CHS).
FIGURE 5.
Decreased BCH plus glutamine-stimulated insulin release in pyruvate carboxylase-deficient cell lines. Insulin release is expressed as a percentage of the control cell lines equal to 100%. In Experiment 1, BCH (10 mm) plus glutamine (10 mm)-stimulated insulin release of the control INS-1 832/13 cell line was 18.4 ± 1.0 (n = 36) milliunits of insulin/mg of cell protein, and in Experiment 2 it was 23.8 ± 1.2 (n = 28) milliunits of insulin/mg of cell protein for the control CHS cell line (mean ± S.E.). Results are the mean ± S.E. of ≥16 replicate incubations for each cell line and each test condition. Insulin release of 65% of the controls or lower was significantly different from any of the three controls with p < 0.001.
FIGURE 6.
Decreased β-hydroxybutyrate plus methyl succinate-stimulated insulin release in pyruvate carboxylase-deficient cell lines. The concentrations of d-β-hydroxybutyrate and succinic acid methyl ester were 5 and 10 mm, respectively. Results are the mean ± S.E. of 4–20 replicate incubations for each cell line and each test condition (PC118/1971, p < 0.01 versus 832/13 parent control or vector control cell line CHS).
Pyruvate Carboxylase Knockdown Does Not Alter the Levels of Control Enzymes, Insulin Content, and Glucose Oxidation—To obtain some assurance that a decrease in insulin release was not due to an off-target effect, the activities of several metabolic enzymes and insulin content were measured in the cell lines showing the largest decreases in pyruvate carboxylase enzyme activity and insulin release. Table 2 shows the relative specific enzyme activities of several control enzymes in four cell lines with the lowest pyruvate carboxylase activity and lowest glucose-stimulated insulin release. Enzyme activities of propionyl-CoA carboxylase, ATP citrate lyase, malic enzyme, NADP isocitrate dehydrogenase, and aspartate aminotransferase in these cell lines, as well as activities of these enzymes in cell lines with lesser decreases in pyruvate carboxylase and insulin release (that are not shown), were not significantly different from the activities in the control parent INS-1 832/13 cell line. In addition, the relative levels of mRNAs of the ATP citrate lyase and glutamate dehydrogenase genes estimated by quantitative reverse transcription-PCR in the pyruvate carboxylase-deficient cell lines were not significantly different from those of the control cell lines (Fig. 1). Furthermore, the insulin content of the pyruvate carboxylase-deficient cell lines was not significantly different from control cell lines. The insulin contents of the cell lines PC118/1971, U6, and CHS were 214.33 ± 9.39 (n = 7), 227.2 ± 10.933 (n = 3), and 216.29 ± 9.4 (n = 10) milliunits of insulin/mg of protein (mean ± S.E.), respectively. The oxidation of [U-14C]glucose to 14CO2 was similar in control and pyruvate carboxylase knockdown cell lines, indicating that lower levels of the enzyme did not interfere with energy consumption of the cell lines (Table 3).
TABLE 2.
Enzyme activities of control enzymes in some of the pyruvate carboxylase-deficient cell lines with the lowest glucose-stimulated insulin release
Enzyme rates are expressed as a percentage of the control parent INS-1 832/13 cell line equal to 100%. Average specific enzyme activities defined as 100% in nmol of product formed/min/mg of protein were 4 for propionyl-CoA carboxylase, 56 for malic enzyme, 89 for NADP isocitrate dehydrogenase, 167 for ATP citrate lyase, and 953 for aspartate amino transferase.
|
Enzyme
|
Relative enzyme activity
|
||||||
|---|---|---|---|---|---|---|---|
| PC118 | PC1971 | PC1973 | PC118/1971 | ||||
| % of INS-1 832/13 | |||||||
| Propionyl-CoA carboxylase | 88 | 89 | 86 | 106 | |||
| Malic enzyme | 75 | 99 | 135 | 103 | |||
| NADP isocitrate dehydrogenase | 117 | 94 | 104 | 105 | |||
| ATP citrate lyase | 102 | 94 | 81 | 112 | |||
| Aspartate aminotransferase | 126 | 120 | 99 | 109 | |||
TABLE 3.
Decreased carbon incorporation from glucose into lipid and no decrease in glucose oxidation to CO2 in pyruvate carboxylase-deficient cells
Cell lines stably transfected with shRNA targeting the pyruvate carboxylase mRNA or a control vector (U6) were incubated for 45 min with 16.7 mm [U-14C]glucose (specific radioactivity 0.75 mCi/mmol glucose). Radioactivity incorporated into a lipid extract and CO2 was measured. Results are the mean ± S.E. of four replicate incubations for each condition.
|
Cell line
|
Glucose incorporation
|
||
|---|---|---|---|
| Into lipid fraction | Into CO2 | ||
| nmol/mg cell protein | |||
| U6 (control) | 0.92 ± 0.06 | 2.2 ± 0.1 | |
| PC118/1971 | 0.73 ± 0.04a | 2.0 ± 0.1 | |
| PC118/3064 | 0.64 ± 0.01b | 2.2 ± 0.1 | |
| PC118/1973 | 0.65 ± 0.01b | 2.4 ± 0.1 | |
p < 0.05 versus control.
p < 0.005 versus control.
Metabolite Levels Are Consistent with a Block at Pyruvate Carboxylase—Cells with knocked down pyruvate carboxylase and cells transfected with the control vectors were incubated with or without 16.7 mm glucose for 30 min, and then malate, citrate, pyruvate, and lactate were measured. Glucose stimulated increases in the levels of malate, citrate, and pyruvate plus lactate in both the control cell lines and the pyruvate carboxylase knockdown cells. With decreased pyruvate carboxylase activity, the expected metabolite pattern in glucose-stimulated cells versus control cells is lower malate and citrate levels and, depending on the rate of flux of pyruvate through the pyruvate carboxylase reaction versus the rate of pyruvate flux through other pathways, possibly higher pyruvate plus lactate levels. This is the metabolite pattern that was seen (Fig. 7). Malate, which is derived directly from oxaloacetate, was lower in each of the two cell lines studied compared with the control cell lines whether the cells were stimulated with glucose or not. The malate level was very low (p < 0.0005) in the PC118/1971 cell line, which is the cell line with the lower pyruvate carboxylase activity of the two cell lines studied. The lower malate level in the glucose-stimulated PC118 cell line did not achieve statistical significance. Levels of citrate, which is also a metabolite derived directly from oxaloacetate (although formed by acetyl-CoA condensing with oxaloacetate, so that the flux of acetyl-CoA will also influence citrate levels), were lower in unstimulated and glucose-stimulated cells than in their respective control cells (p < 0.05 for the PC118/1971 cell line and not significant for the PC118 cell line). The unstimulated and glucose-stimulated pyruvate plus lactate levels in both the PC118 and PC118/1971 cell lines were significantly higher than in their respective control cell lines (p < 0.01 to p < 0.05) (Fig. 7).
FIGURE 7.
Lower malate and citrate and increased pyruvate plus lactate in glucose-stimulated pyruvate carboxylase-deficient INS-1 cells. Cells were incubated without or with 16.7 mm glucose for 30 min. For each condition, results are the mean ± S.E. for four replicate incubations in Experiment 1 and eight replicate incubations in Experiment 2. a, p < 0.0005; b, p ≤ 0.05; c, p < 0.01 for the same metabolite and the same condition for the pyruvate carboxylase-deficient cell line versus the control CHS (Experiment 1) or U6 (Experiment 2) cell line in the same experiment.
In control and pyruvate carboxylase knockdown cell lines incubated with BCH plus glutamine, the levels of citrate, malate, and pyruvate plus lactate were increased. However, in the PC118/1973 and PC118/3064 cell lines, the BCH plus glutamine-induced increases in citrate were severely blunted versus the control cell line (p < 0.005). The level of aspartate, which is formed by the transamination of oxaloacetate with glutamate, was relatively unchanged in the PC118/1973 cell line, but aspartate was decreased in the unstimulated and stimulated PC118/3064 cell line relative to the control cell line (Fig. 8). In contrast to the cells incubated with glucose (Fig. 7), in the BCH plus glutamine-stimulated cells, malate levels were not affected very much by pyruvate carboxylase deficiency. The malate level in the PC118/3064 cell line was decreased compared with the control cell line but did not quite achieve statistical significance (0.05 < p < 0.1). The reason malate levels were not affected by pyruvate carboxylase deficiency is probably that malate is both a precursor of pyruvate and one metabolite removed from oxaloacetate, the product of the pyruvate carboxylase reaction when glutamine is the source of carbon (see “Discussion”). Also consistent with a block at pyruvate carboxylase, the BCH plus glutamine-stimulated concentrations of lactate plus pyruvate were increased in both pyruvate carboxylase-deficient cell lines versus the control cell line, but the differences also did not quite achieve statistical significance (0.05 < p < 0.1). The concentrations of alanine, which can be formed by transamination of pyruvate and glutamate, were similar among the control and pyruvate carboxylase-deficient cell lines (Fig. 8).
FIGURE 8.
Lower citrate and/or aspartate and/or malate and higher pyruvate plus lactate in BCH plus glutamine-stimulated pyruvate carboxylase-deficient cell lines. Cells were incubated without or with 10 mm BCH plus 10 mm glutamine for 30 min. Values are the mean ± S.E. of four replicate incubations per cell line and condition. a, p < 0.005; b, p < 0.01; c, 0.05 ≤ p ≤ 0.1 for the same metabolite and the same condition for a pyruvate carboxylase-deficient cell line versus the vector control cell line CHS.
Effect of Pyruvate Carboxylase Knockdown on NADPH Levels—One of the purposes of a pyruvate malate shuttle (4) or a pyruvate citrate shuttle (17, 18) involving pyruvate carboxylase might be the export of NADPH equivalents from mitochondria to the cytosol (8). Therefore, NADPH levels in pyruvate carboxylase knockdown and control cells were compared. NADPH patterns in individual cell lines were consistent among experiments, and there was no difference in NADPH levels between control cell lines and pyruvate carboxylase knockdown cells when the cells were stimulated with a secretagogue. However, the unstimulated levels of NADPH in two of the three pyruvate carboxylate knockdown cell lines studied were lower in separate experiments performed months apart. NADPH levels in the unstimulated and glucose (16.7 mm)-stimulated pyruvate carboxylase-deficient PC118/1971 cell lines were the same or insignificantly lower than similarly treated control U6 cells after a 30-min incubation (unstimulated, 171 ± 8 (U6) versus 181 ± 6 (PC118/1971); stimulated, 359 ± 13 (U6) versus 314 ± 25 (PC118/1971) pmol of NADPH/mg of cell protein (means ± S.E., n = 6 each)). In contrast, in a separate experiment with two other pyruvate carboxylase cell lines, unstimulated NADPH levels were lower after a 30-min incubation, although glucose (16.7 mm)-stimulated NADPH levels in the control and pyruvate carboxylase knockdown cell lines were similar (unstimulated, 180 ± 3 (CHS control) versus 125 ± 5 (PC118/1973) or versus 136 ± 5 (PC118/3064) (p < 0.001 for each comparison); stimulated, 198 ± 8 (CHS) versus 194 ± 12 (PC118/1973) or versus 188 ± 7 (PC118/3064) pmol of NADPH/mg of cell protein (means ± S.E., n = 4 each)). There was also no difference in NADPH concentrations between pyruvate carboxylase knockdown cells and control cells stimulated with BCH plus glutamine (10 mm each) for 30 min (220 ± 4, 216 ± 8, and 244 ± 11 pmol of NADPH/mg of total cell protein in PC118/1973 cells, PC118/3064 cells, and CHS control cells, respectively (means ± S.E., n = 8 each)). However, in each of the two experiments with the cell lines used for this analysis, unstimulated levels of NADPH were again lower in the pyruvate carboxylase knockdown cell lines after a 30-min incubation (128 ± 3 and 131 ± 8 versus 170 ± 6 pmol of NADPH/mg of cell protein in PC118/1973 cells, PC118/3064 cells, and CHS control cells, respectively (means ± S.E., n = 8 each) p ≤ 0.001).
Effect of Pyruvate Carboxylase Knockdown on NADH Levels in BCH Plus Glutamine-stimulated Cells—When BCH and glutamine are applied to beta cells, α-ketoglutarate is formed by the activation of glutamate dehydrogenase, and through a series of reactions, α-ketoglutarate can be converted to malate (see “Discussion”). Malate can be converted to oxaloacetate directly via the mitochondrial malate dehydrogenase reaction or indirectly via a malic enzyme reaction plus the pyruvate carboxylase reaction. The conversion of malate to oxaloacetate in the malate dehydrogenase reaction should increase NADH, whereas the formation of oxaloacetate via the indirect pathway involving pyruvate carboxylase should not. Increased NADH inhibits several reactions of the citric acid cycle (see “Discussion”), and this could contribute to the causation of inhibited insulin release seen in the pyruvate carboxylase knockdown cell lines stimulated with BCH plus glutamine. Unstimulated NADH levels in the cell lines averaged 400 ± 15 pmol/mg of cell protein, and the level of NAD, the bulk of which resides in the cytosol of most cell types, did not change significantly with BCH plus glutamine stimulation and averaged 2300 ± 80 pmol/mg of cell protein. However, when the cells were incubated in the presence of BCH plus glutamine for 30 min, whole cell NADH levels in the PC118/1973 and PC118/3064 cell lines increased 55 ± 2% (p < 0.001) and 38 ± 2% (p < 0.01), respectively, versus 28 ± 1.6% in the control CHS cell line in one experiment and NADH increased 14 ± 3.5% in the PC118/1973 cell line (p < 0.01) and 18 ± 1.3% in the PC118/3064 cell line (p < 0.001) versus a 4.5 ± 3% decrease in the control CHS cell line in a second experiment (means ± S.E., n = 4 each in each experiment). These small differences in cellular NADH levels between pyruvate carboxylase-deficient and control cell lines stimulated with BCH plus glutamine suggest that a lower rate of NADH production that occurs when mitochondrial oxaloacetate is formed in the pyruvate carboxylase reaction instead of, or in addition to, via malate dehydrogenase might be at most one factor among several for the requirement of pyruvate carboxylase in BCH plus glutamine-induced insulin release.
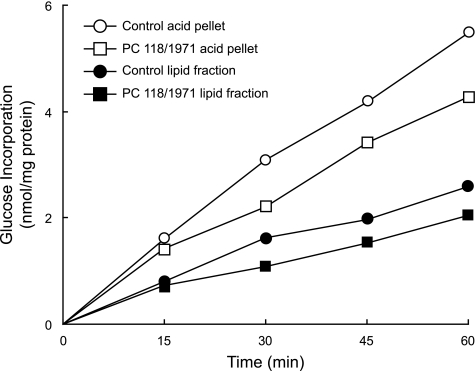
[14C]Glucose Incorporation into Products Of Anaplerosis—Incorporation of radioactive carbon from an insulinotropic concentration of [U-14C]glucose into acid-precipitable material or lipid was decreased 20–30% in pyruvate carboxylase-deficient cell lines compared with the control cell lines when the cell lines were incubated for various time points up to 1 h (Fig. 9) or for 45 min (Table 3) (p < 0.05 or p < 0.005 versus the control).
FIGURE 9.
Decreased incorporation of glucose into acid-precipitable products and lipid in pyruvate carboxylase-deficient cells. Cell lines transfected with shRNA targeting pyruvate carboxylase mRNA (PC118/1971) or a control vector (CHS) were incubated for various times with 16.7 mm glucose (specific radioactivity 0.75 mCi/mmol glucose), and metabolism was stopped with trichloroacetic acid. Radioactivity incorporated into the acid-washed cell pellet, as well as a lipid extract of the pellet, was measured. Each data point is the mean of duplicate incubations for each condition.
DISCUSSION
Knockdown Of Pyruvate Carboxylase mRNA, Enzyme Activity, and Insulin Release—In INS-1 832/13 cell lines with up to 90% reductions in pyruvate carboxylase mRNA (Fig. 1) and enzyme activity, the inhibition of insulin release by various secretagogues was proportional to the degree of lowering of pyruvate carboxylase activity (Figs. 2, 3, 4, 5, 6). The activity of nontargeted metabolic enzymes, the insulin contents, and glucose oxidation were not altered in the pyruvate carboxylase deficient cell lines. In general, a 60% or larger reduction in pyruvate carboxylase enzyme activity was required to produce a significant lowering of insulin release, and in individual cell lines the decrease in the enzyme activity was usually proportionately greater than the decrease in insulin release (Figs. 2 and 3). In addition, the decrease in pyruvate carboxylase mRNA in individual cell lines was usually greater than the decrease in enzyme activity (Fig. 1).
A Metabolite Crossover at the Pyruvate Carboxylase Reaction Confirms the Site of Inhibited Glucose-stimulated Insulin Release—Pyruvate carboxylase catalyzes the carboxylation of pyruvate to oxaloacetate and when oxaloacetate combines with acetyl-CoA to form citrate in the reaction catalyzed by citrate synthase, the level of any citric acid cycle intermediate can be increased (Fig. 10). To test the specificity of the targeting of pyruvate carboxylase in the various cell lines, we measured the levels of pyruvate, a substrate for the reaction catalyzed by the enzyme, and several metabolic intermediates near oxaloacetate, a product of the reaction, in cells stimulated with glucose. When the activity of pyruvate carboxylase is decreased, the level of oxaloacetate and citric acid cycle intermediates near oxaloacetate are likely to be decreased. The very low concentration of oxaloacetate in most cells and its chemical lability make accurate measurements of oxaloacetate difficult. Therefore, we measured the concentrations of malate and citrate, which are each one step removed from oxaloacetate. We also measured lactate, the redox partner of pyruvate. In cells with decreased pyruvate carboxylase activity, the predicted metabolite pattern is a crossover point at the pyruvate carboxylase reaction with decreased malate and citrate and possibly increased pyruvate and lactate (Fig. 10). This pattern was seen in glucose-stimulated cells, and there was even a tendency toward this metabolite pattern in the cells in the absence of added glucose (Fig. 7).
FIGURE 10.
Pathways of insulin secretagogue-derived metabolites and their conversion to anaplerotic products in the pancreatic beta cell. Malic enzyme(s), the cytosolic NADP malic enzyme and/or the mitochondrial NAD(P)- and NAD-specific malic enzymes. NAD isocitrate dehydrogenase is the enzyme of the citric acid cycle. Enzymes mentioned in the plural form indicate mitochondrial and cytosolic isoforms of enzymes that catalyze the same reaction.
Inhibition of Insulin Release in the Presence of Nonglucose Secretagogues—Insulin secretion in the pyruvate carboxylase-deficient cells also showed decreased responses to nonglucose stimuli, including pyruvate (Fig. 4) (which is a potent insulin stimulant in INS-1 cells (23, 25, 47), probably because INS-1 cells possess a plasmalemmal monocarboxylate transporter (46) enabling the rapid uptake of pyruvate into the cells), methyl succinate plus β-hydroxybutyrate (Fig. 6) (which together also form a fairly strong stimulant of insulin release in INS-1 cells (23, 47)), and 10 mm BCH plus 10 mm glutamine (which together are a potent stimulant of insulin in INS-1 cells (48) (Fig. 5)).
Normal Glucose Oxidation but Decreased Carbon Incorporation into Products of Anaplerosis in Pyruvate Carboxylase Knockdown Cells—Glucose and other insulin secretagogues acutely stimulate 20–30% increases in various individual lipids and lipid classes in INS-1 832/13 cells (42). In cell lines with decreased pyruvate carboxylase activity, the rate of incorporation of 14C from [U-14C]glucose into acid-precipitable material and/or lipid was decreased 20–30% compared with control cell lines (Fig. 9 and Table 3) during a 45-min incubation period. This strengthens the idea that normal insulin secretion requires pyruvate carboxylase-derived oxaloacetate that can enable the formation of metabolites that shuttle precursors of anaplerotic products from the mitochondria to the cytosol (8, 9, 17–19, 23, 24, 42, 48). In these same experiments, glucose oxidation to CO2 was not decreased. This is consistent with the lower rate of anaplerosis not being caused by decreased energy production.
Metabolite Patterns in the Presence Of BCH plus Glutamine Also Confirm Pyruvate Carboxylase as the Inhibited Reaction—Although it is easy to understand the involvement of pyruvate carboxylase in anaplerosis from glucose and pyruvate, a role for the enzyme in stimulation of insulin release by noncarbohydrate secretagogues might be somewhat surprising. A very interesting result of our study is the demonstration of a role for pyruvate carboxylase in the stimulation of insulin release by noncarbohydrate secretagogues (e.g. by BCH in the presence of a high concentration of glutamine). BCH is a nonmetabolizable analog of leucine that allosterically activates glutamate dehydrogenase (49, 50), enabling the large amount of glutamate formed from glutamine to be converted to α-ketoglutarate (48–50) (reviewed in Ref. 8) (Fig. 10).
The inhibited BCH plus glutamine-induced insulin release in the pyruvate carboxylase-deficient cells (Fig. 5) suggests that pyruvate carboxylase is necessary for the beta cell to convert glutamate to metabolic intermediates that support insulin secretion. This conversion could occur via the reactions of the citric acid cycle between α-ketoglutarate and malate followed by malate's conversion to pyruvate catalyzed by either the cytosolic or the mitochondrial malic enzyme (Fig. 10) (4, 8, 21). (However, there is evidence that the route of glutamate metabolism in the beta cell preferentially uses the mitochondrial malic enzyme (21).) Pyruvate carboxylase could then convert pyruvate to oxaloacetate. An alternative, even more indirect pathway also culminates with the pyruvate carboxylase reaction. This pathway begins with the conversion of α-ketoglutarate to citrate via the combined actions of mitochondrial and/or cytosolic NADP isocitrate dehydrogenase and aconitase, essentially reversing two reactions of the citric acid cycle. (We have shown that this can occur in beta cell mitochondria4 (8) as it can in other types of cells (51, 52).) ATP citrate lyase would then cleave citrate to acetyl-CoA and oxaloacetate. The oxaloacetate would be reduced to malate catalyzed by cytosolic malate dehydrogenase, and the malate would be oxidatively decarboxylated to pyruvate by a malic enzyme to feed the pyruvate carboxylase reaction. Indeed, in control cells, as well as in pyruvate carboxylase-deficient cells, lactate and pyruvate were increased in the presence of BCH plus glutamine (Fig. 8), indicating that glutamate carbon derived from the glutamine, which was the only metabolizable compound applied to the cells, was converted to pyruvate via the reactions of one or both of the malic enzymes. In addition, in the pyruvate carboxylase-deficient cells, a metabolic crossover point consistent with a block at the pyruvate carboxylase reaction was seen when the cells were stimulated with BCH plus glutamine. There were marked reductions in citrate in both of the pyruvate carboxylase-deficient cell lines compared with the control cell line and a slight reduction in aspartate and malate in one of the pyruvate carboxylase-deficient cell lines studied. In both pyruvate carboxylase-deficient cell lines, pyruvate plus lactate was increased compared with the control cell line (Fig. 8). The decreased citrate level can be explained by decreased oxaloacetate available for condensation with acetyl-CoA in the citrate synthase reaction caused by the lowered pyruvate carboxylase activity (Fig. 10). The decreased level of aspartate in the pyruvate carboxylase-deficient cells is consistent with a low amount of oxaloacetate available for transamination with glutamate.
The reason that malate was not decreased in the pyruvate carboxylase-deficient cell lines when they were stimulated with BCH plus glutamine to the extent it was when the cells were stimulated with glucose is probably that malate derived from glutamate can be both a precursor and a downstream product of the pyruvate carboxylase reaction. In order for pyruvate to feed the pyruvate carboxylate reaction, malate must be formed from α-ketoglutarate, and then malate must be converted to pyruvate via a malic enzyme. Thus, in addition to being one metabolite removed from oxaloacetate, the product of the pyruvate carboxylase reaction, malate is also an immediate precursor of pyruvate the substrate for the enzyme (Fig. 10).
Implications about Anaplerosis in Insulin Release Deduced from Noncarbohydrate Insulin Secretagogues in Control and Pyruvate Carboxylase Knockdown Cells—The results of the experiments with BCH plus glutamine indicate that for insulin secretion, the anaplerotic input of pyruvate carboxylase-generated oxaloacetate into mitochondrial pathways is necessary although a secretagogue carbon has a direct upstream anaplerotic entrance into the citric acid cycle. This other entrance would be at α-ketoglutarate catalyzed by glutamate dehydrogenase for glutamate derived from glutamine in BCH plus glutamine-stimulated cells and at succinate in methyl succinate plus β-hydroxybutyrate-stimulated cells (Fig. 10).
One possible explanation for the necessity of mitochondrial oxaloacetate formation via a pathway involving pyruvate carboxylase versus the more direct conversion via mitochondrial malate dehydrogenase (Fig. 10) is that the latter reaction generates additional NADH in the mitochondria, whereas a pathway of oxaloacetate formation via the malic enzyme and pyruvate carboxylase reactions would generate less NADH. NADH inhibits the pyruvate dehydrogenase complex as well as enzymes of the citric acid cycle, including citrate synthase and three enzymes that utilize NAD as a substrate: isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and malate dehydrogenase itself (Fig. 10). In cells with knocked down pyruvate carboxylase, relatively more mitochondrial oxaloacetate should be formed via malate dehydrogenase, thus further increasing NADH. Although cellular NADH was increased more by BCH plus glutamine in the pyruvate carboxylase knockdown cell lines than in the control cell lines, the differences were relatively small. Thus, the ability to attenuate an increase in NADH levels may be only one factor among several for the necessary involvement of pyruvate carboxylase in BCH plus glutamine-induced insulin release.
In BCH plus glutamine-stimulated cells, the only known pathway for the formation of pyruvate from glutamine-derived glutamate to feed the pyruvate carboxylase reaction involves a malic enzyme reaction. Thus, the BCH plus glutamine results support previous studies that found evidence for a cycle of flux of carbon from malate to pyruvate and then to oxaloacetate and malate through a malic enzyme, pyruvate carboxylase, and the mitochondrial malate dehydrogenase reactions in beta cells (4, 19–21, 53) (Fig. 10) as well as a study by Simpson (54), who found evidence for two anaplerotic entrances into the citric acid cycle in beta cells. In regard to the essentiality of shuttles that utilize pyruvate carboxylase and malic enzyme for the formation of cytosolic NADPH in the beta cell (4, 17–19, 21, 55), this remains an open hypothesis, since there are other cytosolic enzymes that can form NADPH that are present in the beta cell, including NADP-dependent isocitrate dehydrogenase (8, 34, 56) and glucose-6-phosphate dehydrogenase (8, 34). However, it is noteworthy that, although NADPH increased to similar levels in pyruvate carboxylase-deficient cell lines and control cell lines when they were stimulated with glucose or BCH plus glutamine, unstimulated NADPH levels were lower in two of the three pyruvate carboxylase-deficient cell lines studied.
Conclusions—In cell lines with severely reduced pyruvate carboxylase activity, insulin release in response to glucose and noncarbohydrate metabolizable secretagogues was lowered, indicating that many secretagogues require pyruvate carboxylase for stimulating insulin secretion. A metabolic crossover point at the pyruvate carboxylase reaction with decreased citrate and/or malate and/or aspartate and increased pyruvate plus lactate in unstimulated and in glucose- or BCH plus glutamine-stimulated pyruvate carboxylase knockdown cells was consistent with inhibition of pyruvate carboxylase as the cause of the decreased insulin release. Incorporation of carbon from glucose into lipid was decreased in the cell lines with decreased pyruvate carboxylase, indicating that downstream products of anaplerosis support insulin secretion. The results provide direct evidence for the necessity of pyruvate carboxylase and anaplerosis in insulin secretion. The participation of pyruvate carboxylase in anaplerosis from glucose is obvious, since the only known pathway for the net synthesis of citric acid cycle intermediates from glucose involves pyruvate carboxylase. The necessity for the participation of the enzyme in anaplerosis from nonglucose secretagogues is less obvious, but nonetheless the results conclusively show that the enzyme is required for insulin release from nonglucose insulin secretagogues.
Acknowledgments
We thank Jens C. Eickhoff for advice on statistical analysis.
This work was supported, in whole or in part, by National Institutes of Health Grant DK28348. This work was also supported by a grant from the Oscar C. Rennebohm Foundation (to M. J. M.); a Career Development Grant (Faculty of Science, Mahidol University) and Thailand Research Fund RSA Grant 4680002 (to S. J.); and Australian Research Council Grant DP0346807 (to J. C. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: siRNA, small interfering RNA; BCH, 2-aminobicyclo[2,2,1]heptane-2-carboxylic acid; BSA, bovine serum albumin; siRNA, small interfering RNA.
M. J. MacDonald, unpublished observation.
References
- 1.MacDonald, M. J. (1993) Arch. Biochem. Biophys. 300 201–205 [DOI] [PubMed] [Google Scholar]
- 2.MacDonald, M. J. (1993) Arch. Biochem. Biophys. 305 205–214 [DOI] [PubMed] [Google Scholar]
- 3.MacDonald, M. J. (1993) Metabolism 42 1229–1231 [DOI] [PubMed] [Google Scholar]
- 4.MacDonald, M. J. (1995) J. Biol. Chem. 270 20051–20058 [PubMed] [Google Scholar]
- 5.Khan, A., Ling, Z. C., and Landau, B. R. (1996) J. Biol. Chem. 271 2539–2542 [DOI] [PubMed] [Google Scholar]
- 6.Brunengraber, H., and Roe, C. R. (2006) J. Inherit. Metab. Dis. 29 327–331 [DOI] [PubMed] [Google Scholar]
- 7.Jitrapakdee, S., St. Maurice, M., Rayment, I., Cleland, W. W., Wallace, J. C., and Attwood, P. V. (2008) Biochem. J. 413 369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald, M. J., Fahien, L. A., Brown, L. J., Hasan, N. M., Buss, J. D., and Kendrick, M. A. (2005) Am. J. Physiol. 288 E1–E15 [DOI] [PubMed] [Google Scholar]
- 9.Jitrapakdee, S., Vidal-Puig, A., and Wallace, J. C. (2006) Cell. Mol. Life Sci. 63 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald, M. J., Kaysen, J. H., Moran, S. M., and Pomije, C. E. (1991) J. Biol. Chem. 266 22392–22397 [PubMed] [Google Scholar]
- 11.MacDonald, M. J., McKenzie, D. I., Walker, T. M., and Kaysen, J. H. (1992) Horm. Metab. Res. 24 158–160 [DOI] [PubMed] [Google Scholar]
- 12.Louis, N. A., and Witters, L. A. (1992) J. Biol. Chem. 267 2287–2293 [PubMed] [Google Scholar]
- 13.Schuit, F., De Vos, A., Farfari, S., Moens, K., Pipeleers, D., Brun, T., and Prentki, M. (1997) J. Biol. Chem. 272 18572–18579 [DOI] [PubMed] [Google Scholar]
- 14.Hedescov, C. J., Capito, K., and Thams, P. (1984) Biochim. Biophys. Acta 791 37–44 [DOI] [PubMed] [Google Scholar]
- 15.MacDonald, M. J., and Chang, C. M. (1985) Diabetes 34 246–250 [DOI] [PubMed] [Google Scholar]
- 16.Jitrapakdee, S., Gong, Q., MacDonald, M. J., and Wallace, J. C. (1998) J. Biol. Chem. 273 34422–34428 [DOI] [PubMed] [Google Scholar]
- 17.Farfari, S., Schulz, V., Corkey, B., and Prentki, M. (2000) Diabetes 49 718–726 [DOI] [PubMed] [Google Scholar]
- 18.Flamez, D., Berger, V., Kruhoffer, M., Orntoft, T., Pipeleers, D., and Schuit, F. C. (2002) Diabetes 51 2018–2024 [DOI] [PubMed] [Google Scholar]
- 19.Lu, D., Mulder, H., Zhao, P., Burgess, S. C., Jensen, M. V., Kamzolova, S., Newgard, C. B., and Sherry, A. D. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 2708–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cline, G. W., Lepine, R. L., Papas, K. K., Kibbey, R. G., and Shulman, G. I. (2004) J. Biol. Chem. 279 44370–44375 [DOI] [PubMed] [Google Scholar]
- 21.Pongratz, R. L., Kibbey, R. G., Shulman, G. I., and Cline, G. W. (2007) J. Biol. Chem. 282 200–207 [DOI] [PubMed] [Google Scholar]
- 22.Sunyakumthorn, P., Boonsaen, T., Boonsaeng, V., Wallace, J. C., and Jitrapakdee, S. (2005) Biochem. Biophys. Res. Commun. 329 188–196 [DOI] [PubMed] [Google Scholar]
- 23.MacDonald, M. J. (2007) J. Biol. Chem. 282 6043–6052 [DOI] [PubMed] [Google Scholar]
- 24.MacDonald, M. J., Smith, A. D., III, Hasan, N. M., Sabat, G., and Fahien, L. A. (2007) J. Biol. Chem. 282 30596–30606 [DOI] [PubMed] [Google Scholar]
- 25.Antinozzi, P. A., Garcia-Diaz, A., Hu, C., and Rothman, J. E. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 3698–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen, M. V., Joseph, J.W., Ilkayeva, O., Burgess, S., Lu, D., Ronnebaum, S. M., Odegaard, M., Becker, T. C., Sherry, A. D., and Newgard, C. B. (2006) J. Biol. Chem. 281 22342–22351 [DOI] [PubMed] [Google Scholar]
- 27.Shack, S., Miller, A., Liu, L., Prasanna, P., Thibault, A., and Samid, D. (1996) Clin. Cancer Res. 2 865–872 [PubMed] [Google Scholar]
- 28.Samid, D., Wells, M., Greene, M. E., Shen, W., Palmer, C. N., and Thibault, A. (2000) Clin. Cancer Res. 6 933–941 [PubMed] [Google Scholar]
- 29.Hohmeier, H. E., Mulder, H., Chen, G., Henkel-Rieger, R., Prentki, M., and Newgard, C. B. (2000) Diabetes 49 424–430 [DOI] [PubMed] [Google Scholar]
- 30.Asfari, M., Janjic, D., Meda, P., Li, G., Halban, P. A., and Wollhein, C. B. (1992) Endocrinology 130 167–178 [DOI] [PubMed] [Google Scholar]
- 31.MacDonald, M. J., Efendic, S., and Ostenson, C. G. (1996) Diabetes 45 886–890 [DOI] [PubMed] [Google Scholar]
- 32.MacDonald, M. J., Tang, J., and Polonsky, K. S. (1996) Diabetes 45 1626–1630 [DOI] [PubMed] [Google Scholar]
- 33.MacDonald, M. J. (2002) Am. J. Endocrinol. Metab. 283 302–310 [DOI] [PubMed] [Google Scholar]
- 34.MacDonald, M. J., and Marshall, L. K. (2001) Mol. Cell. Biochem. 220 117–125 [DOI] [PubMed] [Google Scholar]
- 35.MacDonald, M. J. (1981) Endocrinology 108 1899–1902 [DOI] [PubMed] [Google Scholar]
- 36.MacDonald, M. J. (1982) Arch. Biochem. Biophys. 213 643–649 [DOI] [PubMed] [Google Scholar]
- 37.MacDonald, M. J. (2003) Biochim. Biophys. Acta 1619 77–88 [DOI] [PubMed] [Google Scholar]
- 38.MacDonald, M. J. (2004) Mol. Cell. Biochem. 258 201–210 [DOI] [PubMed] [Google Scholar]
- 39.MacDonald, M. J., Chaplen, M. J., Triplett, C. K., Gong, Q., and Drought, H. (2006) Arch. Biochem. Biophys. 447 118–126 [DOI] [PubMed] [Google Scholar]
- 40.Queval, G., and Noctor, G. (2007) Anal. Biochem. 363 58–69 [DOI] [PubMed] [Google Scholar]
- 41.Bergmeyer, H. U., Bergmeyer, J., and Grassl, M. (eds) (1985) Methods of Enzymatic Analysis, Vol. VII, 3rd Ed., pp. 261–267, VCH, Weinheim, Germany [Google Scholar]
- 42.MacDonald, M. J., Dobrzyn, A., Ntambi, J., and Stoker, S. W. (2008) Arch. Biochem. Biophys. 470 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald, M. J., Stoker, S. W., and Hasan, N. M. (2008) Mol. Cell. Biochem. 313 195–202 [DOI] [PubMed] [Google Scholar]
- 44.Vickers, T. A., Lima, W. F., Nichols, J. G., and Crooke, S. T. (2007) Nucleic Acids Res. 35 6598–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castanotto, D., Sakurai, K., Lingeman, R., Li, H., Shively, L., Aagaard, L., Soifer, H., Gatignol, A., Riggs, A., and Rossi, J. J. (2007) Nucleic Acids Res. 35 5154–5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antinozzi, P. A., Ishihara, H., Newgard, C. B., and Wollheim, C. B. (2002) J. Biol. Chem. 277 11746–11755 [DOI] [PubMed] [Google Scholar]
- 47.MacDonald, M. J., Longacre, M. J., Stoker, S. W., Brown, L. J., Hasan, N. M., and Kendrick, M. A. (2008) Am. J. Physiol. 294 C442–C450 [DOI] [PubMed] [Google Scholar]
- 48.MacDonald, M. J., Hasan, N. M., and Longacre, M. J. (2008) Biochim. Biophys. Acta 1780 966–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gylfe, E. (1976) Acta Diabetol. Lat. 13 20–24 [DOI] [PubMed] [Google Scholar]
- 50.Sener, A., Malaisse-Lage, F., and Malaisse, W. J. (1981) Proc. Natl. Acad. Sci. U. S. A. 78 5460–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Comte, B., Vincent, G., Bouchard, B., Benderdour, M., and Des Rosiers, C. (2002) Am. J. Physiol. 283 H1505–H1514 [DOI] [PubMed] [Google Scholar]
- 52.Yang, L., Kasumov, T., Kombu, R. S., Zhu, S. H., Cendrowski, A. V., David, F., Anderson, V. E., Kelleher, J. K., and Brunengraber, H. (2008) J. Biol. Chem. 283 21988–21996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, C., Nissim, I., Chen, P., Buettger, C., Najafi, H., Daikhin, Y., Nissim, I., Collins, H. W., Yudkoff, M., Stanley, C. A., and Matschinsky, F. M. (2008) J. Biol. Chem. 283 17238–17249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson, N. E., Khokhlova, N., Oca-Cossio, J. A., and Constantinidis, I. (2006) Diabetologia 49 1338–1348 [DOI] [PubMed] [Google Scholar]
- 55.Guay, C., Madiraju, S. R., Aumais, A., Joly, E., and Prentki, M. (2007) J. Biol. Chem. 282 35657–35665 [DOI] [PubMed] [Google Scholar]
- 56.Ronnebaum, S. M., Ilkayeva, O., Burgess, S. C., Joseph, J. W., Lu, D., Stevens, R. D., Becker, T. C., Sherry, A. D., Newgard, C. B., and Jensen, M. V. (2006) J. Biol. Chem. 281 30593–30602 [DOI] [PubMed] [Google Scholar]