Abstract
Purpose
The authors recently reported that a severe inflammatory response resulting in substantial loss of acinar cells was induced by a single injection of interleukin-1α into the lacrimal gland and that this effect was reversible. The purpose of the present study was to determine the mechanisms involved in lacrimal gland injury and repair.
Methods
Inflammation was induced by direct injection of recombinant human interleukin-1α (IL-1α, 1 μg in 2 μL) into the exorbital lacrimal glands of anesthetized female BALB/c mice. Animals were killed 1, 2, 3, 4, 5, 6, or 7 days after injection. Exorbital lacrimal glands were then removed and processed for measurement of protein secretion, histology, immunohistochemistry, and Western blotting.
Results
The results show that lacrimal gland acinar cells are lost through programmed cell death (apoptosis) and autophagy. They also show that the number of nestin (a stem cell marker)–positive cells increased 2 to 3 days after injury and that some of these cells were also positive for Ki67 (a cell proliferation marker) and α-smooth muscle actin (a marker of myoepithelial cells). Finally, they show that the amount of phosphorylated Smad1/5/8 (effector molecules of bone morphogenetic protein 7 [BMP7]) increased 2 to 3 days after injury and could also be detected in nestin-positive cells.
Conclusions
The lacrimal gland contains stem/progenitor cells capable of tissue repair after injury. Programmed cell death after injury triggers proliferation and differentiation of these cells, presumably through activation of the BMP7 pathway.
The nonkeratinized epithelia of the ocular surface are constantly exposed to and challenged by environmental insults such as smoke, dust, and airborne pathogens. Tears are the sole physical protective barrier for the ocular surface.1,2 They form a complex thin film that fulfills several functions, from forming a smooth refractive surface over the corneal surface and lubricating the eyelids to maintaining an optimal extracellular environment for the epithelial cells of the cornea and conjunctiva.1,2 The tear film consists of three interacting layers. The innermost layer coating the cornea and conjunctiva is a mucous layer.3 The large middle layer is an aqueous layer.4 Finally, the outer layer is a lipid layer that floats on the aqueous layer to retard its evaporation.5 Production of tears in inadequate quantity or of inadequate quality results in constant irritation of the ocular surface, leading to dry eye syndrome. There are two major subtypes of dry eye syndrome: evaporative dry eye, caused by perturbations of the lipid layer of the tear film, and aqueous-deficient dry eye, also known as keratoconjunctivitis sicca (KCS), caused by inadequate production of the aqueous layer of the tear film.6
The mechanisms leading to insufficient lacrimal gland secretion are still poorly understood. It is believed that chronic inflammation of the lacrimal gland is a major contributor to insufficient secretion. Chronic inflammation of the lacrimal gland occurs in several pathologic instances (for a review, see Zoukhri7), such as autoimmune diseases (Sjögren syndrome, sarcoidosis, diabetes) or after organ transplantation (chronic graft versus host disease), or simply as a result of aging. The hallmarks of lacrimal gland inflammation are the presence of focal lymphocytic infiltrates, increased production of proinflammatory cytokines, and destruction of the tear-producing parenchymal cells.7
There is a large body of literature on the regenerative capacity of the salivary glands.8–10 Long-term (7–21 days) ligation of the main excretory ducts of salivary glands leads to atrophy of these glands and has been used as an experimental model of human diseases of the salivary glands.8–10 Salivary glands with ligated ducts show signs of inflammation, edema, and death of the acinar cells.8–10 When the duct ligation is released, the salivary glands go through a process of repair through the proliferation of acinar, ductal, or myoepithelial cells and the synthesis and deposition of extracellular matrix.8,9 Similarly, studies on the pancreas11,12 and mammary glands13 reported self-regenerating capabilities of these tissues. Salivary glands go through a phase of inflammation/destruction of parenchymal cells followed by a period of proliferation/tissue repair.
Remodeling of tissue after injury or trauma often recapitulates the same cellular events that govern embryonic tissue development. Apoptosis, a form of programmed cell death, is crucial during embryonic development and is equally important in adult organs to maintain normal cellular homeostasis.14 Under physiological conditions, the occurrence of apoptosis in tissues is typically a rare event, but apoptotic cells become readily identifiable under pathophysiological conditions such as inflammation.14 The role of apoptosis in tissue atrophy/repair is well documented.14
Another form of cell death has also been implicated in tissue repair. Autophagic cell death (also called type 2 cell death; apoptosis is type 1 and necrosis is type 3) is characterized by the appearance of double- or multiple-membrane delimited cytoplasmic vesicles (autophagic vesicles or vacuoles) engulfing bulk cytoplasm or cytoplasmic organelles such as mitochondria and endoplasmic reticulum.15,16 Autophagic vesicles are then destroyed by the cellular lysosomal system.15,16 Apoptosis and autophagic cell death are not mutually exclusive phenomena; they may occur simultaneously in tissues or even conjointly in the same cell.15,16
The presence of stem cells in adult tissues is a very active area of research because of the potential clinical benefits. Several studies on the salivary glands and the pancreas have shown that stem/progenitor cells are present in these tissues and are involved in their regeneration.9,17,18
In previous studies, we developed an animal model of acute, experimentally induced inflammation to study the role of IL-1 in impairing lacrimal gland function. We reported that a single injection of IL-1 into the lacrimal gland inhibited neurally induced and agonist-induced protein secretion measured 1 day after injection.19 We also reported that IL-1 (IL-1β or IL-1α) injection induced a severe inflammatory response in the lacrimal gland that led to the destruction of the acinar cells and resulted in reduced aqueous tear production.7,20 Furthermore, we showed that the effects of IL-1 on lacrimal gland functions were transient. By the seventh (BALB/c mice) or the thirteenth (C57BL/6 mice) day after IL-1 injection, inflammation resolved and the lacrimal gland recovered.20 The purpose of the present study was to determine the cellular mechanisms involved in lacrimal gland injury and repair in this model of acute, experimentally induced inflammation.
Materials and Methods
Antibodies
The following primary antibodies were used for Western blotting or immunofluorescence studies: rabbit polyclonal antibody against phosphorylated Smad 1/5/8 (1:1000 Western blotting, 1:50 immunofluorescence; Cell Signaling Technologies, Danvers, MA); rat monoclonal antibody against Ki67 (1:100 immunofluorescence; Dako, Carpinteria, CA); goat polyclonal antibody against nestin (1:1000 Western blotting, 1:40 immunofluorescence; R&D Systems, Minneapolis, MN); rabbit polyclonal antibody against poly (ADP-ribose) polymerase-1 (PARP-1; 1:200 Western blotting, 1:50 immunofluorescence; Novus Biologicals Inc., Boulder, CO); mouse monoclonal antibody against β-actin (1:5000 Western blotting; Sigma, St. Louis, MO); rabbit polyclonal antibody against α-smooth muscle actin (1:100 immunofluorescence; Abcam Inc., Cambridge, MA); and rat monoclonal antibody against lysosomeassociated membrane protein-1 (LAMP-1, also known as CD107a; 1:200 Western blotting, 1:100 immunofluorescence) and rabbit polyclonal antibody against microtubule-associated protein 1 light chain 3 (MAP LC3; 1:50 immunofluorescence; Santa Cruz Biotechnology, Santa Cruz, CA). All secondary antibodies were from Invitrogen and were conjugated to Alexa Fluor 488 and Alexa Fluor 594 for immunofluorescence studies or to Alexa Fluor 680 and Alexa Fluor 800 for Western blotting studies.
Animals and Treatment
Female BALB/c mice (10–12 weeks old) were purchased from Taconic (Germantown, NY). Animals were maintained in rooms with constant temperature and fixed 12-hour light/12-hour dark intervals and were fed ad libitum. All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Tufts-New England Medical Center Animal Care and Use Committee.
Animals were anesthetized, and the exorbital lacrimal glands were left untreated (control) or were injected, in a total volume of 2 μL, with either saline (vehicle) or recombinant human IL-1α (1 μg, a generous gift from Craig W. Reynolds, Biological Resources Branch, National Cancer Institute Preclinical Repository, Rockville, MD).
TUNEL Staining and Immunofluorescence
Lacrimal gland pieces were fixed overnight at 4°C in 4% formaldehyde made in phosphate-buffered saline (PBS) containing 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 NaH2PO4 at pH 7.2. Paraffin sections of the lacrimal gland (6 μm) were deparaffinized and rehydrated using graded alcohols. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was performed using the fluorescein in situ apoptosis detection kit (ApopTag; Chemicon, Temecula, CA) according to the manufacturer's recommendations. For immunofluorescence experiments, the slides were first subjected to microwave pretreatment (20 minutes) with antigen-retrieval solution (Dako). After three washes in PBS, nonspecific binding sites were blocked for 30 minutes using 10% rabbit serum diluted in PBS. Slides were incubated overnight at 4°C with the indicated primary antibody diluted in PBS. After three washes in PBS, slides were incubated for 60 minutes at room temperature with the appropriate secondary antibody diluted 1:100 in PBS. After three washes in PBS, coverslips were mounted with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA). Sections were viewed using a microscope equipped for epi-illumination (UFXII; Nikon, Tokyo, Japan). Omission of the primary antibody or incubation with irrelevant immunoglobulins was performed for negative control experiments.
Western Blotting
Lacrimal glands were homogenized in 0.4 mL ice-cold homogenization buffer (30 mM Tris-HCl [pH 7.5], 10 mM EGTA, 5 mM EDTA, 1 mM dithiothreitol, and 250 mM sucrose, supplemented with protease inhibitors). After centrifugation, proteins in the supernatants were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; NuPage 4%–12% Bis-Tris Gels; Invitrogen, Carlsbad, CA) followed by transfer onto polyvinylidene difluoride membranes (Invitrogen). The membranes were blocked for 60 minutes at room temperature or overnight at 4°C in blocking buffer (Odyssey; Li-Cor Biosciences, Lincoln, NE) diluted 1:1 in Tris-buffered saline (TBS; 10 mM Tris-HCl [pH 8.0], 150 mM NaCl). The primary antibody was prepared in blocking buffer (Odyssey; Li-Cor Biosciences) diluted 1:1 in TBST (TBS plus 0.05% Tween 20) and incubated for 1 hour at room temperature or overnight at 4°C. After three washes in TBST, the appropriate secondary antibody (diluted 1:5000) was prepared in blocking buffer (Odyssey; Li-Cor Biosciences) and was diluted 1:1 in TBST, and the membranes were incubated for 30 minutes at room temperature. After three washes in TBST, the membranes were scanned with an imaging system (Odyssey Infrared Imaging; Li-Cor Biosciences). Blotting for the structural protein β-actin was performed to control for gel loading and transfer efficiency. Immunoreactive bands acquired from the membranes were quantified with the Odyssey software or with the National Institutes of Health ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Transmission Electron Microscopy
Saline- and IL-1–treated lacrimal glands, removed 3 days after the injections, were fixed overnight in half-strength Karnovsky fixative (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M cacodylate buffer [pH 7.4]). Tissue was postfixed in 1% buffered osmium tetroxide, dehydrated in a series of graded ethanol solutions, and embedded in Epon 812 resin. Sections were prepared, double stained with lead and uranyl acetate, and examined on an electron microscope (410; Philips, Eindhoven, The Netherlands).
Data Presentation and Statistical Analysis
Data are expressed as mean ± SEM and were statistically analyzed using Student's t-test for paired or unpaired values. P < 0.05 was considered significant.
Results
Several studies on salivary and mammary glands and the pancreas showed that, during the inflammatory process, the epithelial acinar cells are lost through apoptosis.8,13,21 Therefore, we hypothesized that the lacrimal gland acinar cells are eliminated by apoptosis after experimentally induced inflammation. As depicted in Figure 1A, apoptotic figures were scarce in saline-injected lacrimal glands. In contrast, the number of cells showing positive TUNEL staining started to increase the first day after injection of IL-1. By the third day, a large number of cells stained positive. TUNEL staining started to decline on the fourth day after injection of IL-1; by the seventh day, the number of apoptotic cells was similar to that seen in saline-injected lacrimal glands (Fig. 1A).
Figure 1.
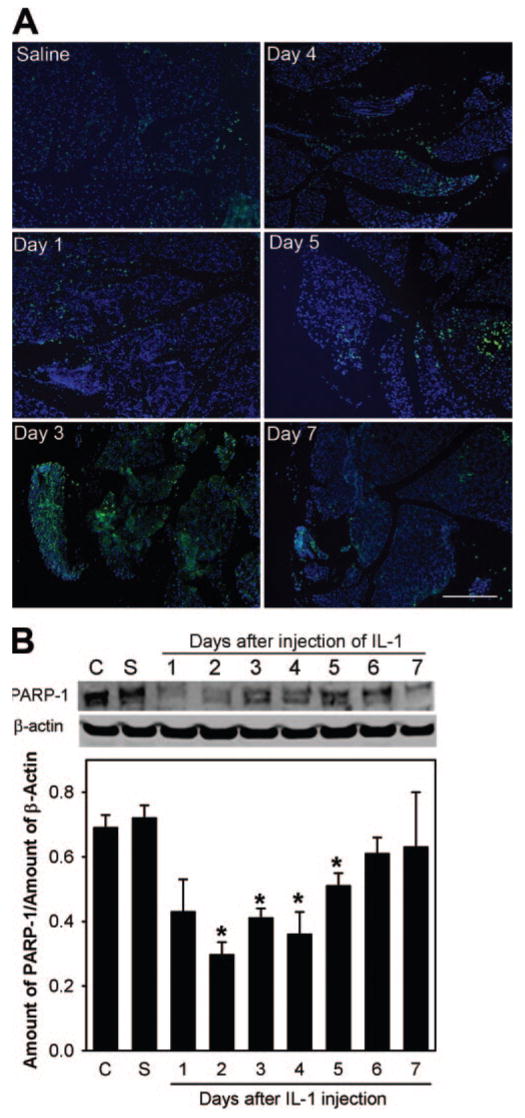
Injection of IL-1 triggers apoptotic programmed cell death in the lacrimal gland. (A) Sections of lacrimal glands removed from saline- and IL-1–injected animals were processed for TUNEL (green). Nuclei were counterstained with DAPI (blue). Scale bar, 200 μm. (B) Lacrimal gland homogenates were processed for Western blotting using an antibody against PARP-1, a caspase 3 substrate. Data in the plot are mean ± SEM from three independent experiments. *Statistically significant difference from control (C) and saline (S).
To substantiate the data obtained with the TUNEL staining, lacrimal gland homogenates were processed for SDS-PAGE followed by Western blotting for PARP-1. PARP-1, a protein involved in DNA repair and DNA stability, is a well-studied cellular substrate of activated caspase 3, and its proteolytic cleavage (from 113 kDa- to 89 kDa- and 24-kDa fragments) has been used as an indicator of caspase 3 activation.22 As shown in Figure 1B, compared with samples from control (C) and saline (S), the amount of the 113 kDa PARP-1 protein decreased 2 to 5 days after the injection of IL-1, suggesting the activation of caspase 3–dependent programmed cell death. The amount of PARP-1 protein returned to control levels by the seventh day after injection (Fig. 1B). With this antibody, we could not detect the 89-kDa or 24-kDa degradation products of PARP-1.
Lacrimal glands were injected with saline or IL-1, removed 3 days later, fixed, and processed for transmission electron microscopy. The purpose of these experiments was to determine whether, besides apoptosis, other types of cell death, such as necrosis and autophagy, were involved in lacrimal gland repair. As shown in Figure 2A, lacrimal gland acinar cells from saline-injected animals had a normal appearance, basally located nuclei, and a cytoplasm filled with secretory granules. In contrast, acinar cells from IL-1–injected lacrimal glands lost their polarity (Figs. 2B–D); their cytoplasm was often devoid of secretory granules and instead contained vacuoles (Figs. 2B, 2C). Some of the vacuoles contained fragmented cellular structures and were double-membrane delimited, a structural characteristic of autophagic vacuoles (Fig. 2B, inset). The electrondense structures (i.e., proteinaceous material) in Figures 2C and 2D might have represented different stages from autophagosomes to lysosomes.
Figure 2.
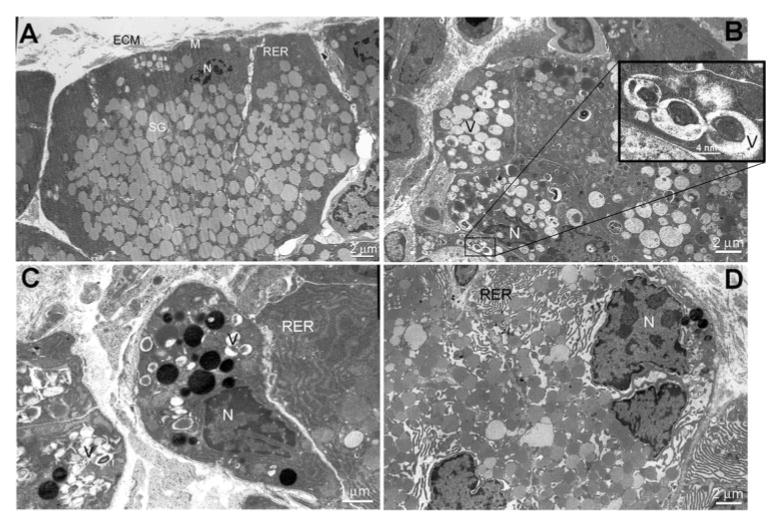
Involvement of autophagic cell death in IL-1–induced injury of the lacrimal gland. Lacrimal glands from saline- and IL-1–injected (3-day) animals were processed for transmission electron microscopy. (A) Normal ultrastructure of a control lacrimal gland. Acinar cells are highly polarized with basally located nuclei (N); they have a very dense, rough endoplasmic reticulum (RER) network and normal mitochondria (M), and the cytoplasm is filled with secretory granule (SG) located toward the apical area. ECM, extracellular matrix. (B–D) Ultrastructure of IL-1–injected lacrimal glands. Most of the acinar cells have lost their polarity, the RER network is disorganized (D), and the cytoplasm is filled with vacuoles (V), some of which are double-membrane delimited (B, inset).
To confirm the involvement of autophagy and the lysosomal compartment in lacrimal gland repair, we used antibodies against LAMP-1 and microtubule-associated protein-1 light chain 3 (MAP LC3, a homolog of Apg8p in yeast associated with autophagosome membranes) and performed Western blotting and immunofluorescence studies. As shown in Figure 3A, the amount of LAMP-1 protein increased significantly, relative to control and saline, starting at 2 days in IL-1–injected glands and gradually returned to control level by day 7. Immunofluorescence experiments showed increased punctate staining for LAMP-1 and the autophagosome marker MAP LC3 in IL-1–injected lacrimal glands (Fig. 3B) and both proteins were often present in the same cells (Fig. 3B, inset).
Figure 3.
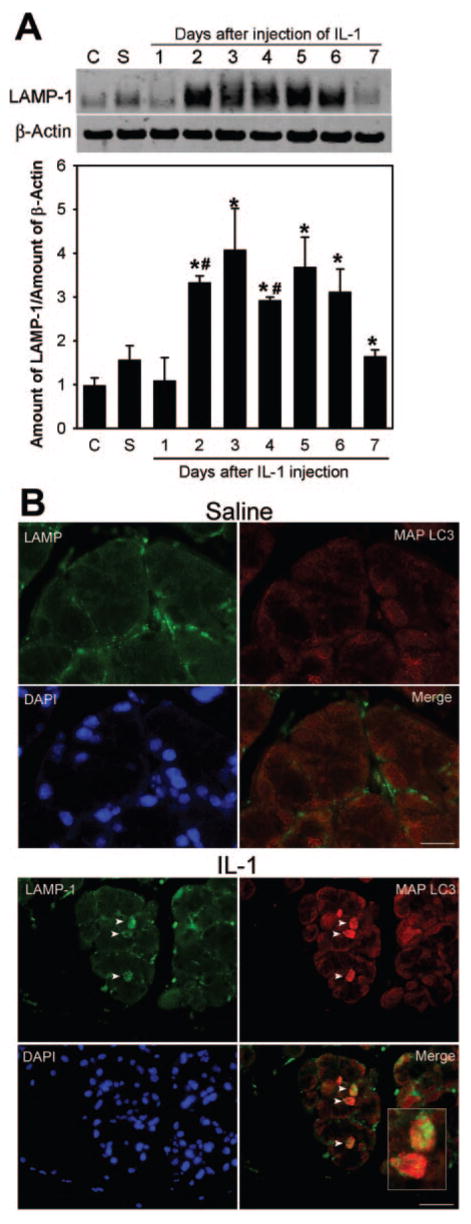
Involvement of autophagy and the lysosomal compartment in acinar cell death. (A) Lacrimal glands removed from control (C), saline-treated (S), or IL-1–treated animals were processed for Western blotting using an antibody against LAMP-1. Data in the plot are mean ± SEM from three independent experiments. *Statistically significant difference from control. #Statistically significant difference from saline. (B) Lacrimal glands removed from saline- or IL-1–treated (3-day) animals were processed for double-immunofluorescence studies using the LAMP-1 antibody (green) and an antibody against MAP LC3, a marker of autophagic vacuoles (red). Nuclei were counterstained with DAPI (blue). Arrowheads and inset highlight cells harboring LAMP-1 and MAP LC3 immunoreactivity. Scale bar, 50 μm.
Involvement of stem/progenitor cells in tissue repair and regeneration is widely documented. Nestin, an intermediate filament protein, is a well-studied and accepted marker for stem/progenitor cells.23 Experiments were conducted to determine whether stem/progenitor cells were present in the murine lacrimal gland and, if so, whether they were involved in repair after experimentally induced inflammation. Lacrimal glands removed from control and IL-1–treated animals were processed for immunofluorescence and Western blotting experiments with an antibody against nestin.
As shown in Figure 4A, nestin-positive cells could be detected in lacrimal glands removed from saline (vehicle)-injected or control noninjected (not shown) animals. It should be noted that these cells were scarce in tissues from both groups. Their spindlelike shape was reminiscent of that of myoepithelial cells. After experimentally induced inflammation, the number of nestin-positive cells increased, especially between days 2 and 3 after injection of IL-1 (Fig. 4A). Not only did the number of nestin-positive cells increase, their shape was altered (there were more nestin-positive, round cells, especially in the 2-day sections; Fig. 4A). After the fourth day following IL-1 injection, the number of nestin-positive cells diminished, and their shape resembled that seen in control glands (Fig. 4A). Brain sections were used as a positive control for nestin staining (Fig. 4B). Omission of the primary antibody resulted in a loss of specific immunoreactivity (Fig. 4B).
Figure 4.
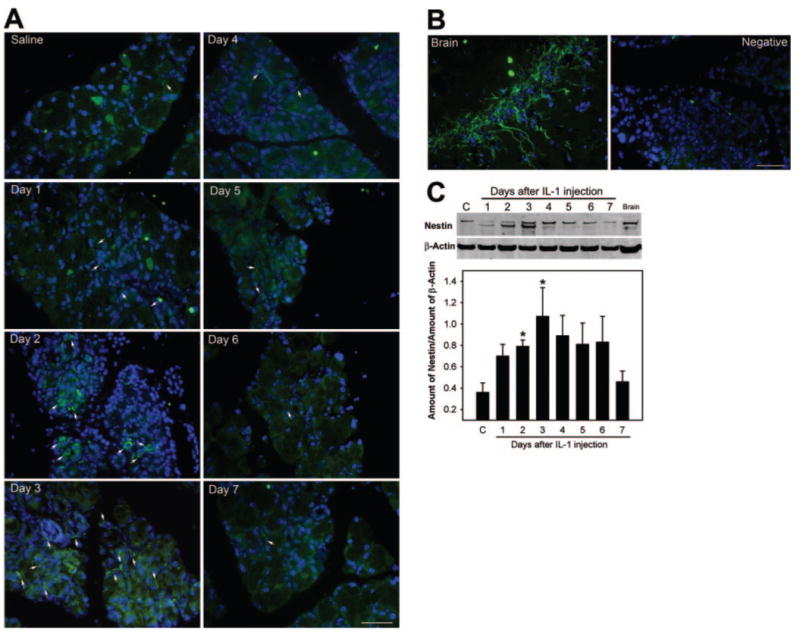
Involvement of stem/progenitor cells in lacrimal gland repair. (A) Lacrimal glands removed from saline (3 days) or IL-1–treated animals were processed for immunofluorescence studies using an antibody against nestin, a stem cell marker (green). Nuclei were counterstained with DAPI (blue). The number of nestin-positive cells (arrows) increased between 2 and 4 days after injury to the lacrimal gland. (B) Brain sections were used as a positive control for nestin staining. Incubation of lacrimal gland sections with irrelevant immunoglobulins (Negative) resulted in a loss of immunoreactivity. Nuclei were counterstained with DAPI (blue) (C) Lacrimal gland homogenates prepared from control (C) and IL-1–treated animals and from brain homogenate (positive control) were processed for Western blotting using anti–nestin or anti–β-actin (to control for gel loading) antibodies. Data in the plot are mean ± SEM from three independent experiments. *Statistically significant difference from control. Scale bar: (A, B) 50 μm.
Western blot data presented in Figure 4C lend further support to the presence of stem/progenitor cells in murine lacrimal glands. Homogenates prepared from lacrimal glands 2 and 3 days after injection showed increased nestin protein immunoreactivity. In these samples, a second, faster migrating protein was also detected (Fig. 4C). This band, though less abundant, was also present in samples from 1- and 4-day injected animals and in brain homogenate (used as a positive control). The function of nestin has recently been shown to be regulated through phosphorylation by cell division cycle 2 (cdc2) kinase and cyclin-dependent kinase 5 (cdk5).24,25 It is possible that the fast-migrating band detected in our samples is a less phosphorylated form of nestin. The amount of nestin protein declined slowly, starting 4 days after injection, and returned to control level by the seventh day (Fig. 4C).
The shape of the cells staining positive for nestin is reminiscent of that of lacrimal gland myoepithelial cells. We tested this possibility in double-labeling experiments using an antibody against α-smooth muscle actin, a marker of lacrimal gland myoepithelial cells. As shown in Figure 5, several, but not all, nestin-positive cells are also positive for α-smooth muscle actin immunoreactivity.
Figure 5.
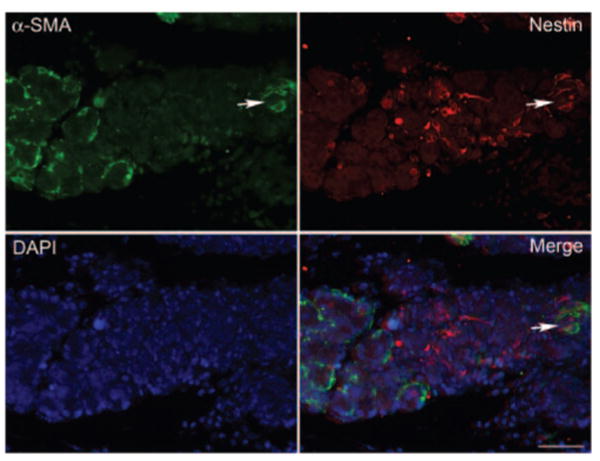
Some myoepithelial cells are nestin positive. Lacrimal glands from IL-1–injected animals (3 day) were processed for double-immunofluorescence studies using an antibody against α-smooth muscle actin (α-SMA, a marker for myoepithelial cells, green) and the anti-nestin antibody (red). Nuclei were counterstained with DAPI (blue). Some cells (arrow) were positive for both α-SMA and nestin. Scale bar, 50 μm.
The increase in the number of nestin-positive cells during repair of the lacrimal gland suggested that these cells might be proliferating. To test this hypothesis, we conducted double-immunofluorescence studies using a rat monoclonal antibody against Ki67, a proliferation-associated protein tightly bound by chromatin in all cells as they progress from the S to the G2 phase of the cell cycle.26 As shown in Figure 6, several nestin-positive cells were also positive for Ki67 staining, suggesting that these cells were proliferating.
Figure 6.
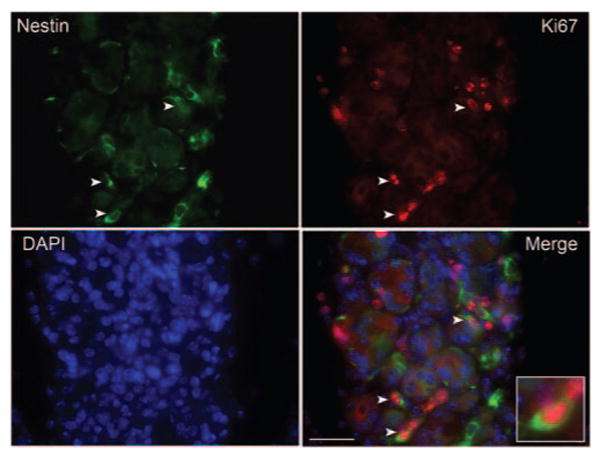
Nestin-positive cells are proliferating. Lacrimal glands from IL-1–injected animals (3 day) were processed for double-immunofluorescence studies using the anti–nestin antibody (green) and an antibody against Ki67, a marker for proliferating cells. Nuclei were counterstained with DAPI (blue). Some cells (arrowheads and inset) were positive for nestin and Ki67, indicating that these cells have entered the cell cycle. Scale bar, 50 μm.
Bone morphogenetic protein 7 (BMP7), a member of the transforming growth factor β (TGFβ) family, is known to play a major role in tissue development and remodeling. BMP7-null mice have smaller lacrimal glands and reduced branching.27 Inhibition of BMP activity has similar effects on lacrimal gland explant cultures.28 Activated BMP receptors, similar to TGFβ receptors, transmit their signals through activation (phosphorylation) of Smad proteins.29 Of the eight Smad proteins identified in mammals, only Smad1, Smad5, and Smad8 are activated by BMP7.29 As shown in Figure 7A, phosphorylated Smad1/5/8 could be detected in control untreated lacrimal glands, suggesting basal activation of the BMP7 pathway. After injection of IL-1, the amount of phosphorylated Smad1/5/8 increased starting at 2 and 3 days after injection (Fig. 7A), when we showed that the lacrimal gland underwent repair.20 There-after, the amount of phosphorylated Smad1/5/8 declined (Fig. 7A). We have also conducted immunofluorescence studies and found that immunoreactivity against phosphorylated Smad1/5/8 was restricted to the nucleus, as expected, and tended to be more intense in sections from 2- and 3-day injected lacrimal glands (Fig. 7B). Furthermore, double-immunofluorescence studies showed that phosphorylated Smad1/5/8 could be detected on some nestin-positive cells (Fig. 7C), suggesting activation of the BMP7 pathway in lacrimal gland stem/progenitor cells during the repair phase.
Figure 7.
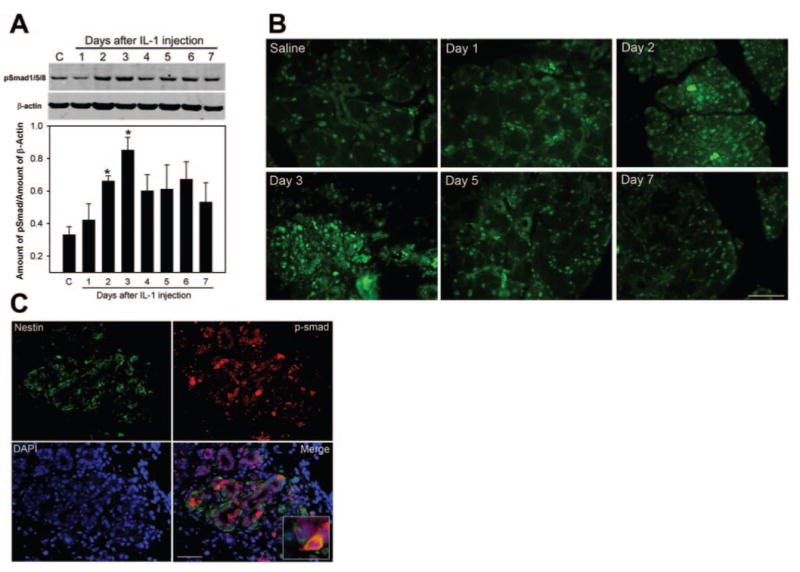
The BMP7 pathway is activated during the repair phase. (A) Lacrimal gland homogenates prepared from control (C) and IL-1–treated animals were processed for Western blotting using an antibody against phosphorylated Smad1/5/8 or against β-actin (to control for gel loading). Data in the plot are mean ± SEM from three independent experiments. *Statistically significant difference from control. (B) Lacrimal glands removed from saline- and IL-1–treated animals were processed for immunofluorescence studies using the antibody against phosphorylated Smad1/5/8. (C) Lacrimal glands removed from IL-1–treated (3-day) animals were processed for double-immunofluorescence studies using anti–nestin (green) or anti–phosphorylated Smad1/5/8 (red). Nuclei were counterstained with DAPI (blue) Most of the nestin-positive cells were also positive for phosphorylated Smad1/5/8 (inset) Scale bar: (B, C) 50 μm.
Discussion
Remodeling of tissues after injury or trauma often recapitulates the same cellular events that govern embryonic tissue development. Apoptosis is crucial during embryonic development and is equally important in adult organs to maintain normal cellular homeostasis.14 The role of apoptosis in tissue atrophy/repair is well documented. In fact, it has been demonstrated that apoptosis of the pancreatic acinar cells is necessary for the induction of cell proliferation in the regenerating tissues.11 The biochemical hallmark of apoptosis is degradation of DNA by endogenous DNase, which cuts the internucleosomal regions into double-stranded DNA fragments of 180 to 200 base pairs.14 These fragments are detectable as a ladder pattern in the electrophoresis of isolated DNA. However, they are most commonly detected, in situ, with the TUNEL assay.30 Other biochemical hallmarks of apoptosis involve the activation of several caspases.14 With the use of TUNEL staining, we showed that lacrimal gland acinar cells actively undergo apoptosis. This conclusion is further supported by the increase in proteolytic cleavage of PARP-1, a caspase 3 substrate, after injury to the lacrimal gland.
Autophagy is an evolutionarily conserved and dynamic process in which cytoplasmic components are sequestered and delivered to the lysosome for degradation and recycling.15,16 Deregulated autophagy has been implicated in several pathologic conditions in humans, including cancer and neurodegenerative disease.15,16 Autophagy starts with the formation of a double-membrane delimited autophagic vacuole (also called autophagosome), which engulfs bulk cytoplasm and cytoplasmic organelles such as mitochondria and endoplasmic reticulum.16 In mammalian cells, autophagosomes undergo a maturation process by fusing with endocytic compartments and lysosomes. Electron microscopy remains the criterion standard for assessing autophagy by identifying the presence of autophagosomes.31 Immunohistochemical analyses of MAP LC3 and the lysosomal marker LAMP-1 have also been successfully used to assess autophagy.31 During the formation of the autophagosome, LC3 is lapidated, and this LC3-phospholipid conjugate (LC3-II) is localized on autophagosome membranes.31 In the present study, we used electron microscopy and immunohistochemistry to demonstrate the involvement of autophagy in lacrimal gland repair after experimentally induced inflammation.
Apoptosis and autophagic cell death are not mutually exclusive phenomena. They may occur simultaneously in tissues and even conjointly in the same cell.15,16,31 Depending on the cellular context and stimulus, autophagy may be indispensable for apoptosis by preceding and further stimulating apoptosis.16,31 During apoptosis, the stimulation of autophagy can be a protective mechanism or a process that contributes to cell death. Autophagy can be protective because of the recycling of long-lived cytosolic macromolecules to support cellular anabolic needs and viability under starvation conditions. In addition, by removing damaged or surplus organelles, including compromised mitochondria, autophagy can protect cells from unscheduled apoptosis. Our data show that both forms of programmed cell death are activated during repair of the lacrimal gland. It is unclear whether both processes operate in the same cell and whether autophagy has a protective role in the lacrimal gland. Future studies investigating the effect of inhibiting each process separately would help answer these questions.
The presence of stem cells in adult tissues is an active area of research because of the potential clinical benefits. In the field of tissue repair, there are two schools of thought: one that advocates repair through expansion of stem/progenitor cells and another that advocates the transdifferentiation of existing cells.32,33 Several studies on the salivary glands and the pancreas have shown that stem/progenitor cells are present and involved in the regeneration of these tissues.9,17,18 However, a major technical difficulty has been to distinguish between the expansion of stem/progenitor cells and transdifferentiation during tissue repair. Using genetic marking for lineage tracing with the insulin promoter, Dor et al.34 showed that during regeneration of the pancreas, new β cells could be generated by replication of existing β cells, arguing against the involvement of stem/progenitor cells. Conversely, several investigators have isolated and expanded stem cells from the salivary glands and the pancreas.9,18 These cells are usually associated with the ductal/periductal fraction of these tissues.9,35 Of note, in studies on salivary stem/progenitor cells, it was reported that the number of these cells increased dramatically after tissue injury.10 We present evidence showing the presence of stem/progenitor cells in murine lacrimal glands and show that their number increased after experimentally induced inflammation. We also show that some of these cells express α-smooth muscle actin, a marker of myoepithelial cells, and that they are capable of proliferation during repair of the lacrimal gland. The fact that not all nestin-positive cells stained positively for α-smooth muscle actin suggests a common source of stem cells shared by myoepithelial and acinar cells in the lacrimal gland. In the future, we plan to isolate and propagate in vitro lacrimal gland stem/progenitor cells and to investigate whether these cells, once transplanted, can accelerate lacrimal gland repair.
Development of the lacrimal gland occurs through a process known as branching morphogenesis.36 This process of morphogenesis is achieved through epithelial-mesenchymal interactions that include a highly coordinated spatiotemporal release of several growth factors and the subsequent activation of critical transcription factors.36 Several growth and transcription factors are shown to be crucial for branching morphogenesis of the lacrimal gland, and BMP7, in particular, is shown to play a pivotal role in murine lacrimal gland development.28,37,38
Members of the BMP family bind to two distinct type 2 and type 1 serine/threonine kinase receptors, both of which are required for signal transduction. On receptor activation, BMPs transmit signals through Smad-dependent and Smad-independent pathways.29 In the developing lacrimal gland, BMP7 is expressed in the epithelial and the mesenchymal components.28 Exogenous addition of BMP7 to mesenchymal cells in culture induces the formation of cell contacts through increased expression of connexin 43 and cadherins and leads to the upregulation of α-smooth muscle actin, which forms wellorganized stress fibers.28 Of relevance to tissue repair, BMP7 has been shown to ameliorate the course of injury through a variety of mechanisms, including the inhibition of apoptosis, the prevention of infarction and cell necrosis, and the downregulation of intracellular adhesion molecule expression.39 In the present study, we showed that the BMP7 pathway is activated in the lacrimal gland and is upregulated during the repair phase. The fact that this pathway is activated in stem/progenitor cells suggests that BMP7 might dictate the fate of these cells. Future studies investigating the effect of exogenous addition of BMP7 or inhibition of BPM7 signaling on lacrimal gland repair would test this hypothesis.
In summary, our data show that during lacrimal gland repair, acinar cells are lost through apoptosis and autophagy. This is followed by an increase in the number of stem/progenitor cells, the stimulation of proliferation, and the upregulation of the BMP7 pathway. Future studies investigating the therapeutic potential of stem/progenitor cells in lacrimal gland abnormalities are warranted.
Acknowledgments
The authors thank Craig Reynolds for the generous gift of recombinant human cytokines; Patricia Pearson for electron microscopy; Darlene Dartt, Robin Hodges, and Barbara Talamo for critical reading of the manuscript; and Fara Sourie for invaluable contributions to this work.
Supported by National Institutes of Health/National Eye Institute Grant RO1-EY12383.
Footnotes
Disclosure: D. Zoukhri, None; A. Fix, None; J. Alroy, None; C.L. Kublin, None
References
- 1.Tiffany JM, Bron AJ. Role of tears in maintaining corneal integrity. Trans Ophthalmol Soc U K. 1978;98:335–338. [PubMed] [Google Scholar]
- 2.Lamberts DW. Physiology of the tear film. In: Smolin G, Thoft RA, editors. The Cornea. Boston: Little, Brown and Company; 1994. pp. 439–483. [Google Scholar]
- 3.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- 4.Dartt DA. Regulation of tear secretion. Adv Exp Med Biol. 1994;350:1–9. doi: 10.1007/978-1-4615-2417-5_1. [DOI] [PubMed] [Google Scholar]
- 5.Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan DA, Wickham LA, Krenzer KL, Rocha EM, Toda I. Aqueous tear deficiency in Sjögren's syndrome: possible causes and potential treatment. In: Pleyer U, Hartmenn C, editors. Oculodermal Diseases. Buren, Netherlands: Aeolus Press; 1997. pp. 95–152. [Google Scholar]
- 7.Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885–898. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi S, Shinzato K, Nakamura S, Domon T, Yamamoto T, Wakita M. Cell death and cell proliferation in the regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004;33:23–29. doi: 10.1111/j.1600-0714.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 9.Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340:544–552. doi: 10.1016/j.bbrc.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Hisatomi Y, Okumura K, Nakamura K, et al. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39:667–675. doi: 10.1002/hep.20063. [DOI] [PubMed] [Google Scholar]
- 11.Scoggins CR, Meszoely IM, Wada M, Means AL, Yang L, Leach SD. p53-Dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am J Physiol Gastrointest Liver Physiol. 2000;279:G827–G836. doi: 10.1152/ajpgi.2000.279.4.G827. [DOI] [PubMed] [Google Scholar]
- 12.Walker NI, Winterford CM, Kerr JF. Ultrastructure of the rat pancreas after experimental duct ligation, II: duct and stromal cell proliferation, differentiation, and deletion. Pancreas. 1992;7:420–434. doi: 10.1097/00006676-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- 14.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 15.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Galluzzi L, Maiuri MC, Vitale I, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 17.Holland AM, Gonez LJ, Harrison LC. Progenitor cells in the adult pancreas. Diabetes Metab Res Rev. 2004;20:13–27. doi: 10.1002/dmrr.430. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YQ, Kritzik M, Sarvetnick N. Identification and expansion of pancreatic stem/progenitor cells. J Cell Mol Med. 2005;9:331–344. doi: 10.1111/j.1582-4934.2005.tb00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoukhri D, Hodges RR, Byon D, Kublin CL. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Invest Ophthalmol Vis Sci. 2002;43:1429–1436. [PMC free article] [PubMed] [Google Scholar]
- 20.Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker NI, Winterford CM, Williamson RM, Kerr JF. Ethionineinduced atrophy of rat pancreas involves apoptosis of acinar cells. Pancreas. 1993;8:443–449. doi: 10.1097/00006676-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiese C, Rolletschek A, Kania G, et al. Nestin expression–a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahlgren CM, Mikhailov A, Hellman J, et al. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276:16456–16463. doi: 10.1074/jbc.M009669200. [DOI] [PubMed] [Google Scholar]
- 25.Sahlgren CM, Mikhailov A, Vaittinen S, et al. Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol. 2003;23:5090–5106. doi: 10.1128/MCB.23.14.5090-5106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 27.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 28.Dean C, Ito M, Makarenkova HP, Faber SC, Lang RA. Bmp7 regulates branching morphogenesis of the lacrimal gland by promoting mesenchymal proliferation and condensation. Development. 2004;131:4155–4165. doi: 10.1242/dev.01285. [DOI] [PubMed] [Google Scholar]
- 29.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 32.Dor Y, Melton DA. How important are adult stem cells for tissue maintenance? Cell Cycle. 2004;3:1104–1106. [PubMed] [Google Scholar]
- 33.Parenteau NL, Rosenberg L, Hardin-Young J. The engineering of tissues using progenitor cells. Curr Top Dev Biol. 2004;64:101–139. doi: 10.1016/S0070-2153(04)64006-3. [DOI] [PubMed] [Google Scholar]
- 34.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic betacells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 35.Kume S. Stem-cell-based approaches for regenerative medicine. Dev Growth Differ. 2005;47:393–402. doi: 10.1111/j.1440-169X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 36.Hogan BL. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 37.Mattiske D, Sommer P, Kidson SH, Hogan BL. The role of the forkhead transcription factor, Foxc1, in the development of the mouse lacrimal gland. Dev Dyn. 2006;235:1074–1080. doi: 10.1002/dvdy.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–286. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Simic P, Vukicevic S. Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 2005;16:299–308. doi: 10.1016/j.cytogfr.2005.02.010. [DOI] [PubMed] [Google Scholar]


