Abstract
The cell surface-expressed γ chain of the high affinity receptor for IgE (FcεRI) can be phosphorylated on two tyrosine residues of the immunoreceptor tyrosine-based activation motif (ITAM), leading to recruitment and activation of spleen tyrosine kinase (Syk), a kinase that is essential for mast cell signaling and allergic responses. However, it is not known whether preferential phosphorylation or dephosphorylation of the two individual FcRγ tyrosines (the N-terminal Tyr47 and C-terminal Tyr58) could regulate Syk activation. Herein we report that phosphorylation of only Tyr58 was able to elicit Syk phosphorylation and a weak rise in intracellular calcium, suggesting that Tyr58 phosphorylation may be distinctively important for Syk activation. In vitro and in vivo studies revealed that both Tyr47 and Tyr58 could be similarly phosphorylated. However, mass spectrometric analysis of the phosphorylated FcεRγ from bone marrow-derived mast cells showed that phosphorylation at Tyr47 was at least 2-fold greater than at Tyr58. This suggested that, once phosphorylated, Tyr58 is preferentially dephosphorylated. In vitro studies demonstrated more efficient dephosphorylation of Tyr58 (by the receptor-associated phosphatases SHP-1 and SHP-2) than of Tyr47. Analysis of Syk binding to wild type and mutant phosphorylated FcεRI revealed that mutation at Tyr58 almost completely ablated Syk binding, whereas mutation at Tyr47 moderately reduced Syk binding. The findings argue for a novel regulatory mechanism, where dephosphorylation of phospho-Tyr58 is likely to promote the down-regulation of Syk activation and suppression of mast cell responses.
Phosphorylation and dephosphorylation are key events in the initiation and regulation of cell signaling and responses. Antigen-dependent stimulation of FcεRI initiates its tyrosine phosphorylation by Lyn kinase on both the β and γ subunit ITAMs4 of this multimeric receptor (1, 2). The phosphorylated receptor is then able to recruit and bind other signaling proteins through their Src homology 2 (SH2) domains, which recognize phosphotyrosines. Among the proteins demonstrated to interact with the phosphorylated FcεRI is the protein tyrosine kinase Syk (3, 4). Syk binding promotes its activation, and it subsequently phosphorylates multiple signaling molecules, thus amplifying the signaling that is essential for cellular responses (5, 6). This fast forward response must be effectively controlled to avoid the detrimental effects of a prolonged inflammatory response.
Protein tyrosine phosphatases are key regulators of cell activation. SHP-1 and -2 (Src homology 2-containing protein tyrosine phosphatases 1 and 2) are expressed in hematopoietic cells or are ubiquitously expressed, respectively. These phosphatases have been described to interact with numerous signaling proteins (such as Vav1, Grb2, Sos, SLP-76, Lyn, and SIRPα1) as well as with many inhibitory receptors containing immunoreceptor tyrosine-based inhibitory motifs, where they impart the inhibitory activity of these receptors (reviewed in Refs. 7 and 8). SHP-1 was detected in immunoprecipitates of FcεRI prior to stimulation, whereas SHP-2 was observed only after FcεRI stimulation (9, 10). Overexpression of SHP-1 caused decreased phosphorylation of FcεRIβ and γ subunits in RBL cells, and similar effects were noted on the phosphorylation of Syk. However, whether SHP-1 or SHP-2 equally dephosphorylate both ITAM tyrosines or whether there is preferential dephosphorylation of ITAM tyrosines is not known. This is an important query, given that the ITAM motifs of both β and γ subunits were shown to potentially interact with multiple signaling proteins (3, 11, 12). Thus, preferential dephosphorylation at one tyrosine residue might cause the loss of interaction with one protein but still allow the subsequent binding of another to the second phosphorylated tyrosine residue. In addition, selective phosphorylation/dephosphorylation might serve to control the binding and activation of Syk kinase. In vitro experiments have shown that both Tyr(P)47 and Tyr(P)58 (herein we use the amino acid designation of the surface-expressed protein, without leader peptide) of FcRγ was required for high affinity Syk binding; however, Tyr(P)58 appeared to have a modestly higher affinity for Syk than Tyr(P)47 (11, 13).
In the current study, we explored whether the FcRγ ITAM tyrosines are preferentially phosphorylated and/or preferentially dephosphorylated. This was based on the observation of multiple phosphorylated species of FcRγ following FcεRI stimulation. We reasoned that these multiple species might represent forms of FcRγ with differing potential in binding Syk. Prior studies already demonstrated that the FcεRIβ ITAM tyrosines differentially bind Lyn (12, 14), but to date no studies address whether differential phosphorylation/dephosphorylation might regulate such binding. Herein, we took the approach of expressing the wild type and individual tyrosine mutants of FcRγ in BMMC derived from FcRγ-deficient mice. Our findings demonstrate that weak phosphorylation of Tyr58 alone can cause a modest phosphorylation of Syk and weak calcium signals. However, Tyr47 and Tyr58 are not differentially phosphorylated when phosphatase activity is inhibited in vivo or when phosphorylated by Lyn kinase in vitro. In contrast, dephosphorylation of Tyr(P)58 by SHP-1 or SHP-2 was more efficient than that of Tyr(P)47. Mass spectrometry revealed that Tyr(P)47 was more abundant than Tyr(P)58 in FcεRI-stimulated wild type cells. Collectively, the data argue that Tyr(P)58 is more important for Syk interaction, phosphorylation, and calcium responses, but this site is more susceptible to dephosphorylation than Tyr(P)47. Thus, the findings suggest a rapid dephosphorylation of FcRγ Tyr58 as a mechanism for controlling the extent of Syk activation.
EXPERIMENTAL PROCEDURES
Antibodies—The cytokinergic (15) monoclonal anti-DNP IgE was prepared from the culture supernatants of the H1 DNP-ε-26.82 hybridoma (16). 125I-Labeled IgE was prepared as described (17). FITC-conjugated rat anti-mouse IgE (clone R35-72), FITC-conjugated rat IgG1 monoclonal Ig isotype control (clone R3-34), and purified rat anti-mouse CD16/CD32 were from BD Biosciences. Goat anti-mouse IgE was purchased from MP Biomedicals. Affinity-purified rabbit anti-mouse IgG (F(ab′)2 fragment-specific) was from Jackson ImmunoResearch Laboratories. Rabbit polyclonal antibody to FcRγ and mouse monoclonal anti-phosphotyrosine (4G10) were from Upstate Biotechnology, Inc. (Lake Placid, NY). Monoclonal mouse anti-FcεRIβ was described else-where (18). Rabbit antibodies to Lyn, glutathione S-transferase (GST), SHP-1, and SHP-2 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Secondary antibodies used for immunoblotting were Alexa Fluor 680-conjugated goat anti-rabbit IgG (Molecular Probes) and IRDye800-conjugated goat anti-mouse IgG (Rockland Immunochemicals).
Mice and Cell Culture—FcRγ-/- (B6.129P2-Fcεr1γtm1Rav) mice were obtained from Taconic Farms. All mice were maintained and used in accordance with National Institutes of Health (NIH) guidelines and the NIAMS (NIH)-approved animal study proposal. BMMCs were grown essentially as previously described (19).
Site-specific Mutagenesis and Retroviral Constructs—The cDNA encoding mouse wild type FcRγ (YY) and the tyrosine to phenylalanine mutant at Tyr47 (FY), at Tyr58 (YF), and both at Tyr47 and Tyr58 (FF) were cloned using the pLenti6/V5 Directional TOPO cloning kit (Invitrogen). The sequences of PCR primers used were as follows: YY, 5′-CACCATGATCTCAGCCGTGATC-3′ and 5′-CTACTGGGGGTGGTTTTTCATG-3′; FY, 5′-AGATGCTGTCTTCACGGGCCTGA-3′ and 5′-TCAGGCCCGTGAAGACAGCATCT-3′; YF, 5′-CCAGGAGACATTTGAGACTCTGA-3′ and 5′-TCAGAGTCTCAAATGTCTCCTGG-3′ (mutation sites are underlined). Cloning was verified by nucleotide sequencing. The wild type and three mutant FcRγ were subcloned into the BamHI and XhoI site of the Moloney murine leukemia virus-based vector pMX-puro.
Expression of GST Fusion Proteins—The cytoplasmic region corresponding to amino acids 27-68 of mouse wild type FcRγ and the three Tyr/Phe mutants were amplified by PCR with primers that included a 5′-BamHI site and a 3′-XhoI site. The PCR products were ligated into pGEX-4T vector (GE Healthcare). These GST fusion proteins were expressed in Escherichia coli strain BL21. To obtain tyrosine-phosphorylated GST proteins, these cDNA constructs were introduced into TKB1 E. coli strain (Stratagene, La Jolla, CA) that contains an inducible Elk1 tyrosine kinase. The tandem SH2 domains of rat Syk corresponding to amino acids 14-260 and the SH2 domain of rat Lyn corresponding to amino acids 127-241 were subcloned between the BamHI and EcoRI multicloning sites of pGEX-2T and expressed in E. coli DH5α. All of the fusion proteins were purified by column chromatography using glutathione-Sepharose (GE Healthcare). Phosphorylation of fusion proteins was confirmed by immunoblotting with anti-phosphotyrosine mAb 4G10.
Retroviral Gene Transduction—Viral supernatants were produced by transfecting the retroviral packaging cells Phoenix-E with wild type or mutated FcRγ in the pMX-puro vector using Lipofectamine 2000 (Invitrogen). The cells were also mock-transfected with an empty pMX-puro vector as a negative control. The viral supernatants were 10× concentrated by centrifugation at 20,000 × g for 4 h. Bone marrow cells from femurs of FcRγ-/- mice were grown in the presence of IL-3 and SCF for 10 days. For infection, the cells were resuspended in the concentrated viral supernatants containing 10 μg/ml Polybrene, spin-infected at 1200 × g for 45 min, and further incubated for 18 h. Infected cells were washed and allowed to grow in IL-3- and SCF-containing medium for 24 h before selection of transduced cells with 2 μg/ml puromycin. After 2 weeks of selection, cells were analyzed for FcεRI expression. Typical cultures used for analysis showed >95% FcεRI expression.
Flow Cytometric Analysis for FcεRI Expression—BMMCs (106/ml) were sensitized with 0.5 μg/ml anti-DNP IgE for 18 h at 37 °C. The sensitized BMMCs were preincubated with 5 μg/ml anti-mouse CD16/CD32 mAb mix for 5 min at 4 °C to prevent Ab binding to FcγRII/III and then stained with 10 μg/ml FITC-conjugated rat anti-mouse IgE or the isotype control FITC-conjugated rat IgG1 for 30 min at 4 °C. Stained cells were analyzed on a FACSCalibur (BD Biosciences) using CellQuest software (BD Biosciences).
Calcium Imaging—To measure changes in the intracellular calcium concentration, two Ca2+ indicator dyes, Fluo-4/Fura Red/AM (Molecular Probes), were used. BMMCs were loaded with Fluo-4/AM (2 μm) and Fura Red/AM (10 μm) for 30 min at 37 °C. The cells were rinsed three times with Hepes-Tyrode buffer. A Zeiss LSM-510 META confocal microscope with a 488-nm wavelength light from an argon laser was used for ratio-metric measurements. The fluorescence of Fluo-4 and Fura Red was detected through a band pass filter (505-530 nm) and a long pass filter (>560 nm), respectively. Fluorescence images were collected every 5 s, and the intensity of fluorescence was quantified using Zeiss LSM-510 META software.
Ag Stimulation, Cell Lysates, Immunoprecipitation, and Immunoblotting—IgE-sensitized BMMCs were washed and resuspended in Tyrode's buffer (135 mm NaCl, 5 mm KCl, 20 mm HEPES, 5.6 mm glucose, 1 mm MgCl2, 1.8 mm CaCl2, 0.05% bovine serum albumin, pH 7.4) at 2 × 107/ml. Unless otherwise indicated, cells were stimulated with 200 ng/ml Ag (DNP-HSA) for 2 min at 37 °C. In some experiments, cells were stimulated with pervanadate (prepared freshly by incubating 100 mm vanadate with 100 mm H2O2) for 15 min at room temperature, as indicated. Cells were then solubilized in lysis buffer containing 0.5% Triton X-100, 50 mm Tris-HCl (pH 7.6), 150 mm NaCl, 5 mm EDTA, 1 mm Na3VO4, 5 mm Na4P2O7, 5 mm NaF, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 μg/ml pepstatin A for 30 min at 4 °C. For immunoprecipitation of Lyn, 0.05% SDS and 0.5% deoxycholate were further added to the buffer (lysis buffer SDC). Lysates were centrifuged at 14,000 rpm for 5 min at 4 °C. For immunoprecipitation of IgE-bound FcεRI, postnuclear supernatants were incubated with goat anti-mouse IgE (10 μl/ml lysate) for 1 h at 4 °C followed by incubation with protein A-Sepharose beads (30 μl/ml lysate; GE Healthcare) for 1 h at 4°C. Beads were washed three times with lysis buffer SDC and boiled in 2× SDS-PAGE sample buffer for 5 min. The recovered proteins were resolved by SDS-PAGE followed by electrophoretic transfer to nitrocellulose membranes. Membranes were blocked in 2× diluted Odyssey blocking buffer (LI-COR, Inc., Lincoln, NE) for 30 min at room temperature. Blots were then incubated with optimal concentrations of various primary Ab, washed three times with the blocking buffer, and incubated with secondary antibodies coupled to infrared fluorescence dye IR800 or Alexa Fluor 680 for 30 min. After a further wash with the blocking buffer, the immunoblots were analyzed using the Odyssey infrared imaging system (LI-COR). Some membranes were blotted with 10 nm GST fusion proteins (far Western) for 18 h at 4 °C followed by rabbit anti-GST antibody. For some experiments, membranes were stripped of bound antibodies by incubating with 0.2 n NaOH for 30 min and subsequently reprobed with other antibodies.
Degranulation and Cytokine Measurements—Degranulation was determined by measuring the enzymatic activity of the granule marker, β-hexosaminidase, as previously described (19). Cytokine secretion (IL-6, IL-13, tumor necrosis factor, and MCP-1) was measured from culture supernatants prior to and after 4-h stimulation with Ag using a specific enzyme-linked immunosorbent assay according to the manufacturer's instructions (R&D Systems).
In Vitro Kinase Assay—Anti-Lyn immunoprecipitates from 107 unstimulated BMMCs were washed twice with kinase buffer (20 mm Tris/HCl, pH 7.5, 10 mm MgCl2, 1 mm Na3VO4) and resuspended in 90 μl of kinase buffer containing 2 mm GST fusion proteins as exogenous substrates. Kinase reaction conditions were previously established to be linear (20) and were initiated by the addition of 10 μl of ATP (final concentration of 1 mm). After incubation for 15 min at 30 °C, the reaction was stopped by the addition of 4 μl of 0.5 m EDTA, and 10 μl of kinase reaction products were separated by SDS-PAGE and subjected to immunoblotting with anti-phosphotyrosine 4G10.
In Vitro PTPase Assay—The activities of recombinant human SHP-1 (Biomol International LP, Plymouth Meeting, PA) and SHP-2 (Upstate Biotechnology) were assayed using p-nitrophenyl phosphate as a substrate at 37 °C in PTPase assay buffer containing 50 mm HEPES, 150 mm NaCl, 5 mm dithio-threitol, and 0.1 μg/ml bovine serum albumin. The assay conditions were linear using an amount of each enzyme whose activity corresponded to ∼200 pmol/min p-nitrophenyl phosphate hydrolysis (1 arbitrary unit) or 0.2 arbitrary units incubated with 100 nm tyrosine-phosphorylated GST-FcRγ in a total volume of 20 μl at 37 °C for 60 min. The PTPase reaction products were then separated by SDS-PAGE and subjected to immunoblotting with anti-phosphotyrosine 4G10.
Large Scale Purification of FcRγ—IgE-sensitized BMMCs (4 × 107/ml) were unstimulated or stimulated with 400 ng/ml DNP-HSA for 2 min at 37 °C and then solubilized similarly as for the immunoprecipitation of FcεRI. Postnuclear supernatants from 1 × 109 cells were precleared by passing through a 0.5-ml Protein A-Sepharose column, and IgE-bound FcεRI was purified by affinity chromatography using 0.5 ml of Protein A-Sepharose beads that were covalently bound to a rabbit anti-mouse IgG, F(ab′)2 fragment-specific, using dimethyl pimelimidate (Pierce). After washing the beads with lysis buffer, the bound material was eluted with lysis buffer containing 0.1% SDS. FcRγ in the eluates from 4-6 × 109 cells was then further purified using 0.5 ml of rabbit anti-FcRγ-bound Sepharose beads. After washing the beads with lysis buffer (0.1% Triton X-100), the bound material was eluted with 0.2 m glycine, pH 2.5. The eluate was neutralized with 1 m Tris, pH 8.0, concentrated with cold acetone, and solubilized in SDS-PAGE sample buffer. The resulting material was separated by SDS-PAGE using 16% Tris-glycine gels. Proteins were stained with the Colloidal Blue staining kit (Invitrogen), and two major bands were identified for mass spectrometry.
LC/MS/MS Analysis of the FcRγ—In order to map which sites on the FcRγ are phosphorylated following FcεRI stimulation, protein bands from the SDS-polyacrylamide gels were digested with trypsin to produce peptides that could be analyzed by LC/MS/MS. LC/MS/MS analysis of tryptic peptides generated from the FcRγ bands identified peptides spanning the carboxyl-terminal region from residue 41 to 68 of the mature processed FcRγ subunit, as seen in Table 1.
TABLE 1.
Phosphopeptides identified by MS/MS analysis of FcRγ tryptic peptides from activated cells
| Residues | m/z | Charge | Mr (experimental) | Mr (calculated) | Mass error | Sequencea |
|---|---|---|---|---|---|---|
| Da | ||||||
| Band 1 | ||||||
| 41-53 | 759.31 | +2 | 1516.61 | 1516.69 | −0.08 | R.EKADAVYTGLNTR.S(Tyr(P)47) |
| 41-53 | 506.59 | +3 | 1516.74 | 1516.69 | 0.05 | R.EKADAVYTGLNTR.S(Tyr(P)47) |
| 43-53 | 630.89 | +2 | 1259.76 | 1259.55 | 0.21 | K.ADAVYTGLNTR.S(Tyr(P)47) |
| 54-62 | 589.88 | +2 | 1177.74 | 1177.49 | 0.25 | R.SQETYETLK.H(Tyr(P)58) |
| Band 2 | ||||||
| 41-53 | 759.53 | +2 | 1517.04 | 1516.69 | 0.35 | R.EKADAVYTGLNTR.S(Tyr(P)47) |
| 41-53 | 506.75 | +3 | 1517.22 | 1516.69 | 0.53 | R.EKADAVYTGLNTR.S(Tyr(P)47) |
| 43-53 | 630.91 | +2 | 1259.81 | 1259.55 | 0.26 | K.ADAVYTGLNTR.S(Tyr(P)47) |
| 54-62 | 589.81 | +2 | 1177.61 | 1177.49 | 0.12 | R.SQETYETLK.H(Tyr(P)58) |
R.E, R.S, K.A, or K.H indicate peptide bond cleavage.
RESULTS
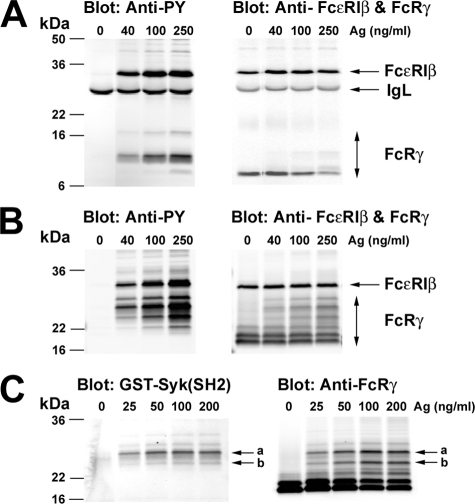
Engagement of FcεRI Induces Multiple Species of Tyrosine-phosphorylated FcRγ with Differing Potential for Syk Binding—FcεRI stimulation of mast cells was demonstrated to elicit multiple species (as defined by differing molecular mass) of FcRγ (21). This was attributed to changes both in the phosphorylation state of the FcRγ and FcεRI-induced ubiquitination. We further explored this observation by analyzing the species of FcRγ detected after FcεRI stimulation using reducing and nonreducing conditions, thus identifying the homodimers of FcRγ. Fig. 1A shows that brief stimulation of FcεRI (2 min) allows resolution of at least three phosphorylated species of FcRγ ranging from 7 to 16 kDa in molecular mass. The major phosphorylated species showed a molecular mass of ∼9 kDa. Total protein blots also revealed three bands (at 250 ng/ml Ag), two apparently coinciding with the 7- and 9-kDa species observed in the phosphotyrosine blots. The major species, however, was ∼7 kDa and coincided with the molecular mass of the unmodified translated FcRγ. Under nonreducing conditions, a minimum of six species can be observed when probed with anti-FcRγ (Fig. 1B, right). Phosphotyrosine blotting revealed that at least four of these six species are thus modified, with the highest phosphorylation level detected in a ∼26-kDa form. In both reducing and nonreducing conditions, the FcεRIβ and its phosphorylation were unaltered. Because nonreducing conditions resolved the largest number of phosphorylated FcRγ species and might reflect multiple species capable of binding Syk, we used these conditions in far Western analysis of Syk-SH2 domain binding to phosphorylated FcRγ. Fig. 1C demonstrates that the ∼26-kDa species (labeled a) had the highest affinity for interacting with Syk. Minor reactivity was observed in the next most well phosphorylated ∼24-kDa species (labeled as b in Fig. 1, B and C). The two dominant protein bands of about 18 and 20 kDa (Fig. 1B, right) in molecular mass were not phosphorylated (Fig. 1B, left) or found to bind the SH2 domains of Syk (Fig. 1C).
FIGURE 1.
Engagement of FcεRI on BMMC induces multiple species of tyrosine-phosphorylated FcRγ. BMMCs from wild type mice were incubated with anti-DNP IgE. The IgE-sensitized cells were stimulated or not with the indicated concentrations of DNP-HSA (Ag) for 2 min at 37 °C. Cells were then solubilized, and FcεRI was immunoprecipitated with anti-IgE. Recovered proteins were resolved by SDS-polyacrylamide gels under reducing (A) or nonreducing (B) conditions and analyzed by immunoblotting with anti-phosphotyrosine. Membranes were stripped and reprobed with anti-FcεRIβ and anti-FcRγ. Molecular mass markers are shown on the left. C, immunoprecipitated FcεRI from IgE and Ag-stimulated BMMCs was resolved by SDS-PAGE under nonreducing conditions and blotted with GST-Syk(SH2) fusion proteins, followed by detection with rabbit anti-GST antibody. The same membrane was stripped and reprobed with anti-FcRγ. One representative of more than five individual experiments is shown.
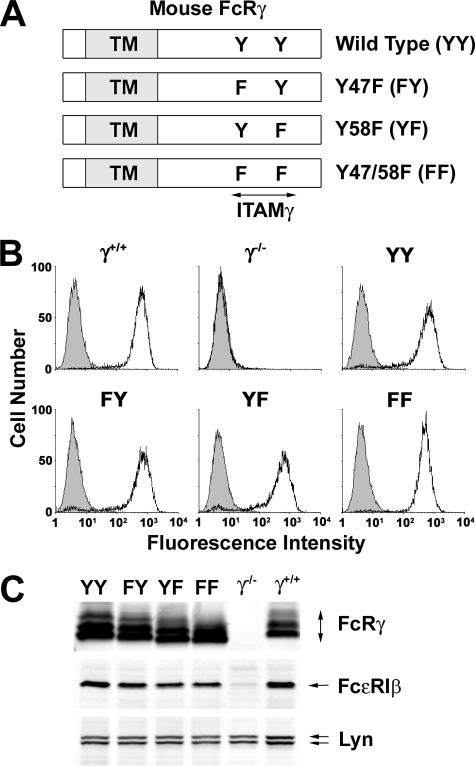
Phosphorylation and Functional Responses of Wild Type and ITAM Tyrosine-mutated FcRγ—To further explore the regulation of phosphorylation of FcRγ in situ, we generated (Fig. 2A) four retroviral constructs encoding the wild type FcRγ (YY), a Y47F mutant (FY), a Y58F mutant (YF), and a Y47F/Y58F double mutant (FF). These constructs were expressed in BMMC derived from FcRγ-deficient mice. As compared with normal BMMCs (γ+/+), FcRγ-deficient (γ-/-) BMMCs showed no expression of FcεRI (Fig. 2B) as previously described (22). However, reconstitution of the deficient cells with wild type or mutant forms of FcRγ restored normal levels of FcεRI expression (Fig. 2, B and C).
FIGURE 2.
Reconstitution of wild type and FcεRIγ ITAM-mutated in FcRγ-deficient BMMC by retroviral gene transduction. A, schematic diagram of mouse FcRγ-ITAM mutations. The N-terminal Tyr47 and C-terminal Tyr58 in the FcRγITAM(YY) were singly mutated to phenylalanine (FY and YF, respectively) or doubly mutated (FF). TM, transmembrane domain. B, flow cytometric analysis of surface FcεRI expression on BMMCs. IgE-sensitized BMMCs from wild type mouse (γ+/+) and retrovirally transfected γ-/- BMMC (γ-/-; mock, YY, FY, YF, and FF) were stained with FITC-conjugated anti-IgE mAb (solid line) or isotype control mAb (shaded) and analyzed by FACSCalibur. C, expression of FcRγ, FcεRIβ, and Lyn. Proteins in whole cell lysates of BMMCs were resolved by SDS-PAGE under nonreducing conditions and immunoblotted with anti-FcRγ, anti-FcεRIβ, and anti-Lyn. Analysis of FcεRI expression was done for all cultures used in this study. One representative is shown.
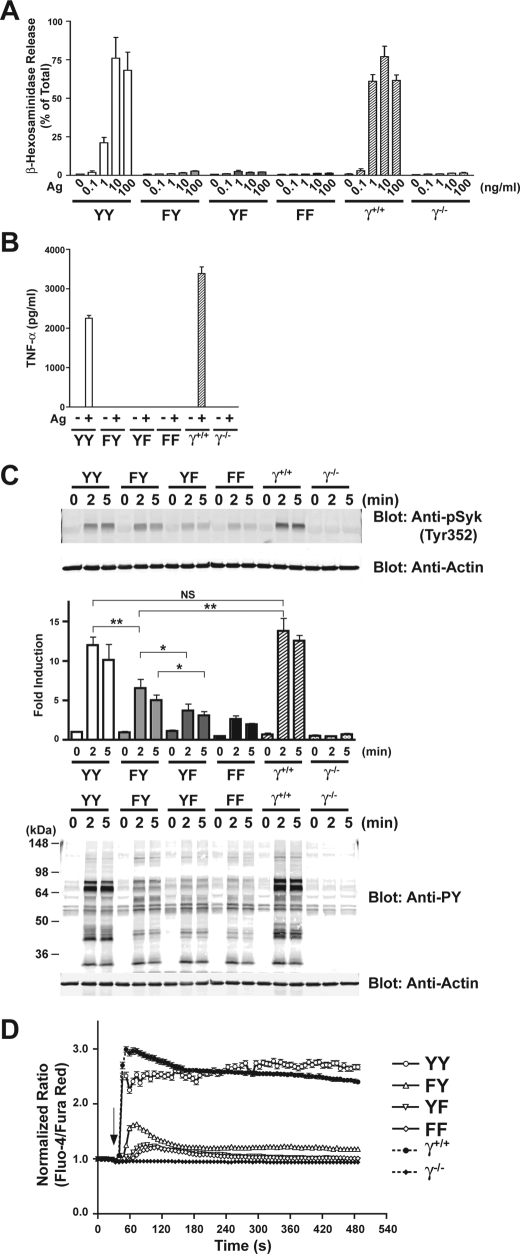
We tested the functional response of the FcRγ-reconstituted cells to assess whether the individual mutations of Tyr47 or Tyr58 might reveal differences in cellular signaling and responses. Fig. 3A shows that in contrast to YY-reconstituted cells or to wild type cells, mutation of either Tyr47 or Tyr58 caused ablation of mast cell degranulation similar to that seen with FF mutants. A test of cytokine responses also revealed that induction of cytokines was impaired, since production of tumor necrosis factor was not observed (Fig. 3B). Production of IL-6 and IL-13 was also impaired, but a trend toward a very weak, but not significant, production of MCP-1 was seen in all mutants relative to FcεRIγ-null cells upon FcεRI stimulation (supplemental Fig. 1). We do not exclude the possibility that tumor necrosis factor was produced and retained in the secretory granule compartment, since this was not examined. However, the failure to secrete substantial amounts of MCP-1, a cytokine that is not stored in secretory granules and that is produced in response to a very weak stimulus (23), suggests a probable general impairment of cytokine transcription. Analysis of Syk phosphorylation revealed a moderate and reproducible increase in Syk Tyr352 phosphorylation in cells carrying wild type Tyr58 (FY) (Fig. 3C). This ranged from 20 to 60% of the phosphorylation seen in YY-expressing cells depending on the experiment. Both YF- and FF-expressing cells also elicited very weak Syk phosphorylation following Ag stimulation but were clearly less efficient than FY-expressing cells. This relative difference in Syk phosphorylation was also reflected at the level of phosphorylation of total proteins from these cells (Fig. 3C, bottom). Single cell calcium imaging revealed that FY-expressing cells had significantly higher intracellular calcium levels when compared with YF- or FF-expressing cells. The average calcium response from a minimum of more than 138 cells is shown in Fig. 3D. Supplemental Fig. 2 shows the individual cellular responses observed in a typical experiment. These findings demonstrated that expression of Tyr58 alone can elicit modest Syk phosphorylation and weak calcium responses upon FcεRI engagement but not degranulation or cytokine secretion.
FIGURE 3.
Functional responses of BMMCs are ablated by mutation of FcRγ ITAM tyrosines, but Syk is still phosphorylated in Y48F mutant, and weak calcium responses are induced. A, wild type or FcRγ-mutant transfected BMMCs were incubated with anti-DNP IgE. The IgE-sensitized cells were stimulated or not with the indicated concentrations of DNP-HSA (Ag) for 30 min at 37 °C. Mast cell degranulation was measured by release of β-hexosaminidase. B, cytokine analysis was performed on cells sensitized with IgE as above and stimulated with Ag (10 ng) for 4 h. C, IgE-sensitized wild type or FcRγ ITAM-mutant bearing BMMCs were stimulated for the indicated time with 100 ng of Ag. Proteins in the whole cell lysates were resolved by SDS-PAGE under reducing conditions and either blotted with anti-phosphotyrosine (Anti-PY) or anti-phospho-Syk (Tyr352). The graph shows the mean from a minimum of four experiments for the 2 min time point and three experiments for all other times. Statistics were done using Student's t test. p values are as follows: *, p < 0.05; **, p < 0.01. NS, no significant difference. D, mean calcium responses observed from an analysis of a minimum of 138 cells using single cell calcium imaging analysis. IgE-sensitized wild type of FcRγ ITAM-mutant cells was stimulated with 10 ng of Ag. The mean is from four individual experiments.
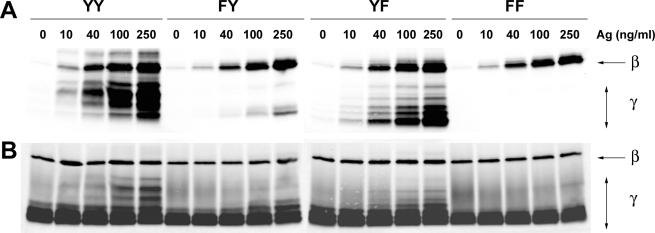
To assess whether the observed effect of Tyr58 on Syk phosphorylation and calcium responses resulted from enhanced phosphorylation of this site relative to Tyr47, the FcεRI-induced phosphorylation of wild type and the FcRγ mutants was analyzed 2 min poststimulation (at peak phosphorylation), using varying concentrations of Ag. Fig. 4A shows the unanticipated result where phosphorylation of the FY mutant, relative to wild type or to the YF mutant, was minimal. The extent of phosphorylation was Ag concentration-dependent and did not correlate with maximal degranulation seen at 10 ng/ml of Ag (Fig. 3A). This may be due to the more effective activation of negative regulators of degranulation, such as SHIP-1 (24, 25) at higher Ag concentrations. As expected, no phosphorylation of the FF mutant was observed under any condition. In contrast, no effect on the phosphorylation of FcεRIβ was observed, and the recovered protein was similar for all immunoprecipitates (Fig. 4B). Thus, these findings suggested that the FY mutant was either weakly phosphorylated upon FcεRI stimulation or was rapidly dephosphorylated.
FIGURE 4.
Tyrosine phosphorylation of FcεRI in wild type and mutant FcεRIγ-reconstituted γ-/- BMMC following Ag stimulation. A, retrovirally transfected γ-/- BMMCs (YY, FY, YF, and FF) were sensitized with IgE and stimulated or not with the indicated concentrations of Ag for 2 min at 37 °C. IgE-occupied FcεRI was immunoprecipitated with anti-IgE, and the proteins resolved, under nonreducing conditions, were analyzed for tyrosine phosphorylation of FcεRIβ (β) and FcRγ (γ) by immunoblotting with anti-phosphotyrosine. B, the same membrane was stripped and reprobed with anti-FcεRIβ and anti-FcRγ. One representative of four individual experiments is shown.
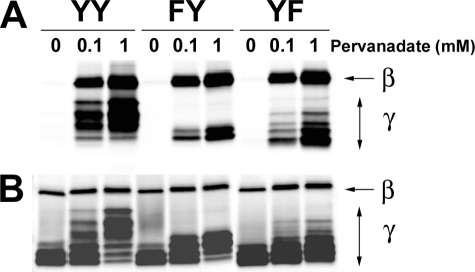
To test these possibilities, we first incubated cells in the presence of pervanadate to inhibit phosphatase activity and then explored the effect on phosphorylation of FcRγ. Fig. 5A demonstrates that under conditions of phosphatase inhibition, the FY mutant was efficiently phosphorylated. Although not all species of FcRγ seen in the wild type or YF transductants were present in the pervanadate-stimulated FY mutant, the species seen (Fig. 5, A and B) were effectively phosphorylated when compared with stimulation via FcεRI (Fig. 4A). Negligible phosphorylation of an FF mutant was observed under these conditions (data not shown). Thus, these results argued for a regulatory mechanism of preferential dephosphorylation of Tyr58 following FcεRI stimulation.
FIGURE 5.
Pervanadate treatment elicits strong tyrosine phosphorylation of wild type and mutant FcεRIγ ITAMs. A, retrovirally transfected γ-/- BMMCs (YY, FY, and YF) were sensitized with IgE and stimulated or not with the indicated concentrations of pervanadate for 5 min at 37 °C. FcεRI was immunoprecipitated, and protein was resolved by SDS-PAGE and analyzed for tyrosine phosphorylation of FcεRIβ (β) and FcRγ (γ) by immunoblotting with anti-phosphotyrosine. B, the same membrane was stripped and reprobed with anti-FcεRIβ and anti-FcRγ. One representative of four individual experiments is shown.
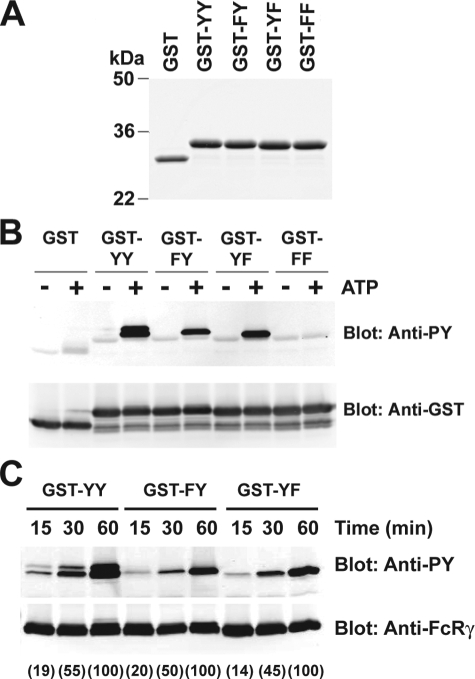
In Vitro Analysis of the Dephosphorylation of FcRγ ITAM Tyrosines—We first tested the possibility, using in vitro kinase assays, of differential phosphorylation of Tyr47 and Tyr58 by endogenous Lyn kinase. For these experiments, GST fusion proteins were generated that encoded the FcRγ cytoplasmic domain for wild type ITAM (YY) or mutant ITAMs (FY, YF, and FF). Fig. 6A shows that these proteins could be efficiently expressed with almost no detected free GST. Using equal amounts of these fusion proteins and endogenous Lyn kinase (immunoprecipitated from wild type cells), minimal phosphorylation of GST was observed upon the addition of ATP (Fig. 6B). In contrast, the addition of ATP led to a marked phosphorylation of the GST-YY. Both GST-FY and GST-YF were also efficiently phosphorylated, whereas GST-FF was not. Moreover, the kinetics of phosphorylation (Fig. 6C) revealed that peak phosphorylation required between 30 and 60 min of incubation. This showed that under the conditions of this experiment, endogenous Lyn phosphorylated both Tyr47 and Tyr58. This is in contrast to the poor phosphorylation of the FcRγ mutant FY observed upon FcεRI stimulation (Fig. 4A).
FIGURE 6.
Tyrosine phosphorylation of wild type and mutant GST-FcRγ fusion proteins by Lyn. A, characterization of the GST fusion proteins. The cytoplasmic domain (4.8 kDa) of wild type (YY) or mutated FcRγ ITAM (FY, YF, and FF) were expressed as GST fusion proteins in E. coli and purified by affinity chromatography on GSH-Sepharose 4B beads. The purified proteins (1 mg) were resolved by SDS-PAGE and stained with Coomassie Brilliant Blue. B, in vitro kinase assay of GST-FcRγ fusion proteins as an exogenous substrate for Lyn kinase. Lyn was immunoprecipitated from BMMCs. The beads were incubated with the indicated GST fusion proteins in the presence or absence of ATP. The kinase reaction products were resolved on SDS-PAGE and subjected to immunoblotting with anti-phosphotyrosine. The same membrane was stripped and reprobed with anti-GST. One representative of three individual experiments is shown.
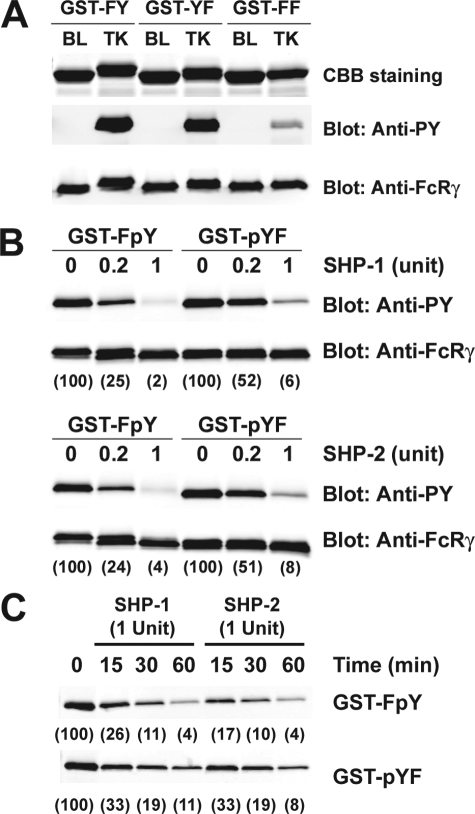
We next examined whether the apparent discrepancy in poor phosphorylation of FcRγ FY mutant (Tyr58), after FcεRI stimulation, and its effective phosphorylation in vitro by Lyn kinase could be consequence of Tyr(P)58 being a better substrate for the phosphatases SHP-1 and SHP-2, which are known to associate with FcεRI (10). To obtain phosphorylated GST-FcRγ cytoplasmic domain constructs, we expressed the GST-FY, GST-YF, and GST-FF in the TKB1 E. coli strain that contains an inducible Elk1 tyrosine kinase. As shown in Fig. 7A, both GST-FY and GST-YF were effectively phosphorylated, whereas minimal phosphorylation of GST-FF was observed (probably reflecting a weak phosphorylation of GST). The activities of recombinant and highly purified SHP-1 and SHP-2 were then normalized using p-nitrophenyl phosphate as a substrate, and the amount of enzyme providing 40 pmol/min (0.2 units) or 200 pmol/min (1 unit) of activity was used in an in vitro dephosphorylation assay. Fig. 7B demonstrates that GST-FpY (Tyr(P)58) was more effectively dephosphorylated (2-fold greater at 0.2 units) than GST-pYF (Tyr(P)47). At this level of enzymatic activity (40 pmol/min) Tyr(P)58 lost 75% of its phosphate, whereas Tyr(P)47 lost ∼50%. At higher enzymatic activity (200 pmol/min), these differences were narrowed, but phosphorylation of Tyr47 was still more readily retained (Fig. 7B). Kinetic analysis (Fig. 7C) demonstrated that the conditions used were linear. Collectively, these experiments showed that both Tyr47 and Tyr58 are targets of Lyn activity, but dephosphorylation of Tyr(P)58 appears to be preferred.
FIGURE 7.
Differential dephosphorylation of the FcεRIγ ITAM C-terminal Tyr58 by SHP-1 and SHP-2. A, characterization of the tyrosine-phosphorylated GST fusion proteins. GST-FY, GST-YF, and GST-FF were expressed in either the E. coli BL21 (BL) or TKB1 (TK) strain. The purified GST fusion proteins were stained with Coomassie Brilliant Blue (CBB). Tyrosine phosphorylation of GST fusion proteins were analyzed by immunoblotting with anti-phosphotyrosine (Anti-PY). The same membrane was stripped and reprobed with anti-FcRγ. B, in vitro PTPase assay with tyrosine-phosphorylated GST-FcRγ fusion proteins as exogenous substrates for SHP-1 and SHP-2. The activities of recombinant human SHP-1 and SHP-2 were assayed and normalized. Each enzyme (0.2 or 1 arbitrary unit) was incubated with 100 nm tyrosine-phosphorylated GST-FcRγ at 37 °C for 60 min. The PTPase reaction products were then resolved by SDS-PAGE, transferred to membranes, and probed with anti-phosphotyrosine. The same membranes were stripped and reprobed with anti-FcRγ. The relative band intensity in the anti-phosphotyrosine immunoblot was quantified and reported in parenthesis. One representative of a minimum of three individual experiments is shown.
LC/MS/MS Analysis of the Phosphorylation State of FcRγ upon FcεRI Stimulation—The in vitro dephosphorylation assay above suggested that the weak phosphorylation of Tyr58 observed in cells (Fig. 4A) was probably a result of dephosphorylation, since Lyn kinase efficiently phosphorylated both Tyr47 and Tyr58 and inhibition of phosphatase activity led to strong Tyr58 phosphorylation. Thus, we postulated that in FcεRI-stimulated cells one might find the phosphorylation of Tyr47 to be more prevalent to that of Tyr58 if indeed Tyr(P)58 is more susceptible to phosphatase activity. Using mass spectrometry we determined the state of phosphorylation of tryptic peptides derived from FcRγ following FcεRI stimulation.
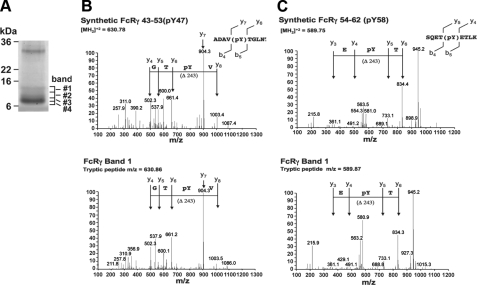
The majority of reduced FcRγ purified from activated cells migrated as a doublet around 7 kDa (Fig. 8A). However, a minor protein doublet of FcRγ was observed with a molecular mass of 9-14 kDa. The minor protein doublet appeared to contain phosphotyrosine, based on immunoblots using an anti-phosphotyrosine antibody (see Fig. 1A) (data not shown). In order to map which sites were phosphorylated under these conditions, the protein bands 1 and 2 from the SDS/PAGE gels were digested with trypsin to produce peptides that could be analyzed by LC/MS/MS. This analysis identified peptides spanning the carboxyl-terminal region from residue 41 to 68 of the mature processed FcRγ subunit (supplemental Fig. 3). In addition to the ions predicted for unmodified peptides from the protein, ions corresponding to the m/z values predicted for peptides containing one phosphate were observed from the two upper bands of FcRγ isolated from FcεRI-stimulated cells (Table 1). MS/MS fragmentation of these ions indicated that Tyr47 and Tyr58 were the phosphorylated residues. In order to verify the phosphopeptide assignments, peptides corresponding to the proposed FcRγ phosphopeptides were synthesized and analyzed by LC/MS/MS under the same conditions. MS/MS fragmentation of synthetic peptides containing either Tyr(P)47 or Tyr(P)58 gave MS/MS spectra that were the same as those observed for peptides isolated from the endogenous FcRγ subunit (Fig. 8, B and C).
FIGURE 8.
Mass spectrometry of FcεRIγ purified from Ag-stimulated wild type BMMCs reveals increased Tyr(P)47 relative to Tyr(P)58. A, Coomassie Brilliant Blue staining of purified FcRγ isolated from Ag-stimulated BMMCs. Four identifiable bands are labeled (bands 1-4). Based on anti-Tyr(P) immunoblots, bands 1 and 2 contained phosphorylated FcRγ. B and C, spectra of peptides resolved by LC/MS/MS derived from bands 1 and 2 in A. The top panels show fragmentation spectra of the doubly charged ions produced from synthetic peptides carrying either a Tyr(P)47 or Tyr(P)58. Localization of the phosphate to the tyrosine is demonstrated by the y6 and y7 ions (m/z = 661.36 and 904.49, respectively) for the Tyr(P)47 peptide and by the y4 and y5 ions (m/z = 490.29 and 733.32, respectively) for the Tyr(P)58 peptide. The complementary b ions diagnostic for phosphotyrosine localization were also observed for both peptides. The bottom panel shows the fragmentation spectra for peptides isolated from bands 1 and 2. The masses and fragmentation pattern for the spectra from the isolated peptides are almost identical to those obtained from the synthetic peptides. Based on relative ion intensities, phosphorylation of Tyr58 appears to be less than that of Tyr47. This was verified as shown in Table 2.
Based on the relative ion intensities of the Tyr(P)47 and Tyr(P)58 peptides, it appeared that Tyr47 was modified to a higher level than Tyr58. In order to determine the molar ratios of the modifications, the relative ionization efficiency for each peptide was determined. Calibration curves for ion intensities were generated using known amounts of synthetic peptide standards corresponding to the phosphorylated and nonphosphorylated peptides. Ionization efficiencies for each phosphopeptide relative to the corresponding nonphosphorylated peptide were determined from the MS ion peak areas for the doubly charged ions. A linear relationship was observed between the peak area ratios and the molar ratios for MS spectra of the standard peptides (supplemental Fig. 4). The slope of the line was calculated and used as a correction factor to convert relative peak areas of the unknowns into molar ratios. For the peptide FcRγ 43-53, the ion intensity of the tyrosine-phosphorylated peptide was almost identical to the nonphosphorylated peptide, with a slope = 0.995 ± 0.022 (supplemental Fig. 4). For the peptide FcRγ 54-62, the ion intensity for the phosphotyrosine peptide was only about 63% of the corresponding nonphosphorylated peptide (slope = 0.634 ± 0.077). These calibration curves were then used to convert phosphopeptide/nonphosphopeptide ion peak area ratios into molar ratios.
Using this approach, a significant difference was observed for the level of tyrosine phosphorylation in different bands of the FcRγ subunit resolved by SDS-PAGE. As indicated on Western blots, using anti-phosphotyrosine antibodies, the top two bands (Fig. 8A,#1 and #2) of the FcRγ from FcεRI-stimulated cells were significantly phosphorylated on both Tyr47 and Tyr58, with the top band having the highest level of phosphorylation. In both of the phosphorylated bands, the level of phosphorylation of Tyr47 was significantly higher than the level of Tyr58 phosphorylation (Table 2). In band 1, 70% of Tyr47 was phosphorylated, while only 35% of Tyr58 was found to be phosphorylated. A similar pattern was observed in band 2, where ∼47% of Tyr47 was phosphorylated compared with only 26% of Tyr58 being phosphorylated. Because trypsin cuts between the two phosphorylated residues (cutting at Arg53), it was not possible to determine what fraction of the protein is phosphorylated on both tyrosine residues compared with phosphorylation on only a single residue. Regardless, the findings demonstrated that phosphorylation of Tyr47 of the FcRγ is found in greater abundance than that of Tyr58 after FcεRI stimulation.
TABLE 2.
Phosphorylation of Tyr47 and Tyr58 in activated FcRγ gel bands
|
FcRγ gel band
|
Ion peak area (×10−8)
|
Calculated molar ratios (Tyr(P)/Tyr peptide)
|
||||
|---|---|---|---|---|---|---|
| FcR 43-53 (Tyr(P)47) | FcR 43-53 | FcR 54-62 (Tyr(P)58) | FcR 54-62 | FcR 43-53 (Tyr(P)47/Tyr47) | FcR 54-62 (Tyr(P)58/Tyr58) | |
| 1 | 5.7 | 3.4 | 1.4 | 6.7 | 1.9 | 0.4 |
| 7.3 | 2.7 | 1.8 | 4.5 | 2.9 | 0.7 | |
| 2 | 5.4 | 8.2 | 1.5 | 6.7 | 0.8 | 0.5 |
| 5.5 | 6.4 | 0.8 | 10.8 | 1.0 | 0.2 | |
| 3 | 0.2 | 39.8 | 0.02 | 8.8 | <0.01 | <0.01 |
| 1.1 | 8.1 | 0.3 | 29.2 | 0.14 | 0.02 | |
| 1.2 | 25.4 | 0.1 | 43.7 | 0.05 | <0.01 | |
| 4 | 0.2 | 41.0 | 0.1 | 67.2 | <0.01 | <0.01 |
| 0.7 | 25.0 | 0.7 | 38.3 | 0.03 | 0.03 | |
| 0.8 | 28.9 | 0.3 | 46.7 | 0.03 | <0.01 | |
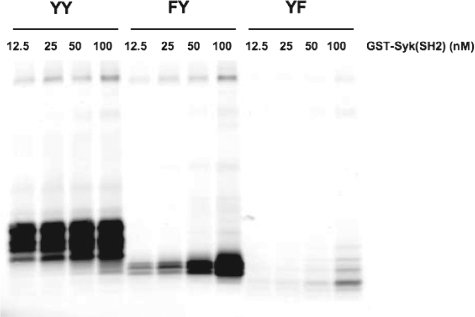
Capture of Wild Type and Mutant FcεRIγ by Syk SH2 Domains—Whereas high affinity Syk binding to the FcRγ requires phosphorylation of both Tyr47 and Tyr58, we sought to address the relative roles of Tyr47 and Tyr58 in Syk binding. We adopted the strategy of immunoprecipitating the intact wild type (YY) or mutant (FY and YF) FcεRI from pervanadate-stimulated cells, which inhibits phosphatase activity and leads to its phosphorylation and determining how much of the phosphorylated FcεRI might be captured by varying concentrations of glutathione bead-bound GST Syk-SH2. The conditions used for pervanadate stimulation of the cells were identical to those in Fig. 5A. As shown in Fig. 9, capture of wild type receptor (YY) was detected at all concentrations of Syk-SH2 used. The mutant FcεRI (FY) was also captured by Syk SH2 even at the lowest concentration, albeit at reduced levels when compared with wild type. In contrast, the mutant FcεRI (YF) was minimally captured by Syk SH2 domains even at the highest concentration used. These findings demonstrated that the phosphorylation of the FcεRIγ Tyr58 alone (FY mutant) results in considerable interaction of this γ chain monophosphorylated receptor with the SH2 domains of Syk. Thus, the findings are consistent with the previous data showing that phosphorylation of Tyr58 alone has activating potential for Syk. Since FcεRI-induced phosphorylation of Tyr58 was less readily detected by LC/MS/MS analysis, our findings argue that dephosphorylation is favored in vivo, and thus this may serve to control Syk activation and mast cell responses.
FIGURE 9.
Capture of wild type and mutant FcεRIγ by Syk SH2 domains, from pervanadate-treated BMMC transductants, demonstrates Syk binding to Tyr(P)58. Retrovirally transfected γ-/- BMMCs (YY, FY, and YF) were sensitized with IgE and treated or not with pervanadate (100 nm) for 5 min at 37 °C. Cells were lysed and incubated with the indicated concentration of glutathione bead-bound GST-Syk (SH2 domain construct). Interacting FcεRI was resolved by SDS-PAGE and transferred to membranes. The membranes were probed with anti-FcRγ to reveal captured FcεRI. One representative of three individual experiments is shown.
DISCUSSION
The essential role of Syk kinase in mast cell activation and function is well recognized (5). Syk deficiency results in cells that are nonresponsive to FcεRI stimulation (26), and reconstitution of such cells with Syk restores responsiveness (27). Syk activation causes amplification of the signals required for mast cell function (5). Once activated, Syk is able to phosphorylate diverse substrates that are important for assembling signaling networks, such as adaptor molecules that function to organize and coordinate the signals leading to the generation of lipid second messengers and activation of calcium responses (28). These are key intermediates in eliciting functional responses, and thus their phosphorylation is likely be tightly regulated. It is also well known that Syk activation in mast cells requires the phosphorylation of the two tyrosine residues found in the FcεRIγ ITAMs (3, 4), where Syk binds via its tandem N-terminal SH2 domains. As shown here, mutation of either tyrosine (Tyr47 or Tyr58) results in the loss of mast cell degranulation. However, our findings also show that minimal phosphorylation of Tyr58, relative to Tyr47, is still sufficient to elicit modest Syk activation and weak calcium responses. This suggests that tight regulation of Tyr58 phosphorylation is needed to avoid spurious activation of Syk. Thus, we set out to explore if there is differential phosphorylation or dephosphorylation of these residues as a mechanism to control the initiation and/or extent of Syk activation.
Regulation of Syk activation occurs at multiple levels. Syk, like its related family member ZAP-70, normally is maintained in an inactive state through intramolecular interactions of its SH2 domains with phosphorylated tyrosine residues in its linker (A and B) regions (29, 30). In B cells, it was shown that a Syk Y126F mutation (which lies in the linker A region) results in an active kinase (31). In mast cells, a Syk Y317F mutant (which lies in the linker B region) showed gain of function, which was readily detected when FcεRI was engaged (32). This suggests that, like ZAP-70, the interdomain interactions with the tandem N-terminal SH2 domains are a key regulatory feature in Syk activation. However, what might lead to loss of these intramolecular interactions is not known. One possibility is phosphatase-mediated dephosphorylation, but to date this has not been demonstrated for Syk. Another plausible mechanism is a shift in the equilibrium toward an open conformation by providing a stable high affinity interaction via phospho-ITAMs. This mechanism is supported by the ability to elicit Syk activation in vitro in the presence of phospho-ITAMs (reviewed in Ref. 5). Thus, Syk activation is highly dependent on ITAM interactions. The recent demonstration that non-ITAM-bearing integrin receptors (like β2 integrins) use ITAMs to activate Syk (reviewed in Ref. 6) supports the notion that this is a highly conserved activatory mechanism shared by varied receptors.
In mast cells, a requirement for FcεRIβ in achieving full activation of Syk was also demonstrated (33). Thus, the absence of FcεRIβ or mutation of its N-terminal or C-terminal cytoplasmic domains caused diminished Syk activation. Presumably, this is a consequence of diminished FcεRIγ phosphorylation, since the loss of FcεRIβ ITAM function reduces the phosphorylation of this receptor (12, 14), probably through the loss of Lyn kinase, which associates with the FcεRIβ ITAM and phosphorylates both β and γ ITAMs. A role for Lyn kinase in the phosphorylation of Syk has also been shown (34), suggesting that Syk activation could be dually influenced by Lyn through the regulation of FcεRIγ phosphorylation/dephosphorylation and through direct phosphorylation of Syk. However, the consequence of direct phosphorylation of Syk by Lyn is not clear.
Studies have demonstrated the binding of Lyn to the phosphorylated Tyr219 of FcεRIβ (12, 14). Mutation of this residue (Y219F) caused loss of phosphorylation of both β and γ chains as well as the loss of Syk phosphorylation. In contrast, mutation of the noncanonical Tyr225 or of the C-terminal canonical Tyr229 did not significantly alter FcεRI or Syk phosphorylation (12, 14). Our findings show, however, that the apparent dephosphorylation of Tyr58 is independent of the state of FcεRIβ phosphorylation, since it occurs under circumstances where FcεRIβ phosphorylation is unaltered (Fig. 4). This suggests that dephosphorylation of the FcεRIβ Tyr219, which binds Lyn, is not a major mechanism for controlling Syk activity.
Once activated, Syk is controlled by multiple mechanisms that govern its activity. One demonstrated mechanism is that of ubiquitylation and degradation. In B cells, Cbl was shown to bind and mediate Syk ubiquitylation, leading to degradation (35). Moreover, in mast cells, the loss of Cbl-b but not c-Cbl caused enhanced FcεRI and Syk phosphorylation as well as enhanced mast cell effector responses (36), suggesting that Cbl-b may dampen directly and/or indirectly the activation of Syk. However, this Cbl-b-mediated regulation does not appear to play a major role in initiation of Syk activity, suggesting that the major mechanism to control the initiation and maintenance of Syk activity is through phosphorylation and dephosphorylation of the ITAM tyrosines to which it binds.
Although much is known about how FcεRI is phosphorylated, much less is known about its dephosphorylation. Co-immunoprecipitation of the protein-tyrosine phosphatases SHP-1 and SHP-2 with this receptor has been shown (3, 4). SHP-1 appeared to constitutively interact with FcεRI, whereas SHP-2 was recruited upon FcεRI stimulation (data that were reproduced in this study but not shown). Previous phosphopeptide pull-down experiments also revealed the association of SHP-2 with the FcεRIβ ITAM but no association of SHP-1 was found with either β or γ ITAMs, suggesting that the observed co-immunoprecipitation may reflect an indirect association (3, 11). Overexpression of wild type or catalytically inactive SHP-1 in RBL-2H3 cells demonstrated decreased or enhanced phosphorylation of FcεRI (37), respectively, and corresponding changes were observed in Syk phosphorylation. However, despite the overexpression of an active or inactive SHP-1, these cells showed no alterations in FcεRI-induced histamine release, whereas enhanced or decreased JNK phosphorylation and tumor necrosis factor-α mRNA expression correlated with active or inactive SHP-1, respectively (37). This suggests that although SHP-1 may be able to dephosphorylate FcεRI and Syk, it may also have its major role further downstream. It should be noted that SHP-1 showed similar inhibitory effects on FcγRIIA and Syk phosphorylation as well as on FcγRIIA-mediated phagocytosis in transfected COS-1 cells (38).
A previous study showed that the FcεRIγ undergoes ubiquitylation upon FcεRI stimulation (21), which might explain the differences we observed in the molecular mass of FcεRIγ. However, our finding showing multiple phosphorylated FcεRIγ bands and a difference in the binding of Syk to these bands as determined by far Westerns suggests that some of these bands have differing states of phosphorylation. This suggested difference in the state of phosphorylation of these phospho-FcεRIγ isoforms was investigated by mass spectrometry with the discovery that less phospho-Tyr58 to phospho-Tyr47 is found upon analysis of FcεRIγ ITAM peptides. However, given the inability to maintain an intact ITAM peptide in the tryptic digest, we could not assess the abundance of dually phosphorylated ITAMs. Nonetheless, based on the expression of wild type and mutant forms of the FcεRIγ (which showed little phosphorylation of Tyr58 in vivo regardless of whether wild type or mutant) and on experiments demonstrating the potential for Tyr58 to be heavily phosphorylated both in vitro (by Lyn) and in vivo (in the presence of a phosphatase inhibitor), we conclude that Tyr58 is more susceptible to dephosphorylation. This is supported by the higher susceptibility of Tyr(P)58, relative to Tyr(P)47, to dephosphorylation by SHP-1 or SHP-2 in vitro. Interestingly, the poor phosphorylation of Tyr58 alone (but not the more abundant phosphorylation of Tyr47) elicited modest Syk activation and weak calcium responses. This suggests that increases in Tyr58 phosphorylation alone could result in unwanted Syk phosphorylation and possible mast cell activation. This view is supported by the demonstration that receptors carrying a phosphorylated Tyr58 alone can bind to Syk. Thus, keeping this site under tight regulatory control may be important. Taken together, these findings argue for the dephosphorylation of phospho-Tyr58 as a key step in controlling the activation of Syk and mast cell responses.
Supplementary Material
Acknowledgments
We thank the Flow Cytometry and Laboratory Animal Care and Use Sections of the Office of Science and Technology, NIAMS, for assistance.
This work was authored, in whole or in part, by National Institutes of Health staff. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1-4.
Footnotes
The abbreviations used are: ITAM, immunoreceptor tyrosine-based activation motif; BMMC, bone marrow-derived mast cells; SH2, Src homology 2; DNP, 2,4-dinitrophenol; HSA, human serum albumin; Ag, antigen; Ab, antibody; mAb, monoclonal antibody; FITC, fluorescein isothiocyanate; GST, glutathione S-transferase; IL, interleukin; LC, liquid chromatography; MS, mass spectrometry.
References
- 1.Paolini, R., Jouvin, M. H., and Kinet, J. P. (1991) Nature 353 855-858 [DOI] [PubMed] [Google Scholar]
- 2.Pribluda, V. S., Pribluda, C., and Metzger, H. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 11246-11250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kihara, H., and Siraganian, R. P. (1994) J. Biol. Chem. 269 22427-22432 [PubMed] [Google Scholar]
- 4.Benhamou, M., Ryba, N. J. P., Kihara, H., Nishikata, H., and Siraganian, R. P. (1993) J. Biol. Chem. 268 23318-23324 [PubMed] [Google Scholar]
- 5.Siraganian, R. P., Zhang, J., Suzuki, K., and Sada, K. (2002) Mol. Immunol. 38 1229-1233 [DOI] [PubMed] [Google Scholar]
- 6.Berton, G., Mocsai, A., and Lowell, C. A. (2005) Trends Immunol. 26 208-214 [DOI] [PubMed] [Google Scholar]
- 7.Neel, B. G., and Tonks, N. K. (1997) Curr. Opin. Cell Biol. 9 193-204 [DOI] [PubMed] [Google Scholar]
- 8.Bruhns, P., Fremont, S., and Daeron, M. (2005) Curr. Opin. Immunol. 17 662-669 [DOI] [PubMed] [Google Scholar]
- 9.Swieter, M., Berenstein, E. H., and Siraganian, R. P. (1995) J. Immunol. 155 5330-5336 [PubMed] [Google Scholar]
- 10.Kimura, T., Zhang, J., Sagawa, K., Sakaguchi, K., Appella, E., and Siraganian, R. P. (1997) J. Immunol. 159 4426-4434 [PubMed] [Google Scholar]
- 11.Kimura, T., Kihara, H., Bhattacharyya, S., Sakamoto, H., Appella, E., and Siraganian, R. P. (1996) J. Biol. Chem. 271 27962-27968 [DOI] [PubMed] [Google Scholar]
- 12.Furumoto, Y., Nunomura, S., Terada, T., Rivera, J., and Ra, C. (2004) J. Biol. Chem. 279 49177-49187 [DOI] [PubMed] [Google Scholar]
- 13.Chen, T., Repetto, B., Chizzonite, R., Pullar, C., Burghardt, C., Dharm, E., Zhao, Z., Carroll, R., Nunes, P., Basu, M., Danho, W., Visnick, M., Kochan, J., Waugh, D., and Gilfillan, A. M. (1996) J. Biol. Chem. 271 25308-25315 [DOI] [PubMed] [Google Scholar]
- 14.On, M., Billingsley, J. M., Jouvin, M. H., and Kinet, J. P. (2004) J. Biol. Chem. 279 45782-45790 [DOI] [PubMed] [Google Scholar]
- 15.Kitaura, J., Song, J., Tsai, M., Asai, K., Maeda-Yamamoto, M., Mocsai, A., Kawakami, Y., Liu, F. T., Lowell, C. A., Barisas, B. G., Galli, S. J., and Kawakami, T. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 12911-12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, F. T., Bohn, J. W., Ferry, E. L., Yamamoto, H., Molinaro, C. A., Sherman, L. A., Klinman, N. R., and Katz, D. H. (1980) J. Immunol. 124 2728-2737 [PubMed] [Google Scholar]
- 17.Mendoza, G. R., and Metzger, H. (1976) Nature 264 548-550 [DOI] [PubMed] [Google Scholar]
- 18.Rivera, J., Kinet, J.-P., Kim, J., Pucillo, C., and Metzger, H. (1988) Mol. Immunol. 25 647-661 [DOI] [PubMed] [Google Scholar]
- 19.Saitoh, S., Arudchandran, R., Manetz, T. S., Zhang, W., Sommers, C. L., Love, P. E., Rivera, J., and Samelson, L. E. (2000) Immunity 12 525-535 [DOI] [PubMed] [Google Scholar]
- 20.Yamashita, T., Mao, S. Y., and Metzger, H. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 11251-11255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paolini, R., and Kinet, J.-P. (1993) EMBO J. 12 779-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takai, T., Li, M., Sylvestre, D., Clynes, R., and Ravetch, J. V. (1994) Cell 76 519-529 [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Espinosa, C., Odom, S., Olivera, A., Hobson, J. P., Martinez, M. E., Oliveira-Dos-Santos, A., Barra, L., Spiegel, S., Penninger, J. M., and Rivera, J. (2003) J. Exp. Med. 197 1453-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, M., Helgason, C. D., Scheid, M. P., Duronio, V., Humphries, R. K., and Krystal, G. (1998) EMBO J. 17 7311-7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, M., Kalesnikoff, J., Reth, M., and Krystal, G. (2002) Immunol. Lett. 82 17-21 [DOI] [PubMed] [Google Scholar]
- 26.Costello, P. S., Turner, M., Walters, A. E., Cunningham, C. N., Bauer, P. H., Downward, J., and Tybulewicz, V. L. J. (1996) Oncogene 13 2595-2605 [PubMed] [Google Scholar]
- 27.Zhang, J., Berenstein, E. H., Evans, R. L., and Siraganian, R. P. (1996) J. Exp. Med. 184 71-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera, J. (2002) Curr. Opin. Immunol. 14 688-693 [DOI] [PubMed] [Google Scholar]
- 29.Sada, K., Shahjahan Miah, S. M., Maeno, K., Kyo, S., Qu, X., and Yamamura, H. (2002) Blood 100 2138-2144 [DOI] [PubMed] [Google Scholar]
- 30.Arias-Palomo, E., Recuero-Checa, M. A., Bustelo, X. R., and Llorca, O. (2007) Biochim. Biophys. Acta 1774 1493-1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi, T., Wienands, J., Tsubata, T., and Kurosaki, T. (2007) Biochem. Biophys. Res. Commun. 364 111-117 [DOI] [PubMed] [Google Scholar]
- 32.Sada, K., Zhang, J., and Siraganian, R. P. (2000) J. Immunol. 164 338-344 [DOI] [PubMed] [Google Scholar]
- 33.Lin, S., Cicala, C., Scharenberg, A. M., and Kinet, J. P. (1996) Cell 85 985-995 [DOI] [PubMed] [Google Scholar]
- 34.El-Hillal, O., Kurosaki, T., Yamamura, H., Kinet, J.-P., and Scharenberg, A. M. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 1919-1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao, N., Ghosh, A. K., Ota, S., Zhou, P., Reddi, A. L., Hakezi, K., Druker, B. K., Wu, J., and Band, H. (2001) EMBO J. 20 7085-7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, J., Chiang, Y. J., Hodes, R. J., and Siraganian, R. P. (2004) J. Immunol. 173 1811-1818 [DOI] [PubMed] [Google Scholar]
- 37.Xie, Z. H., Zhang, J., and Siraganian, R. P. (2000) J. Immunol. 164 1521-1528 [DOI] [PubMed] [Google Scholar]
- 38.Huang, Z.-Y., Hunter, S., Kim, M.-K., Indik, Z. K., and Schreiber, A. D. (2003) J. Leukocyte Biol. 73 823-829 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.