Abstract
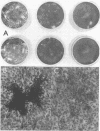
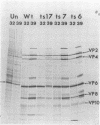
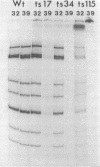
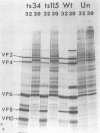
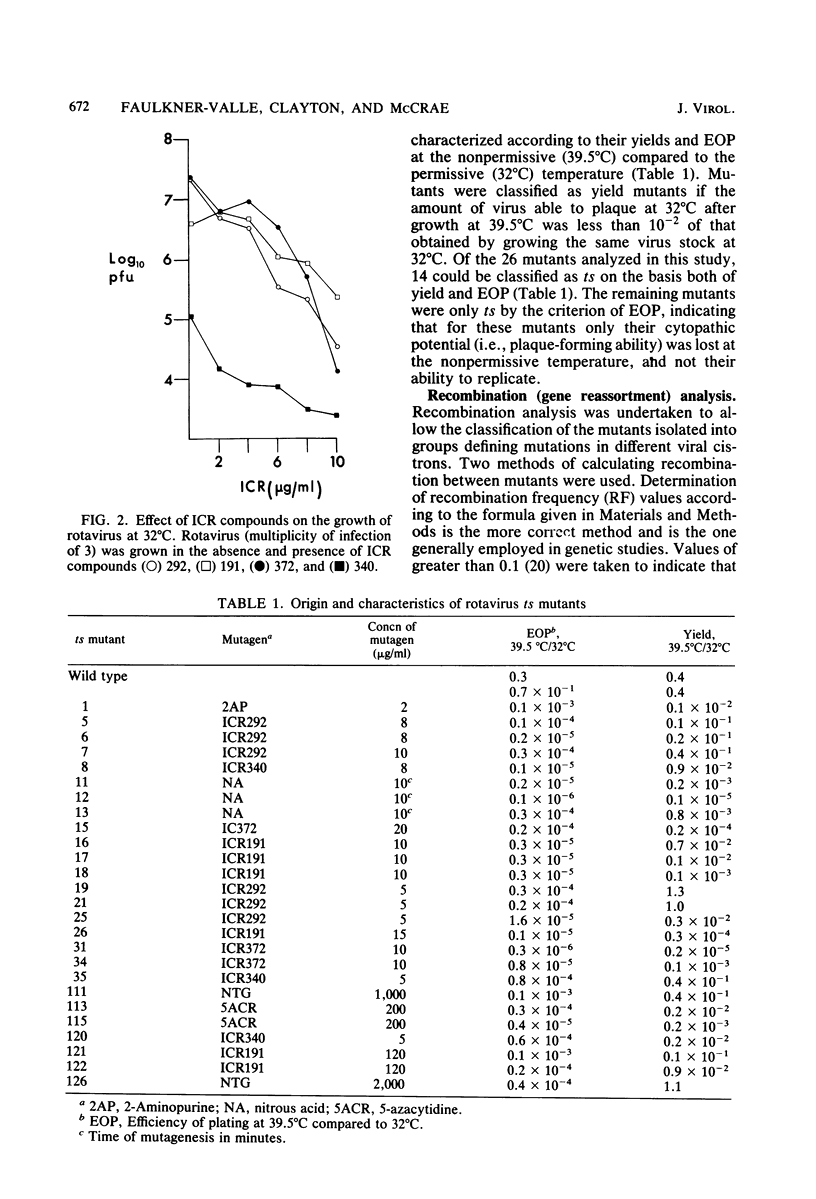
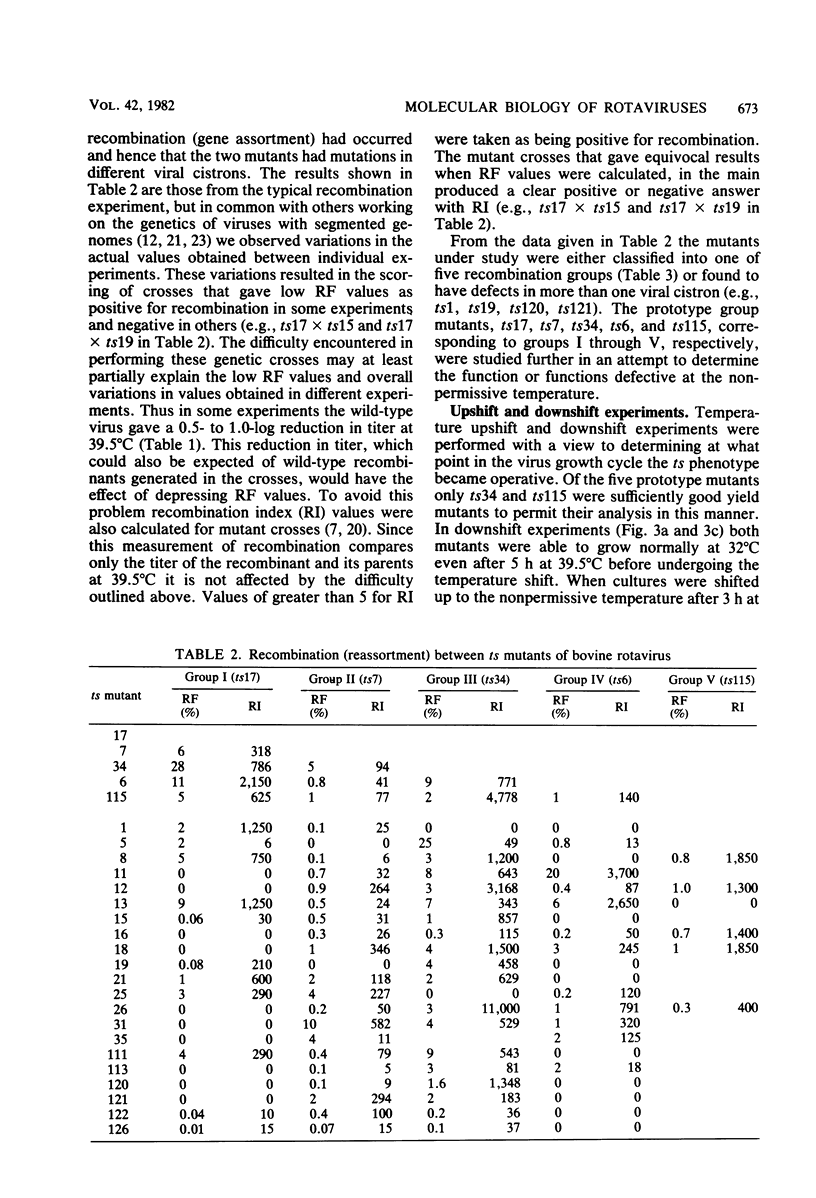
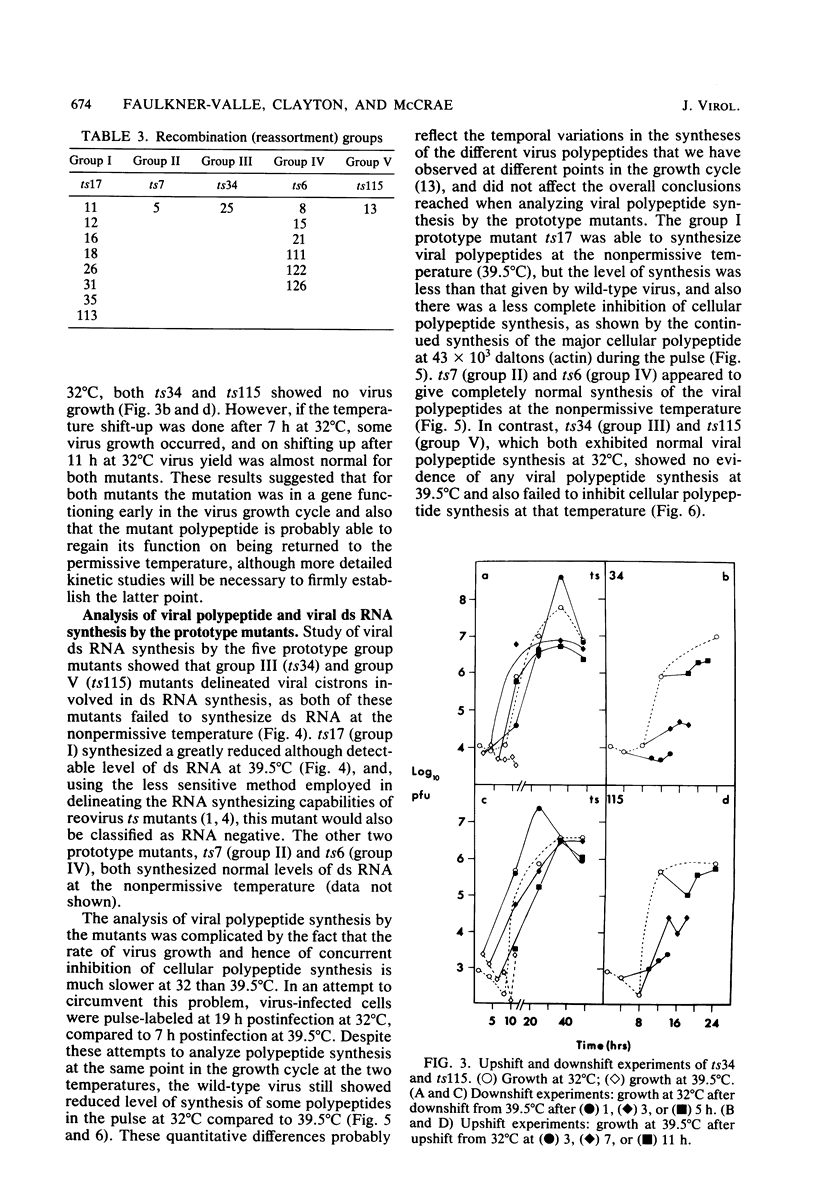
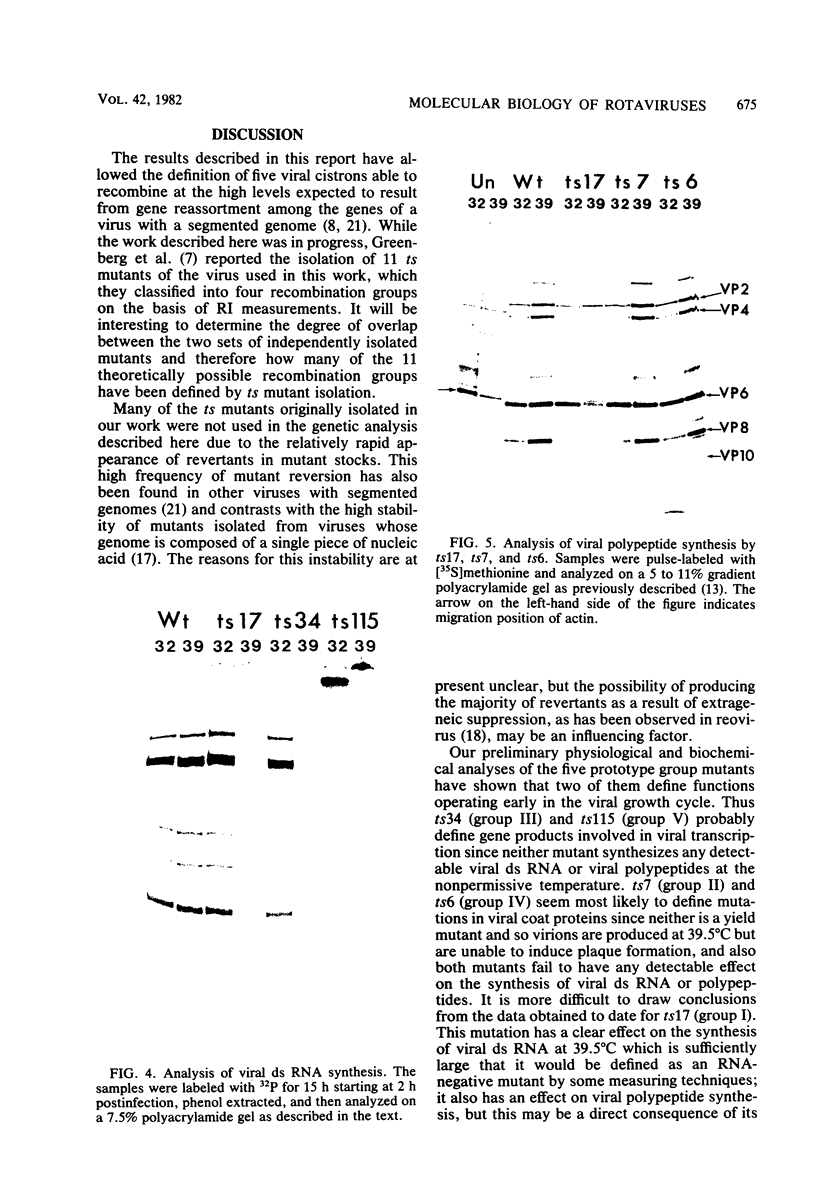
Twenty-six temperature-sensitive (ts) mutants of United Kingdom tissue culture-adapted bovine rotavirus were isolated and characterized. Fourteen of these mutants were determined to be ts both by efficiency of plating and by virus yield at the nonpermissive temperature of 39.5 degrees C as compared with that at the permissive temperature of 32 degrees C. The remaining mutants were only ts by the criterion of efficiency of plating. High-frequency recombination (gene reassortment) was observed when some pairs of mutants were crossed, and this allowed the classification of the mutants into five separate recombination groups. Groups III and V have prototype ts mutants (ts34 and ts115, respectively) that do not synthesize RNA or polypeptides at 39.5 degrees C. The other groups, I, II, and IV, have prototype mutants (ts17, ts7, and ts6, respectively) that synthesize both RNA and polypeptides at 39.5 degrees C, although ts17 does so only at a reduced level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy A. R., Shapiro L., August J. T., Joklik W. K. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. doi: 10.1016/0022-2836(67)90177-5. [DOI] [PubMed] [Google Scholar]
- Clarke I. N., McCrae M. A. A rapid and sensitive method for analysing the genome profiles of field isolates of rotavirus. J Virol Methods. 1981 Mar;2(4):203–209. doi: 10.1016/0166-0934(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Epidemic viral gastroenteritis. Am J Med. 1979 Jun;66(6):1001–1007. doi: 10.1016/0002-9343(79)90457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Thouless M. E., Pilfold J. N., Bryden A. S., Candeias J. A. More serotypes of human rotavirus. Lancet. 1978 Sep 16;2(8090):632–632. doi: 10.1016/s0140-6736(78)92854-4. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. K. Mechanism of influenza recombination. I. Factors influencing recombination rates between temperature-sensitive mutants of strain WSN and the classification of mutants into complementation--recombination groups. Virology. 1973 Sep;55(1):81–93. doi: 10.1016/s0042-6822(73)81010-4. [DOI] [PubMed] [Google Scholar]
- Iroegbu C. U., Pringle C. R. Genetic interactions among viruses of the Bunyamwera complex. J Virol. 1981 Jan;37(1):383–394. doi: 10.1128/jvi.37.1.383-394.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. S. Isolation of temperature-sensitive mutants and the construction of a preliminary genetic map for influenza virus. J Gen Virol. 1970 Jan;6(1):63–75. doi: 10.1099/0022-1317-6-1-63. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Faulkner-Valle G. P. Molecular biology of rotaviruses. I. Characterization of basic growth parameters and pattern of macromolecular synthesis. J Virol. 1981 Aug;39(2):490–496. doi: 10.1128/jvi.39.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- McNulty M. S., Allan G. M., Todd D., McFerran J. B. Isolation and cell culture propagation of rotaviruses from turkeys and chickens. Arch Virol. 1979;61(1-2):13–21. doi: 10.1007/BF01320587. [DOI] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Fields B. N. Revertants of temperature-sensitive mutants of reovirus: evidence for frequent extragenic suppression. Virology. 1979 Jan 15;92(1):155–167. doi: 10.1016/0042-6822(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Ray U. R., Fields B. N. Mutagenic specificity in reovirus. J Virol. 1979 Jun;30(3):913–916. doi: 10.1128/jvi.30.3.913-916.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Bowles A. L. Isolation and characterization of temperature-sensitive mutants of fowl plague virus. Virology. 1975 Oct;67(2):576–587. doi: 10.1016/0042-6822(75)90457-2. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hirst G. K. Temperature-sensitive mutants of influenza A virus: isolation of mutants and preliminary observations on genetic recombination and complementation. Virology. 1968 May;35(1):41–49. doi: 10.1016/0042-6822(68)90303-6. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Herring J. A., Reid H. W., Scott F. M., Gray E. W. Virus infections in cattle and sheep in Scotland 1975-1978. Vet Rec. 1980 Mar 1;106(9):193–194. doi: 10.1136/vr.106.9.193. [DOI] [PubMed] [Google Scholar]
- Sugiura A., Tobita K., Kilbourne E. D. Isolation and preliminary characterization of temperature-sensitive mutants of influenza virus. J Virol. 1972 Oct;10(4):639–647. doi: 10.1128/jvi.10.4.639-647.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H. Serotypes of human rotavirus. Lancet. 1978 Jan 7;1(8054):39–39. doi: 10.1016/s0140-6736(78)90381-1. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J., Hall G. A., Jones J. M., Jackson G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J Med Microbiol. 1976 May;9(2):203–209. doi: 10.1099/00222615-9-2-203. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]