Abstract
BACKGROUND
For patients who have above-target low-density lipoprotein cholesterol (LDL-C) levels while on statin monotherapy, coadministration of a cholesterol absorption inhibitor with the statin may decrease serum LDL-C levels and improve overall lipid profiles.
OBJECTIVES
To assess the effectiveness and safety of ezetimibe 10 mg/day coadministered with a statin in patients with primary hypercholesterolemia who have higher than recommended LDL-C levels while on statin monotherapy.
METHODS
A six-week, prospective, multicentre study of eligible patients who had above-target LDL-C levels while on monotherapy with any statin, regardless of dose, for a minimum of four weeks. All patients were treated for six weeks with 10 mg ezetimibe daily coad-ministered with their current statins.
RESULTS
A total of 1141 patients were screened, 953 (83.5%) fulfilled the study inclusion criteria and 837 (87.8%) completed the study. Reasons for withdrawal included: lost to follow-up (50 patients [5.2%]); protocol violations (45 patients [4.7%]); adverse events (19 patients [2.0%]); and withdrawal of consent (two patients [0.2%]). After six weeks of treatment, statistically significant (P=0.001) mean reductions were observed in LDL-C (30.05%), total cholesterol (20.84%), triglycerides (10.16%), apolipoprotein B (19.84%) and the total cholesterol to high-density lipoprotein cholesterol ratio (19.88%). At six weeks, 674 patients (80.5%) achieved target LDL-C levels. Fifty predominantly mild, nonserious adverse events related to ezetimibe were reported by 32 patients (3.4%). Frequently reported adverse events included constipation (n=7 [0.7% of patients]), diarrhea (n=4 [0.4%]) and dizziness (n=4 [0.4%]).
CONCLUSION
Ezetimibe coadministered with statins is effective in reducing LDL-C in patients who do not attain target LDL-C levels while on statin monotherapy.
Keywords: Ezetimibe, Hypercholesterolemia, Low-density lipoprotein cholesterol, Statin
Abstract
HISTORIQUE
Chez les patients dont les taux de cholestérol à lipoprotéines de basse densité (C-LDL) demeurent trop élevés malgré une monothérapie aux statines, la coadministration d’un inhibiteur de l’absorption du cholestérol pourrait réduire les taux sériques de C-LDL et améliorer le bilan lipidique global.
OBJECTIFS
Évaluer l’efficacité et l’innocuité de 10 mg/jour d’ézétémibe coadministrés avec une statine chez des patients atteints d’hypercholestérolémie primaire dont les taux de C-LDL sont plus élevés que la normale malgré une monothérapie aux statines.
MÉTHODOLOGIE
Étude prospective canadienne multicentre de six semaines auprès de patients admissibles aux taux de C-LDL supérieurs à la normale malgré une monothérapie à une statine d’au moins quatre semaines, quelle qu’en soit la dose. Tous les patients ont reçu un traitement quotidien de 10 mg/jour d’ézétémibe pendant six semaines, coadministrés avec leur statine habituelle.
RÉSULTATS
Un total de 1 141 patients ont subi un dépistage; 953 (83,5 %) respectaient les critères d’inclusion, et 837 (87,8 %) ont terminé l’étude. Les raisons du retrait étaient la perte au suivi (50 patients [5,2 %]), les violations au protocole (45 patients [4,7 %]), les événements indésirables (19 patients [2,0 %]) et le retrait du consentement (2 patients [0,2 %]). Au bout de six semaines de traitement, on a observé des diminutions moyennes statistiquement significatives (p = 0,001) du C-LDL (30,05 %), du cholestérol total (20,84 %), des triglycérides (10,16 %), de l’apoliprotéine B (19,84 %) et du ratio entre le cholestérol total et le cholestérol à lipoprotéines de haute densité (19,88 %). Après six semaines, 674 patients (80,5 %) avaient atteint les taux de C-LDL cibles. Trentedeux patients (3,4 %) ont déclaré 50 événements indésirables plutôt bénins et sans gravité, reliés à l’ézétimibe. Les plus fréquents étaient la constipation (n = 7 [0,7 % des patients]), la diarrhée (n = 4 [0,4 %]) et les étourdissements (n = 4 [0,4 %]).
CONCLUSION
L’ézétimibe coadministrée avec des statines réduit efficacement le C-LDL chez les patients qui n’obtiennent pas les taux de C-LDL cibles malgré une monothérapie aux statines.
According to the 2003 Canadian guidelines for the management of dyslipidemia and the prevention of cardiovascular disease, the recommended low-density lipoprotein cholesterol (LDL-C) target levels for patients at low, moderate and high estimated 10-year risk for coronary artery disease (CAD) are less than 4.5 mmol/L, less than 3.5 mmol/L and less than 2.5 mmol/L, respectively (1). More recently, evidence generated from clinical trials and epidemiological studies has suggested that for patients at high risk for CAD, additional protective benefits may be associated with an LDL-C level reduction to below 2.0 mmol/L (2–8).
Management of patients with dyslipidemia involves a combination of dietary modifications, exercise and drug therapy. The current standard of pharmacological intervention for the reduction of LDL-C is first-line treatment with 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors (statins) (1,9–12). However, for a large number of patients, monotherapy with a statin, even with increasing doses, may be ineffective in achieving target LDL-C levels (13–16), and these patients remain at increased risk for CAD. Recently, combination therapy has emerged as a potential solution to this treatment gap (17–29).
Ezetimibe is a cholesterol absorption inhibitor that prevents the passage of dietary and biliary cholesterol across the intestinal wall (30–40). This mechanism of action is complementary to that of statins in the prevention of serum cholesterol accumulation (41). Evidence generated in controlled clinical trials has demonstrated that coadministration of ezetimibe with currently marketed statins is well tolerated and more effective in reducing LDL-C levels and improving other lipid parameters (total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C] and triglycerides [TGs]) than statin monotherapy (17–27,40,42–48).
The clinical trials that evaluated the effectiveness of the coadministration of ezetimibe and statins in managing dyslipidemia have been based on selected patient populations, and many focused on specific statin regimens. In addition, these trials did not differentiate between patients with significant comorbidities, specifically, diabetes and the metabolic syndrome (49–51). Furthermore, generalization of the results from controlled clinical trials to the target population and a real-life setting is often difficult because of the highly selected sample of patients and controlled treatment protocols used in these studies. There is currently a need for studies that assess real-life effectiveness and safety of lipid-lowering treatments.
The present study addressed these needs by using a prospective cohort design that simulated the real-life setting of family practice in Canada. It was a Phase IV real-life trial conducted on patients who were being treated for hyperlipidemia by Canadian general practitioners. The purpose of the present study was to assess the effectiveness and tolerability of a six-week treatment with 10 mg ezetimibe daily coadministered with any current statin regimen in patients with primary hypercholesterolemia who had difficulty achieving target LDL-C levels. There were no limitations with respect to patient risk profiles, or the type or dose of statin used. Subgroup analyses of patients with diabetes and the metabolic syndrome (51) were performed to assess the effectiveness of treatment in these clinically important patient subgroups.
METHODS
Study design
The present study was a prospective, single-cohort, open-label study. All potentially eligible patients signed an informed consent form before performing any study procedure, including all eligibility assessments. The study was approved by two independent ethics review boards (Institutional Review Board Services, Aurora, Ontario, and the College of Physicians and Surgeons of Alberta, Edmonton). All study assessments were conducted at the treating physician’s office.
During the screening visit, study patients were assessed for eligibility and underwent reviews of medical history and diet, as well as a brief physical examination. Information on the statin used, including daily dose and duration of treatment, was recorded, and each patient’s 10-year risk for CAD was estimated. Patients with confirmed diabetes or cardiovascular disease were automatically classified into the high-risk (20% or greater) category (1). For all other patients, the Framingham model was used to classify patients into three risk categories: high (20% or greater), moderate (11% to 19%) or low (10% or less) (1,52,53). Blood was drawn at local facilities within two weeks of the screening visit for assessment of patient lipid profiles. Specifically, LDL-C, TC, TG and HDL-C levels were measured. Apolipoprotein B (apo B) was assessed in a subgroup of approximately 60% of patients, based on the availability of the test across Canada. When TG levels were greater than or equal to 3.99 mmol/L, LDL-C levels were determined by ultracentrifugation, and when TG levels were less than 3.99 mmol/L, the Friedewald equation was used (54,55). Patients who had higher than the recommended target LDL-C levels and continued to be eligible for inclusion in the study were invited to return for the baseline visit.
During the baseline visit, which took place within two weeks of the screening visit, the study medication was dispensed, and the follow-up blood draw and final assessment visits were arranged. The final study visit took place six weeks (± four days) after the baseline visit. Blood draw for the final assessment of lipid profile took place no more than three days before the final visit. During the final study visit, patients underwent a brief physical examination, which included vital sign measurements, along with reviews of concomitant medication use and dietary changes. The assessment of compliance with treatment was ascertained by the number of missed doses of study medication, as reported by the patient. Details regarding all adverse events occurring during the study were also recorded at the final assessment. Study physicians were instructed to follow their patients for a safety assessment approximately 14 days after the last dose of ezetimibe. Any adverse events that occurred during this two-week period were recorded.
Study sample
The 221 participating physician investigators were selected from a stratified random sample of Canadian general practitioners representative of the Canadian population. Physicians from all 10 Canadian provinces were invited to participate; however, no patients were enrolled from Prince Edward Island.
Eligibility criteria
The following inclusion criteria were applied to potentially eligible patients: age of at least 18 years; confirmed diagnosis of hyper-cholesterolemia and elevated plasma LDL-C levels (2.5 mmol/L or higher for patients at high 10-year CAD risk, 3.5 mmol/L or higher for moderate-risk patients and 4.5 mmol/L or higher for low-risk patients); stable diet and statin regimen for at least four weeks before study entry and for the duration of the study; and use of an effective method of contraception by women of child-bearing potential, beginning at least seven days before the study and continuing for at least 14 days after study completion or discontinuation.
Patients were excluded from the study based on the following criteria: presence of any condition, including poor mental function, substance abuse, unstable psychiatric illness, or pregnancy or lactation, which, in the opinion of the investigator, would render the patient unable to complete the study, or would produce significant risk or not be in the best interest of the patient; treatment with any other investigational drug within 30 days before study entry; diagnosis with, or history of, any illness that would increase risk to the patient, including congestive heart failure of New York Heart Association class III or IV, uncontrolled cardiac arrhythmias or hypertension (systolic blood pressure greater than 180 mmHg and/or diastolic blood pressure greater than 110 mmHg), myocardial infarction, coronary bypass surgery or angioplasty with or without stent use within the past three months, unstable angina pectoris or unstable/severe peripheral vascular disease, uncontrolled endocrine or metabolic disease known to influence serum lipids or lipoproteins (including poorly controlled type 1 or type 2 diabetes mellitus [glycated hemoglobin concentration greater than 9.0%], creatinine level greater than 177 μmol/L or active renal disease with significant proteinuria [more than 1 mg albumin per 1 mg creatinine]), active acute or chronic hepatobiliary disease, levels of aspartate aminotransferase, alanine aminotransferase or creatine kinase more than twice the upper limit of normal, or a positive HIV serostatus; concomitant use, during a period of eight weeks or less before enrolment in the study, of any medications that may have interacted with statins or ezetimibe, or affected serum lipid levels (including therapeutic doses of corticosteroids, antifungal azoles, macrolide antibiotics, nefazodone and protease inhibitors, amiodarone, verapamil and cyclosporine). Patients using fibrates within eight weeks before study entry were also excluded.
Patients treated with cardiovascular medications were included in the study if they were on a stable medication regimen for at least four weeks before study entry and remained on the same regimen for the duration of the study. Allowed cardiovascular medications included beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, nitrates, alpha-adrenergic blockers, thiazide diuretics, clopidogrel and anticoagulants (ie, warfarin). Estrogen replacement therapy in women was allowed if a stable dose had been taken for at least four weeks before screening and was unlikely to change during the study. Patients receiving bile salt-binding resins, other lipid-lowering medications, including niacin and probucol, or regular maintenance doses of psyllium were also eligible for inclusion in the study if they had been on a stable dose for at least six weeks before the study screening visit and the dose remained stable during the trial. Patients were instructed to take ezetimibe at least two hours before or four hours after administration of bile salt-binding resins.
Treatment
All patients were treated with 10 mg ezetimibe (Ezetrol, Merck Frosst/Schering Pharmaceuticals, Canada) per day, coadministered with their current statins at a unaltered dose for a period of six weeks. Patients were instructed not to change their diets during the study. There were no limitations on the type or dose of statin being used.
Outcome measures
The primary efficacy outcome measure was the percentage of change in LDL-C during the six-week treatment period, which was calculated as follows:
Secondary measures of efficacy were the percentage of patients who had achieved the recommended target LDL-C level at the end of the six-week treatment period, and the percentages of change in TC, TGs, HDL-C, apo B and the TC/HDL-C ratio.
Safety and tolerability
Safety was assessed by the incidence of treatment-related adverse events, as reported by the patient during the final study visit. The investigators were also asked to report, as adverse events, any clinically important changes in laboratory test values. However, laboratory tests for the detection of adverse events were not required as part of the study protocol. Testing may have been conducted by the treating investigators if, according to their clinical judgment and routine practice, it was necessary for the management of the patient. Investigators were instructed to report any adverse events that occurred during the six-week study period and to follow up with their patients for safety for an additional 14 days after the last dose of ezetimibe. Study investigators graded the severity of adverse events as mild, moderate or severe on the basis of information provided by the patient. The status of a causal relationship between an adverse event and the study drug was determined by the treating physician. Adverse events were coded and reported according to terminology in the Medical Dictionary for Regulatory Activities (56).
Statistical methods
Statistical significance of the primary and secondary efficacy outcome measures, specifically, the change in lipid parameters between the final and the baseline visits, was assessed using Student’s t test for paired samples. The rate of achieving target LDL-C levels was analyzed as the proportion of patients achieving the recommended target after six weeks of treatment. In accordance with the intent-to-treat principle, all study patients who completed the six-week visit assessment, regardless of compliance with the study protocol, were included in the efficacy analyses. However, patients who were lost to follow-up and did not return for the six-week assessment could not be included in the efficacy analyses. No replacement of missing data was used. All subjects who received at least one dose of ezetimibe, including those who were withdrawn due to an adverse event or any other reason, were included in the safety analyses.
The following four patient subgroups were analyzed: patients with diabetes but without the metabolic syndrome; patients with the metabolic syndrome but without diabetes; patients with both diabetes and the metabolic syndrome; and patients with neither diabetes nor the metabolic syndrome. The presence of diabetes was determined by review of a patient’s medical history. The metabolic syndrome was defined according to the American Heart Association (51) criteria published at the time of the study as the presence of three or more of the following: abdominal obesity (waist circumference of greater than 102 cm in men and greater than 88 cm in women); elevated TG levels (1.7 mmol/L or greater); high serum glucose (6.2 mmol/L or greater), low serum HDL-C levels (less than 1.0 mmol/L in men and less than 1.3 mmol/L in women); and high blood pressure (blood pressure greater than 130/85 mmHg).
RESULTS
A total of 1141 patients were screened, 953 (83.5%) of whom fulfilled the study inclusion criteria and were enrolled in the study. Of these, 837 (87.8%) completed the six-week follow-up. One hundred sixteen patients were discontinued from the study and did not complete the six-week follow up assessments. These included 50 patients (5.2%) who were lost to follow-up, 45 (4.7%) who were withdrawn by the investigators because of a changed or stopped statin treatment, 19 (2.0%) who were withdrawn due to adverse events and two (0.2%) who withdrew consent before initiation of treatment.
Table 1 summarizes the demographics of the study sample. The mean ± SD age of the study population was 62±10.5 years, with a range of 21 to 89 years, and 62.5% were men. With respect to the 10-year risk for CAD, the majority, or 777 subjects (92.8%), were in the high-risk category. Of these, 64 (8.2%) were classified in the high-risk group on the basis of the Framingham model and 713 (91.8%) on the basis of confirmed diabetes or cardiovascular disease. There were 40 (4.8%) and 20 (2.4%) subjects in the moderate- and low-risk categories, respectively.
TABLE 1.
Baseline and demographic characteristics of patients
| Characteristics | |
|---|---|
| Mean age ± SD (range) | 62±10.5 (21–89) |
| Age in years, n (%) | |
| 45 | 45 (5.4) |
| 46–64 | 455 (54.4) |
| 65–74 | 245 (29.3) |
| ≤75 | 89 (10.6) |
| Sex, n (%) | |
| Male | 523 (62.5) |
| Female | 313 (37.4) |
| 10-year coronary artery disease risk, n (%) | |
| Mild (≤10%) | 20 (2.4) |
| Moderate (11% – 19%) | 40 (4.8) |
| High (≥20%) | 777 (92.8) |
|
Comorbidity and risk factor profile | |
| Smoking status, n (%) | |
| Current smoker | 191 (22.8) |
| Past smoker | 268 (32.0) |
| Nonsmoker | 375 (44.8) |
| Hypertension, n (%) | 432 (51.6) |
| Diabetes mellitus (type 2), n (%) | 358 (42.8) |
| The metabolic syndrome, n (%) | 408 (48.7) |
| Diabetes mellitus and the metabolic syndrome, n (%) | 244 (29.2) |
| Coronary artery disease, n (%) | 388 (46.3) |
| Cerebrovascular disease, n (%) | 75 (9.0) |
| Peripheral vascular disease, n (%) | 78 (9.3) |
| Chronic kidney disease, n (%) | 15 (1.8) |
| Family history of cardiovascular disease, n (%) | 504 (60.2) |
| Menopausal status, n (%) (n=313) | |
| Premenopause | 22 (7.0) |
| Menopause | 291 (93.0) |
| Use of hormone replacement therapy | 54 (17.3) |
With respect to related comorbidities, the most frequently reported was hypertension (51.6%), while 358 subjects (42.8%) had type 2 diabetes mellitus, 408 (48.7%) had the metabolic syndrome (51), and 244 (29.2%) had both diabetes mellitus and the metabolic syndrome. There were 388 patients (46.3%) with CAD and 504 (60.2%) who had a known family history of cardiovascular disease.
The data summarized in Table 2 show that more than one-half (50.5%) of the study subjects were taking atorvastatin, followed by simvastatin (20.0%), rosuvastatin (14.2%) and pravastatin (12.3%). Before enrolment in the study, 330 patients (39.4%) were treated with a moderate or high statin dose, defined as 40 mg/day or 80 mg/day, depending on the statin.
TABLE 2.
Statin therapy at baseline (n=837)
| Total daily statin dose, n (%)
|
|||||
|---|---|---|---|---|---|
| Statin | Patients, n (%) | 10 mg | 20 mg | 40 mg | 80 mg |
| Atorvastatin | 423 (50.5) | 105 (12.5) | 154 (18.4) | 117 (14.0) | 41 (4.9) |
| Simvastatin | 167 (20.0) | 20 (2.4) | 58 (6.9) | 67 (8.0) | 14 (1.7) |
| Rosuvastatin | 119 (14.2) | 62 (7.3) | 38 (4.5) | 17 (2.0) | 0 |
| Pravastatin | 103 (12.3) | 6 (0.7) | 39 (4.7) | 56 (6.7) | 2 (0.2)* |
| Lovastatin | 27 (3.2) | 3 (0.4) | 12 (1.4) | 12 (1.4) | 0 |
| Fluvastatin | 9 (1.1) | 0 | 5 (0.6) | 4 (0.5) | 0 |
| Total | 195 (23.3) | 305 (36.4) | 273 (32.6) | 57 (6.8) | |
Four subjects had missing data regarding their statin use; 21 patients reported taking more than one statin; one subject reported taking 15 mg/day of atorvastatin; six subjects reported taking 30 mg/day of a statin; nine subjects reported taking 60 mg/day of a statin.
Both subjects reported taking 80 mg/day of pravastatin even though the recommended maximum dose is 40 mg/day
The data in Table 3 summarize the lipid profile parameters of the study subjects at baseline and at the six-week visit. These results show that, with the exception of HDL-C levels, statistically significant (P=0.001) mean per cent reductions were observed among all lipid profile parameters. More specifically, in the study sample as a whole, LDL-C levels were reduced by 30.1%, TC levels by 20.8%, TG levels by 10.2% and TC/HDL-C ratios by 19.9%; HDL-C levels remained unchanged. Apo B concentration was measured in a subgroup of 506 of the 837 patients (60.5%) who completed the study. Among these patients, the mean per cent reduction in apo B concentration was 19.84% (P=0.001). The subgroup analyses summarized in Table 3 showed similar and consistent results in all subject subgroups with statistically significant reductions in all lipid parameters, excluding HDL-C levels.
TABLE 3.
Baseline and six-week lipid profile
| Patient subgroup | Baseline mean ± SD | Six-week mean ± SD | % Change mean ± SD | P* |
|---|---|---|---|---|
|
All patients (n=837) | ||||
| Low-density lipoprotein cholesterol, mmol/L | 3.43±0.85 | 2.38±0.87 | −30.05±18.40 | 0.001 |
| Total cholesterol, mmol/L | 5.55±1.00 | 4.36±1.01 | −20.84±14.06 | 0.001 |
| HDL-C, mmol/L | 1.22±0.31 | 1.21±0.30 | −0.08±13.20 | 0.856 |
| Triglycerides, mmol/L | 2.06±1.28 | 1.73±0.93 | −10.16±29.25 | 0.001 |
| Apolipoprotein B, g/L (n=506) | 1.17±0.41 | 0.92±0.27 | −19.84±18.13 | 0.001 |
| Total cholesterol/HDL-C ratio | 4.76±1.33 | 3.75±1.08 | −19.88±15.45 | 0.001 |
|
Diabetes, but no metabolic syndrome (n=114) | ||||
| Low-density lipoprotein cholesterol, mmol/L | 3.19±0.56 | 2.20±0.64 | −30.24±20.00 | 0.001 |
| Total cholesterol, mmol/L | 5.28±0.69 | 4.17±0.83 | −20.58±14.33 | 0.001 |
| HDL-C, mmol/L | 1.36±0.31 | 1.33±0.31 | −1.91±10.72 | 0.059 |
| Triglycerides, mmol/L | 1.65±0.82 | 1.44±0.64 | −6.78±30.31 | 0.019 |
| Apolipoprotein B, g/L (n=68) | 1.10±0.34 | 0.88±0.25 | −16.62±23.76 | 0.001 |
| Total cholesterol/HDL-C ratio | 4.04±0.86 | 3.24±0.69 | −18.61±14.42 | 0.001 |
|
Metabolic syndrome, but no diabetes (n=164) | ||||
| Low-density lipoprotein cholesterol, mmol/L | 3.62±1.01 | 2.59±1.00 | −27.71±18.95 | 0.001 |
| Total cholesterol, mmol/L | 5.79±1.12 | 4.64±1.18 | −19.42±15.87 | 0.001 |
| HDL-C, mmol/L | 1.07±0.25 | 1.08±0.22 | 2.00±14.37 | 0.076 |
| Triglycerides, mmol/L | 2.62±1.19 | 2.14±0.97 | −14.84±26.88 | 0.001 |
| Apolipoprotein B, g/L (n=102) | 1.32±0.72 | 0.99±0.32 | −20.32±17.81 | 0.001 |
| Total cholesterol/HDL-C ratio | 5.64±1.57 | 4.44±1.30 | −19.87±17.86 | 0.001 |
|
Metabolic syndrome and diabetes (n=244) | ||||
| Low-density lipoprotein cholesterol, mmol/L | 3.30±0.64 | 2.15±0.68 | −33.80±20.14 | 0.001 |
| Total cholesterol, mmol/L | 5.49±0.84 | 4.18±0.88 | −23.18±15.33 | 0.001 |
| HDL-C, mmol/L | 1.16±0.26 | 1.15±0.27 | −0.14±13.68 | 0.878 |
| Triglycerides, mmol/L | 2.27±1.01 | 1.93±1.00 | −12.18±29.68 | 0.001 |
| Apolipoprotein B, g/L (n=148) | 1.13±0.21 | 0.88±0.23 | −22.94±19.00 | 0.001 |
| Total cholesterol/HDL-C ratio | 4.89±1.08 | 3.76±0.98 | −22.06±16.96 | 0.001 |
|
Neither metabolic syndrome nor diabetes (n=315) | ||||
| Low-density lipoprotein cholesterol, mmol/L | 3.51±0.96 | 2.51±0.94 | −28.29±15.53 | 0.001 |
| Total cholesterol, mmol/L | 5.57±1.11 | 4.44±1.04 | −19.86±11.54 | 0.001 |
| HDL-C, mmol/L | 1.30±0.32 | 1.29±0.33 | −0.47±12.92 | 0.522 |
| Triglycerides, mmol/L | 1.74±1.49 | 1.47±0.81 | −7.38±29.39 | 0.001 |
| Apolipoprotein B, g/L (n=188) | 1.14±0.29 | 0.92±0.25 | −18.30±14.70 | 0.001 |
| Total cholesterol/HDL-C ratio | 4.47±1.25 | 3.58±0.96 | −18.64±12.89 | 0.001 |
P value based on Student’s t test for paired observations. HDL-C High-density lipoprotein cholesterol
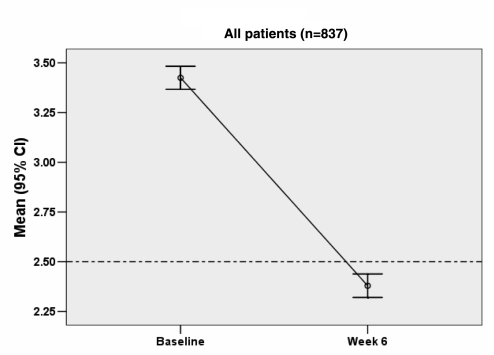
The results in Figure 1 show that at baseline, the mean serum LDL-C level in the study sample was 3.43 mmol/L, with a 95% CI between 3.37 mmol/L and 3.48 mmol/L. At the six-week assessment, the mean serum LDL-C level was reduced to 2.38 mmol/L, with a 95% CI between 2.32 mmol/L and 2.44 mmol/L.
Figure 1.
Low-density lipoprotein cholesterol at baseline and six weeks of treatment (all patients)
After six weeks of treatment with ezetimibe, 674 patients (80.5%) who completed the study had achieved LDL-C levels below the recommended target (Table 4). The subgroup of subjects who had diabetes but not the metabolic syndrome had the highest rate of achieving target LDL-C at the end of treatment (90%), while the lowest rate was observed in subjects with the metabolic syndrome but not diabetes (66%) (Figure 2). Compared with those who had reached target LDL-C levels at six weeks, 163 patients who did not reach the recommended target had significantly higher mean LDL-C levels at baseline (4.03 mmol/L versus 3.28 mmol/L; P=0.001). In addition, the proportion of patients treated with a moderate- to high-dose statin (40 mg/day to 80 mg/day) was significantly higher among the 163 patients with above-target LDL-C levels at six weeks than among those patients with below-target levels (52.8% versus 38.7%; P=0.005). Among these patients, a statistically significant mean per cent reduction in LDL-C level of 12.47% (P=0.001) was observed. The baseline CAD risk classification distribution was similar among the 674 patients who had below-target LDL-C levels and the 163 who had above-target LDL-C levels at six weeks (Table 4). However, this is expected, given that the vast majority of the patients in the study sample were in the high CAD risk category.
TABLE 4.
Comparison of subjects below and above target low-density lipoprotein cholesterol (LDL-C) levels at six weeks
| Patient group
|
|||
|---|---|---|---|
| Below target LDL-C (n=674) | Above target LDL-C (n=163) | P | |
| LDL-C, mmol/L (mean ± SD) | |||
| Baseline | 3.28±0.70 | 4.03±1.12 | 0.001* |
| Six weeks | 2.13±0.57 | 3.46±1.07 | 0.001* |
| Change in LDL-C, % (mean ± SD) | |||
| Baseline to six weeks | −34.10±15.86 | −12.47±18.39 | 0.001* |
| P | 0.001† | 0.001† | |
| Statin dose at baseline, n (%) | |||
| 10 mg/day | 168 (24.9) | 28 (17.2) | 0.005‡ |
| 20 mg/day | 253 (37.5) | 52 (31.9) | |
| 40 mg/day – 80 mg/day | 261 (38.7) | 86 (52.8) | |
| 10-year coronary artery disease risk, n (%) | |||
| Low | 623 (92.4) | 154 (94.5) | 0.825‡ |
| Moderate | 34 (5.0) | 6 (3.7) | |
| High | 17 (2.5) | 3 (1.8) | |
P value based on Student’s t test for independent groups;
†P value based on Student’s t test for paired comparisons;
P value based on χ2 test
Figure 2.
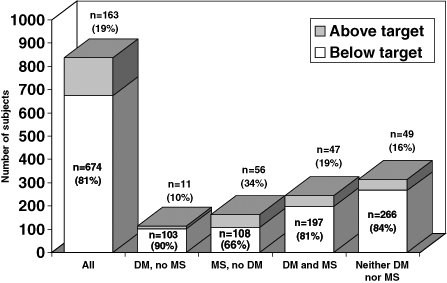
Numbers and proportions of patients below the target low-density lipoprotein cholesterol level at six weeks. DM Diabetes mellitus; MS Metabolic syndrome
Of the 837 patients who completed the six-week study evaluation, 680 (81.2%) had reported not missing any doses of the study medication. Among the 157 patients (18.8%) who reported missing more than one dose, the median number of doses missed was 2.0, with a mean of 3.8 (95% CI 3.12 to 4.52) and a range of between one and 40 doses missed. Overall, 820 of the 837 patients (98%) reported that they took 80% or more of the required ezetimibe and statin doses during the study period.
Table 5 summarizes the results of the safety analyses. A total of 111 nonserious adverse events (NSAEs) were reported by 78 of the 953 patients (8.2%) included in the safety analyses. Of these, 50 events reported by 32 patients (3.4%) were attributed to ezetimibe by the treating physician. The severity of the 50 NSAEs causally associated with ezetimibe was mild for 43 events (86%) reported by 27 patients (2.8%) and moderate for seven events (14%) reported by five patients (0.5%). No severe adverse events were reported that were attributed to ezetimibe. The most frequently reported NSAEs attributed to ezetimibe were constipation (0.7% of patients), diarrhea (0.4%), dizziness (0.4%), flatulence (0.3%), myalgia (0.3%) and headache (0.3%). Less frequently reported NSAEs were dyspepsia (0.2%), nausea (0.2%), fatigue (0.2%) and arthralgia (0.2%). No clinically important laboratory findings or serious adverse events were reported that were attributed to ezetimibe.
TABLE 5.
Treatment-emergent adverse events (n=953)
| Adverse events | Events, n | Patients, n (%) |
|---|---|---|
| Total | ||
| Serious adverse events | 0 | 0 |
| Nonserious adverse events | 111 | 78 (8.2) |
| Causality in relation to ezetimibe | ||
| Definitely not or probably not due to ezetimibe | 61 | 53 (5.6) |
| Probably or definitely due to ezetimibe | 50 | 32 (3.4) |
| Severity of events associated with ezetimibe | ||
| Mild | 43 | 27 (2.8) |
| Moderate | 7 | 5 (0.5) |
| Severe | 0 | 0 |
| Most frequently reported (≥0.2%)* | ||
| Constipation | 7 | 7 (0.7) |
| Diarrhea | 4 | 4 (0.4) |
| Dizziness | 6 | 4 (0.4) |
| Flatulence | 3 | 3 (0.3) |
| Myalgia | 3 | 3 (0.3) |
| Headache | 3 | 3 (0.3) |
| Dyspepsia | 2 | 2 (0.2) |
| Nausea | 2 | 2 (0.2) |
| Fatigue | 2 | 2 (0.2) |
| Arthralgia | 2 | 2 (0.2) |
Only events associated with ezetimibe, as per the investigator’s assessment, are presented
DISCUSSION
There is evidence in the literature that a significant proportion of patients with hypercholesterolemia do not achieve target LDL-C levels with statin monotherapy (13–16). This may be explained by the fact that statins only inhibit cholesterol production in the liver, but have no effect on intestinal absorption of dietary cholesterol. Other factors that may explain the lower observed effectiveness of statin monotherapy include low tolerability of statins (especially at higher doses), suboptimal patient compliance with treatment regimens, inadequate follow-up frequency, and resistance on the part of physicians and patients to the use of higher doses of medications (13–16). Empirical evidence has shown that increasing the statin dose produces only marginal decreases in LDL-C levels (57–59); however, the risk for adverse reactions increases with higher statin doses (17,19–21,58). As a result, health care providers are faced with the challenge of effectively managing patients with persistent hyperlipidemia, while the recommended LDL-C targets are lowered further. Combination therapy of ezetimibe and a statin may provide a solution by targeting the two possible sources of serum cholesterol, specifically, by inhibition of the production of cholesterol in the liver, and by prevention of the absorption of dietary and biliary cholesterol in the intestine (41).
The effectiveness of ezetimibe, administered alone or in combination with a statin, in improving patient lipid profiles and, more specifically, reducing serum LDL-C levels has been supported by the results of controlled clinical trials (17–27,31,32,34,42–47). Ballantyne et al (17) reported a significantly higher reduction in LDL-C levels and improvement in lipid profile parameters in patients treated with ezetimibe coad-ministered with atorvastatin than in patients treated with atorvastatin alone (17,18). Similar results were reported when ezetimibe was coadministered with lovastatin or simvastatin (18–22,24,25,27,42–44,46). The results of pooled analyses assessing the coadministration of ezetimibe with different statins showed evidence of significant reductions in levels of LDL-C, TC and TGs (20,42,46,60). Gaudiani et al (21) reported a 20.8% reduction in LDL-C levels in diabetic patients treated with ezetimibe added to simvastatin 20 mg/day. In the same study, patients treated with double their existing statin dose experienced only a nonsignificant 0.3% reduction in LDL-C levels. Gagne et al (20) reported that patients with homozygous familial hypercholesterolemia who were treated with ezetimibe coadministered with atorvastatin or simvastatin had a significantly higher mean per cent reduction in LDL-C level than when treated with the statins alone. A large, community-based, double-blinded, randomized clinical trial conducted by 303 physicians in a diverse sample of 3030 patients randomly assigned in a 2:1 ratio of ezetimibe or placebo in addition to their current statin was reported (61,62). The patients enrolled in the study had elevated LDL-C levels while on statin monotherapy; after six weeks of treatment, the mean LDL-C level reduction was 25.8% in the ezetimibe-treated patients and 2.7% in the placebo-treated patients (P<0.001). In the same study, 71% of patients treated with ezetimibe had LDL-C levels below the recommended target compared with 20.6% in the placebo group (P<0.001) (61,62).
Our study was conducted in patients with primary hyperlipidemia who had LDL-C levels higher than the target levels recommended by the 2003 Canadian guidelines for the management of hyperlipidemia (1). The study emulated a real-life setting by not imposing limitations on the statin regimen used (other than that it could not be modified during the six-week study follow-up period), administering ezetimibe treatment according to the product monograph and instructing physicians to adhere to their routine care for the management of their patients. Generalization of our study results to the real-life setting is further supported by the fact that minimal limitations were imposed on the selection of subjects for the study and that patients were included in the analysis regardless of compliance with treatment. In the present study sample, the demographic profile and distribution of statin used was similar to that reported in other Canadian studies (13,63,64), confirming that our study sample is representative of the Canadian dyslipidemic population. The results of the present study are in agreement with those reported in literature and provide further evidence in support of combination therapy with ezetimibe and a statin for the management of hypercholesterolemia in patients who fail to achieve target LDL-C levels on statin monotherapy. In the present study, we observed a higher mean LDL-C level reduction than that reported in other studies; however, our results are similar to those reported in the community-based study previously discussed (61,62). Possible explanations for the higher change in LDL-C levels in our study sample include fewer restrictions on patient selection and high compliance with treatment.
In the present study sample, the 95% CI of the mean serum LDL-C levels was between 3.37 mmol/L and 3.48 mmol/L at baseline, or before the addition of ezetimibe to the treatment regimen. This is not different from the recommended target level for patients with moderate CAD risk of 3.5 mmol/L, and it is significantly higher than the 2.5 mmol/L target for patients at high risk for CAD (1). After six weeks of treatment with ezetimibe, the 95% CI of the mean serum LDL-C levels of the study sample was between 2.32 mmol/L and 2.44 mmol/L. For the latter point estimate, the 99% CI was between 2.30 mmol/L and 2.46 mmol/L. The upper 95% and 99% CI values of the mean LDL-C level estimate at six weeks was below the 2.5 mmol/L target for high-risk patients. This is an important observation because inference to a comparable, predominantly high CAD risk population that is above target LDL-C levels while on statin monotherapy shows that 99% of the time, the addition of ezetimibe to a statin regimen reduces mean serum LDL-C levels to below the recommended target levels.
In addition to the significant reductions in LDL-C levels, significant reductions in levels of TC, TGs, apo B and TC/HDL-C ratios were observed in the study sample. Therefore, for this patient population, overall lipid profiles were improved and CAD risk was effectively reduced. An important observation in the present study was the consistent improvement in the lipid profiles of the subgroups of patients with diabetes and/or the metabolic syndrome. These are clinically important patient populations that are at increased risk for CAD, and require aggressive and effective treatment of hypercholesterolemia.
Within six weeks of treatment, the majority of patients in the study (81%) had serum LDL-C levels below the recommended target. Although patients who did not achieve target LDL-C levels experienced statistically significant reductions of this parameter, they had significantly higher baseline LDL-C levels and were more likely to have been treated with higher statin doses. These results suggest that these patients had more resilient dyslipidemia than patients who had below-target LDL-C levels after six weeks of treatment.
An important consideration of our study is the risk profiles of the participating patients. The vast majority of patients were at high (20% or greater) 10-year risk for CAD, and 43% and 49% had diabetes and the metabolic syndrome, respectively. In addition, all patients enrolled in the study had serum LDL-C levels higher than the recommended targets despite treatment with a statin. Approximately 39% of these patients did not achieve target LDL-C levels, despite treatment with a moderate to high statin dose. Among these patients, further increases in the dose of the statin may not have been feasible or tolerated.
The results of the present study have important implications for management of patients who are at increased CAD risk and have LDL-C levels persistently higher than the recommended target levels. Epidemiological studies have produced strong evidence showing that, for a significant proportion of patients, monotherapy with statins is not always effective in achieving reductions of LDL-C levels to recommended target levels. Furthermore, increasing the statin dose in these patients would be relatively ineffective in achieving target LDL-C levels. According to the ‘rule of six’, for every doubling of statin dose, the LDL-C level is reduced by an average of 6% (57–59). Therefore, for a large percentage of these patients, statin monotherapy, even at the highest doses, would not be effective in reaching recommended target LDL-C levels. This observed treatment gap is further accentuated by the reduced tolerability of higher statin doses. Other add-on treatments, such as resins, are available. The data in the literature show that resins produce maximum reductions in LDL-C levels of 10% to 20%. However, because of the formulation and the mechanism of action of the resins, they have been associated with an increased rate of gastrointestinal effects and interference with the absorption of other drugs (65–67).
Ezetimibe coadministered with a statin was well tolerated in the present study. The profile and incidence of adverse events observed were comparable with those reported in controlled clinical trials, with a low incidence of adverse events, which were predominantly mild in severity (48). In the present study, laboratory tests for the detection of adverse events were not required as part of the study protocol. Physicians could, however, perform any test that, according to their clinical judgment, was necessary for patient management. Although this approach may potentially lead to under-reporting of clinically important changes in laboratory values, it closer emulates the real-life setting in which physicians conduct laboratory tests only when clinically indicated. There were no laboratory abnormalities reported. Also of importance is the fact that, in the present study, there were no reports of rhabdomyopathy, gallbladder or liver-related events, including clinically significant elevated aspartate aminotransferase, alanine aminotransferase or creatine phosphokinase levels, which are often of concern in patients being treated for hyperlipidemia with statins alone or combination therapy.
The potential limitations of the present study are related to the single-cohort design and, therefore, lack of a comparator or control group. However, the intent of the study was to assess the effect of adding ezetimibe to an existing statin regimen in patients who had LDL-C levels above the recommended targets while on statin monotherapy. By comparing the change in lipid profiles from baseline, the study effectively addressed the question of whether the addition of ezetimibe among these patients produced incremental benefits with respect to LDL-C level reduction, lipid profile improvement and achievement of target LDL-C levels. Therefore, the results of the present study are applicable to patients who maintain LDL-C levels above target values while on statin monotherapy, and should not be interpreted as comparing ezetimibe and statin combination therapy with other alternatives.
The strengths of the present study include the emulation of the real-life setting achieved by design features that were outlined earlier. In addition, the study sample size was large enough to conduct analyses in clinically important patient subgroups, as well as to provide sufficient statistical power to produce precise estimates of effectiveness and safety that allow valid inference to the target populations.
CONCLUSION
This prospective, single-cohort, open-label study showed that therapy with ezetimibe coadministered with a statin is effective in reducing LDL-C levels, improving the overall lipid profile and achieving target LDL-C levels among patients who have remained above the target LDL-C level while on monotherapy with a statin. Coadministration of ezetimibe with an existing statin is also effective in improving the serum lipid profile in subgroups of patients with hyperlipidemia who also have diabetes and/or the metabolic syndrome. The safety analyses showed that ezetimibe taken in combination with any statin at a wide range of doses is well tolerated and safe. The results of the present study further support the use of ezetimibe in combination with a statin as a safe and effective treatment choice for the management of persistent hyperlipidemia.
ACKNOWLEDGEMENTS
British Columbia: Claire, Daljit T; Craigmyle, John W; Craven, Charles P; Dickey, Rod G; Ebrahim, Shehla; Eng, Aik-Png; Gill, Sukhdev S; Hutchinson, Angela; James, Howard D; Jones, Ralph; Karon, Stanley; Kooy, Jacobus; Lee, Mona; McFetridge, Gerald; Morgan, David C; Nielsen, Anthony; Rasool, Meenaz H; Sear, Andrew I; White, Diana; White, J Patrick; White, Philip A; Wong, Paul CH; Alberta: Ahmed, Ghalib; Campbell, Grant K; Gee, Edward; Hoy, Wayne KF; Lee, Marilyn; Lo, George YK; Loewen, Theodore E; Rosenstock, Carl B; Sacks, Herbert W; Starke, Victor A; Van Heerden, Alida; Yip, David KW; Saskatchewan: Enweani, Cyprian C; Lipsett, William GC; Lok, Winston A; Nayar, Arun; Manitoba: Bedder, Phyllis; Bedi, Bhupinder; Kreml, John; Lam, Charles; Lee, Sandra; Mahay, Raj; Marsh, David; Nyhof, Harold; Patel, Jayshree; Patel, Praful; Pawlak, Jerzy; Schellenberg, Chuck; Sochocka, Elizabeth; Szajkowski, Stan; Ontario: Abramson, Norman; Adno, B; Ah Now, Walter; Aleksiejuk, Janusz; Allison, R Chris; Armogan, Edward D; Awde, Murray; Bartlett, John Malcolm; Boekhoud, John WF; Bridgeo, David B; Bruckschwaiger, Dieter; Bukovy, Brent E; Buttoo, Ajit S; Chan, HM Norman; Chang, Walter WK; Che, Claudius; Chilvers, Martyn; Cho, Kevin Yeon; Csanadi, Michael A; Cutbush, Wesley S; Davies, Edward George; Dhillon, Ripple; Faiers, Alan A; Fan-Lun, Haw Kin Chen; Gamble, Eamon N; Gaur, Shiva K; Goswami, Dibyendu S; Greenspoon, Allen S; Grigore, Emil; Hartford, Brian J; Henein, Sam; Hinnawi, Hanna M; Hirsch, Andrei E; Hong, Tommy; Isenstein, Norman G; Jacques, Raymond; Jacyk, Peter; Kanani, Subodh D; Karalis, Peter; King, Bertram W; Kozak, Joseph H; Kraft, Jouni P; Kundi, K; Kundi, P; Lam, Andrew Wai Keung; Lavelle, Peter R; Leung, CC Michael; Leung, Cho Yau; Leung, Daniel P; Leung, Wilson Wai S; Li Wan Po, James Y L; Liang, William S; Lim, Ka-Chee; Lloyd, Ronald M; Luces, Kevin F; Luterman, Maynard; Maharaj, A; Martinho, Valdemar; Mawji, Altaf ST; Mehan, Upender K; Mithoowani, Mohamed; Mohamed, Mohamed D; Mok, C Albert; Monk, Charles M; Najgebauer, Edward J; Ng-A-Fook, Robert A; Ng Thow Hing, Roland E; Ng, Albert P; Ng, Ken HM; Nguyen, Phuongbich; O’Mahony, William F; Obaji, Hind I; Owsianik, Walter DJ; Pazuki, Keyvan; Petrlich, Steve T; Petrosoniak, Peter; Pinkney, Terence J; Quinn, Gerard; Quon, David WA; Ragaz, Stephan C; Rajani, Mohamed G; Rana, Ranjit S; Rosenberg, David S; Rossman, Lloyd H; Russell, Alan D; Sayad, George R; Simmons, John Morris; Sood, A; Sunderji, Salim; Tschirhart, Jeffrey Dennis; Tung, Frances YY; Tung, Tommy HT; Tytus, Richard H; Vanhoof, Ronald A; Verma, Vinod Kumar; Weinstock, Michael S; Welch, Robert J; William, Hany; Wong, Albert See Chee; Wu, Stephen Tat-Wah; Yeung, Jack SP; Zajner, Michael WJ; Quebec: Auger, Jacques; Bah, Abdoulaye; Barrière, Ginette; Barrs, Gary B; Berjat, Maria; Bérubé, Claude; Bouchard, Clermont; Breton, Jeannot; Champoux, Serge; Charbonneau, Jacques; Dallaire, Gaétan; Dubé, Gilles; Dumais, Michelle; Émond, Sylvie; Fortier, Gérald J; Fruchterman, Lucien; Gagnon, Robert; Goyer, Pierre; Habib, Nader H; Houle, Gaétan; Lalonde, Alain-Paul; Larouche, Pierre; Lauzière, Maurice; Lavigueur, Denis; Lavoie, Régis; Lévesque, Ghislain; Malenfant, Claude; Marsan, Gilbert; Martel, Jean-Marie; Meunier, Michel; Meunier, Pierre; Morin, Gilles PJ; Morissette, Pierre R; Naim, Maurice; Payer, Pierre; Pelland, Marcel; Popescu, Laila-C; Rioux, Denis; Roberge, Claude; Serfaty, Samuel; St-Onge, Michel; Tessier, Jean-Francois; Tremblay, Alain; Trudeau, Pierre; Turcotte, Chantal; Yelle, Jean-Pierre; New Brunswick: Anand, Sanjiv; Boucher, Daniel; Gauthier, Louis-Marie; Lamontagne, Rene; MacKinnon, Brian J; McLaughlin, A Wayne; Nadkarni, Ashok B; Richardson, John D; Srinivasan, Kalyani; Stevenson, Robert N; Nova Scotia: Booth, A William; Fay, Donald F; Ferguson, Linda; Ferguson, Murdo; MacDonald, John W; Nicholson, John D; Newfoundland: Callahan-Dyer, Deborah; Collingwood, John; Rolfe, Anthony; Smolarkieweiz, Marek; Woodland, Heather
REFERENCES
- 1.Genest J, Frohlich J, Fodor G, McPherson R Working Group on Hypercholesterolemia and Other Dyslipidemias. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: Summary of the 2003 update. CMAJ. 2003;169:921–4. (Erratum in 2003;169:1149) [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. (Erratum in 2006;354:778) [DOI] [PubMed] [Google Scholar]
- 3.Colhoun HM, Betteridge DJ, Durrington PN, et al. CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 4.Collins R, Armitage J, Parish S, Sleigh P, Peto R Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 5.LaRosa JC, Grundy SM, Waters DD, et al. Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 6.Jones PH. Lower Is Better: LDL-C Goal Achievement and Statin Efficacy in Coronary Prevention. < www.medscape.com/viewarticle/472518_print> (Version current at August 24, 2006)
- 7.Evans M, Roberts A, Rees A. Pharmacological management of hyperlipidemia. Br J Diabetes Vasc Dis. 2003;3:204–10. [Google Scholar]
- 8.Grundy SM, Cleeman JI, Merz CN, et al. Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 10.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 13.Bourgault C, Davignon J, Fodor G, et al. Statin therapy in Canadian patients with hypercholesterolemia: The Canadian Lipid Study –Observational (CALIPSO) Can J Cardiol. 2005;21:1187–93. [PubMed] [Google Scholar]
- 14.Foley KA, Simpson RJ, Jr, Crouse JR, III, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol. 2003;92:79–81. doi: 10.1016/s0002-9149(03)00474-0. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: Findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003;107:2185–9. doi: 10.1161/01.CIR.0000066320.27195.B4. [DOI] [PubMed] [Google Scholar]
- 16.Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L-TAP): A multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med. 2000;160:459–67. doi: 10.1001/archinte.160.4.459. [DOI] [PubMed] [Google Scholar]
- 17.Ballantyne CM, Houri J, Notarbartolo A, et al. Ezetimibe Study Group. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: A prospective, randomized, double-blind trial. Circulation. 2003;107:2409–15. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 18.Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol. 2004;93:1487–94. doi: 10.1016/j.amjcard.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 19.Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–34. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 20.Gagne C, Bays HE, Weiss SR, et al. Ezetimibe Study Group. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1084–91. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 21.Gaudiani LM, Lewin A, Meneghini L, et al. Efficacy and safety of ezetimibe co-administered with simvastatin in thiazolidinedione-treated type 2 diabetic patients. Diabetes Obes Metab. 2005;7:88–97. doi: 10.1111/j.1463-1326.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 22.Kerzner B, Corbelli J, Sharp S, et al. Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol. 2003;91:418–24. doi: 10.1016/s0002-9149(02)03236-8. [DOI] [PubMed] [Google Scholar]
- 23.Turley SD, Dietschy JM. The intestinal absorption of biliary and dietary cholesterol as a drug target for lowering the plasma cholesterol level. Prev Cardiol. 2003;6:29–33. 64. doi: 10.1111/j.1520-037x.2003.01691.x. [DOI] [PubMed] [Google Scholar]
- 24.Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: The Vytorin Versus Atorvastatin (VYVA) study. Am Heart J. 2005;149:464–73. doi: 10.1016/j.ahj.2004.11.023. (Erratum in 2005;149:882) [DOI] [PubMed] [Google Scholar]
- 25.Bays HE, Ose L, Fraser N, et al. Ezetimibe Study Group. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26:1758–73. doi: 10.1016/j.clinthera.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Stein E, Stender S, Mata P, et al. Achieving lipoprotein goals in patients at high risk with severe hypercholesterolemia: Efficacy and safety of ezetimibe co-administered with atorvastatin. Am Heart J. 2004;148:447–55. doi: 10.1016/j.ahj.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Kosoglou T, Meyer I, Veltri EP, et al. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor ezetimibe and simvastatin. Br J Clin Pharmacol. 2002;54:309–19. doi: 10.1046/j.1365-2125.2002.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurley DL, Isley WL. Getting there: Statin plus ezetimibe for low-density lipoprotein cholesterol goals. Mayo Clin Proc. 2005;80:585–6. doi: 10.4065/80.5.585. [DOI] [PubMed] [Google Scholar]
- 29.Worz CR, Bottorff M. Treating dyslipidemic patients with lipid-modifying and combination therapies. Pharmacotherapy. 2003;23:625–37. doi: 10.1592/phco.23.5.625.32204. [DOI] [PubMed] [Google Scholar]
- 30.Altmann SW, Davis HR, Jr, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 31.Bays HE, Moore PB, Drehobl MA, et al. Ezetimibe Study Group. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: Pooled analysis of two phase II studies. Clin Ther. 2001;23:1209–30. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 32.Dujovne CA, Ettinger MP, McNeer JF, et al. Ezetimibe Study Group. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1092–7. doi: 10.1016/s0002-9149(02)02798-4. (Erratum in 2003;91:1399) [DOI] [PubMed] [Google Scholar]
- 33.Ezzet F, Krishna G, Wexler DB, Statkevich P, Kosoglou T, Batra VK. A population pharmacokinetic model that describes multiple peaks due to enterohepatic recirculation of ezetimibe. Clin Ther. 2001;23:871–85. doi: 10.1016/s0149-2918(01)80075-8. [DOI] [PubMed] [Google Scholar]
- 34.Knopp RH, Gitter H, Truitt T, et al. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24:729–41. doi: 10.1016/s0195-668x(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 35.Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos. 2002;30:430–7. doi: 10.1124/dmd.30.4.430. [DOI] [PubMed] [Google Scholar]
- 36.Sudhop T, Lutjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–8. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 37.van Heek M, France CF, Compton DS, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–63. [PubMed] [Google Scholar]
- 38.van Heek M, Farley C, Compton DS, et al. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000;129:1748–54. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Heek M, Compton DS, Davis HR. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J Pharmacol. 2001;415:79–84. doi: 10.1016/s0014-2999(01)00825-1. [DOI] [PubMed] [Google Scholar]
- 40.Jeu L, Cheng JW. Pharmacology and therapeutics of ezetimibe (SCH 58235), a cholesterol-absorption inhibitor. Clin Ther. 2003;25:2352–87. doi: 10.1016/s0149-2918(03)80281-3. [DOI] [PubMed] [Google Scholar]
- 41.Sudhop T. Inhibition of cholesterol absorption by ezetimibe – A new approach in lowering cholesterol. Cardiovasc Rev Rep. 2004;25:225–8. [Google Scholar]
- 42.Davidson MH, Ballantyne CM, Kerzner B, et al. Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with statins: Randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemia. Int J Clin Pract. 2004;58:746–55. doi: 10.1111/j.1368-5031.2004.00289.x. [DOI] [PubMed] [Google Scholar]
- 43.Feldman T, Koren M, Insull W, Jr, et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol. 2004;93:1481–6. doi: 10.1016/j.amjcard.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg AC, Sapre A, Liu J, Capece R, Mitchel YB. Efficacy and safety of ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia: A randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2004;79:620–9. doi: 10.4065/79.5.620. [DOI] [PubMed] [Google Scholar]
- 45.Kosoglou T, Statkevich P, Yang B, et al. Pharmacodynamic interaction between ezetimibe and rosuvastatin. Curr Med Res Opin. 2004;20:1185–95. doi: 10.1185/030079904125004213. [DOI] [PubMed] [Google Scholar]
- 46.Lipka L, Sager P, Strony J, Yang B, Suresh R, Veltri E Ezetimibe Study Group. Efficacy and safety of coadministration of ezetimibe and statins in elderly patients with primary hypercholesterolaemia. Drugs Aging. 2004;21:1025–32. doi: 10.2165/00002512-200421150-00005. [DOI] [PubMed] [Google Scholar]
- 47.Mauro VF, Tuckerman CE. Ezetimibe for management of hypercholesterolemia. Ann Pharmacother. 2003;37:839–48. doi: 10.1345/aph.1C209. [DOI] [PubMed] [Google Scholar]
- 48.Nutescu EA, Shapiro NL. Ezetimibe: A selective cholesterol absorption inhibitor. Pharmacotherapy. 2003;23:1463–74. doi: 10.1592/phco.23.14.1463.31942. [DOI] [PubMed] [Google Scholar]
- 49.Protopsaltis ID, Konstantinopoulos PA, Kamaratos AV, Melidonis AI. Comparative study of prognostic value for coronary disease risk between the U.K. prospective diabetes study and Framingham models. Diabetes Care. 2004;27:277–8. doi: 10.2337/diacare.27.1.277. [DOI] [PubMed] [Google Scholar]
- 50.Cziraky MJ. Management of dyslipidemia in patients with metabolic syndrome. J Am Pharm Assoc (Washington DC) 2004;44:478–88. doi: 10.1331/1544345041475643. [DOI] [PubMed] [Google Scholar]
- 51.American Heart Association. The metabolic syndrome. 2006 < www.americanheart.org/presenter.jhtml?identifier=4756> (Version current at August 24, 2006)
- 52.Gotto J. Management of dyslipidemia. Am J Med. 2002;112(Suppl 8A):10S–8S. doi: 10.1016/s0002-9343(02)01085-9. [DOI] [PubMed] [Google Scholar]
- 53.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 54.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 55.Akanji AO. Direct method for the measurement of low-density lipoprotein cholesterol levels in patients with chronic renal disease: A comparative assessment. Nephron. 1998;79:154–61. doi: 10.1159/000045018. [DOI] [PubMed] [Google Scholar]
- 56.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Medical Dictionary for Regulatory Activities Terminology (7.1). 2004.
- 57.Maeder M, Blank R, Feucht J, Darioli R, Rickli H. Effect of ezetimibe co-administered with statin therapy in Swiss outpatients: Results of patients with coronary artery disease and diabetes mellitus. Kardiovaskuläre Medizin. 2005;8:399–409. [Google Scholar]
- 58.Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol. 1997;80:106–7. [PubMed] [Google Scholar]
- 59.Stone N. Combination therapy: Its rationale and the role of ezetimibe. Eur Heart J. 2002;4(Suppl J):J19–J22. [Google Scholar]
- 60.Simons L, Tonkon M, Masana L, et al. Effects of ezetimibe added to on-going statin therapy on the lipid profile of hypercholesterolemic patients with diabetes mellitus or metabolic syndrome. Curr Med Res Opin. 2004;20:1437–45. doi: 10.1185/030079904x2321. [DOI] [PubMed] [Google Scholar]
- 61.Pearson T, Denke M, McBride P, Battisti WP, Brady WE, Palmisano J. Effectiveness of the addition of ezetimibe to ongoing statin therapy in modifying lipid profiles and attaining low-density lipoprotein cholesterol goals in older and elderly patients: Subanalyses of data from a randomized, double-blind, placebo-controlled trial. Am J Geriatr Pharmacother. 2005;3:218–28. [PubMed] [Google Scholar]
- 62.Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: The ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005;80:587–95. doi: 10.4065/80.5.587. [DOI] [PubMed] [Google Scholar]
- 63.Cooke C, Nissen L, Sketris I, Tett SE. Quantifying the use of the statin antilipemic drugs: Comparisons and contrasts between Nova Scotia, Canada, and Queensland, Australia. Clin Ther. 2005;27:497–508. doi: 10.1016/j.clinthera.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Mamdani MM, Tu JV. Did the major clinical trials of statins affect prescribing behaviour? CMAJ. 2001;164:1695–6. [PMC free article] [PubMed] [Google Scholar]
- 65.Ast M, Frishman WH. Bile acid sequestrants. J Clin Pharmacol. 1990;30:99–106. doi: 10.1002/j.1552-4604.1990.tb03447.x. [DOI] [PubMed] [Google Scholar]
- 66.McKenney JM. Lipid management: Tools for getting to the goal. Am J Manag Care. 2001;7(9 Suppl):S299–S306. [PubMed] [Google Scholar]
- 67.Steiner G. The need for a different cholesterol lowering drug. Can J Clin Pharmacol. 2003;10(Suppl A):4A–6A. [PubMed] [Google Scholar]