Abstract
Hepatitis B virus X protein (pX) is implicated in hepatocellular carcinoma pathogenesis by an unknown mechanism. Employing the tetracycline-regulated pX-expressing 4pX-1 cell line, derived from the murine AML12 hepatocyte cell line, we demonstrate that pX induces partial polyploidy (>4N DNA). Depletion of p53 in 4pX-1 cells increases by 5-fold the polyploid cells in response to pX expression, indicating that p53 antagonizes pX-induced polyploidy. Dual-parameter flow cytometric analyses show pX-dependent bromodeoxyuridine (BrdUrd) incorporation in 4pX-1 cells containing 4N and >4N DNA, suggesting pX induces DNA re-replication. Interestingly, pX increases expression of endogenous replication initiation factors Cdc6 and Cdtl while suppressing geminin expression, a negative regulator of rereplication. In comparison to a geminin knockdown 4pX-1 cell line used as DNA re-replication control, the Cdt1/geminin ratio is greater in 4pX-1 cells expressing pX, indicating that pX promotes DNA re-replication. In support of this conclusion, pX-expressing 4pX-1 cells, similar to the geminin knockdown 4pX-1 cells, continue to incorporate BrdUrd in the G2 phase and exhibit nuclear Cdc6 and MCM5 co-localization and the absence of geminin. In addition, pX expression activates the ATR kinase, the sensor of DNA re-replication, which in turn phosphorylates RAD17 and H2AX. Interestingly, phospho-H2AX-positive and BrdUrd -positive cells progress through mitosis, demonstrating a link between pX-induced DNA re-replication and polyploidy. Our studies high-light a novel function of pX that likely contributes to hepatocellular carcinoma pathogenesis.
Chronic hepatitis B virus (HBV)4 infection results in the development of hepatocellular carcinoma (HCC) by the fourth or fifth decade (1) by an unknown mechanism. In HBV-mediated HCC the rate of chromosomal aberrations is significantly increased in comparison to HCC associated with other risk factors (2-4). However, the mechanism by which genomic changes initiate HCC development is not yet understood (5-7). Herein, employing the HBV X protein (pX) as the oncogenic signal, we investigate whether pX expression induces chromosomal abnormalities, resulting in HCC pathogenesis.
The link between HBV-mediated HCC and pX is derived both from clinical evidence (8) as well as animal and cell culture transformation studies (9). Specifically, integration of HBV DNA into the host genome occurs at early steps of clonal tumor expansion, with most tumors displaying sustained expression of pX (8). Importantly, pX, which is essential for the viral life cycle (10), is a multifunctional protein inducing activation of the cellular mitogenic ras-raf-MAPK, c-Jun NH2-terminal kinase, and p38MAPK pathways (11) and transcription of select viral and cellular genes (9). These pX activities deregulate cellular gene expression, resulting either in unscheduled cell cycle progression (12) or apoptosis (13), depending on the growth conditions. Specifically, pX expression sensitizes the less-differentiated 4pX-1 hepatocyte cell line (14) to p53-mediated apoptosis only when X-expressing cells are challenged with additional pro-apoptotic stimuli (13, 15). By contrast, in optimal growth factor conditions, pX induces unscheduled cell cycle progression, a transient S phase pause, activation of the G2/M checkpoint, and eventual progression through the cell cycle (12).
Studies by others have demonstrated that overexpression of cyclin E, Cdc25A, and E2F1 leads to unscheduled entry into the S-phase and activation of the ATM/ATR kinases (16). Likewise, overexpression of the cellular oncogene c-myc (17) or expression of pX in apoptotic conditions activate ATM or ATR, respectively, leading to p53 activation and p53-mediated apoptosis (15). Interestingly, pre-neoplastic human specimen exhibit markers of DNA damage and apoptosis, including phosphorylated ATM, Chk2, H2AX, and p53 (16, 18). These results suggest that unscheduled S-phase entry induces via ATM/ATR the activation of p53, which acts as a barrier to cancer development. In agreement with these observations, our hypothesis is that in response to pX expression, the hepatocyte undergoes unscheduled S-phase entry, leading to replication stress and DNA damage, activation of ATR, and induction of p53-mediated apoptosis, thereby constraining oncogenesis. Conversely, deregulation of the G2/DNA damage checkpoint, inhibition of DNA repair, or inactivation of p53 would rescue infected hepatocytes from X-induced apoptosis, allowing the generation of genomic aberrations and progression to malignancy.
In all cell types DNA replication and segregation of the chromosomes is a highly regulated process, ensuring that both daughter cells inherit a complete and intact complement of the genome (19, 20). The process ensuring that DNA replication occurs only once per cell cycle is called replication licensing. Deregulation of replication licensing results in re-replication or partial replication of the genome, leading to chromosomal abnormalities characteristic of cancer (21, 22). Replication licensing involves assembly of the pre-replicative complex (pre-RC) at the origin of replication, comprised of the origin recognition complex, Cdc6, Cdt1, and MCM2-7 proteins (19, 23). The binding of MCM2-7 proteins to pre-RC completes the process of replication licensing, limiting DNA replication to once per cell cycle. The functional licensing of the origin, i.e. the initiation of replication, occurs once cyclin-dependent kinases become active at the onset of S phase (22).
In metazoans, regulation of replication licensing or pre-RC assembly is mediated by down-regulating Cdt1 activity, required for the recruitment of the MCM2-7 proteins to the replication origin. Cdt1 is expressed in early G1 and is degraded at the late G1 and early S phase (24, 25). In addition, Cdt1 interacts directly with geminin, the main inhibitor of replication licensing in S and G2 phases (26-28), resulting in dissociation of the MCM2-7 complex from chromatin (29). Geminin is absent in G1, accumulating during the S and G2/M phases. Geminin is degraded at the end of mitosis, consistent with being a substrate of the anaphase-promoting complex (30), thus permitting the onset of a new round of replication (22). Cyclin-dependent kinases also regulate replication licensing, demonstrated by the induction of re-replication after elimination of the mitotic Cdc2 kinase (27, 31-33). Cyclin-dependent kinase inactivation promotes re-accumulation of Cdt1 on chromatin (34). Moreover, overexpression of both Cdt1 and Cdc6 in p53-negative cells induces re-replication and polyploidy (35, 36). Likewise, inhibition of geminin expression induces re-replication (35-37), supporting that failure to control pre-RC formation results in re-replication, leading to chromosomal abnormalities and cancer development (21, 22).
Herein, employing the less-differentiated 4pX-1 hepatocyte cell line, a tetracycline regulated pX-expressing cell line (14), we demonstrate that pX expression induces DNA re-replication, DNA damage, and polyploidy, identifying a likely mechanism for the genomic instability characteristic of HBV-mediated HCC (2-4).
EXPERIMENTAL PROCEDURES
Cell Culture—Cell lines 4pX-1 (14) and 4pX-1-p53kd (15) were used. pX expression was initiated by removal of tetracycline (14). The following reagents were used: nocodazole (250 ng/ml), Sigma; SB 202190 (5 μm), Calbiochem; and BrdUrd (20 mm), Invitrogen.
Construction of Clonal 4pX-1-gemininkd Cell Line—4pX-1 cells were transfected with shRNAmir for geminin in retroviral vector derived from pSM2C, purchased from Open Biosystems. Clonal stable cell lines were isolated by puromycin (1.0 μg/ml) selection and screened by Western blot assays employing geminin antibody (Santa Cruz Biotechnology, Inc.).
Flow Cytometry and Live Cell Sorting—4pX-1 and 4pX-1-p53kd cell lines were growth factor-deprived for 18 h as described (14) and subsequently grown for 10 h with 10% fetal calf serum followed by removal of tetracycline for an additional 10 h; nocodazole (250 ng/ml) was added for the last 6 h. Cells harvested by trypsin were washed in PBS, fixed in 70% ethanol containing 0.1% Triton X-100 for 20 min, and incubated for 2 h in Vindelov reagent containing PBS, 3.5 units/ml RNase A, 75 mg/ml propidium iodide, and 0.1% Nonidet P-40. Cells were analyzed by Cytomics FC-500 (Beckman-Coulter) at a flow rate of <1000 cells/s using WinList 5.0 software (Verity Software House). Calibration (not shown) was performed using chick erythrocytes for each assay. For live cell sorting, 4pX-1 cells were grown with and without tetracycline as described above, harvested by trypsin treatment, resuspended in Dulbecco's modified Eagle's medium/F-12 medium, and stained with the Hoechst 33422 (500 ng/ml) 1 h before cell sorting. Cells were sorted for DNA content employing the Epics Altra cell sorter (Beckman-Coulter). For dual parameter flow cytometry, 4pX-1 cells grown as described above were incubated with 20 mm BrdUrd for 30 min before fixation in 70% ethanol, Triton X-100. Cells were washed 3 times with PBS and incubated in blocking buffer containing PBS and 10% goat serum (Sigma) for 30 min followed by a 2-h incubation with BrdUrd-fluorescein isothiocyanate-conjugated antibody (1:300) and propidium iodide (75 mg/ml). Cells were analyzed by Cytomics FC-500 (Beckman-Coulter) with compensation adjustments standardized for each experiment.
Double Thymidine Block—4pX-1 and 4pX-1-gemininkd cell lines at 20% confluence were grown for 19 h in growth medium (14) containing 5 μg/ml tetracycline and 2 mm thymidine (Sigma) followed by an additional incubation for 9 h in the same growth medium without thymidine. The second thymidine block was performed for an additional 16 h of incubation ± 5 μg/ml tetracycline. After release from the double thymidine block, cells were grown for 0-14 h and processed for flow cytometry, cell sorting, immunofluorescence microscopy, or whole cell extract (WCE) preparation.
Immunofluorescence Microscopy—Cells were fixed in 4% paraformaldehyde (Sigma) for 20 min, washed 3 times in PBS, and incubated for 30 min in blocking buffer containing 130 mm NaCl, 7 mm Na2HPO4, 3.5 mm NaH2PO4, 7.7 mm NaN3, 0.1% bovine serum albumin, 1.0% Triton-X-100, 0.05% Tween 20, and 10% goat serum. Incubation with primary antibody was for at least 2 h at room temperature at the following dilutions: 1:1000 for α-tubulin (Sigma), 1:1000 for Cdc6 (MBL International), 1:700 for MCM5 (Abcam), 1:700 for geminin (Santa Cruz), 1:500 for phosphohistone 3 (Upstate Biotechnology), and 1:1000 for γ-H2AX (Calbiochem). The BrdUrd kit was from Roche Applied Science. Incubation with secondary antibodies AlexaFluor488 (Invitrogen) and AlexaFluor568 (Invitrogen) was for 1 h. Cells were visualized by Nikon TE300 at 60× or by Nikon E800 confocal microscope.
Real-time PCR Analysis—Total RNA was isolated by the Trizol method and processed for real-time PCR quantification as described (12). PCR primers are listed: Cdc6, forward, 5′-TCCCAGACACAAGCTACC-3′, reverse, 5′-ATTTTACGTCCACACACG-3′; Cdt1, forward, 5′-CCATGTGTCGAGAAAGCTCC-3′, reverse, 5′-CAATGGTGTCCATGC TGC-3′; geminin, forward, 5′-CCATCGGAAGAGGAAGACAC-3′, reverse, 5′-AAGTGGCTGAGCACGTACA; MCM4, forward, 5′-TCAAGTCAGACCTTTTAATGCG-3′, reverse, 5′-TCTGATGACCATGCCACTG-3′; MCM5, forward, 5′-ACATGCAGCTTTATTGTGACAG-3′, reverse, 5′-TTCAAGCCAAACTTCTTGATGG-3′; MCM6, forward, 5-′ AATGATGAAGTAAAACGCGGTG-3′, reverse, 5-TGCAAACATTTATATCCCCACG-3′; proliferating cell nuclear antigen, forward, 5′-GTATTCGAAGCACCAAATCAAG-3′, reverse, 5′-AGCTGTACTCCTGTTCTGG-3′; thymidylate synthase, forward, 5′-GGCCCAGTTTATGGTTTCC-3′, reverse, 5′-GTTGTTTTGATGGTGTCAATC-3′.
Comet Assay—4pX-1 cultures were trypsinized for obtaining single-cell suspension and embedded in 1% agarose on a slide covered by a coverslip and chilled at 4 °C. Slides were placed in lysis buffer (10 mm Tris-HCl, pH 10, 2.5 m NaCl, 0.1 m EDTA, 1% Triton-X) at 4 °C and electrophoresed for 40 min at 25 V. After electrophoresis, slides were incubated for 15 min in neutralization buffer (0.4 m Tris-HCl, pH 7.5), stained with SYBRGREEN (1:1000), and visualized by fluorescence microscopy. Cells were scored for DNA damage when the DNA tailing was discernable at 20×. Quantification is derived from three independent experiments.
Western blot analyses employed WCE isolated from synchronized 4pX-1 cells, sorted by DNA content using the Epics Atra cell sorter (Beckman-Coulter) or in a time course after release from double thymidine block. WCEs (20 μg) were prepared in radioimmune precipitation assay buffer containing PBS, pH 7.4, 0.5% deoxycholate, 1% Nonidet P-40, 1 mm EDTA, 0.4 mm EGTA, 10% glycerol, 0.1% SDS, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml of pepstatin A, 1 mm sodium orthovanadate, and 1 mm sodium fluoride were analyzed by SDS-PAGE. Antibodies used: phospho-ATR (1:1000) and total ATR (1:1000) from Cell Signaling; p-Cdc2 (1:750) and Cdc2 (1:1000) from Santa Cruz Research Biotechnology; p53 (CM5) (1:1000) from Vector Laboratories; γ-H2AX (1:1000) and H2AX from Cell Signaling; Geminin (1:400) from Santa Cruz Research Biotechnology; phospho-Rad17 (1:700) and Rad17 (1:700) from Cell Signaling; Chk1 (1:1000) and phospho-Chk1 (1:600) from Cell signaling; Cdc6 (1:800) and Cdt1 (1:500) both from Santa Cruz Research Biotechnology.
RESULTS
HBV pX Induces Polyploidy—Expression of pX in the immortalized mouse hepatocyte 4pX-1 cell line (14) induces accelerated cell cycle progression, unscheduled S phase entry followed by a transient S phase pause, activation of the G2/M checkpoint, and eventual progression through the cell cycle (12). Similar to human T-cell lymphotrophic virus, type I Tax-expressing cells (38), pX-expressing 4pX-1 cells become multiand micro-nucleated upon treatment for 24 h with a low concentration of nocodazole (250 ng/ml) (39).
To understand how pX causes these chromosomal abnormalities, the tetracycline-regulated pX-expressing 4pX-1 cell line was synchronized by growth factor withdrawal as described (14); to re-enter the cell cycle, 4pX-1 cells were first treated for 10 h with 10% fetal calf serum followed by removal of tetracycline for 10 h to allow pX expression; nocodazole was added for the last 6 h of cell growth (Fig. 1A). Employing flow cytometry, we quantified that 20% of the cells were in the G2/M phase at 20 h after growth in 10% FCS in the presence of pX expression; nocodazole treatment for an additional 6 h increased the cell number in the G2/M phase to 45% (see the table in Fig. 1. Employing the growth protocol shown in the diagram of Fig. 1A, we quantified by flow cytometry the percent of cells progressing through the cell cycle (Fig. 1, table) and those displaying >4N DNA (Fig. 1A). pX expression increases by 2.5-fold the cell population containing >4N DNA, which we refer to as partially polyploid. Importantly, nocodazole treatment increased this pX-dependent polyploid cell population by 3-fold. Nocodazole acts as a mitotic stressor when used at low concentration by activating the p38MAPK pathway (39). Because pX also activates the p38MAPK pathway, which inhibits the Cdc25 phosphatase (40) required for activation of the mitotic Cdc2 kinase (12), we examined the effect of inhibition of the p38MAPK pathway on pX-induced polyploidy. Treatment of 4pX-1 cells with the p38MAPK inhibitor SB202190 (41, 42) resulted in only a 1.6-fold increase in pX-induced polyploidy in the presence of nocodazole (Fig. 1A), an increase that is statistically insignificant (p < 0.1). We interpret these results to mean that pX expression together with nocodazole treatment synergistically activate the p38MAPK pathway, enhancing the pX-induced polyploidy. In supplemental Fig. 1 we show that nocodazole (250 ng/ml) increases the inhibitory phosphorylation of Cdc2 on Tyr-15 in the presence of pX via a p38MAPK-dependent mechanism (supplemental Fig. 1a) without disrupting microtubule polymerization and mitotic spindle assembly (supplemental Fig. 1b).
FIGURE 1.
A, the diagram illustrates the growth conditions of 4pX-1 cells. 4pX-1 cells were serum-starved for 18 h (14) followed by the addition of 10% FCS for 10 h, expression of pX by tetracycline (5 μg/ml) removal for an additional 10 h, and nocodazole (250 ng/ml) treatment for the last 6 h of growth. Table 1, flow cytometric profiles of 4pX-1 cells grown according to the diagram in A. +10 h indicates growth for 10 h in 10% FCS; +20 h indicates growth for 20 h in 10% FCS with 10-h expression of pX; +26 indicates growth for 26 h in 10% FCS, 16 h growth with pX expression, and 6 h with nocodazole treatment. Quantification is from at least three independent flow cytometric experiments. Histogram is the quantification by flow cytometry of 4pX-1 cells containing >4N DNA, grown with (+) or without (-) pX expression by tetracycline removal, as represented in the diagram; nocodazole (250 ng/ml) was added for the last 6 h; SB202190 (5 μm) was added for the last 16 h. Results are from at least three independent experiments. B, flow cytometric profiles of 4pX-1 cells grown as in Fig. 1A, ±pX, sorted for cells containing 2N, 4N, and >4N DNA. The circled area indicates 4pX-1 cells with >4N DNA. AU, absorbance units. C, Hoechst staining of sorted 4pX-1 cells containing 2N, 4N, and >4N DNA. Nuclear size of sorted cells quantified from ∼500 cells, employing Image J software. Results are from three independent experiments. D, Western blot analysis of p53 in WCE isolated from 4pX-1 cells, grown as in Fig. 1A with (+) or without (-) pX and with (+) or without (-) nocodazole (250 ng/ml).
To further confirm the existence of the pX-induced polyploid cell population, we employed fluorescence-activated cell sorting and isolated these pX-induced polyploid cells (Fig. 1B). The increased nuclear size of the sorted cells containing >4N DNA further demonstrates that these pX-expressing cells have aberrant DNA content (Fig. 1C). The integrity of the genome is maintained by activation of p53, which induces cell cycle arrest or apoptosis (43). Because our results indicated that pX induces polyploidy, we examined the level of p53 in 4pX-1 cells. A pX-dependent induction in the p53 protein level is detected in 4pX-1 cells with or without nocodazole treatment, suggesting that this p53 increase is the cellular response to pX-induced polyploidy (Fig. 1D).
p53 Antagonizes pX-mediated Polyploidy—To determine whether p53 negatively regulates pX-mediated polyploidy, we employed the 4pX-1-p53kd cell line displaying more than 80% depletion of endogenous p53 (Ref. 15 and Fig. 2A) and quantified the polyploid cell population as a function of pX expression (Fig. 2, B and C). In comparison to the 4pX-1 cell line expressing pX, the 4pX-1-p53kd cell line displays a 6-fold pX-dependent increase in X-mediated polyploidy. Nocodazole treatment together with pX expression further increased the polyploid cell population to 8.5-fold. The pX-dependent increase in polyploid cells in the 4pX-1-p53kd cell line with or without nocodazole addition clearly demonstrates that pX induces polyploidy, and p53 antagonizes this pX effect, maintaining the genomic integrity of the hepatocyte.
FIGURE 2.
A, Western blot analysis of p53 in WCE isolated from 4pX-1-p53kd and 4pX-1 cells grown as described in Fig. 1A, with (+) or without (-) pX and with (+) or without (-) nocodazole (250 ng/ml). B, flow cytometric profiles of 4pX-1-p53kd cells grown as in Fig. 1A. Arrows indicate the end of the symmetrical G2 peak. Cells to the right of the arrow are quantified as partially polyploid (>4N in DNA). C, quantification of polyploid cells from the flow cytometric analyses shown in Fig. 3B. Results are from at least three independent experiments.
pX Induces DNA Re-replication—To investigate the mechanism by which pX induces polyploidy, 4pX-1 and 4pX-1-p53kd cells were incubated for 30 min with BrdUrd after the 6-h nocodazole treatment. Employing dual parameter flow cytometry, we quantified the percent of cells in each phase of the cell cycle by staining the DNA with propidium iodide and the replicating cells by BrdUrd -fluorescein isothiocyanate fluorescence (supplemental Fig. 2). In 4pX-1 cells the majority of the BrdUrd -positive cells were in the S phase with or without pX expression (supplemental Fig. 2a). In the presence of pX, 20% of BrdUrd -positive cells had 4N DNA (G2 phase) content, and interestingly, ∼8% of BrdUrd -positive cells had >4N DNA, i.e. were partially polyploidy (supplemental Fig. 2a). Interestingly, 4pX-1-p53kd cells treated with nocodazole display a 3-fold pX-dependent increase in the BrdUrd -positive cells containing >4N DNA (supplemental Fig. 2b). Because BrdUrd labeling was performed 30 min after the 6-h nocodazole treatment, the incorporation of BrdUrd in cells containing >4N DNA suggests continuing DNA replication or re-replication in the presence of pX.
pX Increases Cdt1 and Cdc6 Expression Required for Pre-RC Assembly and Suppresses Geminin Expression—To determine whether pX induces re-replication, we quantified by real time PCR the mRNA expression level of Cdc6, Cdt1, and geminin. RNA was isolated from growth factor depleted 4pX-1 cells (14) in a time course after the onset of pX expression by tetracycline removal. In the 4pX-1 cell line, 6 h after pX expression, Cdc6 and Cdt1 mRNA levels increased by 12- and 15-fold, respectively, relative to time 0 h. In the absence of pX expression, Cdc6 and Cdt1 are also induced in the 2-16-h interval, but to statistically significant lower levels (Fig. 3A).
FIGURE 3.
A, real time PCR quantification of Cdc6, Cdt1, and geminin mRNAs using total RNA isolated from 4pX-1 cells serum-starved for 18 h and grown for the indicated time course with (+) or without (-) pX in 10% FCS. Quantification of Cdt1/geminin ratio is shown in the bottom right panel. Results are from at least three independent RNA preparations, normalized to 18 S rRNA. B, Western blot analyses of Cdc6, Cdt1, and geminin using WCE from 4pX-1 cells sorted at the G1/S and G2 phases, grown with (+) or without (-) pX. Actin is the loading control. A representative assay is shown from three independent extract preparations. Quantification was performed by Scion software. C, top panel, Western blot analyses of WCE isolated from 4pX-1 and 4pX-1-gemininkd cell lines, both grown without pX. Lower panel; Western blot analyses of Cdc6, Cdt1, and geminin using WCE from 4pX-1 and 4pX-1-gemininkd cells grown with (+) or without (-) pX for 16 h. A representative assay is shown. Quantification was performed by scion software.
Importantly, pX suppresses the mRNA level of geminin. In the 4pX-1 cell line in the absence of pX, the geminin mRNA level is progressively increased from 2-fold to nearly 4-fold in the 2-16-h interval. By contrast, pX expression suppresses the level of geminin mRNA by nearly 50% (Fig. 3A). Because the balance between Cdt1 and geminin has been proposed to be a crucial determinant for the occurrence of re-replication (44), we quantified the Cdt1/geminin ratio (Fig. 3A). In 4pX-1 cells, pX increased the Cdt1/geminin ratio in the 2-16-h interval, displaying a maximal ratio of 9-fold at 6 h after pX expression. Next, we examined by Western blots the protein level of Cdc6, Cdt1, and geminin using lysates from 4pX-1 cells sorted at the G1/S and G2 phases of the cell cycle (Fig. 3B). pX expression increased the protein level of both Cdt1 and Cdc6 in the G1/S and G2 phases and suppressed the protein level of geminin in the G2 phase, in agreement with the mRNA results (Fig. 3A).
To further confirm these results, we constructed a geminin knockdown cell line in the 4pX-1 background (4pX-1-gemininkd), displaying nearly 80% depletion of geminin (Fig. 3C). Earlier studies by others (36-37, 44) have shown that geminin depletion results in re-replication. Accordingly, we employed the 4pX-1-gemininkd cell line as the positive control for re-replication. The Cdt1/geminin ratio was quantified by Western blot analyses using lysates from the 4pX-1 and 4pX-1-gemininkd cell lines as a function of pX expression (Fig. 3C). pX-expressing 4pX-1 cells have Cdt1/geminin ratio ≈ 4.0, whereas 4pX-1-gemininkd cells without pX expression have Cdt1/geminin ratio ≈ 2.7, indicating that pX expression sufficiently changes the Cdt1/geminin ratio in favor of DNA re-replication.
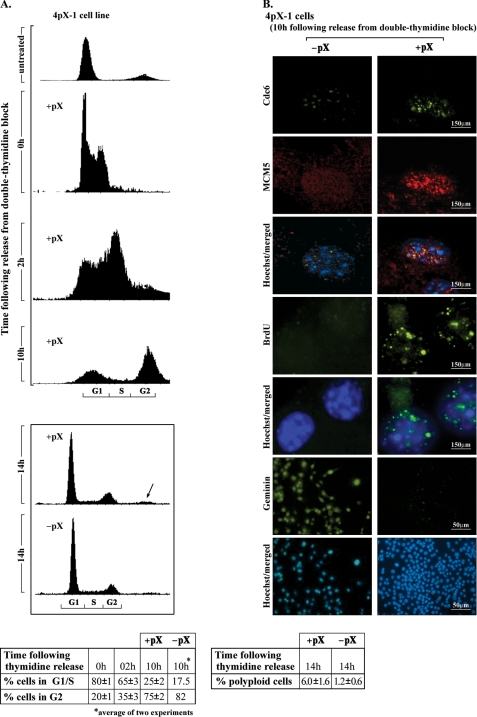
pX Promotes BrdUrd Incorporation in the G2 Phase—To obtain further evidence that pX induces DNA re-replication, we synchronized 4pX-1 cells by the double-thymidine block (Fig. 4A). Expression of pX was initiated during the second thymidine block and was continued after release from the block. Employing flow cytometry, we determined that immediately after release from the thymidine block, 80% of the cells were in the G1/S phase; by 10 h, 25% of the cells were in G1/S and 75% were in the G2 phase; by 14 h we detect a pX-dependent polyploid cell population, comprising 6% of the total cell population (Fig. 4A).
FIGURE 4.
A, flow cytometric profiles of 4pX-1 cells synchronized by the double thymidine block (0 h) and after release from the second thymidine block at 2, 10, and 14 h. Quantification is from three independent experiments except where indicated by an asterisk. B, immunofluorescence microscopy of Cdc6, MCM5, geminin, and BrdUrd using 4pX-1 cells grown for 10 h after release from the double thymidine block. pX expression was initiated 10 h before release of the second thymidine block and continued for an additional 10 h. BrdUrd (BrdU, 20 mm) was added at 30 min before cell fixation. Magnification was 60× for Cdc6 and MCM5 and 10× for geminin and BrdUrd.
Employing this synchronization protocol, we examined by immunofluorescence microscopy the localization of Cdc6 and MCM5, the expression of geminin, and the incorporation of BrdUrd at 10 h after release from the block, when nearly 80% of the cells were in G2 phase (Fig. 4B). Earlier studies (45, 46) demonstrated that Cdc6 and MCM5 dissociate from chromatin in the G2 phase. In agreement with these studies, in the absence of pX, Cdc6 and MCM5 display only background immunostaining at 10 h after release from the block, indicating that these proteins have dissociated from the chromatin (Fig. 4B and supplemental Fig. 3). Surprisingly, pX-expressing cells at 10 h after release from the block display nuclear co-localization of Cdc6 and MCM5 and an absence of geminin immunostaining (Fig. 4B), indicating that the replicative complexes remain assembled. Moreover, these G2 phase cells exhibit pX-dependent BrdUrd incorporation (Fig. 4B), demonstrating that the assembled replicative complexes are functional.
Next, we compared the kinetics of BrdUrd incorporation as a function of pX expression in the 4pX-1 and 4pX-1-gemininkd cell lines (Fig. 5, A and D). BrdUrd was added 30 min before cell fixation in a time course spanning 2-12 h after release from the double thymidine block. In addition, the same cells were immunostained for phospho-H3, a marker for progression into mitosis. In the absence of pX, 4pX-1 cells incorporate BrdUrd for 6-7 h after release from the block, entering mitosis by 10 h. Interestingly, pX-expressing cells continue to incorporate BrdUrd even at 11.5 h after release from the block, whereas phospho-H3 immunostaining is detected starting at 10.5 h (Fig. 5, A and C). Because the kinetics of BrdUrd incorporation in pX-expressing 4pX-1 cells are similar to those of the 4pX-1-gemininkd cell line without pX expression (Fig. 5, D and E), we interpret these results to mean that the continued BrdUrd incorporation observed in the 8-11.5-h interval is due to DNA rereplication. Moreover, ∼20% of X-expressing 4pX-1 cells at 10-10.5 h after release from the block are positive for both BrdUrd and phospho-H3 (Fig. 5, B and C), indicating that in the presence of pX these BrdUrd-positive cells progress to mitosis.
FIGURE 5.
Shown are 4pX-1 (A) and 4pX-1-gemininkd (D) cells synchronized in G1/S by the double thymidine block, immunostained for BrdUrd (BrdU) incorporation (green) and phospho-H3 (red) in the indicated time course after release from the block with (+) or without (-) pX expression. pX expression was initiated by tetracycline removal 10 h before release from the second block and continued for the indicated time course. BrdUrd was added 30 min before fixation. B, confocal microscopy of BrdUrd-positive and phospho-H3-positive cells from the 10.5-h time point with pX expression. C and E, 4pX-1 (C) and 4pX-1-gemininkd (E) cells, quantification of three independent experiments of BrdUrd-positive (green) and phospho-H3-positive (red) cells by ImageJ software, counting at least 1000 cells for each histogram.
To conclusively demonstrate that BrdUrd incorporation was due to re-replication occurring in G2 phase, 4pX-1 cells were synchronized by the double thymidine block; 10 h after release, cells were treated with nocodazole for 6 h to arrest progression into mitosis. BrdUrd was added in the last hour before cell harvesting. Because the goal was to monitor re-replication occurring in the G2 phase, the total cell population was gated on cells containing 4N DNA (Fig. 6A, indicated by a blue color). Employing dual parameter (BrdUrd/pH3) flow cytometric analyses, we quantified in the gated cell population the percent of cells positive for BrdUrd and phospho-H3 (Fig. 6B). Approximately 25% of X-expressing 4pX-1 cells with 4N DNA were positive for both BrdUrd and phospho-H3 (Fig. 6C). By contrast, in the absence of pX, less than 5% of this cell population was positive for both markers. We interpret these data as conclusive evidence that pX-mediated re-replication occurs in the G2 phase in cells containing 4N DNA. Moreover, the dual staining with BrdUrd and phospho-H3 suggests that these cells proceed to mitosis, excluding the possibility that they represent cells in late S phase.
FIGURE 6.
4pX-1 cells, synchronized in G1/S by the double thymidine block, were grown with (+) or without (-) pX for 10 h after release from the block; nocodazole was added for an additional 6 h. BrdUrd was added to cultures 1 h before fixation. Cells were immunostained for BrdUrd (labeled with fluorescein isothiocyanate) and phospho-H3 (pH3) (labeled with AlexaFluor568). A, left panel, flow cytometric analyses of the total cell population gated for DNA content (4,6-diamidino-2-phenylindole staining) to select cells with 4N DNA, indicated by M1 (Marker1) bracket. Right panel, gated 4N DNA-containing cells are indicated in blue on the dual parameter plot for forward scatter (cell size) and side scatter (granulosity). B. Gated cells grown with (+) or without (-) pX expression (indicated in blue in subsequent plots) were analyzed by dual parameter flow cytometry for BrdUrd and phospho-H3 detection. C. Quantification from three independent experiments of gated BrdUrd +/pH3+, BrdUrd +/pH3-, BrdUrd -/pH3+, and BrdUrd -/pH3- cells. Results are from three independent experiments.
pX-dependent ATR Activation in G2 Phase—The cellular sensor of replication stress and re-replication is the ATR kinase (47). Downstream targets of ATR include Rad17 and H2AX. Loss of Rad17 abrogates ATR-dependent signaling (48-52) and results in defects in homologous recombination (53). H2AX becomes phosphorylated at the C-terminal Ser-139 by activated ATR at sites of stalled replication (54). Accordingly, we examined by immunoblotting whether ATR displays pX-dependent activation, employing lysates prepared from G1/S and G2 cell populations, sorted at 0 and 10 h, respectively, after release from the double thymidine block (Fig. 7A). Employing a phospho-ATR-specific antibody, a 5-fold pX-dependent activation of ATR was detected in the G2 phase of pX-expressing cells, whereas only minimal ATR activation was detected in the G1/S phase (Fig. 7A). In agreement with this pX-dependent ATR activation, we also demonstrate increased pX-dependent phosphorylation of Rad17 and H2AX, indicated as γH2AX (Fig. 7A).
FIGURE 7.
A, Western blot assays of pATR/ATR, pRAD17/RAD17, γ-H2AX/H2AX, and actin (internal control) using WCEs from sorted 4pX-1 cells at 0- and 10-h after release from the double thymidine block. pX was expressed 10 h before release from the second block (0-h sample) and an additional 10 h after release from the thymidine block (10-h sample). Quantification is by the Scion software. A representative assay is shown from at least two independent experiments. B, Western blot assays pATR/ATR, γ-H2AX/H2AX, pChk1/Chk1, and actin (internal control) using WCE from 4pX-1 cells grown with (+) or without (-) pX. Expression of pX was initiated by tetracycline removal 10 h before release from the second block and continued for the indicated time course. C, quantification is from three independent WCE preparations.
In addition, the kinetics of ATR activation by pX expression was determined using lysates isolated in a time course after release from the double thymidine block (Fig. 7B). In the absence of pX, the activation of ATR was minimal; by contrast with pX expression, ATR activation was detected starting at 6 h and continued to 11 h after release from the block (Fig. 7B). This pX-dependent ATR activation paralleled the pX-dependent phosphorylation of H2AX (Fig. 7B), supporting the occurrence of pX-induced DNA replication stress. This observation was further supported by the pX-dependent activation of the DNA damage checkpoint kinase Chk1 (Fig. 7B). Activation of Chk1 by pX expression was observed during the 6-8-h interval, in agreement with the activation of ATR and the phosphorylation of H2AX (Fig. 7B). Interestingly, despite the continued presence of γ-H2AX in the 10-11-h interval, indicative of DNA damage, Chk1 was no longer activated (Fig. 7B), suggesting that pX deregulates the DNA damage checkpoint, allowing survival of cells with DNA damage.
pX Propagates DNA Damage—In support of the interpretation that pX promotes DNA damage, we performed the comet assay, a method that detects single or double strand DNA breaks in single cells (55, 56). pX expressed for 12-24 h increased to about 15% the number of cells displaying DNA tailing (Fig. 8A), an indicator of DNA damage at the single cell level. To determine whether the pX-induced DNA damage is linked to DNA re-replication, we performed immunofluorescence microscopy, monitoring BrdUrd incorporation and γ-H2AX immunostaining. BrdUrd was added 5 h (S phase) and 10 h (G2/M phase) after release from the double thymidine block for 30 min before cell fixation. As expected, 4pX-1 cells with or without pX expression progress through S phase at 5 h after release from the block and incorporate BrdUrd without displaying γ-H2AX immunostaining (Fig. 8B). By contrast, in pX-expressing cells at 10 h after release from the block, BrdUrd and γ-H2AX co-localize (Fig. 8B), indicating that DNA re-replication is linked to DNA damage. Co-localization of BrdUrd and γ-H2AX immunostaining was quantified in 60% of X-expressing cells (Fig. 8B) at 10 h after release from the double thymidine block, i.e. although X-expressing cells were in G2/M. Importantly, co-staining for both BrdUrd and γ-H2AX was observed as X-expressing cells progress through mitosis, shown in the confocal images of Fig. 8C, indicating that pX propagates DNA damage to daughter cells.
FIGURE 8.
A, comet assay of 4pX-1 cells grown with (+) or without (-) pX expression for 12 and 24 h. Arrows point to DNA tailing, indicative of DNA damage. Quantification of cells displaying DNA tailing is from three independent experiments, counting a total of 600 cells. B, 4pX-1 cells synchronized in G1/S by the double thymidine block were immunostained for BrdUrd incorporation (green) and γH2AX (red), 5 and 10 h after release from the block, with (+) or without (-) pX expression. BrdUrd (BrdU) was added 30 min before fixation. Images shown are by confocal microscopy. The right panel shows quantification of BrdUrd-positive (green) and γH2AX-positive (red) 4pX-1 cells by ImageJ software, counting at least 1000 cells for each histogram. C, confocal microscopy of mitotic 4pX-1 cells at 10 h after release from the double thymidine block with pX expression using the indicated markers.
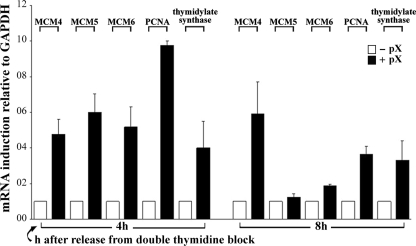
To investigate the relevance of this mechanism of re-replication and DNA damage induced by pX to events occurring in HBV-HCC, we used the microarray data reported by Chen et al. (57) derived from more than 200 clinical HCC samples. One cluster of genes highly expressed in human HCC samples, including HBV-HCC, compared with non-tumor liver tissue, is the proliferation cluster (58), which includes genes for the replication factors MCM4-6 proteins, proliferating cell nuclear antigen, and thymidylate synthase. Accordingly, we investigated by real-time PCR whether these genes are also induced by pX in the 4pX-1 cell line (Fig. 9). Indeed, pX increases the expression of these genes at 4 and 8 h after release from the double thymidine block, in agreement with the microarray analyses of clinical HCC samples, including HBV-HCC (57).
FIGURE 9.
Real-time PCR analysis of total RNA isolated from 4pX-1 cells at 4 and 8 h after release from the double thymidine block, grown with (+) or without (-) pX expression. pX expression was initiated by tetracycline removal 10 h before release from the second block and continued for the indicated time course. Results are from two independent RNA preparations. Each PCR reaction was carried out in identical triplicates and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. PCNA, proliferating cell nuclear antigen.
DISCUSSION
In this study we demonstrate that the weakly oncogenic pX deregulates the expression of the endogenous replication licensing factors Cdc6, Cdt1, and geminin, inducing DNA rereplication, DNA damage, and partial polyploidy. Although earlier studies have determined the role of Cdc6, Cdt1 (34), and geminin (35-37) in DNA re-replication, those studies employed either overexpression or gene silencing approaches, respectively. By contrast, our studies demonstrate that it is the expression of the viral oncoprotein pX that perturbs the balance of the endogenous Cdt1/geminin in favor of DNA re-replication.
The significance of these conclusions is that since the 4pX-1 cell line is immortalized as opposed to the transformed hepatocyte cell lines such as HepG2 or Huh7, our studies link DNA re-replication induced by pX to partial polyploidy, known to be associated with cancer pathogenesis. Interestingly, the 4pX-1 cell line is oval cell-like, exhibiting characteristics of less-differentiated hepatocytes (14, 59), including expression of the CD34 antigen (supplemental Fig. 4), a marker of the oval cell of the liver (60). Oval cells or hepatic progenitors participate in liver regeneration after chronic liver injury from exposure to hepatocarcinogens, toxins, or persistent viral infection (61), and importantly, CD34+ cells are likely involved in the pathogenesis of HBV-mediated HCC (62, 63). Thus, the evidence that pX induces re-replication, DNA damage, and polyploidy in the context of the immortalized, oval cell-like 4pX-1 cell line identifies a mechanism that might be relevant to liver cancer pathogenesis in chronic HBV-infected patients. Another important feature of the 4pX-1 cell line is the low expression level of pX (12, 14, 64), resembling physiologically relevant pX expression levels that occur in viral infection (11, 65).
pX Induces Polyploidy—In contrast to the acutely transforming animal viruses, the slow transforming human oncogenic viruses human T-cell lymphotrophic virus, type I, human papilloma virus, and HBV are replication competent and establish chronic infections linked to cancer (66). The viral oncoproteins Tax of human T-cell lymphotrophic virus, type I (38) and E6/E7 of human papilloma virus (67) act by deregulating the G2/M checkpoint, leading to formation of aberrant mitotic spindles, genomic instability, and oncogenic transformation. Herein we demonstrate that the HBV X protein, which is considered a weak oncogene or a co-factor in HBV-mediated hepatocarcinogenesis (68), induces DNA re-replication (Figs. 3, 4, 5, 6 and 7) and partial polyploidy (Figs. 1 and 2).
The evidence that pX induces partial polyploidy (>4N DNA content) is derived from three independent approaches. 1) First is isolation by cell sorting of a partially polyploid cell population after treatment of pX-expressing 4pX-1 cells with nocodazole (Fig. 1B). In the supplemental data we provide evidence that nocodazole, used at a concentration of 250 ng/ml for 6 h, further activates the G2/M checkpoint via the p38MAPK pathway, thereby increasing the number of cells in the G2/M phase and the effect of pX on polyploidy. 2) Second is the demonstration of the involvement of p53 in suppressing pX-induced polyploidy (Fig. 2). In the 4pX-1-p53kd cell line without nocodazole treatment, the pX-induced polyploid cell population is increased by 6-fold relative to 4pX-1 cells. Because p53 maintains the integrity of the genome and pX increases the protein level of p53 in 4pX-1 cells (Fig. 1D), we interpret these results to mean that pX-induced polyploidy is suppressed by p53. 3) Third, a pX-induced polyploid cell population, representing 6% of the total cell population, has been reproducibly identified 14 h after release from the double thymidine block, excluding the effects of nocodazole treatment (Fig. 4A). Together, these results conclusively demonstrate that pX induces polyploidy, which is antagonized by p53.
pX Deregulates Replication Licensing Factors Cdc6, Cdt1, and Geminin—The pX-induced polyploidy is a partial duplication of the genome (Figs. 1B and 2B), generating cells with more than 4N DNA, but not 8N, 16N, etc. Accordingly, we reasoned that the mechanism of polyploidy by pX is not due to failure of cytokinesis but, rather, due to deregulation of the duplication of the genome. Initial indications that pX deregulates DNA replication were based on the increased pX-dependent incorporation of BrdUrd in 4pX-1 cells containing >4N DNA (supplemental Fig. 2). Next, we examined the expression of the molecules that regulate DNA replication to once per cell cycle, including Cdt1, Cdc6, and geminin (19, 20). Re-replication is regulated by modulating Cdt1 activity. Cdt1 expressed in early G1 recruits MCM2-7 proteins to the pre-RC promoting replication licensing. By contrast, Cdt1 degradation occurs in late G1/S. Geminin, expressed in S and G2/M phases, inhibits replication licensing and re-replication by interacting with Cdt1, leading to dissociation of the MCM2-7 complex from the chromatin. Thus, the balance of Cdt1/geminin in the cell has been proposed to be a critical determinant of replication licensing and re-replication (44). Indeed in the 4pX-1 cell line, pX expression increased the ratio of the endogenous Cdt1/geminin, both at the mRNA and protein levels (Fig. 3). pX increased the mRNA level of Cdc6 and Cdt1 by 12- and 15-fold, respectively, and suppressed the expression of geminin mRNA by nearly 50%. Importantly, at the protein level, the Cdt1/geminin ratio in pX-expressing 4pX-1 cells is larger than the Cdt1/geminin ratio of the 4pX-1-gemininkd cell line without pX expression. This larger Cdt1/geminin ratio in the presence of pX is mediated by both increased expression of Cdt1 as well as inhibition of geminin expression by pX (Fig. 3A). Importantly, the increased Cdt1/geminin ratio in pX-expressing 4pX-1 cells indicates that pX deregulates replication licensing.
pX Induces DNA Re-replication and DNA Damage—We determined by BrdUrd incorporation the duration of S and G2 phases in synchronized 4pX-1 cells not expressing pX. S phase lasts 6-7 h after release from the double thymidine block followed by the G2 phase in the 7-10-h interval (Fig. 5C). In the geminin knockdown 4pX-1-gemininkd cell line, BrdUrd incorporation continues past the 6-7-h interval, demonstrating continued replication or re-replication in G2 phase (Fig. 5D). Based on these controls, the evidence that pX induces DNA re-replication includes the following. 1) 4pX-1 cells exhibit pX-dependent nuclear co-localization of Cdc6 and MCM5, absence of geminin immunostaining, and incorporation of BrdUrd at 10 h after release from the double thymidine block (Fig. 4B), indicating the pre-replicative complexes are assembled and functional in the G2 phase in the presence of pX. 2) The kinetics of BrdUrd incorporation in pX-expressing 4pX-1 cells is similar to BrdUrd incorporation in the 4pX-1-gemininkd cell line without pX, i.e. BrdUrd incorporation continues past the 6-7-h point and overlaps with entry into mitosis at 10.5 h (Fig. 5, C and D). 3) As described earlier, pX-expressing 4pX-1 cells exhibit a larger Cdt1/geminin ratio relative to the 4pX-1-gemininkd cell line without pX expression, indicating that the pX-mediated deregulation of the replication licensing molecules is sufficient to promote DNA re-replication. 4) The results of Fig. 6 conclusively demonstrate that BrdUrd incorporation occurs in G2 phase cells which continue to mitosis since they also co-stain with phospho-H3 and not to cells that are in late S phase. We quantified the BrdUrd-positive and phospho-H3-positive 4pX-1 cells by gating for cells containing 4N DNA (Fig. 6). Nearly 25% of these cells with 4N DNA were positive for both markers in the presence of pX, whereas less than 5% were positive for both markers in the absence of pX (Fig. 6). 5) Western blot analyses of lysates from cells sorted in G1/S and G2 phases demonstrate a 5-fold pX-dependent reduction in geminin (Fig. 3C), a pX-dependent activation of ATR, the sensor of re-replication, and phosphorylation of the ATR substrates Rad17 and H2AX (Fig. 7A).
Interestingly, pX-expressing cells not only continue to incorporate BrdUrd to nearly 11 h after release from the block, but BrdUrd-positive cells also immunostain for the mitotic marker phospho-H3 (Fig. 5B). We interpret these results to mean that despite the activation of ATR by pX, X-expressing cells with re-replicated DNA proceed to mitosis. In support of this interpretation, our results show that despite the presence of γ-H2AX, a marker of DNA damage, pX-expression suppresses Chk1 activation, the DNA damage checkpoint (Fig. 7B). These results suggest that pX expression allows cells with DNA damage to survive. In fact, by the comet assay, we demonstrate DNA damage in single cells in response to pX expression (Fig. 8A). In addition, by co-staining for BrdUrd and γ-H2AX at 5 and 10 h after release from the double thymidine block, we show that γ-H2AX is only detected at 10 h with pX expression, i.e. during the interval of pX-induced DNA re-replication. Moreover, at 10 h after release from the block, nearly 60% of the BrdUrd-positive X-expressing cells immunostain with γ-H2AX (Fig. 8B). More importantly, BrdUrd-positive and γ-H2AX-positive cells do proceed through mitosis as shown by confocal microscopy (Fig. 8C). These results directly demonstrate that pX propagates DNA damage to daughter cells. We conclude that pX induces DNA re-replication and DNA damage.
Attempting to link this mechanism to HBV-HCC pathogenesis, we found microarray databases that have analyzed tumor samples from HCC patients. We found notable the study of Chen et al. (57) that compared the gene expression profile of various types of human liver cancers (HBV- and HCV-mediated). These data are deposited in the Stanford Microarray Data base. More than 200 samples were analyzed, 102 samples of primary HCC from 82 patients and 74 non-tumor samples. A cluster of genes highly expressed in HCC samples compared with non-tumor liver tissue is the proliferation cluster, which includes genes for the replication factors MCM4-6 proteins, proliferating cell nuclear antigen, and thymidylate synthase. Interestingly, these genes are also up-regulated by pX as 4pX-1 cells progress through the S and G2 phases (Fig. 9). Importantly, microarray analyses of human fibroblasts treated with or without DNA damaging agents demonstrated that genes up-regulated in conditions of DNA damage are the same genes induced in normal S phase progression (58). We propose that the mechanism we report herein, employing the model 4pX-1 cell line and pX expression, provides a link to the gene expression profile of clinical HBV-HCC samples (57).
Supplementary Material
Acknowledgments
We thank the Purdue Cytometry Laboratory.
This work was supported, in whole or in part, by National Institutes of Health Grant 044533 (to O. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1-4.
Footnotes
The abbreviations used are: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; pre-RC, pre-replicative complex; pX, X protein; MAPK, mitogen-activated protein kinase; ATM, ataxia telangiectasia mutated; ATR, ATM- and Rad3-related; PBS, phosphate-buffered saline; WCE, whole cell extract; FCS, fetal calf serum; BrdUrd, bromodeoxyuridine; MCM, minichromosome maintenance; H2AX, histone H2A family, member X.
References
- 1.Beasley, R. P., Hwang, L. Y., Lin, C. C., and Chien, C. S. (1981) Lancet 2 1129-1133 [DOI] [PubMed] [Google Scholar]
- 2.Cougot, D., Neuveut, C., and Buendia, M. A. (2005) J Clin Virol 34 (Suppl. 1) S75-S78 [DOI] [PubMed] [Google Scholar]
- 3.Marchio, A., Meddeb, M., Pineau, P., Danglot, G., Tiollais, P., Bernheim, A., and Dejean, A. (1997) Genes Chromosomes Cancer 18 59-65 [PubMed] [Google Scholar]
- 4.Zhang, S.-H., Cong, W.-M., Xian, Z. H., Dong, H., and Wu, M.-C. (2004) J. Cancer Res. Clin. Oncol. 130 757-761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai, H., Suda, T., Aoyagi, Y., Isokawa, O., Mita, Y., Waguri, N., Kuroiwa, T., Igarashi, M., Tsukada, K., Mori, S., Shimizu, T., Suzuki, Y., Abe, Y., Takahashi, T., Nomoto, M., and Asakura, H. (2000) Hepatology 31 1246-1250 [DOI] [PubMed] [Google Scholar]
- 6.Thorgeirsson, S. S., and Grisham, J. W. (2002) Nat. Genet. 31 339-346 [DOI] [PubMed] [Google Scholar]
- 7.Wilkens, L., Flemming, P., Gebel, M., Bleck, J., Terkamp, C., Wingen, L., Kreipe, H., and Schlegelberger, B. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 1309-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su, Q., Schroder, C. H., Hofmann, W. J., Otto, G., Pichlmayr, R., and Bannasch, P. (1998) Hepatology 27 1109-1120 [DOI] [PubMed] [Google Scholar]
- 9.Andrisani, O. M., and Barnabas, S. (1999) Int. J. Oncol. 15 373-379 [DOI] [PubMed] [Google Scholar]
- 10.Zoulim, F., Saputelli, J., and Seeger, C. (1994) J. Virol. 68 2026-2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard, M. J., and Schneider, R. J. (2004) J. Virol. 78 12725-12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, S., Tarn, C., Wang, W. H., Chen, S., Hullinger, R. L., and Andrisani, O. (2002) J. Biol. Chem. 277 8730-8740 [DOI] [PubMed] [Google Scholar]
- 13.Wang, W. H., Gregori, G., Hullinger, R. L., and Andrisani, O. M. (2004) Mol. Cell. Biol. 24 10352-10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarn, C., Bilodeau, M. L., Hullinger, R. L., and Andrisani, O. M. (1999) J. Biol. Chem. 274 2327-2336 [DOI] [PubMed] [Google Scholar]
- 15.Wang, W. H., Hullinger, R. L., and Andrisani, O. M. (2008) J. Biol. Chem. 283 25455-25467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartkova, J., Horejsi, Z., Koed, K., Kramer, A., Tort, F., Zieger, K., Guldberg, P., Sehested, M., Nesland, J. M., Lukas, C., Orntoft, T., Lukas, J., and Barted, J. (2005) Nature 434 864-870 [DOI] [PubMed] [Google Scholar]
- 17.Pusapati, R. V., Rounbehler, R. J., Hong, S., Powers, J. T., Yan, M., Kiguchi, K., McArthur, M. J., Wong, P. K., and Johnson, D. G. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 1446-1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorgoulis, V. G., Vassiliou, L.-V. F., Karakaidos, P., Zacharatos, P., Kotsinas, A., Liloglou, T., Venere, M., Ditullio, R. A., Jr., Kastrinakis, N. G., Levy, B., Kletsas, D., Yoneta, A., Herlyn, M., Kittas, C., and Halazonetis, T. D. (2005) Nature 34 907-913 [DOI] [PubMed] [Google Scholar]
- 19.Blow, J. J., and Dutta, A. (2005) Nat. Rev. Mol. Cell Biol. 6 476-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diffley, J. F. X. (2004) Curr. Biol. 14 778-786 [Google Scholar]
- 21.Mihaylov, I. S., Kondo, T., Jones, L., Ryzhikov, S., Tanaka, J., Zheng, J., Higa, L. A., Minamino, N., Cooley, L., and Zhang, H. (2002) Mol. Cell. Biol. 22 1868-1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishitani, H., and Lygerou, Z. (2004) Front. Biosci. 9 2115-2132 [DOI] [PubMed] [Google Scholar]
- 23.Prasanth, S. G., Mendez, J., Prasanth, K. V., and Stillman, B. (2004) Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359 7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishitani, H., Sugimoto, N., Roukos, V., Nakanishi, Y., Saijo, M., Obuse, C., Tsurimoto, T., Nakayama, K. I., Nakayama, K., Fujita, M., Lygerou, Z., and Nishimoto, T. (2006) EMBO J. 25 1126-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishitani, H., Lygerou, Z., and Nishimoto, T. (2004) J. Biol. Chem. 279 30807-30816 [DOI] [PubMed] [Google Scholar]
- 26.Hodgson, B., Li, A., Tada, S., and Blow, J. (2002) Curr. Biol. 12 678-683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tada, S., Li, A., Maiorano, D., Mechali, M., and Blow, J. J. (2001) Nat. Cell Biol. 3 107-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlschlegel, J. A., Dwyer, B. T., Dhar, S. K., Cvetic, C., Walter, J. C., and Dutta, A. (2000) Science 290 2271-2273 [DOI] [PubMed] [Google Scholar]
- 29.Lee, C., Hong, B., Choi, J. M., Kim, Y., Watanabe, S., Ishimi, Y., Enomoto, T., Tada, S., Kim, Y., and Cho, Y. (2004) Nature 430 913-917 [DOI] [PubMed] [Google Scholar]
- 30.McGarry, T. J., and Kirschner, M. W. (1998) Cell 93 1043-1053 [DOI] [PubMed] [Google Scholar]
- 31.Ballabeni, A., Melixetian, M., Zamponi, R., Masiero, L., Marinoni, F., and Helin, K. (2004) EMBO J. 23 3122-3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita, M., Yamada, C., Tsurumi, T., Hanaoka, F., Matsuzawa, K., and Inagaki, M. (1998) J. Biol. Chem. 273 17092-17101 [DOI] [PubMed] [Google Scholar]
- 33.Itzhaki, J. E., Gilbert, C. S., and Porter, A. C. (1997) Nat. Genet. 15 258-265 [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto, N., Tatsumi, Y., Tsurumi, T., Matsukage, A., Kiyono, T., Nishitani, H., and Mujita, M. (2004) J. Biol. Chem. 279 19691-19697 [DOI] [PubMed] [Google Scholar]
- 35.Vaziri, D., Saxena, S., Geon, Y., Lee, C., Murata, K., Machida, Y., Wagle, N., Hwang, D. S., and Dutta, A. (2003) Mol. Cell 11 997-1008 [DOI] [PubMed] [Google Scholar]
- 36.Melixetian, M., Ballabeni, A., Masiero, L., Gasparini, P., Zamponi, R., Bartek, J., Lukas, J., and Helin, K. (2004) J. Cell Biol. 165 473-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu, W., Chen, Y., and Dutta, A. (2004) Mol. Cell. Biol. 24 7140-7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin, D. Y., Spencer, F., and Jeang, K. T. (1998) Cell 93 81-91 [DOI] [PubMed] [Google Scholar]
- 39.Kurata, S.-I. (2000) J. Biol. Chem. 275 23413-23416 [DOI] [PubMed] [Google Scholar]
- 40.Tarn, C., Zou, L., Hullinger, R. L., and Andrisani, O. M. (2002) J. Virol. 76 9763-9772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemoto, S., Xiang, J., Huang, S., and Lin, A. (1998) J. Biol. Chem. 273 16415-16420 [DOI] [PubMed] [Google Scholar]
- 42.Karahashi, H., Nagata, K., Ishii, K., and Amano, F. (2000) Biochim. Biophys. Acta 1502 207-223 [DOI] [PubMed] [Google Scholar]
- 43.Fei, P., and El-Deiry, W. S. (2003) Oncogene 2 5774-5783 [DOI] [PubMed] [Google Scholar]
- 44.Saxena, S., and Dutta, A. (2005) Mutat. Res. 569 111-121 [DOI] [PubMed] [Google Scholar]
- 45.Bell, S. P., and Dutta, A. (2002) Annu. Rev. Biochem. 76 333-374 [DOI] [PubMed] [Google Scholar]
- 46.Mendez, J., and Stillman, B. (2000) Mol. Cell. Biol. 20 8602-8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abraham, R. T. (2001) Genes Dev. 15 2177-2196 [DOI] [PubMed] [Google Scholar]
- 48.Bao, S, Tibbetts, R. S., Brumbaugh, K. M., Fang, Y., Richardson, D. A., Ali, A., Chen, S. M., Abraham, R. T., and Wang, X. F. (2001) Nature 411 969-974 [DOI] [PubMed] [Google Scholar]
- 49.Bao, S., Lu, T., Wang, X., Zheng, H., Wang, L. E., Wei, Q., Hittelman, W. N., and Li, L. (2004) Oncogene 23 5586-5593 [DOI] [PubMed] [Google Scholar]
- 50.Wang, X., Zou, L., Zheng, H., Wei, Q., Elledge, S. J., and Li, L. (2003) Genes Dev. 17 965-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss, R. S., Enoch, T., and Leder, P. (2000) Genes Dev. 14 1886-1898 [PMC free article] [PubMed] [Google Scholar]
- 52.Zou, L., Cortez, D., and Elledge, S. J. (2002) Genes Dev. 16 198-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budzowska, M., Jaspers, I., Essers, J., De Waard, H., Van Drunen, E., Hanada, K., Beverloo, B., Hendriks, R. W., de Klein, A., Kanaar, R., Hoeijmakers, J. H., and Maas, A. (2004) EMBO J. 23 3548-3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward, I. M., and Chen, J. (2001) J. Biol. Chem. 276 47759-47762 [DOI] [PubMed] [Google Scholar]
- 55.Frenzilli, G., Scarcelli, V., Fornai, F., Paparelli, A., and Nigro, M. (2006) Ann. N. Y. Acad. Sci. U. S. A. 1074 478-481 [DOI] [PubMed] [Google Scholar]
- 56.Olive, P. L. (2002) Methods Mol. Biol. 203 179-194 [DOI] [PubMed] [Google Scholar]
- 57.Chen, X., Cheng, S.T., So, S., Fan, S. T., Barry C., Higgins, J., Lai, K.-M., Ji, J., Dudoit, S., Ng, I. O. L., van de Rijn, M., Botstein, D., and Brown, P.O. (2002) Mol. Biol. Cell 13 1929-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho, R.J., Huang, M., Campbell, M. J., Dong, H., Steinmetz, L., Sapinoso, L., Hampton, G., Elledge, S. J., Davis, R. W., and Lockhart, D. J. (2001) Nat. Genet. 27 48-54 [DOI] [PubMed] [Google Scholar]
- 59.Dan, Y. Y., Riehle, K. J., Lazaro, C., Teoh, N., Hague, J., Campbell, J.S., and Fausto, N. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 9912-9917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crosby, H. A., Kelly, D. A., and Strain, A. J. (2001) Gastroenterology 120 534-544 [DOI] [PubMed] [Google Scholar]
- 61.Thorgeirsson, S. S. (1996) FASEB J. 10 1249-1256 [PubMed] [Google Scholar]
- 62.Lee, E. S., Han, E. M., Kim, Y. S., Shin, B. K., Kim, C. H., Kim, H. K., Won, N. H., Yeom, B. W., Kim I., and Leong, A. S. (2005) Am. J. Clin. Pathol. 124 31-36 [DOI] [PubMed] [Google Scholar]
- 63.Ohmori, S., Shiraki, K., Sugimoto, K., Yamanaka, Y., Yamaguchi, Y., Saitou, Y., Fujikawa, K., Murata, K., and Nakano, T. (2004) Int. J. Mol. Med. 14 179-184 [PubMed] [Google Scholar]
- 64.Tarn, C., Lee, S., Hu, Y., Ashendel, C., and Andrisani, O. (2001) J. Biol. Chem. 276 34671-34680 [DOI] [PubMed] [Google Scholar]
- 65.Dandri, M., Schirmacher, P., and Rogler, C. E. (1996) J. Virol. 70 5246-5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butel, J. S. (2000) Carcinogenesis 21 405-426 [DOI] [PubMed] [Google Scholar]
- 67.Duensing, S., Lee, L. Y., Duensing, A., Basile, J., Piboonniyam, S., Gonzalez, S., Crum, C. P., and Münger, K. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 10002-10007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madden, C. R., Finegold, M. J., and Slagel, B. L. (2001) J. Virol. 75 3851-3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.