Abstract
Ethanol experiences, during late gestation as well as during nursing, modify the behavioral dynamics of the dam/pup dyad, and leads to heightened ethanol intake in the offspring. This study focuses on: a) behavioral and metabolic changes in intoxicated dams with previous exposure to ethanol during pregnancy and b) infantile consumption of milk when the dam is either under the effects of ethanol or sober. Pregnant rats received water, 1.0 or 2.0 g/kg ethanol, and were administered with water or ethanol during the postpartum period. Intoxication during nursing disrupted the capability of the dam to retrieve the pups and to adopt a crouching posture. These disruptions were attenuated when dams had exposure to ethanol during pregnancy. Ethanol experiences during gestation did not affect pharmacokinetic processes during nursing, whereas progressive postpartum ethanol experience resulted in metabolic tolerance. Pups suckling from intoxicated dams, with previous ethanol experiences, ingested more milk than did infants suckling from ethanol-intoxicated dams without such experience. Ethanol gestational experience results in subsequent resistance to the drug’s disruptions in maternal care. Consequently, better maternal care by an intoxicated dam with ethanol experience during gestation facilitates access of pups to milk which could be contaminated with ethanol.
Keywords: Ethanol, Gestation, Lactation, Maternal care, Metabolism, Suckling
1. Introduction
Maternal care exerts profound effects on the structure of a wide array of behaviors in the developing organism (Kinsley, 1994; Stamps, 2003). In non-human mammals the dam-infant interaction can be viewed as a synchronized relationship that serves to maintain the well-being of the infant and aids in the establishment and development of its behavioral repertoire (Alberts and Gubernick, 1983; Fleming et al., 1999; Gonzalez et al., 2000). Focusing on the rat, dams build a nest, retrieve and gather pups in this habitat, lick the anogenital region of the infants to stimulate defecation and micturition and also adopt a crouching posture over the progeny to facilitate nipple attachment and suckling (Rosenblatt and Snowdon, 1996). Disruptions of this behavioral repertoire modify developmental processes that affect various behavioral, physiological and morphological traits of the progeny (Blass, 1990; Roth et al., 2004; Stamps, 2003).
When rat dams are exposed to narcoleptic doses of ethanol, prolactin and oxytocin levels -the principal hormones that regulate synthesis and ejection of milk during lactation- are dramatically disrupted. Milk production decreases as a consequence of repeated states of ethanol intoxication with a negative impact upon offspring’s growth rates (Heil and Subramanian, 2000; Subramanian, 1995, 1997, 1999; Vilaro et al., 1987). Studies performed in lactating women also indicate that even moderate ethanol doses have a negative impact upon milk production (Mennella, 1997) and are sufficient to disrupt the hormonal milieu in terms of the balance between prolactin and oxytocin levels (Mennella et al., 2005).
The literature regarding behavioral alterations in maternal care when dams are exposed to moderate concentrations of ethanol is rather scarce. Recent experiments performed in genetically heterogeneous rats have shown, however, that a subnarcoleptic ethanol dose (2.5 g/kg, yielding peak ethanol levels in milk equivalent to 175 mg/dL) administered to dams during the first postnatal week (postpartum day 3, PPD 3) is sufficient to disrupt fixed action patterns relevant to maternal care (Pepino et al., 2002; Pueta et al., 2005).
Rat pups (Molina et al., 2000, 2007; Pepino et al., 1998) and human infants (Faas et al., 2000; Mennella and Beauchamp, 1991; Menella, 1997) seem capable of processing low ethanol concentrations present in the amniotic fluid during late gestation or in milk during the nursing period. Rats also seem capable of acquiring sensory-related ethanol information derived from non-metabolic excretion of ethanol via respiration, salivation, urine or perspiration (Pepino et al., 1998), age-counterparts’ (Hunt et al., 2000) or themselves (Molina and Chotro, 1989a, b; Molina et al., 1995; also see Kalant, 1996). The infant rat’s perception of ethanol in milk or through non-metabolic routes of elimination appears to result in familiarization with ethanol’s odor or taste, a process which in turn facilitates the expression of ethanol preferences as assessed through olfactory or intake tests (Bachmanov et al., 2003; Spear and Molina 2005). The hedonic content of the memories derived from the perception of ethanol in the amniotic fluid, milk or in social counterparts can also be modulated by explicit association of the drug’s chemosensory cues with a variety of biologically relevant events (for reviews see Molina et al., 2007; Spear and Molina, 2005). In other words, the motivational value of memories derived from social and nutritional interactions involving ethanol perception in the developing rat can be determined by mere familiarization processes or the contingency between the drug’s sensory attributes with various unconditioned stimuli. This would include appetitive effects of nipple suckling (Hunt et al., 1993), positive reinforcing properties of ethanol’s postabsorptive effects (Abate et al., 2002; Nizhnikov et al., 2006; Petrov et al., 2003), and even stressful perception of disruptions in maternal care promoted by the state of intoxication (Molina et al., 2000; Pepino et al., 1999, 2002). Relative to this last issue, our previous work has shown that prior exposure to the drug during late gestation seems to ameliorate deleterious behavioral effects of ethanol in the nursing dam (Pueta et al., 2005).
To our knowledge, Pueta et al.’s (2005) experimental approach constitutes one of the few studies considering the interaction between pre- and postpartum experiences with moderate ethanol doses, subthreshold in terms of evident signs of teratological effects (Abate et al., 2008; Dominguez et al., 1998), that can determine specific patterns of ethanol responsiveness in the progeny. The topic under consideration appears particularly relevant when considering that in humans, maternal ingestion during pregnancy is likely to continue during the breastfeeding period (Jacobson, 1997). This leads to sequential experiences with the drug and interactions between them which can modulate subsequent detection, discrimination, or even acceptance patterns of ethanol (Spear and Molina, 2005). The impact of these interactions upon ethanol responsiveness was evident in Pueta et al.’s study (2005; also see Dominguez et al., 1999). Pups that were exposed to ethanol in the amniotic fluid during late gestation (gestational days 17–20, GDs 17–20) and subsequently in maternal milk (PPDs 3–13) exhibited heightened predisposition to accept a minimal concentration of ethanol (175 mg/dL or its equivalent: 0.22 % v/v) as assessed through an intake test at PPDs 15 and 16. As mentioned, ethanol treatment during pregnancy decreased ethanol’s disruptive effects upon maternal care, particularly when dams received the first ethanol dose during the postpartum period (PPD 3). The lack of ethanol-related alterations in maternal behavior of the dam could be a problem if it facilitates early postnatal exposure of the offspring to milk contaminated with ethanol during nursing.
The present study pursues several goals associated with potential interactions between ethanol experiences during late gestation and nursing. The first experiment was conceived to validate prior observations of reduced ethanol-derived disruptions in maternal care among dams previously exposed to the drug during late gestation. In the original study conducted by Pueta et al. (2005) dams were treated with ethanol (2.0 g/kg) from GD 17 to GD 20 and the first two weeks of nursing (2.5 g/kg). Experiment 1 of the present study included a similar sequence of ethanol administration procedures. We added to the design a lower ethanol dose (1.0 g/kg) administered during pregnancy which has been observed to recruit fetal sensory processing of the drug (Arias and Chotro, 2005; Dominguez et al., 1998). In humans, blood ethanol concentrations (40 –50 mg/dL), similar to that observed with the 1.0 g/kg ethanol dose in pregnant females, are observed after consumption of two to four standard drinks, being this is a rough correspondence, and being this subject to error induced (Abate et al., 2008). In this experiment, maternal latencies to retrieve pups and to adopt a crouching position in commencement (PPD 3) and termination (PPD 13) of postnatal treatments were examined. Pepino et al. (2002) reported that repeated exposures to ethanol during the first two weeks of the postpartum period lead to maternal metabolic tolerance. Nevertheless, it remains unclear whether ethanol experiences during late gestation affect ethanol pharmacokinetics during the postpartum period. In this study (Experiment 1), changes in maternal ethanol metabolism were tested as a function of pre- and postpartum ethanol treatments.
Pueta et al. (2005) reported that ethanol exposure during late gestation attenuates maternal behavioral disruptions generated by the drug soon after parturition (PPD 3). The goal in Experiment 2 was to determine whether exposure to ethanol during late gestation resulted in maternal metabolic tolerance that could help explain initial postpartum resistance to its deleterious effects upon maternal care. Taking into account that ethanol’s presence in milk promotes early ethanol-related memories (Pepino et al., 1998; Ponce et al., 2004) and that maternal behavior linked with the process of nursing is modulated by ethanol experiences during late gestation (Pueta et al., 2005), we evaluated (Experiment 3a) infantile feeding patterns during the first nursing bout (PPD 3) as a function of the state of acute maternal ethanol intoxication. Specifically, we examined whether infantile consumption of milk contaminated with ethanol, as evaluated through body weight gains, would vary as a function of differential maternal experiences with the drug during late pregnancy. In the follow-up experiment (3b) we also tested milk intake patterns of newborns from dams treated with 0.0, 1.0 or 2.0 g/kg ethanol during late gestation. In this Experiment 3b, dams were sober during the course of the nursing bout.
Beyond the specific goals of each experiment, ethanol treatments during gestation and nursing were selected on the basis of the following factors: (a) These treatments provide the opportunity for fetal and infantile detection and learning about ethanol-related sensory cues, phenomena that have also been observed in human fetuses and breastfeeding babies (Faas et al., 2000; Molina et al., 2007). (b) Sequential exposure to the drug seems to have an impact upon maternal behavior and ethanol metabolism. When considering human literature, there is practically no information related to these processes, although the likelihood of alcohol consumption during pregnancy and breastfeeding is still relatively high (see e.g. Giglia and Binns, 2007; Little et al., 2002; McLeod et al., 2002; Parackal et al., 2007; Pepino and Mennella, 2004). (c) These prenatal and nursing treatments are not sufficient to cause gross teratological effects in the offspring (e.g. facial dismorphologies, alterations in the size of different central nervous system structures, delayed growth rates) or evident neurobehavioral alterations that impede sensory, perceptual or learning capabilities (for reviews see Abate et al., 2008; Molina et al., 2007; and Spear and Molina, 2005).
2. General Methods
2.1. Subjects
All animals employed in this study were Wistar-derived rats born and reared at the vivarium of the Instituto Ferreyra (INIMEC-CONICET), Córdoba, Argentina. The animal colony was kept at 22–24 °C and under artificial lighting conditions (lights were on between 0 8:00–20:00 h). Maternal enriched lab chow (Cargill, Buenos Aires, Argentina) and water (delivered through automatic dispenser valves) were available ad libitum. Vaginal smears of adult female rats were microscopically analyzed on a daily basis. On the day of proestrus, females (pre-pregnancy body weight: 200–300 g) were housed during the dark cycle with males (three females per male). Vaginal smears were checked the following morning (10:00–12:00 h) and the day of sperm detection was considered as GD 0. Births were checked daily (10:00–12:00 h) and the day of parturition was considered as PPD 0. On PPD 1, each litter was randomly culled to eight pups (four males and four females, if possible). Pregnant females or litters were individually placed in standard maternity cages filled with wood shavings. At all times, animals used in this study were maintained and treated according to the guidelines for animal care established by the Guide for Care and Use of Laboratory Animals (National Institute of Health, Institute of Laboratory Animal Resources, 1996).
2.2. Maternal Treatments
2.2.1 Treatments during gestation
From GDs 17 to 20, pregnant females were weighed and intragastrically intubated with either 1.0 (group EtOH 1) or 2.0 g/kg ethanol (group EtOH 2). These doses were delivered on a daily basis and were achieved by administering 0.015 ml/g of an 8.4 % v/v or a 16.8% v/v ethanol solution, respectively. The vehicle used was room temperature tap water. Control females (group Water) were only administered with mentioned vehicle. Ethanol doses and the days of administration were selected on the basis of prior studies demonstrating fetal chemosensory processing of the drug under similar experimental circumstances, and general lack of deleterious effects of ethanol upon different infantile morphological and behavioral parameters (Domínguez et al., 1996, 1998; Molina et al., 1995; Pueta et al., 2005). Intragastric intubations were performed employing a polyethylene cannula (PE 50; Clay Adams, Parsippany, New Jersey, U.S.A.) attached to a disposable 5-ml syringe.
2.2.2. Postpartum treatments
On PPDs 3, 5, 7, 9, 11, and 13, half of the mothers in each randomly assigned gestational treatment were weighed and intragastrically administered with a 2.5 g/kg ethanol dose (Postpartum EtOH Group). The ethanol dose was achieved by administering 0.015 ml of a 21% v/v ethanol solution per gram of maternal body weight. The remaining dams were administered an equivalent volume of tap water (Postpartum Water Group).
2.3. Behavioral evaluation of nursing females
During postpartum period, 15-min after dams were administered either ethanol or water, the nest was disarranged and pups were scattered to different sections of the home cage. Dams were then placed back with their corresponding litter and were videotaped for 2 hours. To this aim, the standard wire aluminum cover of each maternity cage was replaced with an acrylic transparent cover to allow better video recording of the dam/pup interaction. Trained observers, blind to maternal treatment, measured maternal behavior (inter-rater reliability according to Pearson’s correlation coefficient ranged between 0.85 and 0.92; p’s < 0.01). Latencies to retrieve all pups and to adopt the upright crouching posture (kyphotic position, a posture that facilitates nipple attachment by the young) were scored on PPDs 3 and 13. These postpartum days correspond to the beginning and the end of each maternal drug treatment (EtOH or Water).
2.4. Determination of maternal blood ethanol concentrations
The analysis of ethanol pharmacokinetic profiles as a function of maternal experience with the drug was executed on PPD 17 (Experiment 1) or on PPD 3 (Experiment 2). This analysis was accomplished through sequential blood ethanol sampling from the jugular vein. To achieve the latter, dams were subjected to a surgical procedure that allowed the implant of a catheter in the jugular vein 24 hours before blood sampling. Hence, dams were anesthetized with an intraperitoneal administration of ketamine (75 mg/kg) and xylazine (13.5 mg/kg) on PPD 16 (Experiment 1 ) or PPD 2 (Experiment 2). Anesthetized dams were placed over a heating pad to maintain presurgical body temperatures. Access to the jugular vein was accomplished through a 1-cm incision of the ventral right portion of the neck, which was previously shaved. A clear vinyl catheter (I.D. 0.58 mm, O.D. 0.96 mm, Dural Plastics & Engineering Auburn, Australia) filled with heparinized saline was inserted into the vein until reaching the atrium. The free end of the catheter was subcutaneously conducted until appearing through a small incision in the nape of the neck. Besides, an insect pin was employed to occlude the free end of the catheter. Following completion of the surgical procedure, and in order to guarantee the animal’s well being, a subcutaneous administration of buprenorphine (0.03 mg/kg) was performed.
On PPD 17 (Experiment 1) or PPD 3 (Experiment 2) dams representative of each particular treatment received an i.g. administration of a 2.5 g/kg ethanol dose. Dams used in Experiment 1 were those previously evaluated in terms of maternal care on PPDs 3, 5, 7, 9, 11 and 13. We chose to evaluate BECs at PPD 17 for two reasons. The most important reason was to explicitly avoid possible disruptive effects of the intrajugular surgical procedure and of handling procedures, related to sequential blood sampling upon maternal behavioral assessments. The second reason was that pups representative of the different treatments were used on PPDs 15 and 16 in an independent study related to infantile affinity for ethanol ingestion (data not shown). A separate group of females was employed for BEC determinations on PPD 3 (Experiment 2).
Females were deprived of food 4 hours prior to ethanol administration to reduce sources of variance attributable to the presence of food in their stomachs. Pups were also removed from the maternity cages four hours prior to blood sampling procedures. Six 200 μl blood samples were obtained from each female. Sampling procedures took place 5, 15, 30, 75, 120 and 240 minutes after ethanol was administered.
Maternal blood samples were subjected to head-space gas chromatography analysis (Pepino et al., 1998; Pepino et al., 2002). Each blood sample was fractioned in order to obtain two 100 μl samples. These samples were then placed in microvials containing 20 μl of a butanol solution (51 mg/dL) that served as an internal standard. The manipulation of the samples was performed in containers filled with crushed ice. Microvials were hermetically sealed and incubated in a water bath at 60°C for 30 minutes. Gas-tight syringes (Hamilton, 10 μl) were used to collect the volatile component of the samples and to inject them into the gas chromatograph (Hewlett-Packard, Model 5890). Column (Carbowax 20M; 10 m x 0.53 mm x 1.33 μm film thickness), injector and detector temperatures were as follows: 60, 150, and 250°C, respectively. Nitrogen served as the carrier gas (flow rate: 15 ml/min).
2.5. Statistical analysis of maternal behavior and blood ethanol concentrations
Maternal body weight changes during pregnancy were subjected to a mixed analysis of variance (ANOVA) defined by gestational treatment and gestational day. Maternal postpartum weights, litter size and infantile body weights were analyzed via ANOVAs that took into account gestational and postpartum drug treatments, and whenever necessary, postpartum days.
Mixed analyses of variance were conducted to determine how each behavior (retrieval and crouching latencies) varied as a function of drug treatment during gestation and lactation. Latencies to perform behavior on PPDs 3 and 13 served as repeated measures. Newman-Keuls’ post-hoc tests were used to further analyze significant main effects or interactions. The critical value for significance was p < 0.05.
Blood ethanol concentrations (BECs) were averaged across both samples obtained during each post-administration interval. All values were expressed as milligrams of ethanol per deciliter of blood (mg/dL). BECs were analyzed using a mixed ANOVA with group as the between-subject factor and ethanol post-administration time (5, 15, 30, 75, 120 and 240 minutes) as repeated measures.
3. Experiment 1
The goal was to analyze behavioral disruptions in maternal care as a function of repeated exposure to ethanol during late gestation and the first two postpartum weeks. In this experiment dams received either water or 1.0 or 2.0 g/kg ethanol from GD 17 to GD 20. Animals representative of the three gestational treatments were either treated with vehicle or 2.5 g/kg ethanol during nursing. This ethanol dose has been observed to cause severe disruptions in maternal care. These disruptions are observable after the first postpartum administration of ethanol, but seem to be markedly attenuated as a function of prior exposure to the drug during pregnancy, or repeated ethanol administrations during lactation (Pepino et al., 2002; Pueta et al., 2005). In relation to motor activities not necessarily involved in maternal care, Pepino et al. (2002) found no ethanol-induced disruptions in self-grooming and locomotion patterns. According to Pepino et al. (2002) metabolic tolerance to ethanol develops as a function of repeated drug administration during lactation. In the present experiment we also examined the possibility of maternal pharmacokinetic tolerance being dependent upon the interaction of gestational and postpartum ethanol exposure.
3.1. Methods
3.1.1. Subjects and procedures
Forty four adult female rats were used in behavioral evaluations. These animals were randomly distributed into six groups defined by gestational (0.0, 1.0 or 2.0 g/kg ethanol; groups Water, EtOH 1 or EtOH 2, respectively) and postpartum treatments (0.0 or 2.5 g/kg ethanol; groups Water or EtOH, respectively). The number of subjects in each group was as follows: Water-Water, n = 8; Water-EtOH, n = 6; EtOH 1-Water, n = 7; EtOH 1-EtOH, n = 8; EtOH 2-Water, n = 9 and EtOH 2-EtOH, n = 6.
Behavioral evaluations (PPD 3 and PPD 13) and blood ethanol determinations (PPD 17) were conducted as described in General Methods. On PPD 16 a total of 28 dams were randomly chosen for subsequent determination of blood ethanol concentrations. Each of the six groups defined by the factorial design was constituted by 4–5 dams.
3.2. Results
3.2.1. Maternal and infantile body weights, litter size and pup viability
In these and all subsequent experiments of this study, maternal body weight gain, litter size, pup viability and the average birth weight of pups were not significantly affected by ethanol treatment during pregnancy. In the present experiment all females delivered viable offspring (litter size collapsed across treatments was equivalent to 13.3 ± 0.1 pups; mean ± SEM). Along treatments, pups’ mean body weight on PPD1 was 6.24 ± 0.7 g.
Maternal weights during pregnancy were found to vary significantly as a function of gestational day [F(3,123) = 320.4, p < 0.01]. As it was expected, body weights increased as a function of the passage of time. Body weights, collapsed across drug treatments during late pregnancy, were as follows: GD 17, 336.7 ± 4.7 g; GD 18, 348.2 ± 4.7 g; GD 19, 359.3 ± 5.1 g and GD 20, 370.0 ± 5.3 g. When focusing on maternal body weight changes across postpartum days the corresponding ANOVA showed a significant main effect of day [F(5,190) = 7.6, p < 0.01]. As expected, dams progressively gained weight throughout the postpartum period. The following means corresponding to the first, third and sixth day of postnatal treatment illustrate the gradual increase in maternal body weight during the first two weeks of lactation: PPD 3, 286.0 ± .4.5 g; PPD 7, 291.7 ± 4.7 g and PPD13, 296.3 ± 4.5 g (values being collapsed across prenatal and postpartum drug treatments).
3.2.2. Maternal Behavior
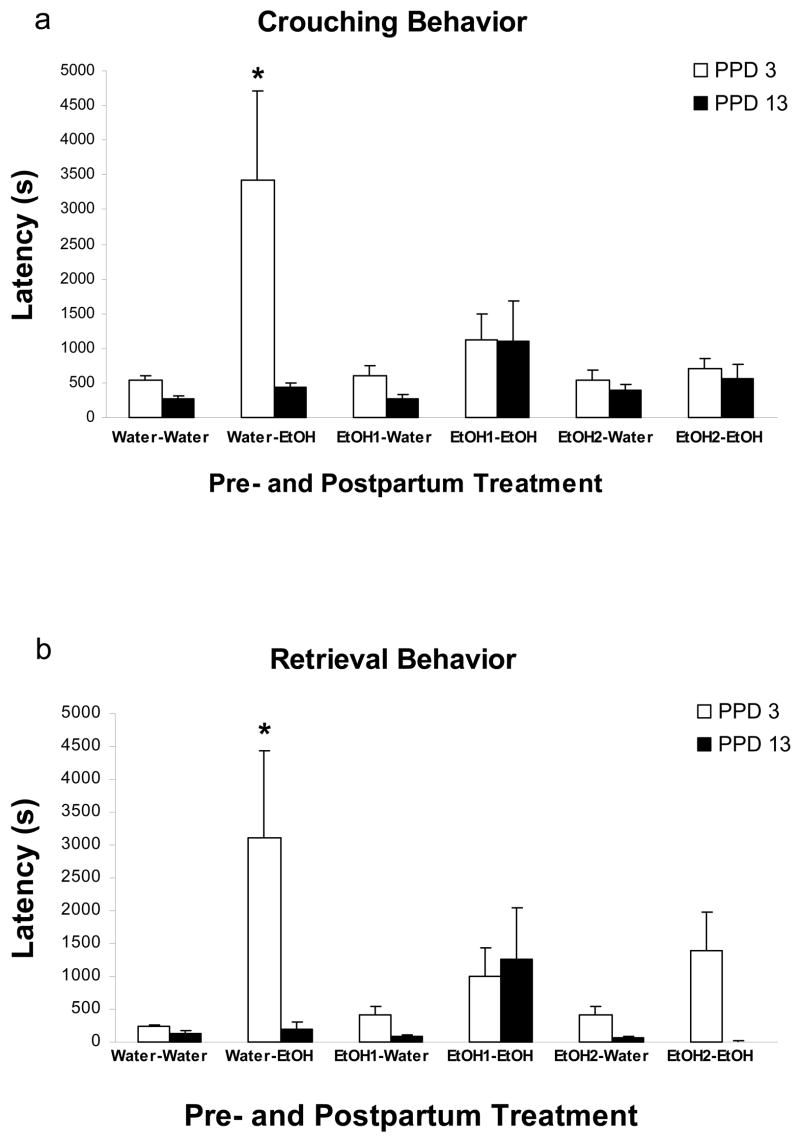
Latency to exhibit maternal crouching behavior: maternal latency to adopt the crouching posture was analyzed via a 3-way mixed ANOVA (Gestational Treatment x Postpartum Treatment x Day of Assessment). The analysis showed that crouching performance was significantly affected by postpartum treatment and by day of evaluation, [F(1,38) =11.19 and F(1,38) = 7.54, respectively; both p’s < 0.01]. The interaction between manipulation during late gestation and day of evaluation also achieved significance [F(2,38) = 4.11, p < 0.05]. Furthermore, this analysis indicated a three-way significant interaction comprising gestational, postpartum treatments, and day of evaluation [F(2,38) = 4.06, p < 0.05]. Post–hoc analysis indicated that on PPD 3 dams intoxicated with ethanol, but without prior experience with the drug (Water-EtOH group), greatly exhibited higher latencies to adopt the crouching posture than any remaining groups. Dams exposed to 1.0 or 2.0 g/kg ethanol during gestation, which were also intoxicated with the drug on PPD 3 (EtOH 1-EtOH and EtOH 2-EtOH), exhibited very low latencies to adopt the crouching posture, as well as did dams that had never experienced the drug (Water-Water), or had only been exposed to ethanol during pregnancy (EtOH 1-Water and EtOH 2-Water). Additionally, those mothers gestationally exposed to ethanol were not significantly different from dams that had never been administered ethanol. On PPD 13 crouching latencies were low and similar across all groups. This recovery phenomenon, which apparently reflects tolerance in ethanol treated dams, was even evident in ethanol inexperienced females prior to lactation (Water-EtOH group). In this group, crouching latencies on PPD 13 were significantly lower than those recorded on PPD 3 (Figure 1a).
Figure 1.
Maternal latencies (s) to exhibit a) crouching behavior and b) retrieval of all pups at PPDs 3 and 13. The data are depicted as a function of gestational and postpartum treatments. The symbol * denotes significant differences between dams that received water versus those that received ethanol (1.0 or 2.0 g/kg) prenatally. Vertical lines illustrate SEM.
Maternal latency to retrieve all pups: the corresponding three-way mixed ANOVA (Gestational Treatment x Postpartum Treatment x Days of Assessment) showed a significant main effect of postpartum treatment as well as day of evaluation, [F(1,38) = 11.61, F(1,38) = 8.06, respectively; both p’s < 0.01]. This analysis also revealed a significant three-way interaction [F(2,38) = 4.09, p < 0.025]. Post-hoc tests indicated a pattern of results similar to that observed when evaluating crouching latencies: dams exposed to ethanol during both late gestation and nursing did not differ in retrieval latencies from dams given ethanol only during gestation, or from those not being administered. On PPD 13 latency scores were very similar across all pre- and postnatal groups (Figure 1b).
3.2.3. Maternal blood ethanol concentrations on PPD 17
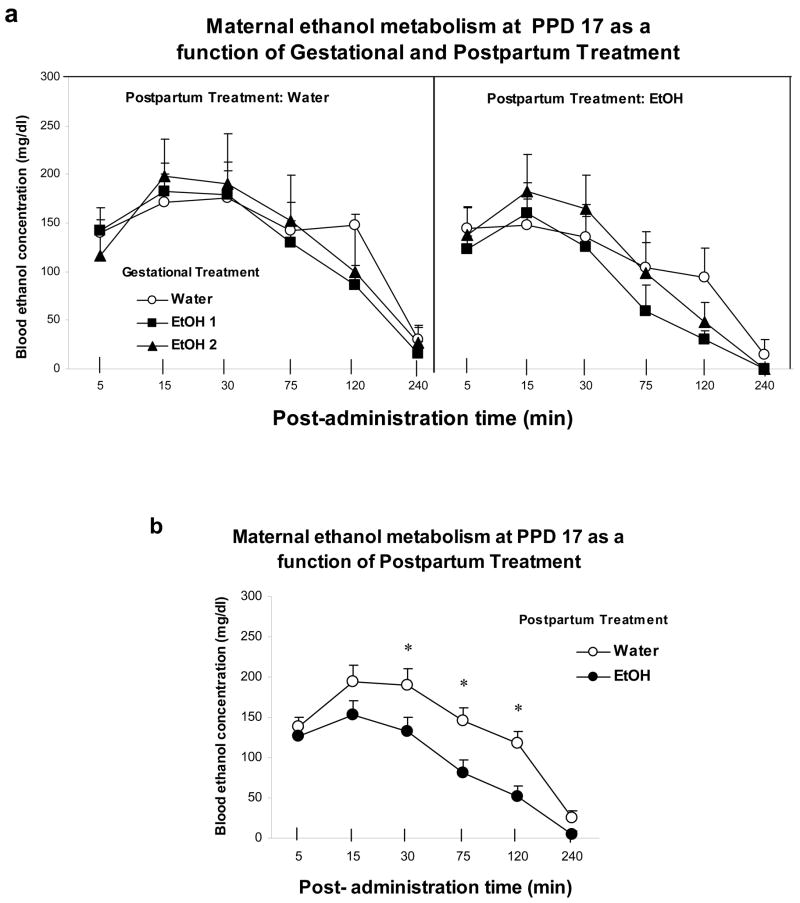
Females’ BECs were analyzed through a three-way mixed ANOVA defined by Gestational Treatment (Water, EtOH1 or EtOH2), Postpartum Treatment (Water or EtOH) and Post-administration sampling time (5, 15, 30, 75, 120 and 240 min). The analysis revealed significant main effects of post-administration time [F(5,110) = 85.20, p < 0.01] and Postpartum Treatment [F(1,22) = 5.0, p < 0.05]. Gestational treatment was not found to exert a significant main effect, or to significantly interact with postpartum treatment or post-administration time (Figure 2a). The ANOVA also revealed a significant interaction comprising Postpartum treatment and Post-administration time, [F(5,110) = 3.14, p < 0.025]. Post hoc tests indicated that, independently of gestational history, dams exposed to ethanol during nursing significantly showed lower BECs than dams given water during the same period. These differences were particularly observable at post-administration times 30, 75, and 120 min (Figure 2b).
Figure 2.
a) Maternal blood ethanol concentrations (mg/dL) as a function of gestational treatments, postpartum treatment and ethanol post-administration time. b) Maternal blood ethanol concentrations (mg/dL) as a function of postpartum treatment and ethanol post-administration time. The symbol * denotes significant differences between groups at post-administration time 30, 75 and 120 min. Vertical lines illustrate SEM.
3.2.4. Summary of results
Exposure to low or moderate ethanol doses (1.0 or 2.0 g/kg) during late gestation did not affect maternal body weight gain during said period, or the number of viable offspring delivered, or neonatal body weight. This regimen of moderate daily ethanol administrations from GD 17 to GD 20 was also found not to affect maternal behavior such as crouching and pup retrieval during the first two weeks of the lactation period. Along all gestational conditions, non-intoxicated females on postpartum days 3 and 13 exhibited similar latencies to perform behavior, but maternal care was markedly disrupted when females were first exposed to 2.5 g/kg ethanol during the initial phase of the postpartum period (PPD 3). Nevertheless, this disruptive effect was only observed in dams that had no prior history of ethanol exposure. Dams pretreated with 1.0 or 2.0 g/kg ethanol during pregnancy appeared to exhibit behavioral tolerance when first given the drug during lactation (PPD 3). This apparent tolerance effect also seemed to develop as a function of repeated postpartum ethanol administrations. On PPD 13 (6th administration of 2.5 g/kg ethanol) intoxicated females, independently of gestational treatment, exhibited latencies to retrieve the offspring and to adopt a crouching position that were similar to those of water-treated dams. In other words, and in accordance with previous results reported by Pueta et al. (2005), on PPD13 dams no longer exhibited abnormal retrieval or crouching latencies when exposed to ethanol. Subsequent assessments of ethanol pharmacokinetic profiles as a function of pre- and postnatal treatments indicated that repeated postpartum ethanol experiences resulted in lower blood ethanol concentrations, a result in accordance to prior studies of metabolic tolerance in nursing females (Pepino et al., 2000). This effect was not influenced by prior gestational treatment with lower ethanol doses than the one used during nursing.
4. Experiment 2
The results of Experiment 1 indicated that gestational treatment with low or moderate ethanol doses significantly attenuated disruptions in maternal care when dams were exposed to ethanol for the first time during the postpartum period. The results suggested development of tolerance, expressed through metabolic and behavioral indexes, as a function of progressive postpartum treatment with ethanol. There were no indications that gestational history with ethanol interacted with the development of postpartum metabolic tolerance. Is it possible that this null effect is simply due to the fact that postpartum tolerance overshadows pre-existing pharmacokinetic changes derived from gestational exposure to relative low ethanol doses, at least when considering metabolic changes? To answer this question, maternal pharmacokinetic profile as a function of gestational treatments were examined at the start (PPD 3) rather than at the end (PPD 17) of the postpartum regimen of ethanol administration procedures. The intention was to assess if behavioral tolerance derived from ethanol exposure during late gestation is also associated with changes in maternal metabolism; as it seems to be the case with repeated postpartum experiences with the drug.
4.1. Methods
4.1.1. Subjects
Twenty one Wistar-derived female rats were tested.
4.1.2. Maternal treatments and blood ethanol determinations on PPD 3
Dams representative of the three prenatal treatments (0.0, 1.0 or 2.0 g/kg ethanol, n = 7 per treatment) were administered 2.5 g/kg ethanol on PPD 3. Blood sampling procedures took place at 5, 15, 30, 75, 120 and 240 min after ethanol was administered.
4.2. Results
4.2.1. Blood ethanol concentrations
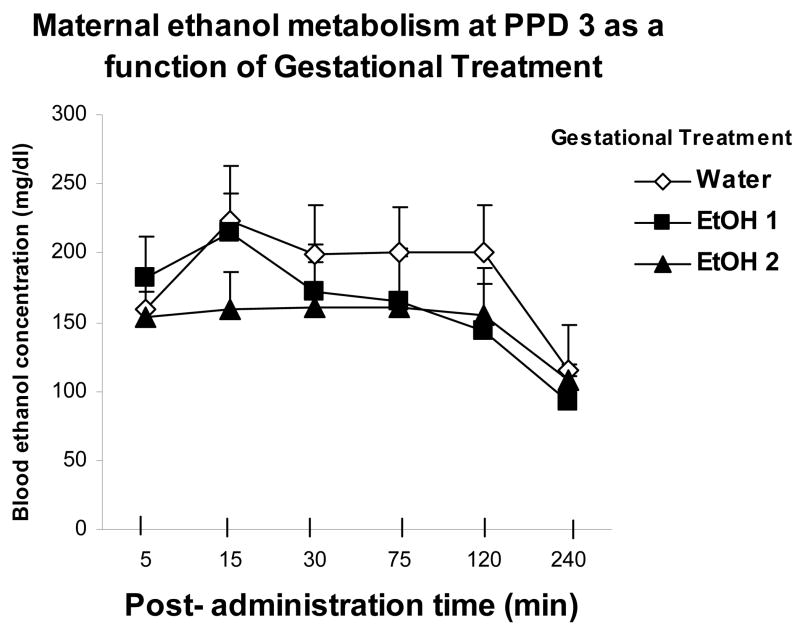
Blood ethanol levels were analyzed through a two-way mixed ANOVA defined by treatment during late gestation (Water, EtOH 1 or EtOH 2) and post-administration sampling times (5, 15, 30, 75, 120 and 240 min). The analysis revealed a main effect of post-administration time [F(5,90) = 9.78, p < 0.01]. Post-hoc tests indicated that BECs were relatively stable between 5 and 120 min. At 240 min BECs were significantly lower than those recorded during the preceding post-administration intervals. There were, however, no significant effects of gestational treatment, or of the interaction between this factor and sampling time (Figure 3).
Figure 3.
Maternal blood ethanol concentrations (mg/dL) as a function of gestational treatments and ethanol post-administration time. Vertical lines illustrate SEM.
4.2.2. Summary of results
According to the present results, ethanol doses equivalent to 1.0 or 2.0 g/kg administered during late gestation do not affect blood ethanol levels in dams treated with 2.5 g/kg on PPD 3. This result appears to be in agreement with the null effects of gestational treatment upon ethanol metabolism on PPD 17 as observed in Experiment 1, following multiple drug administrations during lactation.
In light of the results of Experiments 1 and 2, coupled with those reported by Pueta et al. (2005), it appears that maternal exposure to ethanol during the last days of pregnancy is sufficient to promote maternal tolerance operationalized through behavioral resistance to deleterious effects of ethanol. This result is even observed when employing gestational ethanol treatments yielding minimal blood ethanol concentrations (with 1.0 g/kg ethanol, peak BECs at GD 20: 50 mg/dL; Dominguez et al., 1996). In turn, tolerance to the drug’s behavioral effects derived from gestational exposure to the drug does not appear to be associated with changes in ethanol metabolism.
The pharmacokinetic profiles obtained in the present experiment and those corresponding to Experiment 1 seem to differ. On PPD17, even in dams that had never experienced ethanol before (Water-Water group, see Fig. 2a), i.g. administration of 2.5 g/kg ethanol resulted in peak BECs of approximately 200 mg/dL. This peak content was detected at 15 and 30 min post-administration time, followed by a rapid decrease in BECs. Four hours following ethanol administration, mean ethanol levels were equivalent to 30 mg/dL. On PPD 3 dams prenatally exposed to Water (Figure 3) exhibited peak BECs similar to those detected on PPD 17, but in this case BECs were stable until 120 min post-administration time and were still above 100 mg/dL four hours following ethanol intubation. As it can be observed, this comparison between BECs on PPDs 3 and 17 are made between groups that had no prior experience with the drug. These comparisons in whole suggest that the capability to metabolize ethanol increases during the course of the postpartum period.
5. Experiment 3a
The primary goal of this experiment was to assess whether infantile consumption of milk contaminated with ethanol would vary as a function of prenatal exposure to ethanol. As previously demonstrated, maternal blood ethanol concentrations are very similar to those encountered in milk (Pepino et al., 1998). According to the results in Experiment 1, ethanol treatment during late gestation mitigates deleterious behavioral effects of the drug on dams intoxicated in an early stage of the postpartum period (PPD 3) and without affecting their BECs. Taking these considerations into account we assessed milk ingestion by offspring during the nursing bout, when given the first opportunity to interact with ethanol-intoxicated mothers that differed in terms of their ethanol history during gestation. A second goal of this experiment was to assess if ethanol was traceable in infant blood after the first nursing bout in the context of maternal ethanol intoxication.
5.1. Methods
5.1.1. Subjects and maternal treatments
One hundred and eighty-six pups representative of 22 litters were used. From GD 17 to GD 20, dams received a daily intragastric administration of water (n = 8 dams), 1.0 (n = 6 dams) or 2.0 g/kg ethanol (n = 8 dams). On PPD 1 litters were culled to 9 pups whenever possible (4–5 males and 4–5 females).
5.1.2. Assessment of milk ingestion during a single nursing bout
On PPD 3, four hours prior to maternal ethanol administration, all pups from a given litter were deprived of maternal care. This was accomplished by placing the pups in a similar cage containing clean wood shavings and kept under temperature-controlled conditions through the use of adjustable heating pads (ambient temperature: 32 ºC). During this time period, dams were also deprived of solid food. Dams subsequently received a 2.5 g/kg ethanol dose that was intragastrically administered. Before returning the pups to the mother, they were gently stimulated in the anogenital area through the use of a cotton swab in order to induce urination and defecation. Pups were then weighed (± 0.01 g; pre-suckling body weight).Thirty minutes following maternal ethanol administration, pups were returned to their corresponding maternity cage and allowed to freely interact with their biological mother during 45 min. Prior research indicates that at this age a suckling bout lasts approximately 30–45 min (Thiels et al., 1990). Pups were then removed from the cage and post-suckling body weight was recorded. Percentage body weight gain (% BWG), being an indirect index of ingestion, served as the dependent variable under consideration. This value was calculated as follows: 100 x [(post-suckling body weight – pre-suckling body weight)/pre-suckling body weight)]. Immediately after recording post-suckling body weights, pups were sacrificed in order to obtain blood samples (100 μl per pup). Blood samples were analyzed using headspace gas chromatography. To avoid litter overrepresentation, average intake scores and BECs, corresponding to pups representative of the same litter, served as units of analysis. Scores of a given litter were collapsed across gender since this factor was not observed to exert significant effects.
5.2. Results
5.2.1. Assessment of milk ingestion during a single nursing bout
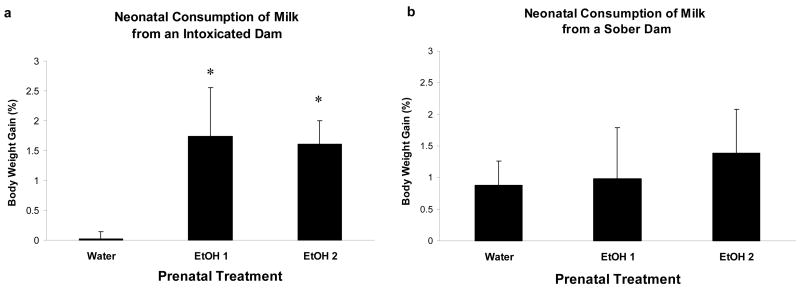
Pre-suckling body weights on PPD 3 were very similar along prenatal treatments (data not shown). Analysis of post-suckling percent body weight gain with a one-way ANOVA showed a significant difference between groups of pups [F(2,19) = 4.57, p < 0.025].Post-hoc tests revealed that pups from dams gestationally exposed to 1.0 or 2.0 g/kg ethanol significantly exhibited higher body weight gain than those whose mothers were treated with water during late gestation. Pups whose dams were given only the vehicle (Water) from GD 17 to GD 20 and so had no previous exposure to ethanol showed negligible intake from the intoxicated dam, whereas pups given either dose of ethanol during late gestation consumed large amounts from the intoxicated dam (Figure 4a).
Figure 4.
a) Pup body weight gains when interacting with an ethanol-intoxicated (2.5 g/kg) dam at PPD 3. The data is shown as a function of prenatal ethanol treatments. The symbol * denotes significant differences relative to pups reared by dams that were gestationally administered water. b) Pup body weight gains when interacting with a sober (Water intubated) dam at PPD 3. Vertical lines illustrate SEM.
On PPD 3 we detected only minimal amounts of ethanol in 8 pups obtained from two litters. One of these litters was prenatally exposed to 1.0 g/kg ethanol and the other to 2.0 g/kg ethanol (BECs range in these pups: 11–27 mg/dL). BECs were undetectable in all the remaining subjects. For this reason, BECs were not subjected to inferential analysis.
6. Experiment 3b
As a function of the results of Experiment 3a, we decided to conduct a follow-up experiment testing whether prenatal ethanol would affect milk ingestion during a single nursing bout of newborn animals with a dam given only vehicle. As will be further discussed (see Discussion), several factors can help explain the reason why newborns prenatally exposed to ethanol significantly ingested higher amounts of milk when their dams were experiencing an acute state of ethanol intoxication (Experiment 3a). In the present Experiment we simply evaluated pup milk intake patterns during a nursing bout when dams were in a state of sobriety.
6.1. Methods
6.1.1. Subjects and maternal treatment
One hundred and sixty-four pups representative of 20 litters were used. The number of dams assigned to each gestational treatment was as follows: Water (0.0 g/kg ethanol), n = 7; EtOH 1 (1.0 g/kg ethanol), n = 6; and EtOH 2 (2.0 g/kg ethanol), n = 7.
6.1.2. Assessment of milk ingestion during a single nursing bout
All experimental details are the same as described in Experiment 3a, except for dams were administered water only. Scores of a given litter were collapsed across gender since this factor was not observed to exert significant effects.
6.2. Results
6.2.1. Assessment of milk ingestion during a single nursing bout
A one-way ANOVA indicated that pre-suckling body weight did not differ across pups representative of the different prenatal conditions (data not shown). As can be observed in Figure 4b, all groups gained similar body weight after interacting for 45 minutes with sober dams. In other words, intake during nursing from a sober dam did not differ as a function of prenatal treatment.
7. Discussion
This study indicates several effects of ethanol exposure during late gestation and postpartum period upon the interaction of the dam with their pups. These effects were a consequence of relatively moderate ethanol doses during pregnancy (1.0 or 2.0 g/kg which result in approximately 50 or 150 mg/dL peak BECs in maternal and fetal blood, respectively; Dominguez et al., 1996) and a subnarcoleptic ethanol dose (2.5 g/kg) during the first two weeks of the postpartum period.
Within the present experimental framework, the results of Experiment 1 can be summarized as follows: a) ethanol administration to the dam on PPD 3 impairs maternal behavior by increasing latency to retrieve the pups and to adopt a kyphotic or crouching posture inherently related with lactation; b) ethanol’s deleterious effects upon maternal care subside when dams are repeatedly exposed to ethanol during pregnancy; c) metabolic profiles of ethanol at the end of the nursing treatment (PPD 17) were not affected by ethanol experience during pregnancy; and d) resistance to ethanol’s disruptive effects indicating development of behavioral tolerance.
These results validate and extend prior behavioral and pharmacokinetic observations relevant to the impact of ethanol exposure during gestation and nursing (Pepino et al., 2002; Pueta et al., 2005). In accordance with these studies, subnarcoleptic ethanol doses are capable of disrupting a variety of maternal behavior during the early course of the postpartum period. Not only pup retrieval and crouching are strongly affected by ethanol doses yielding BECs that range between 150 and 230 mg/dL, but also other maternal-related behavior appear to be similarly sensitive to the drug’s detrimental effects (e.g. decreased time spent on the nest and on licking the anogenital region of the offspring to induce micturition and defecation), while locomotion and self-grooming elicited by handling and administration procedures seem to be relatively resistant to ethanol’s effects (Pepino et al., 2002).
In the case that dams are given ethanol during nursing for the first time, it is likely that ethanol-induced disruption of the maternal repertoire might be associated with neuroendocrinological processes involving prolactin and oxytocin. These hormones are critical for the initiation, maintenance, and re-emergence of maternal behavior in female rats, cows, sheep, and humans (Bridges and Ronsheim, 1990; Bridges et al., 1997; Pedersen and Prange, 1987; Rosenblatt, 1987; Svennersten-Sjaunja and Olsson, 2005). Serum levels of both hormones have been observed to be modified in lactating rats by either acute or chronic ethanol administration (Heil et al., 1998; Subramanian et al., 1990; Subramanian, 1997). It has been recently reported that in nulliparous and lactating women, a moderate ethanol dose (0.4 g/kg resulting in peak BECs ranging between 30 and 75 mg/dL) is sufficient to disrupt the hormonal balance comprising prolactin and oxytocin levels. Ethanol consumption in these women increased plasma prolactin levels while decreased oxytocin levels, effects that appear to be particularly pronounced during lactation. The magnitude of this hormonal disbalance seems to positively correlate with subjective feelings of postabsorptive effects of ethanol (i.e. feelings of intoxication measured through levels of euphoria, sedation, dysphoria and somatic effects) (Mennella et al., 2005; Mennella and Pepino, 2006).
Ethanol’s detrimental effects upon maternal behavior were no longer observed on PPD 13 (i.e. after females had received six i.g. administrations of 2.5 g/kg between PPD3 and 13). This apparent development of ethanol tolerance has been previously reported in a longitudinal evaluation of similar maternal behavior (Pepino et al., 2002). Gradual development of ethanol tolerance in lactating female rats has also been reported when focusing on a physiological index of expression such as thermoregulation. Ethanol-induced hypothermic effects progressively decrease in magnitude during lactation as a function of repeated administrations of the drug (Pepino et al., 2000).
Disruptive effects of ethanol upon maternal behavior and thermoregulatory processes as a function of repeated administrations during postpartum period appear to coincide with changes in metabolic capabilities (Pepino et al., 2002; Pueta et al., 2005). As reported in Experiment 1, BECs in dams that had been repeatedly exposed to ethanol during nursing were significantly lower than those recorded in control dams not given this previous ethanol exposure. Nevertheless, it is important to mention that in Experiment 1 there were two procedural differences related to the assessment of behavioral performance and pharmacokinetic profiles. Behavioral tolerance was detected on PPD 13 while metabolic tolerance was observed on PPD 17. As stated above (see General Methods), this discrepancy can be traced to the requirement to test females for maternal care, while avoiding possible disruptive effects of intrajugular cannulation procedures and sequential blood extractions. Another difference was that metabolic profiles were assessed after the dam was deprived of food during 4 hours while behavioral testing was conducted without any food deprivation. Ethanol absorption is more rapid following food deprivation and may potentially lead to more severe behavioral disruptions than those observed in non-deprived dams. As a function of these observations, the possibility that behavioral tolerance may be mediated by adaptive metabolic processes should only be considered as a hypothesis.
According to the pharmacokinetic results of Experiments 1 and 2, moderate ethanol exposure during late gestation did not modify BECs in dams subjected to the drug soon after giving birth (PPD 3) and was not observed to interact with subsequent effects of repeated ethanol administrations during lactation. The doses given during gestation, as well as the number of administrations (1.0 and 2.0 g/kg, four i.g. administrations from GD 17 to GD 20), were lower than those used after delivery (2.5 g/kg, six administrations between PPDs 3 and 13). These parametric differences may help explain why the metabolic outcomes, associated with ethanol exposure during pregnancy and lactation, were not equivalent. Multiple physiological differences between these stages can determine or modulate metabolic adaptations to the effects of ethanol.
In this respect, it is notable that ethanol metabolism apparently changes even within the course of the nursing stage. This statement is supported by comparing ethanol pharmacokinetic profiles obtained in Experiments 1 and 2. Although maternal BECs at 240 min. were practically undetectable in Experiment 1 (PPD 17), in Experiment 2 (PPD 3) they were still relatively high. Changes in ethanol elimination rates during the course of the postpartum period may partially explain varying effects of ethanol upon maternal care, especially when taking into account that behavior was evaluated between 15 and 120 min. post-administration time. Yet, we cannot dismiss alternative possibilities based on various interactions during the course of the postpartum period between ethanol and hormones (prolactin and oxytocin) or neurotransmitters (e.g. GABA; see below) known to modulate maternal behavior.
Both human and animal studies indicate that during lactation there are profound changes in drug pharmacokinetics. In rats, lactation is associated with intestinal growth (Hammond, 1997). The liver expands during the postpartum period and its metabolic capabilities also increase (da-Silva et al., 1996; David et al., 2000; DeSantiago et al., 1998). This lactation-induced liver hypertrophy results in faster elimination rates of ethanol (Abel et al., 1979; Gordon et al., 1985). According to these observations and as a function of our results, it can be hypothesized that maternal capability to eliminate ethanol increases during the course of the postpartum period. Beyond this observation and according to our knowledge, the literature does not provide adequate empirical data to explicitly compare changes in ethanol metabolism across the postpartum period nor between this stage and the course of gestation.
Despite the fact that ethanol pre-exposure during gestation did not affect ethanol metabolism during lactation, it was clear that this treatment was sufficient to increase behavioral resistance to the drug’s deleterious effects upon maternal care and infantile responsiveness in terms of milk intake. Administration of even a low ethanol dose (1.0 g/kg) during gestation was sufficient to counteract ethanol-induced (2.5 g/kg) deficits in pup retrieval and crouching for nursing. As a whole these results imply neuroadaptative mechanisms during pregnancy that lead to maternal ethanol tolerance during early postpartum life.
In Experiment 3a the progeny of dams pretreated with ethanol exhibited higher milk ingestion levels when suckling under the effects of maternal ethanol intoxication than progeny whose mother only received water during gestation. This difference across gestational treatments was not observed when pups had the opportunity to interact with a sober dam. Under this circumstance, pups delivered by dams treated with water or ethanol during gestation exhibited similar body weight gain during a nursing bout. Treatments during late gestation did not seem to alter overall nursing capabilities of dams or capabilities of offspring in nipple suckling and milk consumption.
So it seems clear that prenatal treatment effects upon pup’s milk ingestion levels during a nursing bout emerged only when the dam was intoxicated with ethanol. Pups prenatally exposed to ethanol (1.0 or 2.0 g/kg) ingested amounts of milk during nursing in this circumstance equivalent to those suckling from a sober dam. This was not the case in infants prenatally exposed to water. These pups had negligible ingestion from an intoxicated dam. Modulation or determination of behavioral maternal ethanol tolerance through prior gestational experiences with the drug may help explain these results. If ethanol pre-exposure attenuates subsequent detrimental effects of the drug upon infantile care and nutrition, it is logical to expect an impact of such attenuation upon infantile suckling patterns. In other words, the level of maternal disruption caused by ethanol as a function of prior experiences with the drug can be linked with the likelihood of effective infantile nipple attachment and suckling behavior that will ultimately determine milk consumption.
Yet, alternative hypotheses should not be dismissed. A first possibility is based on ethanol’s effects upon secretion and distribution of neurohormones (prolactin and oxytocin) that help control maternal behavior and are critical for the production, mammary accumulation, and ejection of milk. Since ethanol significantly affects release, serum levels, and balance among these hormones, (Fuchs, 1969; Heil et al., 1998; Mennella et al., 2005; Mennella and Pepino, 2006) it is possible that even normally effective nipple attachment may not be sufficient to obtain milk (also see Mennella, 2001a, 2001b). Development of maternal ethanol tolerance at a neuroendocrinological level, affecting bioavailability of milk, has yet to be systematically investigated. Nevertheless, there exists biophysical evidence supporting development of ethanol tolerance in terms of oxytocin release in isolated neurohypophysial terminals in rats (Knott et al., 2002). In light of these considerations we cannot dismiss the possibility of maternal exposure to ethanol during pregnancy resulting in the development of tolerance related to oxytocin release. In other words, the development of hormonal tolerance to ethanol’s effects may facilitate subsequent milk ejection processes when pups attach to and suckle from the nipples.
A second alternative hypothesis of the reason why infantile body weights in Experiment 3a varied as a function of maternal pretreatment with ethanol focuses more on behavior of the pups than the repertoire of the dams. Near term fetuses become familiar with ethanol’s chemosensory cues present in the amniotic fluid or fetal-maternal blood. This familiarization process has been detected in various mammalian species, including humans, for a variety of chemosensory stimuli. Fetal experience with olfactory and gustatory cues is sufficient to shape flavor preferences during postnatal life (for reviews see Bachmanov et al., 2003; Schaal et al, 2001, 2004; Smotherman and Robinson, 1985). In the case of fetal ethanol experiences, it has also been observed that near term rat fetuses are capable of associating ethanol’s sensory cues with the drug’s reinforcing effects (Abate et al., 2008; Chotro et al., 2007; Molina et al., 2007; Spear and Molina, 2005). As a function of these perceptual and learning experiences, pups prenatally exposed to moderate ethanol doses show heightened affinity for ethanol ingestion and predisposition to seek and accept the drug’s sensory cues. When dams are acutely intoxicated with ethanol, the drug accumulates in milk reaching levels comparable or even higher than those encountered in maternal blood (Pepino et al., 1998). Even when milk ethanol levels are low e.g. 175 mg/dL in rat milk (Pepino et al. 1998, 1999) and 50 mg/dL in human milk (Mennella 1997; Mennella and Beauchamp, 1991, 1993)] infants are capable of detecting the drug’s presence in this nutrient.
Other sources of ethanol presence in the suckling context are derived from maternal non-metabolic excretion of ethanol via respiration, salivation, perspiration, and micturition (Kalant, 1996; Pepino et al., 1998). Taking these observations into account, it is possible to speculate that infants prenatally exposed to ethanol are more predisposed to seek and ingest maternal milk contaminated with ethanol than pups with no prior experience with the drug. This information is in accordance with the evidence showing that experiences with the flavor of a food in mother’s milk increases preference for that food in both human infants and rat pups (Galef and Henderson, 1972; Galef and Sherry, 1973; Mennella and Beauchamp, 1997). An alternative, almost mirror image of this alternative, is that pups not exposed prenatally to ethanol and so not familiar with ethanol’s chemosensory properties may be less likely to accept maternal milk when this nutrient is contaminated with novel sensory cues; in this particular case, ethanol’s flavor.
Chronic ethanol treatment is also known to modulate the expression of the major subunits of GABA-A receptors in the brain (Cagetti et al., 2003; Marutha Ravindran et al., 2007). Liang et al. (2007) have demonstrated mechanisms of GABA-A receptor plasticity after ethanol intoxication that suggests functional tolerance involving this receptor. GABA neurons in the medial preoptic area and bed nucleus of the stria terminalis (MPOA and BNST; respectively) mediate aspects of maternal behavior through projections to diverse neural sites (Numan et al., 2005; Stack et al., 2002). Injection of GABA receptor agonists in the MPOA and BNST results in dose-dependent deficits in all components of maternal behavior (Arrati et al., 2006).Therefore, ethanol could exert its acute detrimental effects on maternal behavior via its action on GABA-A receptors (Arrati et al., 2006). In turn, the consequence of prior ethanol exposure during pregnancy upon maternal behavior could be mediated by a desensitization process of these GABA-A receptors. Moreover, GABA innervation provides the major inhibitory synaptic input to oxytocin neurons via GABA-A receptors (Brunton and Russell, 2008; Widmer et al., 2003). Therefore, it is plausible that ethanol exposure during late pregnancy exerts similar desensitization effects of GABA-A receptors in oxytocin neurons, as those observed in neurons corresponding to the MPOA and BNST.
It is clearly observed that various mechanisms (behavioral or neuroendocrinological tolerance in the dam or fetal perceptual and learning capabilities) may help explain heightened consumption of milk contaminated with ethanol among infants given late gestational exposure to the drug. Nevertheless, the empirical evidence of this study implies a higher probability of ingestion from an intoxicated dam, following ethanol exposure during gestation. However, this ingestion does not seem to result in pharmacologically relevant levels of ethanol in the pup. Indeed, we were able to detect the presence of ethanol in blood after interacting with an intoxicated mother in very few infants.
Still, rat pups and human infants are sensitive to the presence of minimal amounts of ethanol in milk (Molina et al., 2007). Like fetal ethanol experiences, the presence of the drug in the nursing context provides the opportunity for familiarization with ethanol’s sensory cues. It is also likely to occur the associative learning comprising these cues and the reinforcing effects of milk and suckling (Hunt et al., 1993), or the emotional consequences derived from the perception of maternal ethanol intoxication (Mennella and Garcia, 2000; Molina et al., 2000). Several studies have indicated that these non-associative and associative learning processes increase subsequent affinity for ethanol ingestion - a phenomenon which is in turn potentiated by the interaction between prenatal and nursing ethanol experiences (Molina et al., 2007; Pueta et al., 2005; Spear and Molina, 2005). This information should be taken into account when clinical or epidemiological studies examine the consequences of early ethanol experiences upon later affinity for the drug; since the likelihood of ethanol consumption during pregnancy and breastfeeding is still relatively high in different countries of the world (Giglia and Binns, 2007; Little et al., 2002; McLeod et al., 2002; Parackal et al., 2007; Pepino and Mennella, 2004).
Acknowledgments
This research, a collaborative project between the Center for Developmental Psychobiology of Binghamton University and Instituto Ferreyra, was supported by grants from NIAAA (AA11960, AA015992 and AA013098) and NIMH (MH035219) (to NES), a grant from Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) (to JCM), and a fellowship from CONICET (to MP). MP is a student of the PhD program of Doctorado en Ciencias Biologicas (FCEF y N-UNC). The authors wish to express their gratitude to Teri Tanenhaus and Erika Heindl for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: Evidence against “safe” amounts of prenatal exposure. Exp Biol Med. 2008;233:139–54. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate P, Varlinskaya EI, Cheslock SJ, Spear NE, Molina JC. Neonatal activation of alcohol-relate prenatal memories: Impact upon the first suckling response. Alcohol Clin Exp Res. 2002;26:989–98. doi: 10.1097/01.ALC.0000034668.93601.8F. [DOI] [PubMed] [Google Scholar]
- Abel EL, Greizerstein HB, Siemens AJ. Influence of lactation on rate of disappearance of ethanol in the rat. Neurobehav Toxicol. 1979;1:185–86. [PubMed] [Google Scholar]
- Alberts JR, Gubernick DJ. Reciprocity and resource exchange. A symbiotic model of parent-offspring relations. In: Rosenblum LA, Moltz H, editors. Symbiosis in Parent-Offspring Interactions. New York: Plenum; 1983. pp. 7–44. [Google Scholar]
- Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005;29:337–46. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS. GABA receptor agonist in the medial preoptic area and maternal behavior in lactanting rats. Physiol Behav. 2006;87:51–65. doi: 10.1016/j.physbeh.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences and consumption. Alcohol Clin Exp Res. 2003;27:220–31. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass EM. Suckling: Determinants, Changes, Mechanisms, and Lasting Impressions. Dev Psychology. 1990;26:520–33. [Google Scholar]
- Bridges RS, Ronsheim PM. Prolactin (PRL) regulation of maternal behavior in rats: brocriptine treatment delays and PRL promotes the rapid onset of behavior. Endocrinology. 1990;126:837–48. doi: 10.1210/endo-126-2-837. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RPC, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity and behavioral potencies in rat prolactin and rat placental lactogen I. Endocrinology. 1997;138:756–63. doi: 10.1210/endo.138.2.4921. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatments changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosc Biobehav Reviews. 2007;31:181–91. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- da-Silva VA, MaLean AE, Beales D. Ethanol elimination by rats as a function of reproductive state, gender and nutricional status. Braz J Med Biol Res. 1996;26:1097–103. [PubMed] [Google Scholar]
- David AL, Kotecha M, Girling JC. Factors influencing postnatal liver function test. Br J Obstet Gynaecol. 2000;107:1421–26. doi: 10.1111/j.1471-0528.2000.tb11659.x. [DOI] [PubMed] [Google Scholar]
- DeSantiago S, Torres N, Tovar AR. Leucine catabolism in mammary tissue, liver and skeletal muscle of dam rat during lactation and weaning. Arch Med Res. 1998;29:25–32. [PubMed] [Google Scholar]
- Domínguez HD, López MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol’s chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiol Learn Mem. 1996;65:103–12. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- Domínguez HD, López MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–17. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Interactions between perinatal and neonatal associative learning defined by contigous olfactory and tactile stimulation. Neurobiol Learn Mem. 1999;71:272–88. doi: 10.1006/nlme.1998.3882. [DOI] [PubMed] [Google Scholar]
- Faas AE, Spontón ED, Moya PR, Molina JC. Differential responsiveness to alcohol odor in human neonates: effects of maternal consumption during gestation. Alcohol. 2000;22:7–17. doi: 10.1016/s0741-8329(00)00103-8. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O’Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosc Biobehav Reviews. 1999;23:673–85. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Fuchs A. Ethanol and the inhibition of oxytocin release in lactating rats. Acta Endocrinologica. 1969;62:546–59. doi: 10.1530/acta.0.0620546. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Henderson PW. Mother’s milk: a determinant of the feeding preferences of weaning rat pups. J Comp Physiol Psychol. 1972;2:213–19. doi: 10.1037/h0032186. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Sherry DF. Mother’s milk: a medium for transmission of cues reflecting the flavor of mother’s diet. 1973;3:374–78. doi: 10.1037/h0034665. [DOI] [PubMed] [Google Scholar]
- Giglia RC, Binns CW. Patterns of alcohol intake of pregnant and lactating women in Perth, Australia. Drug Alcohol Rev. 2007;26:493–500. doi: 10.1080/09595230701499100. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality on female rats. Dev Psychobiol. 2001;38:11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gordon BH, Barahona E, Miyakawa H, Finkelman F, Liebre CS. Exaggerated acetaldehyde response after ethanol administration during pregnancy and lactation in rats. Alcohol Clin Exp Res. 1985;9:17–22. doi: 10.1111/j.1530-0277.1985.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Hammond KA. Adaptation of the maternal intestine during lactation. J Mammary Gland Biol Neoplasia. 1997;2:243–52. doi: 10.1023/a:1026332304435. [DOI] [PubMed] [Google Scholar]
- Heil SH, Marappa G, Subramanian MG. Alcohol and the hormonal control of lactation. Alcohol Health Res World. 1998;22:178–84. [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Subramanian MG. Chronic alcohol exposure and lactation: extended observations. Alcohol. 2000;21:127–32. doi: 10.1016/s0741-8329(00)00081-1. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Kraebel KS, Rabine H, Spear LP, Spear NE. Enhanced ethanol intake in preweanling rats following exposure to ethanol in a nursing context. Dev Psychobiol. 1993;26:133–53. doi: 10.1002/dev.420260302. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Lant GM, Carroll CA. Enhanced intake of ethanol in preweanling rats following interactions with intoxicated siblings. Dev Psychobiol. 2000;37:90–9. [PubMed] [Google Scholar]
- Jacobson SW. Assessing the impact of maternal drinking during and after pregnancy. Alcohol Health Res World. 1997;21:199–203. [PMC free article] [PubMed] [Google Scholar]
- Kalant A. Pharmacokinetics of ethanol: absorption, distribution and elimination. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. New York: Oxford University Press; 1996. pp. 15–58. [Google Scholar]
- Kinsley CH. Developmental Psychobiological Influences on Rodent Parental Behavior. Neurosc Biobehav Reviews. 1994;18:269–80. doi: 10.1016/0149-7634(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Knott TK, Dopico AM, Dayanithi G, Lemos J, Treistman SN. Integrated channel plasticity contributes to alcohol tolerance in neurohypohysial terminals. Mol Pharmacol. 2002;62:135–42. doi: 10.1124/mol.62.1.135. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Zinder B, Olsen RW, Spigelman I. Mechanism of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–77. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RE, Northstone K, Golding J ALSPAC Study Team. Alcohol, breastfeeding, and development at 18 months. Pediatrics. 2002;109:E72–77. doi: 10.1542/peds.109.5.e72. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of the δ-subunit of GABAA receptors in the rat brain. Brain Res. 2007;1174:47–52. doi: 10.1016/j.brainres.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod D, Pullon S, Cookson T, Cornford E. Factors influencing alcohol consumption during pregnancy and after giving birth. N Z Med J. 2002;115:U29. [PubMed] [Google Scholar]
- Mennella JA. Infant’s suckling responses to the flavor of alcohol in mother’s milk. Alcohol Clin Exp Res. 1997;21:581–5. [PubMed] [Google Scholar]
- Mennella JA. Alcohol’s effect on lactation. Alcohol Res Health. 2001a;21:230–40. [PMC free article] [PubMed] [Google Scholar]
- Mennella JA. Regulation of milk intake after exposure to alcohol in mother’s milk. Alcohol Clin Exp Res. 2001b;25:590–93. [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Beauchamp GK. The transfer of alcohol to human milk. Effects on flavor and the infant’s behavior. N Engl J Med. 1991;325:981–5. doi: 10.1056/NEJM199110033251401. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Beauchamp GK. Effects of beer on breast-fed infants. JAMA. 1993;269:1635–36. doi: 10.1001/jama.1993.03500130051027. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Beauchamp GK. Mothers’ milk enhances the acceptance of cereal during weaning. Pediatr Res. 1997;41:188–92. doi: 10.1203/00006450-199702000-00006. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Garcia PL. Children’s hedonic response to the smell of alcohol: Effects of parental drinking habits. Alcohol Clin Exp Res. 2000;24:1167–71. [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Teff KL. Acute alcohol consumption disrupts the hormonal milieu of lactating women. J Clin Endocrinol Metab. 2005;90:1979–85. doi: 10.1210/jc.2004-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY. Short-term effects of alcohol consumption on the hormonal milieu and mood states in nulliparous women. Alcohol. 2006;38:29–36. doi: 10.1016/j.alcohol.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Chotro MG. Acute alcohol intoxication paired with appetitive reinforcement: effects upon ethanol intake in infant rats. Behav Neural Biol. 1989a;51:326–45. doi: 10.1016/s0163-1047(89)90974-6. [DOI] [PubMed] [Google Scholar]
- Molina JC, Chotro MG. Acute alcohol intoxication paired with aversive reinforcement: ethanol odor as a conditioned reinforcer in rat pups. Behav Neural Biol. 1989b;52:1–19. doi: 10.1016/s0163-1047(89)90122-2. [DOI] [PubMed] [Google Scholar]
- Molina JC, Chotro MG, Domínguez HD. Fetal alcohol learning derived from ethanol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1995. pp. 295–315. [Google Scholar]
- Molina JC, Pepino MY, Johnson J, Spear NE. The infant rat learns about alcohol through interaction with an intoxicated mother. Alcohol Clin Exp Res. 2000;24:428–37. [PubMed] [Google Scholar]
- Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. Alcohol and development: Beyond Fetal Alcohol Syndrome. Dev Psychobiol. 2007;49:227–42. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Health. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. Institute of Laboratory Animal Resources, Commission on Life Sciences. National Research Council. [Google Scholar]
- Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol increases postnatal ethanol reinforcement. Alcohol Clin Exp Res. 2006;30:34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neumer CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Parackal S, Ferguson E, Harraway J. Alcohol and tobacco consumption among 6–24 months postpartum New Zealand women. Matern Child Nutr. 2007;3:40–51. doi: 10.1111/j.1740-8709.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr . Evidence that central oxytocin plays a role in the activation of maternal behavior. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal Development: A Psychobiological Perspective. Orlando, Florida: Academic Press; 1987. pp. 299–320. [Google Scholar]
- Pepino MY, Kraebel KS, López MF, Spear NE, Molina JC. Behavioral detection of low concentrations of ethanol in the preweanling rat. Alcohol. 1998;15:337–53. doi: 10.1016/s0741-8329(97)00154-7. [DOI] [PubMed] [Google Scholar]
- Pepino MY, López MF, Spear NE, Molina JC. Infant rats respond differently to alcohol after nursing from an alcohol-intoxicated dam. Alcohol. 1999;18:189–201. doi: 10.1016/s0741-8329(99)00003-8. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Land CI, Campbell J, Spear NE, Molina JC. Infantile experience with an alcohol-intoxicated mother: responsiveness to isolation and to specific social interactions (abstract 486) Alcohol Clin Exp Res. 2000;5:87A. [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Disruption of maternal behavior by alcohol intoxication in the lactating rat: a behavioral and metabolic analysis. Alcohol Clin Exp Res. 2002;26:1205–14. doi: 10.1097/01.ALC.0000025884.74272.BC. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Mennella JA. Advice given to women in Argentina about breastfeeding and the use of alcohol. Rev Panam Salud Publica. 2004;16:408–14. doi: 10.1590/s1020-49892004001200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27:1583–591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcohol Clin Exp Res. 2004;28:1039–50. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Pueta M, Abate P, Spear NE, Molina JC. Interaction between pre- and early postnatal alcohol-related memories: Impact upon alcohol acceptance patterns. Int J Comp Psychol. 2005;18:207–24. [Google Scholar]
- Rosenblatt JS, Snowdon CT. Advances in the study of behavior. Vol. 25. New York: Academic Press; 1996. Parental care: Evolution, mechanisms and adaptative significance. [Google Scholar]
- Rosenblatt JS. Biological and behavioral factors underlying in the onset and maintenance of maternal behavior in the rat. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal Development: A Psychobiological Perspective. Orlando, Florida: Academic Press; 1987. pp. 323–41. [Google Scholar]
- Roth TL, Wilson DA, Sullivan RM. Neurobehavioral development of infant learning and memory: Implications for infant attachment. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ, Brockman HJ, Naguib M, editors. Advances in the study of behavior. San Diego: Academic Press; 2004. pp. 103–33. [Google Scholar]
- Schaal B, Coureaud G, Marlier L, Soussignan R. Fetal olfactory cognition preadapts neonatal behavior in mammals. In: Marchlewska-Koj A, Lepri JL, Muller-Schwarze, editors. Chemical Signals in Vertebrates 9. New York: Kluwer Acxademic/Plenum Publishers; 2001. pp. 197–204. [Google Scholar]
- Schaal B, Hummel T, Soussignan R. Olfaction in the fetal and premature infant: functional status and clinical implications. Clin Perinatol. 2004;31:261–285. doi: 10.1016/j.clp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The rat fetus in its environment: behavioral adjustements to novel, familiar, aversive and conditioned stimuli presented in utero. Behav Neurosci. 1985;99:521–30. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: A theoretical review. Alcohol Clin Exp Res. 2005;29:909–29. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial proptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131:17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- Stamps J. Behavioural processes affecting development: Tinbergen’s fourth question comes of age. Animal Behaviour. 2003;66:1–13. [Google Scholar]
- Subramanian MG. Effects of chronic alcohol administration on lactational performance in the rat. Alcohol. 1995;12:137–43. doi: 10.1016/0741-8329(94)00073-5. [DOI] [PubMed] [Google Scholar]
- Subramanian MG. Evaluation of lactational parameters after alcohol administration for four days during early or midlactation in the rat. Alcohol Clin Exp Res. 1997;21:799–803. [PubMed] [Google Scholar]
- Subramanian MG. Alcohol inhibits suckling-induced oxytocin release in the lactating rat. Alcohol. 1999;19:51–5. doi: 10.1016/s0741-8329(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Subramanian MG, Chen XG, Bergeski BA. Pattern and duration of the inhibitory effect of alcohol administered acutely on suckling-induced prolactin in lactanting rats. Alcohol Clin Exp Res. 1990;14:771–5. doi: 10.1111/j.1530-0277.1990.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Svennersten-Sjaunja K, Olsson K. Endocrinology of milk production. Domest Anim Endocrinol. 2005;29:241–58. doi: 10.1016/j.domaniend.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thiels E, Alberts JR, Cramer CP. Weaning in Rats: II. Pup behavior patterns. Dev Psychobiol. 1990;23:495–510. doi: 10.1002/dev.420230605. [DOI] [PubMed] [Google Scholar]
- Vilaro S, Viñas O, Remesar X, Herrera E. Effects of chronic ethanol consumption on lactational performance in rat: Mammary gland and milk composition and pups’ growth and metabolism. Pharmacol Biochem Behav. 1987;27:333–9. doi: 10.1016/0091-3057(87)90577-6. [DOI] [PubMed] [Google Scholar]
- Widmer H, Ludwing M, Bancel F, Leng G, Dayanithi G. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. J Physiol. 2003;548:233–44. doi: 10.1113/jphysiol.2002.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]