Abstract
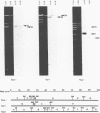
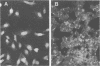
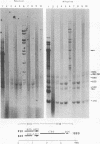
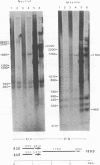
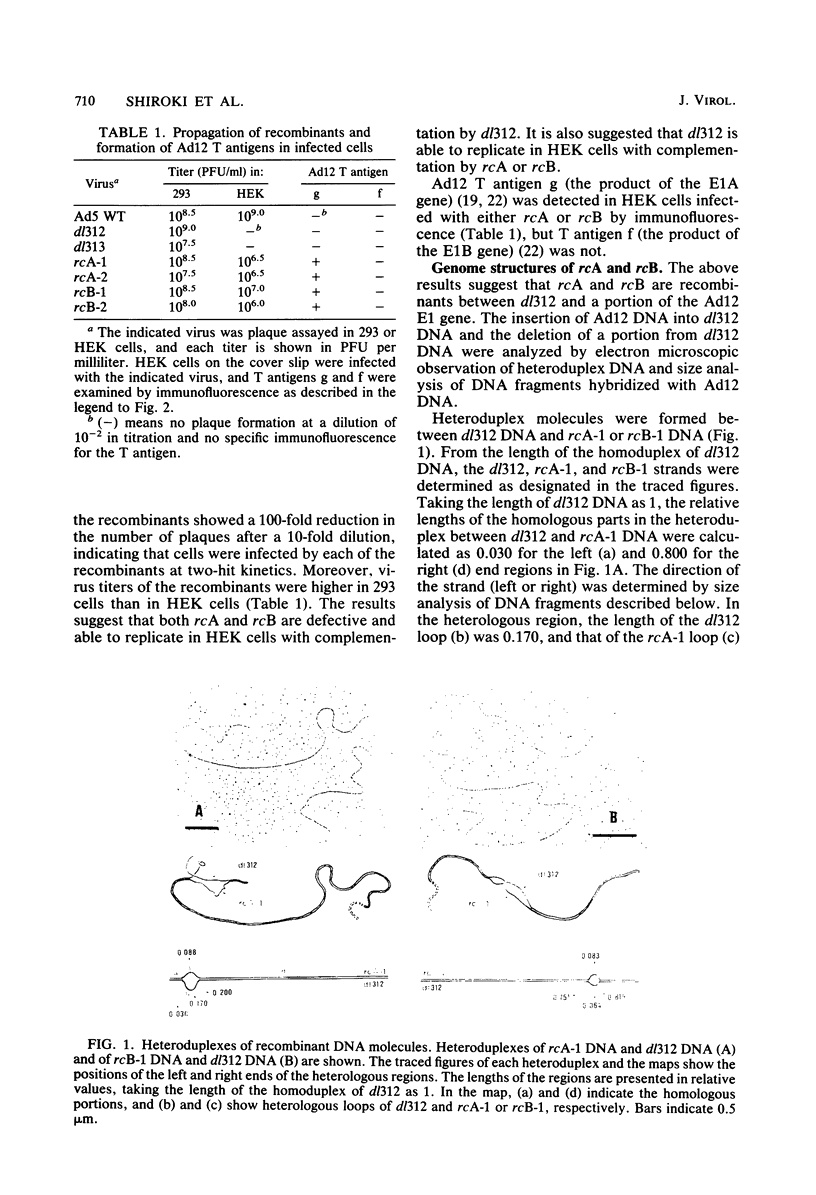
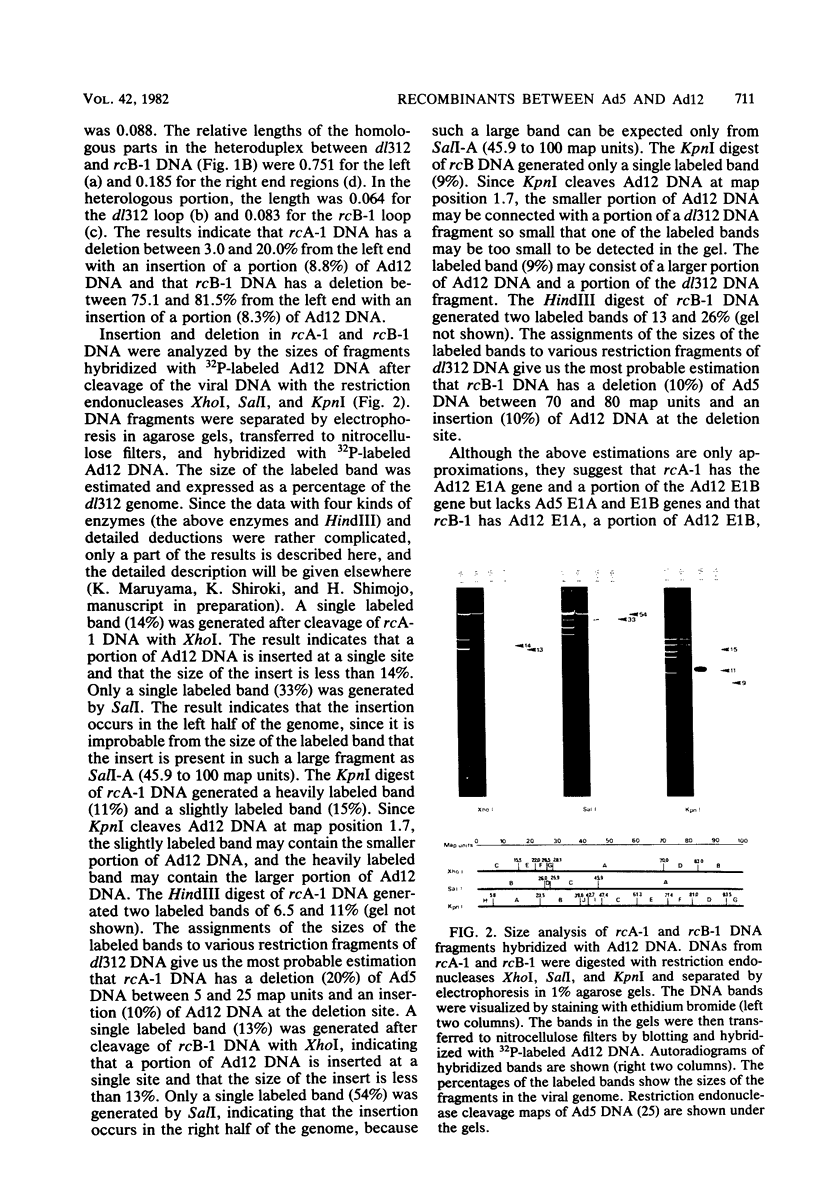
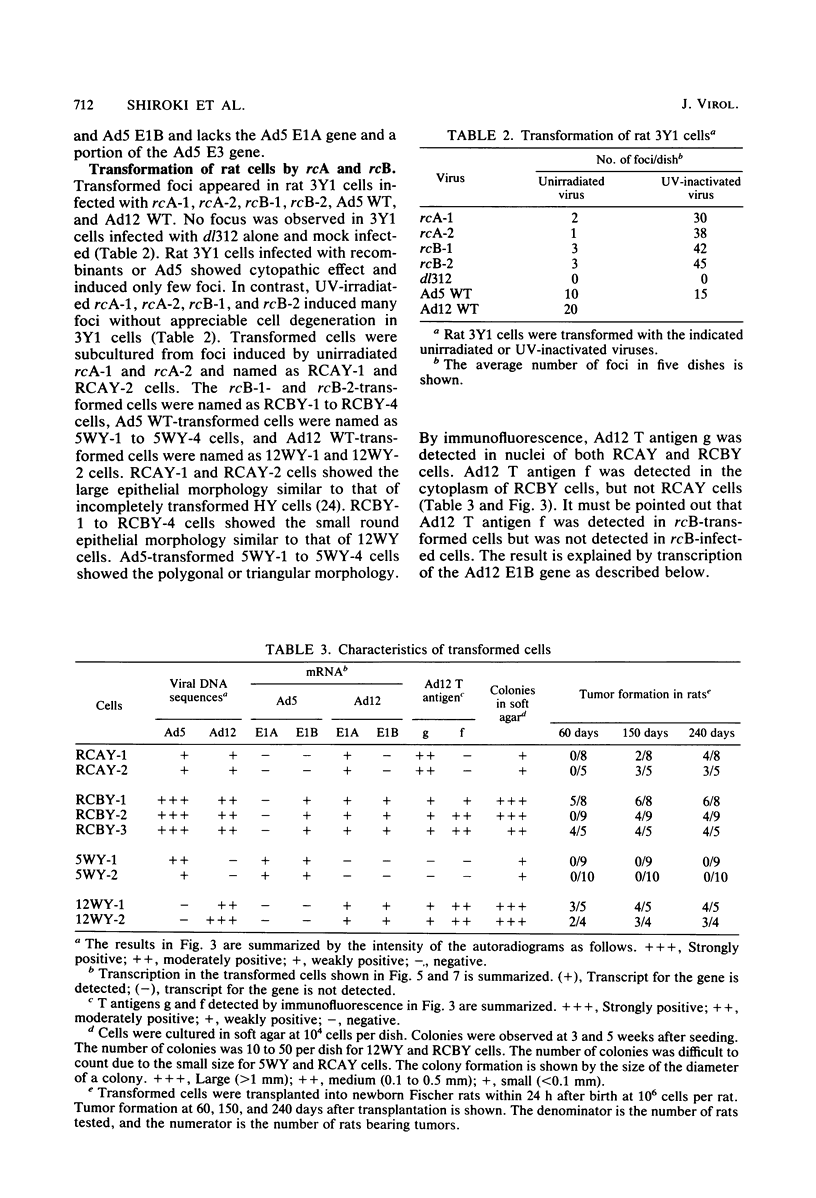
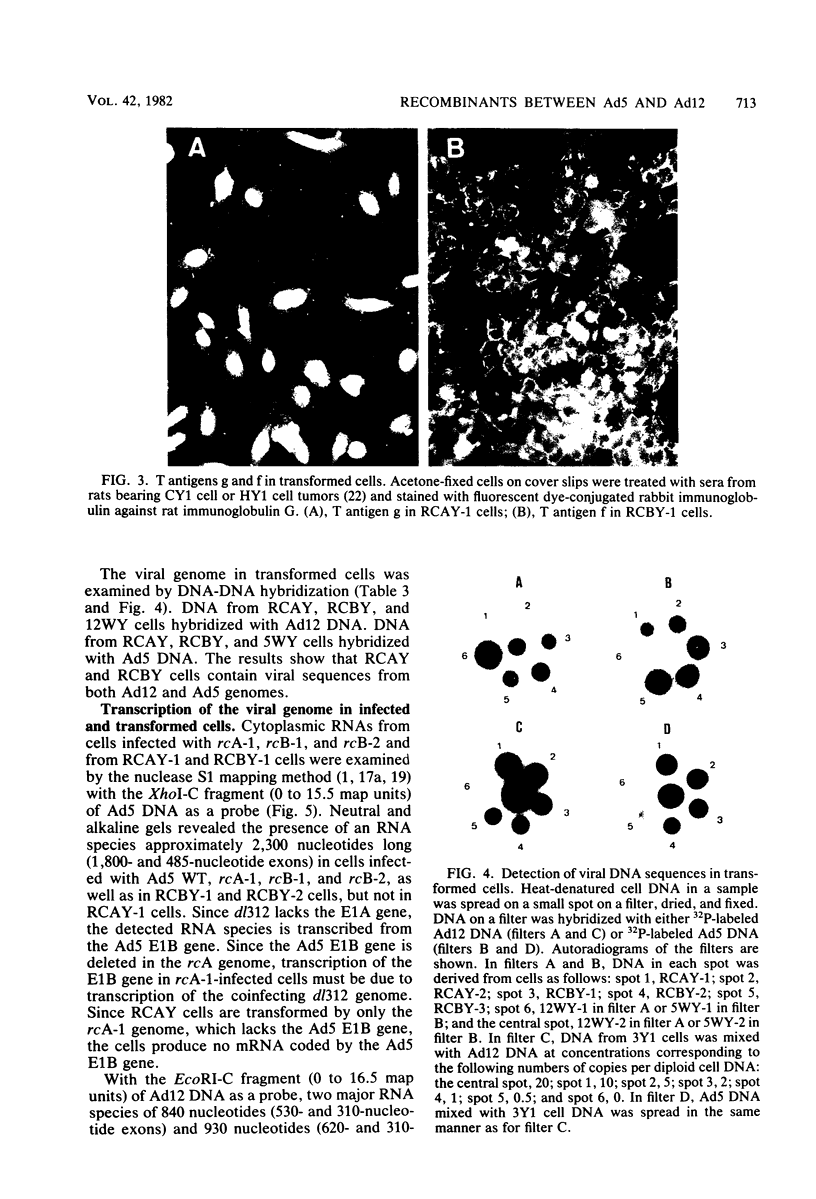
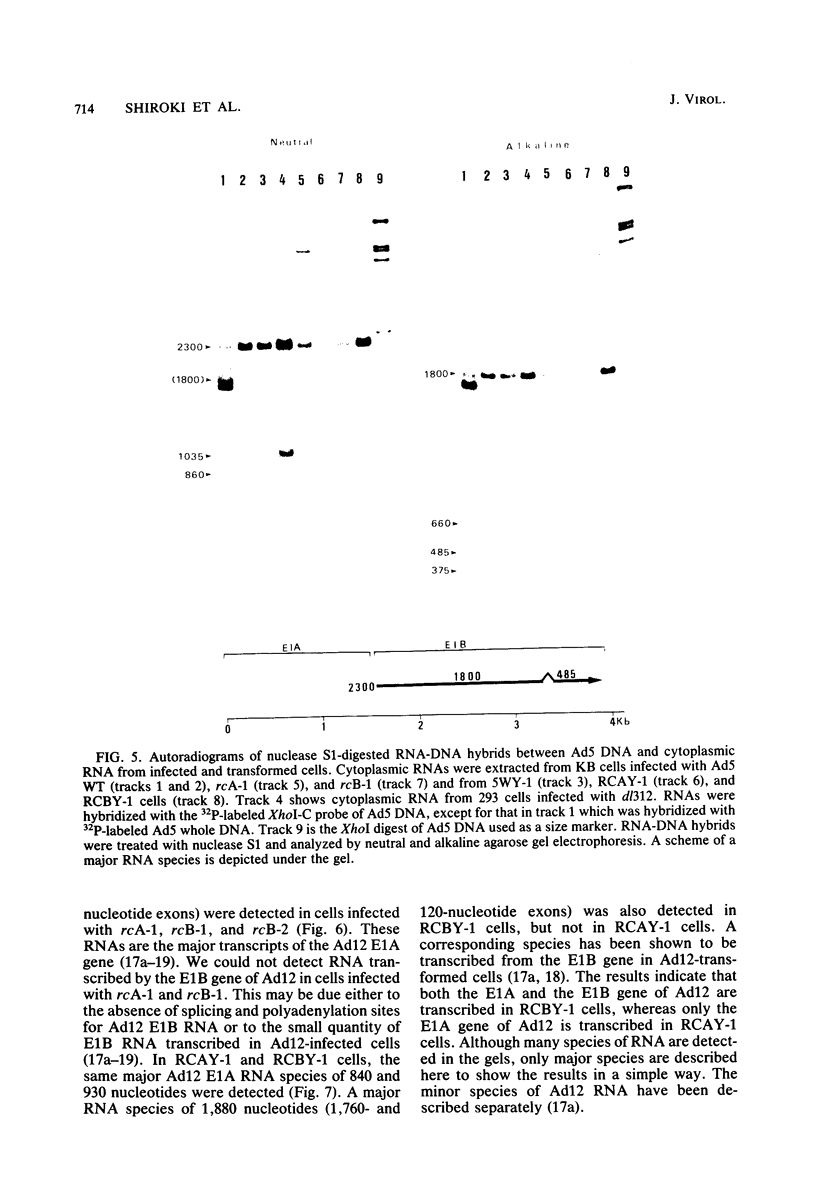
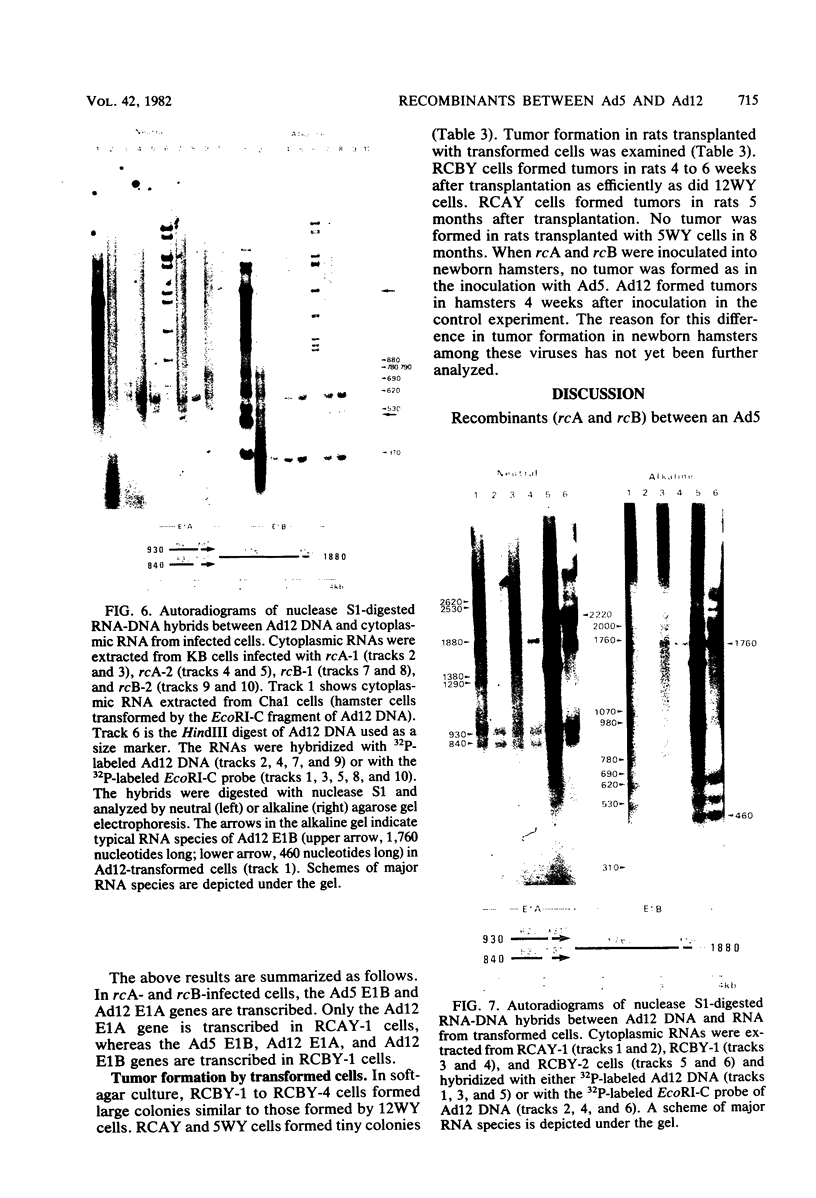
Recombinants between an adenovirus type 5 (Ad5) deletion mutant and the Ad12 DNA fragment containing early region 1 (E1) were isolated from cells cotransfected with the EcoRI-C fragment of Ad12 DNA and Ad5 dl312 (deletion in E1A) DNA (rcA) and from cells cotransfected with the SalI-C fragment of Ad12 DNA and Ad5 dl312 DNA (rcB). No recombinant was isolated from cells cotransfected with Ad5 dl313 (deletion in E1B) DNA and restriction fragments of Ad12 DNA. Both rcA and rcB are defective and able to replicate in human embryo kidney (HEK) and KB cells with complementation by dl312. Both rcA and rcB formed Ad12 T antigen g, but not T antigen f, in infected HEK and KB cells. In rcA- and rcB-infected cells, Ad5 E1B and Ad12 E1A genes are transcribed. Heteroduplex and size analyses of rcA-1 or rcB-1 DNA fragments hybridized with Ad12 DNA revealed that rcA-1 DNA has a deletion between 5 and 15 map units with an insertion of a portion of Ad12 DNA (10%) and that rcB-1 DNA has a deletion between 70 and 80 map units with an insertion of a portion of Ad12 DNA (10%). The transformed cell lines, RCAY and RCBY, were established after infection of rat 3Y1 cells with rcA and rcB, respectively. Both Ad5 and Ad12 DNA sequences are contained in these cells. In RCAY cells, Ad12 T antigen g is detected, but Ad12 T antigen f is not. In RCBY cells, both Ad12 T antigen g and f are detected. Only the Ad12 E1A gene is transcribed in RCAY cells, whereas Ad5 E1B, Ad12 E1A, and Ad12 E1B genes are transcribed in RCBY cells. In soft-agar cultures, RCBY cells form large colonies, whereas RCAY cells form only tiny colonies. RCBY cells form tumors as efficiently as 12WY cells in transplanted rats. RCAY cells formed tumors inefficiently. Ad5-transformed 5WY cells do not form tumors. These observations indicate that the efficient tumor formation by RCBY cells is dependent on the expression of the Ad12 E1A and E1B genes, whereas the inefficient tumor formation by RCAY cells is due to the expression of only the Ad12 E1A gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Brusca J. S., Chinnadurai G. Transforming genes among three different oncogenic subgroups of human adenoviruses have similar replicative functions. J Virol. 1981 Jul;39(1):300–305. doi: 10.1128/jvi.39.1.300-305.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Vanderpool E. A., Henry P. H., Austin J. B., Huebner R. J. Transformation of primary rat embryo cells by adenovirus type 2. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1205–1212. doi: 10.1073/pnas.58.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Wolford R., Huebner R. J. Adenovirus type 12-rat embryo transformation system. J Virol. 1967 Apr;1(2):362–367. doi: 10.1128/jvi.1.2.362-367.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Sawada Y., Uemizu Y. A rapid screening for the specific DNA sequence: analysis of transforming DNA segments in adenovirus-transformed cells. Gan. 1979 Apr;70(2):239–243. [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Hiwasa T., Oda K. I. Characterization of flat revertant cells isolated from simian virus 40-transformed mouse and rat cells which contain multiple copies of viral genomes. J Virol. 1981 Mar;37(3):1028–1043. doi: 10.1128/jvi.37.3.1028-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M. O., Lewis A. M., Jr, Macpherson I., Huebner R. J. Transformation of rat embryo cells by adenovirus type 1. J Gen Virol. 1969 Jan;4(1):29–36. doi: 10.1099/0022-1317-4-1-29. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Complementation of adenovirus type 5 host range mutants by adenovirus type 12 in coinfected HeLa and BHK-21 cells. J Virol. 1981 Apr;38(1):191–197. doi: 10.1128/jvi.38.1.191-197.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Sato J., Handa H., Shiroki K., Shimojo H. Mapping of RNAs transcribed from adenovirus type 12 early and VA RNA regions. Virology. 1981 Oct 30;114(2):379–398. doi: 10.1016/0042-6822(81)90219-1. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Fujinaga K. Mapping of adenovirus 12 mRNA's transcribed from the transforming region. J Virol. 1980 Dec;36(3):639–651. doi: 10.1128/jvi.36.3.639-651.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Saito I., Shiroki K., Shimojo H. In vitro translation of adenovirus type 12-specific mRNA complementary to the transforming gene. Virology. 1980 Nov;107(1):61–70. doi: 10.1016/0042-6822(80)90272-x. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Handa H., Shimojo H., Yano S., Ojima S., Fujinaga K. Establishment and characterization of rat cell lines transformed by restriction endonuclease fragments of adenovirus 12 DNA. Virology. 1977 Oct 15;82(2):462–471. doi: 10.1016/0042-6822(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Maruyama K., Saito I., Fukui Y., Shimojo H. Incomplete transformation of rat cells by a deletion mutant of adenovirus type 5. J Virol. 1981 Jun;38(3):1048–1054. doi: 10.1128/jvi.38.3.1048-1054.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Segawa K., Shimojo H. Two tumor antigens and their polypeptides in adenovirus type 12-infected and transformed cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2274–2278. doi: 10.1073/pnas.77.4.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Maeta Y., Hamada C. Tumor-specific transplantation and surface antigen in cells transformed by the adenovirus 12 DNA fragments. Virology. 1979 Nov;99(1):188–191. doi: 10.1016/0042-6822(79)90053-9. [DOI] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Williams J., Ho Y. S., Galos R. Evidence for functional relatedness of products encoded by the transforming sequences of human adenovirus types 5 and 12. Virology. 1981 Apr 15;110(1):208–212. doi: 10.1016/0042-6822(81)90023-4. [DOI] [PubMed] [Google Scholar]