Abstract
Stigmatellin, a QP site inhibitor, inhibits electron transfer from iron-sulfur protein (ISP) to cytochrome c1 in the bc1 complex. Stigmatellin raises the midpoint potential of ISP from 290 mV to 540 mV. The binding of stigmatellin to the fully oxidized complex, oxidized completely by catalytic amounts of cytochrome c oxidase and cytochrome c, results in ISP reduction. The extent of ISP reduction is proportional to the amount of inhibitor used and reaches a maximum when the ratio of inhibitor to enzyme complex reaches unity. A g = 2.005 EPR peak, characteristic of an organic free radical, is also observed when stigmatellin is added to the oxidized complex, and its signal intensity depends on the amount of stigmatellin. Addition of ferricyanide, a strong oxidant, to the oxidized complex also generates a g = 2.005 EPR peak that is oxidant concentration-dependent. Oxygen radicals are generated when stigmatellin is added to the oxidized complex in the absence of the exogenous substrate, ubiquinol. The amount of oxygen radical formed is proportional to the amount of stigmatellin added. Oxygen radicals are not generated when stigmatellin is added to a mutant bc1 complex lacking the Rieske iron-sulfur cluster. Based on these results, it is proposed that ISP becomes a strong oxidant upon stigmatellin binding, extracting electrons from an organic compound, likely an amino acid residue. This results in the reduction of ISP and generation of organic radicals.
The cytochrome (cyt)2 bc1 complex, also known as ubiquinol-cytochrome c reductase or complex III, is the central segment of the energy-conserving, electron transfer chain of mitochondria and many respiratory and photosynthetic bacteria (1). This enzyme complex catalyzes electron transfer from ubiquinol to cyt c (c2 in bacteria) with concomitant translocation of protons across the membrane to generate a proton electrochemical gradient required for ATP synthesis by ATP synthase. The cyt bc1 complexes from all species contain three core subunits, cyt b, cyt c1, and Rieske iron-sulfur protein (ISP), that house two b-type cyts (b566 and b562), one c-type cyt (c1), and a high potential Rieske [2Fe-2S] cluster, respectively. Depending on the source, the cyt bc1 complexes contain various numbers (0–8) of nonredox group-containing subunits, also called supernumerary subunits.
Recently, the three-dimensional crystal structures of mitochondrial bc1 complexes from bovine (2, 3), chicken (4), and yeast (5), which contain seven or eight supernumerary subunits in addition to the three core subunits, have been obtained. The structure of the cyt b6f complex, a complex analogous to the cyt bc1 complex, which provides an electronic connection between photosystems I and II, has also been established for the thermophilic cyanobacterium Mastigocladus laminosus (6), and for the algae Chlamydomonas reinhardtii (7), respectively. The three-dimensional structural information for the mitochondrial bc1 complex establishes the location of the redox centers, the number of trans-membrane helices, quinone binding at the QN site, and inhibitor binding at both the QP and QN sites (2–4, 8, 9). Moreover, these crystal structures suggest mobility of the extrinsic head domain of ISP during bc1 catalysis. Strong evidence in support of this movement has been reported (10–13, 15–19).
The proton-motive Q-cycle model (20, 21) has been favored for describing electron and proton translocation in the cyt bc1 complex. The key feature of this model is the presence of two separate ubiquinol/ubiquinone binding sites; a ubiquinol oxidation site (QP) near the P (intermembranes space) side of the mitochondrial inner membrane, and a ubiquinone reduction site (QN) near the N (matrix) side. X-ray crystallographic analyses revealed binding of ubiquinone at the QN site (5, 8). Unfortunately, ubiquinone or ubiquinol binding at the QP site has not been observed in native crystal structures, thereby preventing establishment of the putative QP site. Therefore, discussions on the mechanisms of electron transfer are based primarily on the positions occupied by inhibitors, and assumptions are made to reflect a similar site for binding of the natural substrate, ubiquinol. Detailed bifurcation of ubiquinol oxidation, the key step in the Q-cycle mechanism, is difficult to establish, although several models have been proposed (22–28). Stigmatellin, a Pf inhibitor (29) of the cyt bc1 complex, binds to cyt b at a domain distal from heme bL. It interacts with the Rieske ISP through the formation of a H-bond with His-161, and with cyt b by H-bonding with Glu-272, whose side chain undergoes a rotation of ∼160° from its native position (9). The H-bonded configuration of quinone and stigmatellin in the bc1 complex were shown to be similar (27). Hence, it is likely that the binding of stigmatellin resembles that of a quinol oxidation intermediate (5, 30).
Herein, we report the effect of stigmatellin on the redox state of oxidized cyt bc1 complexes. The importance of phospholipids for binding of stigmatellin was established. Generation of free radicals, upon addition of stigmatellin, was explored by analysis of the g = 2.005 EPR signal. Production of superoxide by the bc1 complex in the presence of stigmatellin was determined in wild-type cyt bc1 complex and compared with a mutant complex lacking the Rieske iron-sulfur cluster (ISC).
EXPERIMENTAL PROCEDURES
Materials—Cyt c (horse heart, type III), stigmatellin, 6-(4-methoxyphenyl)-2-methyl-3,7-dihydroimidazo[1,2-a]pyrazin-3 (7H)– one hydrochloride (MCLA), superoxide dismutase, and thermolysin were from Sigma. Azolectin was obtained from Associate Concentrate and purified according to the method of Kagawa and Racker (31). Octyl-Sepharose and DEAE-Sepharose CL-6 were obtained from GE Healthcare. n-Dodecyl-β-d-maltopyranoside (DM) and n-dodecyl-β-d-glucopyranoside were from Anatrace. Nickel-nitrilotriacetic acid gel and a Qiaprep Spin Miniprep kit were from Qiagen. 2,3-Dimethoxy-5-methyl-6-bromodecyl-1,4-benzoquinol (Q0C10BrH2) was prepared in our laboratory as reported previously (11). All other chemicals were of the highest purity commercially available.
Enzyme Preparations and Activity Assay—Chromatophores and intracytoplasmic membranes were prepared as described previously (10), and stored at –80 °C in the presence of 20% glycerol. The His6-tagged cyt bc1 complexes were purified from frozen intracytoplasmic membranes or chromatophores by the method of Tian et al. (10). Purified cyt bc1 complexes were stored at –80 °C in the presence of 10% glycerol. To assay ubiquinol-cyt c reductase activity, membrane preparations or purified cyt bc1 complexes were diluted with 50 mm Tris-Cl, pH 8.0, containing 200 mm NaCl and 0.01% DM to a final cyt b concentration of 1 μm.5 μl of the diluted samples were added to 1 ml of assay mixture containing 100 mm Na+/K+ phosphate buffer, pH 7.4, 0.3 mm EDTA, 50 μm ferricytochrome c, and 25 μm Q0C10BrH2. Activities were determined by measuring the reduction of cyt c, by following the increase in the absorbance at 550 nm, in a Shimadzu UV-2450 PC spectrophotometer at 23 °C using a millimolar extinction coefficient of 18.5 mm–1 cm–1 for calculations. The nonenzymatic oxidation of Q0C10BrH2 determined under similar conditions in the absence of the enzyme was subtracted during calculations for specific activity. Potassium cyanide was added to a final concentration of 30 μm in the assay mixture to inhibit cyt c oxidase, when determining the cyt bc1 activity in intracytoplasmic membranes or chromatophores.
EPR Experiments—The redox state of the Rieske ISP was determined by EPR (32) using a Bruker EMX spectrometer equipped with an Air Products flow cryostat. Cyt bc1 complex diluted in 50 mm Tris-Cl, pH 8, containing 200 mm NaCl and 0.01% DM, was incubated with 5 mm ascorbate on ice for 20 min and frozen in liquid nitrogen. Cyt bc1 complexes were oxidized by overnight incubation with 1 μm cyt c oxidase and 1 μm cyt c. Variable amounts of stigmatellin were added to oxidized bc1 complexes, and the mixture was incubated on ice for 15 min. EPR spectra were recorded at 100 K with the following instrument settings: microwave frequency, 9.39 GHz; modulation frequency, 100 kHz; modulation amplitude, 6.3 G; time constant, 655.4 ms; and sweep time, 167.8 s. Each spectrum represents an average of three different traces.
Isolation of Rieske ISP from Rhodobacter sphaeroides bc1 Complex—Wild-type cyt bc1 complex was diluted to 50 μm cyt b in 50 mm Tris-Cl, pH 8, containing 100 mm NaCl and 0.1% DM. The mixture was gently shaken at 4 °C for 90 min and applied to an octyl-Sepharose column previously equilibrated with 50 mm Tris-Cl, pH 8, containing 500 mm NaCl, 0.01% DM, and 1 mm dithiothreitol (DTT). The column was washed with 1 gel volume of 50 mm Tris-Cl, pH 8, 100 mm NaCl, 0.1% DM, and 1 mm DTT followed by a stepwise decrease in NaCl concentration to 75 mm and 50 mm. The column was then washed with 50 mm Tris-Cl, pH 8, 50 mm NaCl, 0.05% DM, and 1 mm DTT followed by the same buffer containing only 0.01% DM. The eluates from all five washes were combined and subsequently passed through a nickel-nitrilotriacetic acid column equilibrated with 50 mm Tris-Cl, pH 8, containing 1 mm MgSO4 and 1 mm DTT. The column was then washed with 1 gel volume of 50 mm Tris-Cl, pH 8, containing 100 mm NaCl and 1 mm DTT. The eluates were combined and passed through a DEAE-Sepharose CL-6 column from GE Healthcare that was previously equilibrated with 50 mm Tris-Cl, pH 7.5, containing 0.01% DM and 1 mm DTT. The column was washed with 50 mm Tris-Cl, pH 8, containing 100 mm NaCl, 0.01% DM, and 1 mm DTT. The Rieske ISP was finally eluted with 50 mm Tris-Cl, containing 0.01% DM, 500 mm NaCl, and 1 mm DTT.
Measurement of Superoxide Anion Generation—Superoxide
generation by the cyt bc1 complex was determined by
measuring the chemiluminescence of MCLA-superoxide anion
( )
adduct in an Applied Photophysics stopped-flow reaction analyzer SX.18MV, by
leaving the excitation light off and registering light emission
(33). Reactions were carried
out at 23 °C, by mixing solutions A and B in a 1:1 ratio. Solution A
contained 100 mm Na+/K+ phosphate buffer, pH
7.4, 1 mm EDTA, 1 mm KCN, 1 mm
NaN3, 0.1% bovine serum albumin, 0.01% DM, and 6 μm
cyt bc1 complex. Solution B was the same as solution A
except that the cyt bc1 complex was replaced with either
50 μm Q0C10BrH2 or 30
μm stigmatellin and 4 μm MCLA.
)
adduct in an Applied Photophysics stopped-flow reaction analyzer SX.18MV, by
leaving the excitation light off and registering light emission
(33). Reactions were carried
out at 23 °C, by mixing solutions A and B in a 1:1 ratio. Solution A
contained 100 mm Na+/K+ phosphate buffer, pH
7.4, 1 mm EDTA, 1 mm KCN, 1 mm
NaN3, 0.1% bovine serum albumin, 0.01% DM, and 6 μm
cyt bc1 complex. Solution B was the same as solution A
except that the cyt bc1 complex was replaced with either
50 μm Q0C10BrH2 or 30
μm stigmatellin and 4 μm MCLA.
Preparation of Phospholipid-deficient Mitochondrial bc1 Complex—Phospholipid and Q-depleted preparation of mitochondrial bc1 complex was prepared by repeated ammonium sulfate fractionation in the presence of 0.5% sodium cholate in 50 mm Na+/K+ phosphate buffer, pH 7.4. Cyt bc1 complex was diluted to a protein concentration of 10 mg/ml in 50 mm Na+/K+ phosphate buffer, pH 7.4, containing 0.5% sodium cholate. Neutralized saturated ammonium sulfate solution was added to 48% saturation. After stirring for 5 min, the solution was centrifuged at 44,000 × g for 10 min. The precipitate thus obtained was dissolved in 50 mm Na+/K+ phosphate buffer containing 0.5% cholate. Neutralized ammonium sulfate solution was added to 45% saturation, stirred for 5 min followed by centrifugation at 44,000 × g for 10 min. This process was repeated three more times with 40% ammonium sulfate saturation. The precipitate was finally dissolved in 50 mm Na+/K+ phosphate buffer, pH 7.4, containing 10% glycerol.
Extraction of Ubiquinone/Ubiquinol from cyt bc1 Complex—A semimicro assay was employed for extraction of ubiquinol/ubiquinone from mitochondrial and bacterial bc1 complexes (34). 1 ml of solution containing 2–5 mg of protein was placed in a 45-ml glass centrifuge tube. Rapid addition of 4 ml of cold methanol using a hypodermic needle was immediately followed by the addition of 5 ml of hexane. The mixture was vortexed for 1 min and centrifuged for 2 min to separate the layers. The upper layer of hexane was transferred to another glass centrifuge tube, and 3 ml of hexane was added to the lower layer for further extraction. After vortexing and centrifugation as described above, the upper hexane layer was collected and combined with the first extraction. The hexane extract was placed in a vacuum desiccator to evaporate the hexane. Residual lipid was dissolved in spectroscopically pure ethanol. The amount of ubiquinone/ubiquinol extracted was determined spectroscopically in a Shimadzu UV-2450 PC spectrophotometer.
Other Biochemical and Biophysical Techniques—Protein concentration was determined by the method of Lowry et al. (35). Cyt b (36) and cyt c1 (37) concentrations were determined according to published methods. SDS-PAGE was performed according to Laemmli (38) using a Bio-Rad mini-protean dual slab vertical cell.
RESULTS AND DISCUSSION
Stigmatellin-induced Reduction of the Rieske ISC—The EPR spectrum of reduced ISC shows rhombic symmetry with three characteristic g values; gz = 2.02, gy = 1.89, and gx = 1.80 (39). In the presence of ascorbate, the EPR spectrum of the cyt bc1 complex from R. sphaeroides displays the characteristic g values signaling ISC reduction (Fig. 1, trace A). Interestingly, the addition of stigmatellin to R. sphaeroides bc1 complex, oxidized by incubation overnight with enzymatic amounts of cyt c oxidase and cyt c, results in reduction of ISC in the absence of an apparent electron source (Fig. 1, trace B). Reduction of ISC is also observed in oxidized cyt bc1 complexes from bovine mitochondria upon the addition of stigmatellin (data not shown). Moreover, addition of stigmatellin to the cyt bc1 complex results in narrowing of EPR line width and, shifts in gy and gx values, as has been reported previously (40). Complete oxidation of the cyt bc1 complex before the addition of stigmatellin is confirmed by the absence of the characteristic EPR signals of reduced ISC (Fig. 1, trace C).
FIGURE 1.
A and B, EPR spectra of wild-type R. sphaeroides bc1 complex in the presence of ascorbate (A) and stigmatellin (B). Cytochrome bc1 complexes were diluted to 100 μm cyt b in 50 mm Tris-Cl, pH 8, containing 200 mm NaCl and 0.01% DM and incubated with 5 mm ascorbate on ice for 30 min and frozen in liquid nitrogen. Cyt bc1 complexes were oxidized by overnight incubation with 1 μm cyt c oxidase and 1 μm cyt c before 200 μm stigmatellin was added to the bc1 complex. C, EPR spectrum of the oxidized bc1 complex only. EPR spectra were recorded at 100 K with the following instrument settings: microwave frequency, 9.39 GHz; modulation frequency, 100 kHz; modulation amplitude, 6.3 G; time constant, 655.4 ms; and sweep time, 167.8 s. Each spectrum represents the average of three separate traces.
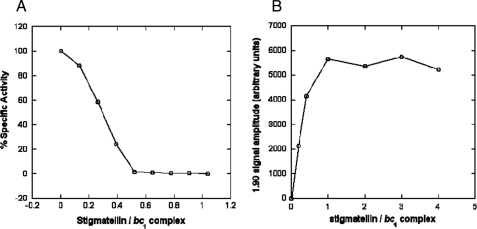
Stigmatellin contains a 5,7-dimethoxy-8-hydroxychromone system with a hydrophobic alkenyl chain at position 2 (29). Binding of stigmatellin to cyt bc1 complex increases the redox midpoint potential of the Rieske ISP from 290 mV to 540 mV (40). Moreover, stigmatellin arrests the head domain of ISP at the “fixed” or b-position, proximal to cyt b, as shown by the anomalous diffraction signals indicating dramatic reduction in the mobility of the ISC (9). Fig. 2A shows the ubiquinol-cyt c reductase activity of R. sphaeroides bc1 complex in the presence of varying amounts of stigmatellin. Stigmatellin inhibits the complex with a stoichiometry of 0.5 mol of inhibitor per mol (monomer) of bc1 complex. Similar stoichiometric values have been demonstrated in cyt bc1 complexes from yeast (41) and in submitochondrial particles (40). This indicates that the binding of stigmatellin to only one monomer per dimer is sufficient to abolish bc1 complex activity. Binding also exhibits negative cooperativity; i.e. stigmatellin binds to a monomer of every inhibitor-free dimer before it binds to the second monomer (41). ISP reduction induced by stigmatellin binding to the fully oxidized bc1 complex at various concentrations of the inhibitor is shown in Fig. 2B. Maximal reduction is obtained at a stoichiometry of 1 inhibitor per bc1 complex monomer, indicating that binding to every monomer is necessary for complete ISC reduction. Similarly, one molecule of stigmatellin per bc1 complex monomer was required to obtain maximal spectral shift of dithionite-reduced cyt b (40). Binding of stigmatellin to the cyt bc1 complex elevates the midpoint potential of the Rieske ISP, thereby generating a strong electron sink that results in reduction of the ISC in the absence of an apparent electron source.
FIGURE 2.
Inhibition of ubiquinol-cyt c reductase activity (A) and reduction of the Rieske ISC of oxidized cyt bc1 complexes by varying amounts of stigmatellin (B). For specific activity measurements, cytochrome bc1 complex was diluted to 1 μm cyt b in 50 mm Tris-Cl, pH 8, containing 200 mm NaCl and 0.01% DM. Stigmatellin was added in varying amounts to the enzyme complex, and the mixture was incubated on ice for 15 min. For determining reduction of the Rieske ISP, the bc1 complexes were diluted to 100 μm cyt b in 50 mm Tris-Cl, pH 8, containing 200 mm NaCl and 0.01% DM before addition of stigmatellin. The mixture was incubated on ice for 15 min and subsequently frozen in liquid nitrogen. The extent of ISP reduction induced by the addition of varying amounts of stigmatellin was determined by the intensity of the gy EPR signal. Sample preparations and instrument settings were the same as in Fig. 1.
Upon increasing the potential from – 0.272 to 0.708 V, electrochemically induced Fourier transform infrared spectroscopy difference spectra of stigmatellin-treated Saccharomyces cerevisiae cyt bc1 complex indicated that the inhibitor itself is redox active: it can undergo reduction but not oxidation (42). Therefore, it is not likely that stigmatellin can serve as an electron donor for ISP when the oxidized complex is treated with the inhibitor. The possibility that the 8-hydroxy group of the chromone ring system of stigmatellin may directly reduce the Rieske ISC was investigated by adding stigmatellin to pure Rieske ISP isolated from R. sphaeroides bc1 complex. The EPR spectrum of isolated ISP was recorded in the presence of either stigmatellin or ascorbate (Fig. 3B). In the presence of ascorbate, the characteristic EPR spectrum for reduced Rieske ISP with its distinct g values is observed, indicating that the ISC is not lost during purification of ISP from the cyt bc1 complex. The gx signal of the isolated Rieske ISP is broad with g = 1.75–1.77. Similar broadening of the gx signal has been reported in bc1 complexes with either an empty QP site, with reduced ubiquinol present or in the presence of a beta-methoxyacrylate type inhibitor (40). Addition of stigmatellin to oxidized ISP does not cause any reduction of the ISC as evidenced by a lack of the characteristic EPR signals (Fig. 3B). Therefore, stigmatellin does not reduce the ISC directly, but upon binding to the bc1 complex induces electron transfer to the ISC. Stigmatellin, like other Pf inhibitors of the cyt bc1 complex, does not bind to isolated Rieske ISP, but binds to the cyt b subunit at the QP site proximal to ISP and distal from heme bL (43).
FIGURE 3.
Characterization of purified Rieske ISP isolated from R. sphaeroides bc1 complex. A, Rieske ISP was isolated from the cyt bc1 complex as detailed under “Experimental Procedures” and subjected to SDS-PAGE. The purified Rieske ISP (lane 3) and cyt bc1 complex (lane 2) were digested with 1% SDS and 0.4% β-mercaptoethanol at 23 °C for 15 min before loading into the gel. Protein standards: myosin (195 kDa), β-galactosidase (115.6 kDa), bovine serum albumin (97.3 kDa), ovalbumin (53.5 kDa), carbonic anhydrase (37.2 kDa), soybean trypsin inhibitor (29.4 kDa), lysozyme (20.4 kDa), and aprotinin (6.92 kDa). B, EPR spectra of the isolated ISP in the presence of stigmatellin (upper trace) and ascorbate (lower trace) were recorded. The Rieske ISP was fully oxidized by addition of ferricyanide. x, unknown signal. Instrument settings were as described in Fig. 1.
The necessity of stigmatellin binding to the cyt bc1 complex for ISC reduction was further evaluated in phospholipid-deficient bovine cyt bc1 complexes where 90% of the phospholipids and ubiquinone/ubiquinol molecules are removed by repeated cholate-ammonium acetate fractionation (44). Delipidation of mitochondrial bc1 complex results in loss of ubiquinol-cyt c reductase activity, but reconstitution with exogenous phospholipids restores a significant amount of the inhibitor-sensitive activity (data not shown). The EPR spectrum of phospholipid-deficient bc1 complex in the presence of stigmatellin shows no ISC reduction, whereas ISC reduction is observed in the presence of ascorbate (data not shown). This indicates that stigmatellin is unable to induce ISC reduction in phospholipid-deficient cyt bc1 complexes. However, incubation of the phospholipid-deficient cyt bc1 complex with azolectin, phospholipids from soybean, before adding stigmatellin restores a significant amount of the ISC reduction (data not shown). This demonstrates that phospholipids are essential for binding of stigmatellin to the cyt bc1 complex, and correct binding is necessary for the stigmatellin-induced ISC reduction. Furthermore, endogenous ubiquinol can effectively be ruled out as the electron source for ISP reduction induced by stigmatellin because ISP reduction is observed in phospholipids-supplemented, ubiquinol-deficient mitochondrial bc1 complex.
It is possible that the Tris used during purification of the bc1 complex is responsible for stigmatellin-induced reduction of the Rieske ISC. However, stigmatellin-induced reduction of the Rieske ISC is also observed in bc1 complexes purified using Na+/K+ phosphate buffer, thereby unambiguously eliminating the -OH groups of Tris as a possible source of electrons (data not shown).
Characterization of Free Radicals Generated by the Cyt bc1 Complex in the Presence of Stigmatellin or Ferricyanide—In addition to reduction of the Rieske ISC, an EPR peak with a symmetrical line shape and g value centered at g = 2.005, typical characteristics of an organic free radical are observed when stigmatellin is added to oxidized cyt bc1 complex. The intensity of the g = 2.005 EPR signal increases with varying amounts of stigmatellin (Fig. 4A). The maximal signal is observed when 15 mol of inhibitor is added per mol of cyt bc1 complex. Stigmatellin increases the midpoint potential of the Rieske ISP by ∼260–540 mV, resulting in the generation of a strong oxidizing agent. Because of its acquired oxidative nature, we speculate that the Rieske ISC extracts an electron from nearby organic residues, leading to its reduction and the subsequent generation of organic free radicals. The need for such a large amount of stigmatellin to obtain the maximum free radical signal is not well understood at this point. We speculate that electrons from the reduced ISC may leak to the environment, possibly reducing molecular oxygen and generating superoxide, because the reduced ISP is unable to move to c-position to be oxidized by cytochrome c1 in the presence of the inhibitor. Also, formation of free radical might modify the binding affinity of the cyt bc1 complex. Hence, a single ISC might extract more than one electron from multiple organic residues. Future studies leading to the identification of the radical source in stigmatellin-treated cyt bc1 complexes should shed more light on this issue. When stigmatellin is added to the cyt bc1 complex at molar ratio of 15 or higher, the mixture turns turbid because of partial precipitation of the protein complex. It is speculated that excessive oxidation of organic residues induced by the high potential Rieske ISC eventually leads to denaturation and unfolding of the native enzyme. Interestingly, the addition of ferricyanide, a strong oxidant (Em ∼ 420 mV), to oxidized cyt bc1 complex also results in generation of the g = 2.005 EPR signal (Fig. 4B). A similar g = 2.005 signal is observed when ferricyanide is added to purified Rieske ISP (data not shown). Radical production increases with increasing concentrations of ferricyanide. When ferricyanide is added at a 10–15-fold molar excess of bc1 complex, the g = 2.005 signal intensity reaches a maximum, indicating that possible oxidizable residues in the bc1 complex become limiting. Moreover, it is speculated that ferricyanide might begin oxidizing some of the generated organic radicals resulting in quasi-equilibrium between oxidation of organic residues and organic radicals. It must be noted that the amount of radicals produced in the ferricyanide-treated complex is ∼10-fold greater than that observed in the presence of stigmatellin. Ferricyanide oxidation of organic residues is directly proportional to the concentration used, whereas stigmatellin-induced oxidation is limited by the amount of ISC, which is no more than 1 mol of ISC per mol of cyt bc1 complex.
FIGURE 4.
Effect of stigmatellin (A) or ferricyanide (B) concentration on the g = 2.005 EPR signal. Cyt bc1 complex was diluted to 50 μm cyt b in 50 mm Tris-Cl, pH 8, containing 200 mm NaCl and 0.01% DM. Stigmatellin and ferricyanide were added to the desired concentrations, and the mixtures were incubated on ice for 15 min before being frozen in liquid nitrogen. Instrument settings were as described in Fig. 1.
Because a symmetrical g = 2.005 EPR signal is often attributed to a ubisemiquinone radical at the QN site upon the reduction of ubiquinone by heme bH (20), it is of interest to determine whether the stigmatellin-induced radical is the QN radical or not. The ubisemiquinone anion generated at the QN site is sensitive to the inhibitor, antimycin A, and it can be distinguished from other organic radicals of the cyt bc1 complex (45). Comparison of EPR spectra indicates that pretreatment of the bc1 complex with antimycin A before addition of stigmatellin reduces the amount of radicals by ∼30% (Fig. 5). Moreover, the addition of antimycin A results in a slight shift of the g = 2.005 EPR signal to the lower field (Fig. 5). Therefore, the majority of organic radicals generated upon addition of stigmatellin to the cyt bc1 complex can be attributed to antimycin-insensitive organic radicals and hence different from ubisemiquinone radicals originating at center N. Addition of stigmatellin to the oxidized bc1 complex induces the reduction of the Rieske ISC as well as ubiquinone at center N as evidenced by the generation of a small population of antimycin-sensitive radicals. Whether they are both reduced by the same organic residue/radical or two separate species remains to be elucidated. If indeed they are reduced by the same species, it raises profound questions regarding the distance of electron transfer from the organic residue to these two centers. Furthermore, it would shed some light into where the residue might be located. Addition of ascorbate to the stigmatellin-treated complex results in the decrease of the g = 2.005 EPR signal by ∼85% (data not shown). Ascorbate likely donates an electron and reduces the organic radicals formed in the stigmatellin-treated complex, resulting in a substantial decrease of the g = 2.005 EPR signal.
FIGURE 5.
Characterization of the g = 2.005 EPR signal in wild-type cyt bc1 complexes. Cyt bc1 complex was diluted to 50 μm in 50 mm Tris-Cl, pH 8, containing 200 mm NaCl and 0.01% DM and oxidized by overnight incubation in the presence of 1 μm cyt c oxidase and cytochrome c. Solid curve shows oxidized complex treated with 500 μm stigmatellin; broken curve is for 250 μm antimycin A plus 500 μm stigmatellin. Instrument settings were as described in Fig. 1.
To demonstrate further that the organic radicals generated by addition of stigmatellin to the complex are different from the ubisemiquinone radical at the QN site, power saturation profiles were determined (Fig. 6). The g = 2.005 signal produced when cyt bc1 complex is treated with stigmatellin is monophasic and shows saturation at ∼0.420 milliwatt. The QN radical in the ascorbate-treated wild-type bc1 complex shows saturation at ∼0.211 milliwatt. Similar power saturation at 0.251 milliwatt has been reported previously for the QN radical generated in mitochondrial bc1 complex (46). The power saturation profiles of the two free radical species indicate differences in interactions with paramagnetic cofactors in the aqueous milieu and also provide information on the relative location of the radical species within the enzyme complex. The significant difference in the power saturation properties confirms that the organic radical generated by stigmatellin is different from the Q-radical at the QN site.
FIGURE 6.
Power saturation properties of the g = 2.005 EPR signal generated in the cyt bc1 complex upon treatment with stigmatellin is shown as closed circles (•) and in the presence of ascorbate as open circles (○). Cyt bc1 complexes were diluted to 50 μm in 50 mm Tris-Cl, pH 8, containing 200 mm NaCl and 0.01% DM and incubated with either 500 μm stigmatellin or 5 mm ascorbate on ice for 20 min before freezing in liquid nitrogen. Instrument settings were the same as in Fig. 1 except various microwave powers were used.
Production of Superoxide in Cyt bc1 Complexes Treated with
Stigmatellin—Approximately 1–2% of oxygen consumed during
normal respiration is not involved with oxidative phosphorylation but with the
formation of superoxide (47).
Complex I and complex III (bc1 complex) were identified as
two segments responsible for much of the superoxide generated in mitochondria
(48–51).
Superoxide
( )
production, detected as the fluorescence of
MCLA-
)
production, detected as the fluorescence of
MCLA- adduct, is observed upon adding the substrate, ubiquinol, to the cyt
bc1 complex (Fig.
7, trace A). Addition of stigmatellin, in the absence of
ubiquinol, also generates
adduct, is observed upon adding the substrate, ubiquinol, to the cyt
bc1 complex (Fig.
7, trace A). Addition of stigmatellin, in the absence of
ubiquinol, also generates
 (trace B), and its production depends on the amount of stigmatellin
added. Similarly, when ferricyanide is added to the bc1
complex,
(trace B), and its production depends on the amount of stigmatellin
added. Similarly, when ferricyanide is added to the bc1
complex,
 is generated (trace C). However, the rate of fluorescence decay is
much more rapid than that observed for
is generated (trace C). However, the rate of fluorescence decay is
much more rapid than that observed for
 formed in the presence of either ubiquinol or stigmatellin. It is likely that
the
formed in the presence of either ubiquinol or stigmatellin. It is likely that
the
 formed is consumed by the rapid reaction with ferricyanide, analogous to the
rapid reduction of cyt c by
formed is consumed by the rapid reaction with ferricyanide, analogous to the
rapid reduction of cyt c by
 .
In the presence of superoxide dismutase,
.
In the presence of superoxide dismutase,
 .
is not observed when either ubiquinol or stigmatellin is added to the
wild-type bc1 complex, confirming production of
.
is not observed when either ubiquinol or stigmatellin is added to the
wild-type bc1 complex, confirming production of
 as a by-product of the reaction. It is speculated that the
as a by-product of the reaction. It is speculated that the
 is generated as a result of interaction between molecular oxygen and organic
free radicals generated in stigmatellin- and ferricyanide-treated
bc1 complexes. To test this speculation,
is generated as a result of interaction between molecular oxygen and organic
free radicals generated in stigmatellin- and ferricyanide-treated
bc1 complexes. To test this speculation,
 generation was measured in the double mutant H131N/H152N in the Rieske ISP
(52). This mutant complex
lacks the Rieske ISC, although the apoprotein remains unaffected
(52). In the presence of
ubiquinol,
generation was measured in the double mutant H131N/H152N in the Rieske ISP
(52). This mutant complex
lacks the Rieske ISC, although the apoprotein remains unaffected
(52). In the presence of
ubiquinol,
 production is observed in the wild-type but not in the mutant complex
(Fig. 8A). In the
normal catalytic cycle of the cyt bc1 complex,
ubisemiquinone radical at the QP site
(48) and reduced cyt
b566 (49)
are postulated to be the autoxidizable factors causing superoxide production.
Absence of Rieske ISC in the mutant complex abolishes bifurcated electron
transfer at the QP site, preventing formation of the two individual
species responsible for
production is observed in the wild-type but not in the mutant complex
(Fig. 8A). In the
normal catalytic cycle of the cyt bc1 complex,
ubisemiquinone radical at the QP site
(48) and reduced cyt
b566 (49)
are postulated to be the autoxidizable factors causing superoxide production.
Absence of Rieske ISC in the mutant complex abolishes bifurcated electron
transfer at the QP site, preventing formation of the two individual
species responsible for
 production. Similarly, addition of stigmatellin to the mutant
bc1 complex does not generate any
production. Similarly, addition of stigmatellin to the mutant
bc1 complex does not generate any
 (Fig. 8B), although
the mutant bc1 complex is able to bind stigmatellin as
evidenced by the red shift in the absorption spectra of reduced cyt b
(data not shown). Binding of stigmatellin to ISC-deficient mutant complex does
not result in the generation of a strong oxidizing agent, i.e. a
Rieske ISC with a highly elevated midpoint potential. Consequently,
stigmatellin binding to the mutant complex does not induce extraction of an
electron from an organic residue by the Rieske ISC. Therefore, organic
radicals are not generated, and no electron source is available for molecular
oxygen to form
(Fig. 8B), although
the mutant bc1 complex is able to bind stigmatellin as
evidenced by the red shift in the absorption spectra of reduced cyt b
(data not shown). Binding of stigmatellin to ISC-deficient mutant complex does
not result in the generation of a strong oxidizing agent, i.e. a
Rieske ISC with a highly elevated midpoint potential. Consequently,
stigmatellin binding to the mutant complex does not induce extraction of an
electron from an organic residue by the Rieske ISC. Therefore, organic
radicals are not generated, and no electron source is available for molecular
oxygen to form
 .
.
FIGURE 7.
Tracings of superoxide generation by 6 μm wild-type cyt bc1 complex upon addition of 50 μm ubiquinol (A), 60 μm stigmatellin (B), 60 μm ferricyanide (C), or 60 μm stigmatellin in the presence of superoxide dismutase (D). Superoxide generation was measured at 23 °C in an Applied Photophysics stopped-flow reaction analyzer SX18MV as described under “Experimental Procedures.”
FIGURE 8.
Tracings of superoxide generation in 6 μm wild-type and mutant (Δ2Fe-2S) cyt bc1 complexes in the presence of 50 μm ubiquinol (A) or 60 μm stigmatellin (B). Solid lines denote the wild-type complex, and broken lines represent the mutant complex. Sample preparation and instrument settings were as described in Fig. 7.
In Conclusion—The Rieske ISP is in the fixed
b-position when stigmatellin is added to the cyt
bc1 complex. As a result, the redox midpoint potential of
the Rieske ISP increases by 250 mV, creating a strong oxidant. This elevated
redox potential serves as the driving force for electron transfer from organic
residues to the oxidized Rieske ISC, resulting in its reduction and the
subsequent generation of organic radicals. The organic radicals in turn can
react with molecular oxygen to yield
 .
The generation of organic radicals by stigmatellin-treated cyt
bc1 complex is severely retarded upon addition of
ascorbate, whereby the organic radicals are neutralized. Ferricyanide, a
strong oxidant, also induces the generation of organic radicals and reduction
of cyt b.
.
The generation of organic radicals by stigmatellin-treated cyt
bc1 complex is severely retarded upon addition of
ascorbate, whereby the organic radicals are neutralized. Ferricyanide, a
strong oxidant, also induces the generation of organic radicals and reduction
of cyt b.
 is not observed in cyt bc1 mutant complexes lacking the
Rieske ISC, providing further evidence that the elevated midpoint potential of
ISC drives reduction of ISC and radical production. Identification of amino
acid residue(s), particularly tyrosines that potentially serve as electron
donors to the ISC when stigmatellin is bound, is currently under investigation
in our laboratory. The g value for the stigmatellin-induced radical is 2.005,
and several sources characterizing tyrosyl radicals list g values at 2.004
(53,
54). Moreover, a broad singlet
EPR spectrum with a line width of ∼10 G reported for tyrosyl radicals
(14,
53,
54) is also observed when
stigmatellin is added to oxidized cyt bc1 complex.
Finally, stigmatellin-induced reduction of the oxidized Rieske ISP might be
used as a means to identify cyt bc1 mutations that confer
resistance to the inhibitor, in addition to causing a red shift in the cyt
b absorption spectrum.
is not observed in cyt bc1 mutant complexes lacking the
Rieske ISC, providing further evidence that the elevated midpoint potential of
ISC drives reduction of ISC and radical production. Identification of amino
acid residue(s), particularly tyrosines that potentially serve as electron
donors to the ISC when stigmatellin is bound, is currently under investigation
in our laboratory. The g value for the stigmatellin-induced radical is 2.005,
and several sources characterizing tyrosyl radicals list g values at 2.004
(53,
54). Moreover, a broad singlet
EPR spectrum with a line width of ∼10 G reported for tyrosyl radicals
(14,
53,
54) is also observed when
stigmatellin is added to oxidized cyt bc1 complex.
Finally, stigmatellin-induced reduction of the oxidized Rieske ISP might be
used as a means to identify cyt bc1 mutations that confer
resistance to the inhibitor, in addition to causing a red shift in the cyt
b absorption spectrum.
Acknowledgments
We thank Dr. Roger Koeppe for a critical review of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM 30721 (to C.-A. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: cyt, cytochrome; DM,
n-dodecyl-β-d-maltoside; DTT, dithiothreitol; ISC,
iron-sulfur cluster; ISP, iron-sulfur protein; MCLA,
6-(4-methoxyphenyl)-2-methyl-3,7-dihydroimidazo[1,2-a]pyrazin-3 (7H)–
one hydrochloride;
 ,
superoxide anion; Q0CB10BrH2,
2,3-dimethoxy-5-methyl-6-bromodecyl-1,4-benzoquinol.
,
superoxide anion; Q0CB10BrH2,
2,3-dimethoxy-5-methyl-6-bromodecyl-1,4-benzoquinol.
References
- 1.Trumpower, B. L., and Gennis, R. B. (1994) Annu. Rev. Biochem. 63 675–716 [DOI] [PubMed] [Google Scholar]
- 2.Xia, D., Yu, C. A., Kim, H., Xia, J. Z., Kachurin, A. M., Zhang, L., Yu, L., and Deisenhofer, J. (1997) Science 277 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwata, S., Lee, J. W., Okada, K., Lee, J. K., Iwata, M., Ramussen, B., Link, T. A., Ramaswamy, S., and Jap, B. K. (1998) Science 281 64–71 [DOI] [PubMed] [Google Scholar]
- 4.Zhang, Z., Huang, L., Shulmeister, V. M., Chi, Y. I., Kim, K. K., Huang, L. W., Crofts, A. R., Berry, E. A., and Kim, S. H. (1998) Nature 392 677–684 [DOI] [PubMed] [Google Scholar]
- 5.Hunte, C., Koepke, J., Lange, C., Rossmanith, T., and Michel, H. (2000) Structure (Lond.) 8 669–684 [DOI] [PubMed] [Google Scholar]
- 6.Kurisu, G., Zhang, H., Smith, J. L., and Cramer, W. A. (2003) Science 302 1009–1014 [DOI] [PubMed] [Google Scholar]
- 7.Stroebel, D., Choquet, Y., Popot, J. L., and Picot, D. (2003) Nature 426 413–418 [DOI] [PubMed] [Google Scholar]
- 8.Gao, X., Wen, X., Esser, L., Quinn, B., Yu, L., Yu, C. A., and Xia, D. (2003) Biochemistry 42 9067–9080 [DOI] [PubMed] [Google Scholar]
- 9.Esser, L., Quinn, B., Li, Y. F., Zhang, M., Elberry, M., Yu, L., Yu, C. A., and Xia, D. (2004) J. Mol. Biol. 341 281–302 [DOI] [PubMed] [Google Scholar]
- 10.Tian, H., Yu, L., Michael, W., and Yu, C. A. (1998) J. Biol. Chem. 273 27953–27959 [DOI] [PubMed] [Google Scholar]
- 11.Tian, H., White, S., Yu, L., and Yu, C. A. (1999) J. Biol. Chem. 274 7146–7152 [DOI] [PubMed] [Google Scholar]
- 12.Xiao, K., Yu, L., and Yu, C. A. (2000) J. Biol. Chem. 275 38597–38604 [DOI] [PubMed] [Google Scholar]
- 13.Darrouzet, E., Valkova-Valchanova, M., Christopher, C., Moser, P., Dutton, L., and Daldal, F. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 4567–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, Y.-R., Chen, C.-L., Chen, W., Zweier, J. L., Augusto, O., Radi, R., and Mason, R. P. (2004) J. Biol. Chem. 279 18054–18062 [DOI] [PubMed] [Google Scholar]
- 15.Kim, H., Xia, D., Yu, C. A., Xia, J., Kachurin, A. M., Zhang, L., Yu, L., and Deisenhofer, J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 8026–8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry, E. A., Guergova-Kuras, M., Huang, L. S., and Crofts, A. R. (2000) Annu. Rev. Biochem., 69 1005–1075 [DOI] [PubMed] [Google Scholar]
- 17.Nett, J. H., Hunte, C., and Trumpower, B. L. (2000) Eur. J. Biochem. 267 5777–5782 [DOI] [PubMed] [Google Scholar]
- 18.Obungu, V. H., Wang, Y., Amyot, S. M., Gocke, C. B., and Beattie, D. S. (2000) Biochim. Biophys. Acta 1457 36–44 [DOI] [PubMed] [Google Scholar]
- 19.Ghosh, M., Wang, Y., Ebert, C. E., Vadlamuri, S., and Beattie, D. S. (2001) Biochemistry 40 327–335 [DOI] [PubMed] [Google Scholar]
- 20.Mitchell, P. (1976) J. Theor. Biol. 62 327–367 [DOI] [PubMed] [Google Scholar]
- 21.Crofts, A. R., Meinhardt, S. W., Jones, K. R., and Snozzi, M. (1983) Biochim. Biophys. Acta 723 202–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link, T. A. (1997) FEBS Lett. 412 257–264 [DOI] [PubMed] [Google Scholar]
- 23.Brandt, U., and Okun, J. G. (1997) Biochemistry 36 11234–11240 [DOI] [PubMed] [Google Scholar]
- 24.Jünemann, S., Heathcote, P., and Rich, P. R. (1998) J. Biol. Chem. 273 21603–21607 [DOI] [PubMed] [Google Scholar]
- 25.Osyczka, A., Moser, C. C., Daldal, F., and Dutton, L. P. (2004) Nature 427 607–612 [DOI] [PubMed] [Google Scholar]
- 26.Covian, R., Gutierrez-Cirlos, E. B., and Trumpower, B. L. (2004) J. Biol. Chem. 279 15040–15049 [DOI] [PubMed] [Google Scholar]
- 27.Crofts, A. R. (2004) Biochim. Biophys. Acta 1655 77–92 [DOI] [PubMed] [Google Scholar]
- 28.Snyder, C. H., Gutierrez-Cirlos, E. B., and Trumpower, B. L. (2000) J. Biol. Chem. 275 13535–13541 [DOI] [PubMed] [Google Scholar]
- 29.Thierbach, G., Kunze, B., Reichenbach, H., and Höfle, G. (1984) Biochim. Biophys. Acta 765 227–235 [Google Scholar]
- 30.Hunte, C. (2001) FEBS Lett. 504 126–132 [DOI] [PubMed] [Google Scholar]
- 31.Kagawa, Y., and Racker, E. (1971) J. Biol. Chem. 246 5477–5487 [Google Scholar]
- 32.McCurley, J. P., Miki, T., Yu, L., and Yu, C. A. (1990) Biochim. Biophys. Acta 1020 176–186 [DOI] [PubMed] [Google Scholar]
- 33.Denicola, A., Souza, J. M., Gatti, R. M., Augusto, O., and Radi, R. (1995) Free Radic. Biol. Med. 19 11–19 [DOI] [PubMed] [Google Scholar]
- 34.Pumphrey, A. M., and Redfearn, E. R. (1960) Biochem. J. 76 61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193 265–275 [PubMed] [Google Scholar]
- 36.Berden, J. A., and Slater, E. C. (1970) Biochim. Biophys. Acta 216 237–249 [DOI] [PubMed] [Google Scholar]
- 37.Yu, L., Dong, J. H., and Yu, C. A. (1986) Biochim. Biophys. Acta 852 203–211 [DOI] [PubMed] [Google Scholar]
- 38.Laemmli, V. K. (1970) Nature 277 680–685 [DOI] [PubMed] [Google Scholar]
- 39.Orme-Johnson, N. R., Hansen, R. E., and Beinert, H. (1974) Biochem. Biophys. Res. Commun. 45 871–878 [DOI] [PubMed] [Google Scholar]
- 40.von Jagow, G., and Ohnishi, T. (1985) FEBS Lett. 185 311–315 [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez-Cirlos, E. B., and Trumpower, B. L. (2002) J. Biol. Chem. 277 1195–1202 [DOI] [PubMed] [Google Scholar]
- 42.Ritter, M., Palsdottir, H., Abe, M., Mäntele, W., Hunte, C., Miyoshi, H., and Hellwig, P. (2004) Biochemistry 43 8439–8446 [DOI] [PubMed] [Google Scholar]
- 43.Snyder, C. H., Merbitz-Zahradnik, T., Link, T. A., and Trumpower, B. L. (1999) J. Bioenerg. Biomembr. 31 235–242 [DOI] [PubMed] [Google Scholar]
- 44.Yu, L., Yu, C. A., and King, T. E. (1978) J. Biol. Chem. 253 2657–2663 [PubMed] [Google Scholar]
- 45.Yu, C. A., Nagaoka, S., Yu, L., and King, T. E. (1978) Biochem. Biophys. Res. Commun. 82 1070–1078 [DOI] [PubMed] [Google Scholar]
- 46.Miki, T., Yu, L., and Yu, C. A. (1992) Arch. Biochem. Biophys. 293 61–66 [DOI] [PubMed] [Google Scholar]
- 47.Boveris, A., Oshino, N., and Chance, B. (1972) Biochem. J. 128 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turrens, J. F., Alexandre, A., and Lehninger, A. L. (1985) Arch. Biochem. Biophys. 237 408–414 [DOI] [PubMed] [Google Scholar]
- 49.Nohl, H., and Jordan, W. (1986) Biochem. Biophys. Res. Commun. 138 533–539 [DOI] [PubMed] [Google Scholar]
- 50.Turrens, J. F., and Boveris, A. (1980) Biochem. J. 191 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cadenas, E., Boveris, A., Ragan, C. I., and Stoppani, A. O. (1977) Arch. Biochem. Biophys. 180 248–257 [DOI] [PubMed] [Google Scholar]
- 52.Gurung, B., Yu, L., Xia, D., and Yu, C. A. (2005) J. Biol. Chem. 280 24895–24902 [DOI] [PubMed] [Google Scholar]
- 53.Barry, B. A., and Babcock, G. T. (1987) Proc. Natl. Acad. Sci. U. S. A. 84 7099–7103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Debus, R. J., Barry, B. A., Babcock, G. T., and McIntosh, L. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]