Abstract
Aberrant regulation of the translation initiation is known to contribute to tumorigenesis. eIF3 plays an important role in translation initiation. eIF3f is the p47 subunit of the eIF3 complex whose function in cancer is not clear. Initial studies from our group indicated that eIF3f expression is decreased in pancreatic cancer. Overexpression of eIF3f induces apoptosis in pancreatic cancer cells. The eIF3f gene is located at chromosome band region 11p15.4. Loss of 11p15.4 is a common event in many tumors including pancreatic cancer. In order to investigate the molecular mechanism of the decreased expression of eIF3f in pancreatic cancer, we performed loss of heterozygosity (LOH) analysis in 32 pancreatic cancer specimens using three microsatellite markers encompassing the eIF3f gene. We showed that the prevalence of LOH ranged from 71% to 93%. We also performed eIF3f gene copy number analysis using quantitative real time PCR to further confirm the specific allelic loss of eIF3f gene in pancreatic cancer. We demonstrated a statistically significant decrease of eIF3f gene copy number in pancreatic tumors compared with normal tissues with a tumor/normal ratio of 0.24. Furthermore, RNA in situ hybridization and tissue microarray immunohistochemistry analysis demonstrated that eIF3f expression is significantly decreased in human pancreatic adenocarcinoma tissues compared to normal pancreatic tissues. These data provides new insight into the understanding of the molecular pathogenesis of eIF3f during pancreatic tumorigenesis.
Keywords: eukaryotic initiation factor 3f, loss of heterozygosity, apoptosis, pancreatic cancer
INTRODUCTION
Cancer of the pancreas is the fourth leading cause of cancer death in the United States. This year approximately 32 300 Americans will die from cancer of the pancreas (American Cancer Society). Ductal adenocarcinoma is by far the most common pancreatic neoplasm, involving around 90% of all pancreatic malignancies. However, there are other types of tumors that arise from both the exocrine or endocrine cellular components [1]. The disease is not only common; it is also extremely difficult to treat. Surgical intervention is possible in only about 10% of cases and adjuvant therapies are virtually ineffective [2]. For these and other reasons, cancer of the pancreas has been called “the challenge of the twenty-first century” [3]. In spite of the fact that enormous advances have been made in our knowledge of the molecular alterations commonly present in ductal cancer and other pancreatic malignancies, an improved understanding of pancreatic cancer genetics is the only means to provide new markers for early diagnosis and to identify potential targets for therapeutic intervention.
Altering the expression of genes involved in cell proliferation or apoptosis may cause abnormal cell growth and malignant transformation. Although most studies focused on the transcriptional regulation of gene expression, recent studies indicate that deregulation of protein translation may also contribute to cancer development [4,5]. Translation initiation factor eIF4F activation is an essential component of the malignant phenotype in breast carcinoma [6]. eIF4E controls the translation of various malignancy-associated mRNAs which are involved in polyamine synthesis, cell cycle progression, activation of proto-oncogenes, angiogenesis, autocrine growth stimulation, cell survival, invasion and communication with the extracellular environment. eIF4E-mediated translational modulation of these mRNAs plays a pivotal role in both tumor formation and metastasis [7]. It was also reported that abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells [8]. These observations indicate the importance of translational regulation in oncogenesis.
The rate of synthesis of any given protein is determined by the level of translation initiation [9]. The eukaryotic initiation factor 3 (eIF3) is a multi-protein complex containing at least 13 non-identical subunits, that plays a central role in translation initiation. The function of eIF3 is not only linked to prevent premature association of the 40S with the 60S ribosomal subunits but also is linked to prevent the premature association of the 40S ribosome to the mRNA by binding to eIF4F complex generating a pre-initiation complex called 48S. When the expression or level of eIF3 is altered, this may influence the synthesis of some growth-regulating proteins [4]. Recent studies by Zhang et al. [4] indicated that overexpression of five individual subunits of eIF3 promotes malignant transformation of NIH3T3 cells. Several lines of evidence suggest that alterations in eIF3 can contribute to tumorigenesis. Aberrant mRNA and protein levels of several eIF3 subunits have been detected in several different solid tumors and cancer cell lines. Different isoforms of eIF3a are over-expressed in human breast, cervical, lung, and gastric cancer, as well as mouse melanoma and Hela cells [10,11]. eIF3a mRNA is increased in proliferate tissues such as bone marrow, thymus, and developing fetal tissues. eIF3b is over-expressed in human breast cancer [10]. eIF3c is also found over-expressed in testicular seminomas [12]. High-level amplification of eIF3h has been found in prostate tumors [13]. In addition, over-expression of eIF3i has been reported to induce malignant transformation of NIH 3T3 cells [14]. Insertion of the mouse mammary tumor virus (MMTV) genome into the eIF3e gene induces neoplasia in mice, suggesting a tumor suppressor role for eIF3e [15,16].
The eIF3f gene, mapped to human chromosome band region 11p15.4, is one of the 13 non-identical subunits of the eIF3 complex. The function of eIF3f is not clear. We can demonstrate that eIF3f mRNA expression is significantly decreased in many human tumors including pancreatic cancer using a cancer profiling array and real time reverse transcription polymerase chain reaction (RT-PCR) analysis [17]. Previous studies from our group identified eIF3f subunit as a protein associated with the caspase-processed isoform of the cyclin dependent kinase11(CDK11p46) which appears to be a down stream effector in apoptotic signaling using a yeast two-hybrid screening strategy [18]. This interaction occurs in vitro and in vivo. eIF3f can be phosphorylated by CDK11p46 during apoptosis [18]. We can demonstrate that the enforced expression of eIF3f inhibits translation, cell growth, cell proliferation and induces apoptosis in melanoma and pancreatic cancer cells [17]. Knockdown of eIF3f prevents apoptosis in tumor cells [17]. Recent studies from Zhang et al. [4] also showed decreased cell growth, cell proliferation, colony formation and increased apoptosis in eIF3f-overexpressing NIH3T3 cells. These findings suggest that eIF3f may be an important negative regulator of cell growth and proliferation. However the molecular mechanisms by which decrease the expression of eIF3f in pancreatic cancer is not known. In the present study, we address whether there is eIF3f gene allele loss in pancreatic cancer using loss of heterozygosity (LOH) and gene copy number analysis. Decreased eIF3f expression in pancreatic cancer was also confirmed at the mRNA and protein level in human tissue specimens.
MATERIALS AND METHODS
Tumor Samples
Thirty-two specimens of pancreatic cancer were obtained from archived fixed human tissue bank of the Department of Pathology at the University of Arizona with IRB approval. Tumor tissues were reviewed by the same pathologist to confirm histopathologic status for a detailed selection of cells to be microdissected. Twenty eight tissue specimens were formalin fixed paraffin-embedded and four specimens were frozen pancreatic cancer tissues. Pancreatic cancer tissue microarray (TMA) was kindly provided by Ray Nagle, Thomas Grogan, Ronald Schifman, Walden Browne and Yvette Frutiger. The TMA contained 36 pancreatic adenocarcinoma samples (well-differentiated, moderately differentiated or poorly differentiated). One section from the TMA was stained with haematoxylin and eosin to confirm the histopathological diagnosis.
Laser Capture Microdissection (LCM)
Pancreatic cells were microdissected out of 8 μ sections, stained with HistoGene staining solution, under direct microscopic visualization using the Arcturus PixCell II Laser Capture Micro-dissection System (Arcturus Molecular Devices Co, Sunnyvale, CA) available at the Arizona Cancer Center. For each case at least 2 caps were obtained including one cap with normal tissue as control and another with tumor cells. The conditions for the microdissection were: laser power: 50–70 mW, laser pulse: 2000–2700, spot size of >2 mm2.
LOH Analysis
After microdissection, the caps were placed in 0.5 ml microfuge tubes containing 50 μ of fresh lysis buffer (Proteinase K in reconstitution buffer from Arcturus Bioscience, Inc.). The tubes were placed upside down and incubated at 65°C overnight. Then they were centrifuged for 1 min at 3000 rpm and heated at 95°C for 10 min in order to inactivate the Proteinase K. Genomic DNA was extracted using DNeasy kit (Qiagen, Valencia, CA). Three micro-satellite markers (D11S1318, D11S1338, and D11S1331) located at 11p15.4 were amplified by polymerase chain reaction (PCR) using 6FAM, HEX and NED-labeled forward primers and reverse primers flanking these markers (D11S1318 Forward: 5′-CCCGTATGGCAACAGG-3′, Reverse: 5′-TGTGCATGTNCATGAGTG-3′; D11S1338 Forward: 5′-GACGGTTTAACTGTATATCTAAGAC-3′, Reverse: 5′-TAATGCTACTTATTTGGAGTGTG-3′; and D11S1331 Forward: 5′-GCTGCTTCCATGAGAGGATACTG-3′, Reverse: 5′-GCAGAGCCCTTTGCAGTCTT-3′). The markers were chosen according to the Genebridge4 and Stanford G3 and for maximal heterozygosity. The PCR reaction contained 100 ng of DNA, 1 μl of each primer in a final concentration of 1 μM, 1 μl of a mixture of deoxynucleotide triphosphate (dNTP) containing 25 mM of each one (Invitrogen, Carlsbad, CA), 1 μl of 10X reaction buffer (New England BioLabs, Inc., Ipswich, MA), 1 μl of 10X PCR enhancer solution (Invitrogen), and 0.25 μl of 5 U/μl Taq DNA polymerase (New England BioLabs) in a final volume of 10 μl. The reactions were performed as follows: denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 1 min, annealing at the requested temperature for 1 min and extension at 72°C for 1 min. Lastly, a 10 min final extension at 72°C was carried out. The DNA fragments were separated by capillary electrophoresis (ABI 3100, Applied Biosystem, Foster City, CA). To assess LOH, the peak areas of both alleles were measured using Genescan software (Applied Bio-system, version 3.1) and the ratios of the normal and tumor samples were compared. LOH index: (PT2/PT1)/(PN2/PN1) of <0.6 or >1.7 were considered positive [19]. Each LOH positive case was repeated at least twice.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed on a PRISM 5700 sequence detector (Applied Biosystem) using iTaq™ SYBR Green Supermix with ROX (Bio-Rad Laboratories, Inc., Hercules, CA). This method is based on the binding of the dye to double stranded DNA. When SYBR Green binds to double stranded DNA, the intensity of the fluorescent emissions increases as previously described [20]. Primer sets were designed to span intron–exon boundaries in order to eliminate the contamination of pseudogenes and RNA. Primers used were: β-actin, Forward: 5′-TGACATGGTGTATCTCTGCC-3′, Reverse: 5′-AGTCCATCACGATGCCAG-3′; eIF3f, Forward: 5′-TAGACAGGA GTCCACCACTG-3′, Reverse: 5′-CCTCTCGGCTGTAGTACTCG-3′. Relative quantification was calculated using β-actin as an endogenous control (User bulletin #2: Relative Quantitation of gene Expression, P/N 4303859, Applied Biosystems, 1997). The PCR reaction mixture (total volume of 25 μl) was composed of the following: 150 ng DNA, 1x iTaq SYBR green Supermix with ROX and 1 μM final concentration of forward and reverse primers. The cycling conditions were: 95°C 10 m, 95°C 15 s, and 60°C 1 m for 40 cycles. The target and the control genes amplification was conducted in separate tubes. The reactions were performed in triplicate. Dissociation curves were performed to determine the specificity of the amplicon. Relative quantification was calculated as the target gene/β-actin ratio [21].
In Situ Hybridization
In situ hybridization (ISH) was performed as reported previously [22,23]. Briefly, 10-μm paraffin embedded tissue sections were treated with proteinase K (Boehringer Mannheim) at a concentration of 10 μg/μl for 10 m at 37°C, and then they were postfixed with 4% paraformaldehyde in PBS for 20 m. After washing in 2× SSC, the samples were prehybridized at 56.5°C for at least 1 h in 50% formamide (v/v), 4× SSC, 2× Denhardt’s solution, and 250 μg of RNA/ml. Hybridization was performed overnight at 56.5C in 50% (v/v) formamide, 4× SSC, 2× Denhardt’s solution, 500 μg of in vitro transcribed eIF3f anti-sense or sense cRNA/ml, and 10% dextran sulfate (w/v). The final concentration of the digoxigenin-labeled—probe (antisense and sense) was approximately 2 ng/μl. After hybridization, the sections were washed and treated with RNase (Roche Applied Science, Indianapolis, IN). The samples were then incubated with an antidigoxigenin antibody conjugated with alkaline phosphatase (Roche Diagnostics; dilution, 1:500). For color reaction, 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Sigma, Buchs, Switzerland) were used. The slides were then counterstained with methylen green. For control experiments, the slides were pre-incubated with RNase or with the eIF3f sense probes. Pretreatment of the slides with RNase abolished the hybridization signal produced by the anti-sense probe. Furthermore, incubation with the sense probe failed to produce ISH signals.
For ISH, anti-sense and sense eIF3f cRNA probes were generated by in vitro transcription using pBluescript-eIF3f construct. Full length eIF3f cDNA was cloned into pBluescript II KS vector using EcoRI/XhoI restriction sites. pBluescript vector carries T3 and T7 promoters for generating sense and antisense eIF3f cRNA probes. After linearization, the eIF3f cRNA was transcribed using the Ribomax system (Promega Corporation, Madison, WI). The sense and antisense eIF3f cRNA probes were labeled with digoxigenin [22,24,25]. The digoxigenin-labeled antisense and sense probes were stored at −80°C until further use.
Immunohistochemistry Analysis
eIF3f antibody was developed by Rockland as described previously [18]. Biotinylated anti-goat antibody raised in horse was purchased from Vector Laboratories (Burlingame, CA). Paraffin-embedded pancreatic cancer tissue sectioned at 5 μm or pancreatic cancer TMA were deparaffinized in xylene, followed by rehydration in graded series of ethanol, ending with water immersion. Antigen retrieval was performed by microwave exposure in sodium citrate buffer (0.01 M, pH 6.1). Endogenous peroxidase blocking was performed with 3% H2O2 in methanol and sections were blocked with 1.5% normal horse serum (Vector Laboratories). The sections were then incubated with a goat polyclonal anti-eIF3f antibody (1:200), followed by a biotinylated secondary anti-goat antibody (1:200) (Vector Laboratories). Sections were then treated with Vectastain Elite ABC Reagent, diaminobenzidine (DAB) activated with H2O2 used according to the manufacturer’s instructions (Vector Laboratories). The slides were finally counterstained with hematoxylin and coverslipped.
The tissue array was evaluated by light microscopic examination and the intensity of immunostaining in each core was assessed by a pathologist (AB) using the scoring system described. The intensity of immunostaining in each core was graded as (0) negative, (1) weak, (2) moderate, or (3) strong [26]. The proportion of cells staining positively was also assessed as percentage. The score was then calculated as the numbers representing intensity times the percentage of cells stained.
Statistical Analysis
Most data are reported as mean ± s.d. When appropriate, differences between two groups were compared by the t-test. Differences were considered significant at P < 0.05. Gene copy number analysis data was reported as medians and was compared between normal tissues and pancreatic cancer tissues using the two-sample Wilcoxon rank sum (Mann–Whitney) test and considered significant at P < 0.05.
RESULTS
Allelic Loss of the Chromosome Region Encompassing eIF3f Gene in Human Pancreatic Cancer Specimens
We studied 32 pancreatic cancer tissues. The clinical and pathological characteristics of the primary pancreatic tumors and derived metastasis are described in Table 1. We captured tumor and normal samples using LCM, an example of micro-dissected tissues is shown in Figure 1. In order to explore allelic loss of eIF3f gene on chromosome 11p15.4, three microsatellite markers were chosen in close proximity to the eIF3f gene. These markers were chosen according to the Genebridge4 and Stanford G3 and for maximal heterozygosity. The results of LOH for each microsatellite are shown in Table 2. A representative genotyping profile of D11S1338 is shown in Figure 2. All cases were informative for at least two microsatellite markers. Despite one sample that we had difficulty to obtain PCR product for two of the markers, we found that the highest prevalence LOH was 93% observed on D11S 1318 microsatellite marker whereas the lowest was 71% on D11S1331. Out of 31 samples studied on D11S1318 marker, all were informative, and out of the informative cases, 29 samples (93%) were positive. Out of 31 samples studied on D11S1338 marker, 28 were informative, and out of the informative cases, 21 samples (75%) were positive; whereas out of 32 samples studied on D11S1331 marker, all were informative, and out of the informative cases, 23 samples (71%) were positive. The average fractional allele loss (FAL) was 0.78, calculated as the number of chromosomal arms having allelic loss divided by the total number of informative chromosomal arms [19]. These results indicate the chromosome region between D11S1318 and D11S1338 microsatellite markers encompassing eIF3f gene could be the region of allelic loss on chromosome band region 11p15.4 in human pancreatic cancer.
Table 1.
Clinical and Pathological Characteristics of the Human Pancreatic Cancer Specimens
| Pancreatic cases | Local invasion | Metastasis | Type of pancreatic cancer
(other than adenocarcinoma) |
|---|---|---|---|
| Frozen samples | |||
| 1 | Yes | Yes | |
| 2 | Yes | Yes | |
| 3 | Yes | Yes | |
| 4 | Yes | Yes | |
| Paraffin-embedded cases | |||
| 5 | No | No | |
| 6 | Yes | No | |
| 7 | Yes | No | Mucinous cystic carcinoma |
| 8 | Yes | Yes | |
| 9 | Yes | Yes | |
| 10 | No | Yes | |
| 11 | Yes | No | |
| 12 | Yes | Yes | |
| 13 | Yes | Yes | |
| 14 | No | No | Papillary carcinoma |
| 15 | Yes | No | |
| 16 | Yes | No | |
| 17 | No | No | |
| 18 | Yes | Yes | |
| 19 | Yes | Yes | Acinar cell carcinoma |
| 20 | No | No | |
| 21 | Yes | Yes | |
| 22 | Yes | No | Islet cell carcinoma |
| 23 | Yes | Yes | Islet cell carcinoma |
| 24 | No | Yes | |
| 25 | No | Yes | |
| 26 | Yes | Yes | |
| 27 | Yes | Yes | |
| 28 | No | Yes | |
| 29 | Yes | Yes | |
| 30 | Yes | Yes | |
| 31 | Yes | Yes | |
| 32 | Yes | Yes | |
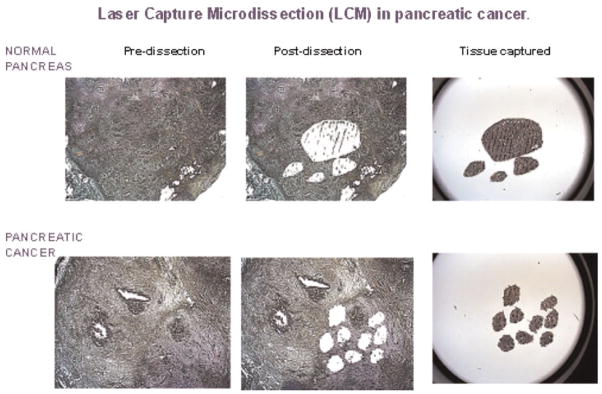
Figure 1.
Representative examples of LCM in pancreatic cancer and normal tissues captured on the caps and the remaining tissues. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table 2.
Detailed LOH Analysis of Three Different Microsatellite Markers Encompassing eIF3f Gene Locus at 11p15.4 in Pancreatic Cancer

|
Figure 2.

A representative example of the genotyping profile at the D11S1338 locus. The electropherograms show the disappearance of one allele peak area in pancreatic cancer tissue.
Decreased eIF3f Gene Copy Number in Pancreatic Cancer Tissues
To further investigate whether eIF3f gene is specifically lost in pancreatic cancer, eIF3f gene copy number was studied in 12 pancreatic cancer samples and 10 normal tissues using quantitative real time PCR analysis [21,27]. The relative gene copy number of eIF3f gene was normalized against β-actin gene copy number. The median of the eIF3f relative gene copy in the pancreatic cancer samples was 0.24, whereas the median of the eIF3f relative gene copy in the normal tissues was 1.0 with a tumor/normal ratio of 0.24 (Figure 3). There was a statistically significant decrease of eIF3f relative gene copy number in pancreatic cancer compared to normal tissues (P = 0.021). Taken together with the LOH results, we are able to confirm the allele loss of eIF3f gene in human pancreatic cancer.
Figure 3.
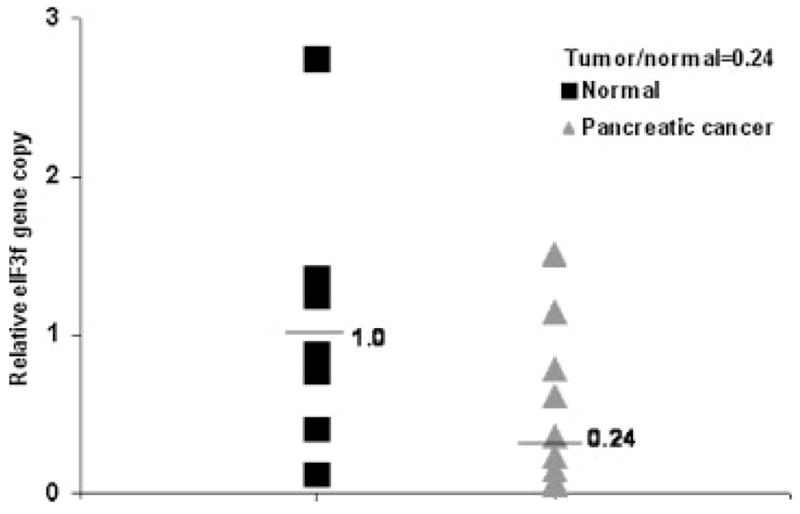
Decreased eIF3f gene copy number in pancreatic cancer specimens. Laser captured microdissected pancreatic cancer tissues were collected and genomic DNAs were isolated. Relative eIF3f gene copy number was analyzed by quantitative real time PCR. The numbers are expressed as a ratio of eIF3f gene copy number over β-actin gene. We analyzed 10 normal samples and 12 pancreatic cancer tissues in triplicate.
Previous studies by our group did not identify any mutations in the eIF3f gene exons, except exon 1 in melanoma cells (data not shown). We also sequenced the exon 1 and promoter region of the eIF3f gene in two pancreatic cancer cell lines (BxPC3 and MiaPaCa-2) and five pancreatic cancer tissue specimens. We did not find any mutation in the cell lines and tissues, except a deletion of T at position −224 in the promoter region of eIF3f gene in one case of pancreatic cancer tissue. However, this deletion also exists in normal skin and lung tissues (data not shown). These data suggest that mutation does not appear to be the mechanism of decreased eIF3f expression in pancreatic cancer.
Decreased eIF3f mRNA in Pancreatic Cancer Tissues
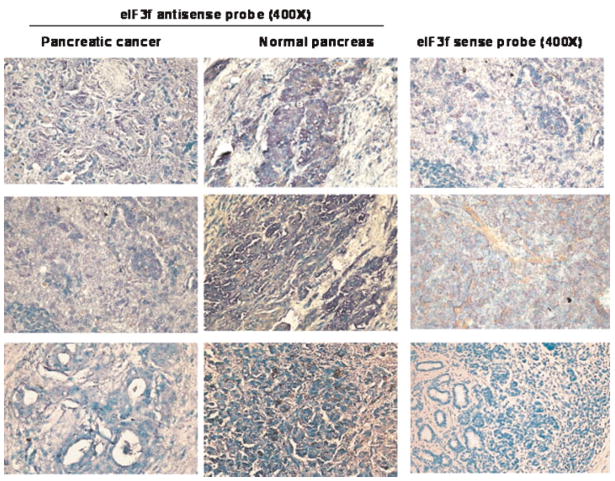
We have shown that eIF3f expression is decreased in pancreatic cancer using a cDNA cancer profiling array and quantitative real time RT-PCR [17]. To further confirm that eIF3f mRNA is decreased in human pancreatic cancer in situ, RNA ISH was performed in normal and pancreatic cancer tissue specimens. It is clear that in the normal pancreas, moderate to strong eIF3f mRNA signals were present in the cytoplasm of normal pancreas cells (Figure 4, middle panels, eIF3f mRNA signal is shown in blue color). In pancreatic cancer samples, eIF3f mRNA was only weakly present in the cytoplasm of cancer cells (Figure 4, left panels). In the control experiments, no eIF3f mRNA signals were obtained using the eIF3f sense probe (Figure 4, right panels). These results further support our previous observations that the expression of eIF3f is decreased in pancreatic cancer tissues.
Figure 4.
eIF3f mRNA is decreased in pancreatic cancer. RNA in situ hybridization was performed in normal and pancreatic cancer tissue specimens. eIF3f sense probe was served as negative control. The positive signal is shown in blue. The slides were counterstained with methylene green. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Decreased eIF3f Protein Level in Pancreatic Cancer Tissues
A pancreatic cancer TMA was used for the immunohistochemistry analysis of eIF3f protein expression using eIF3f specific antibody. The mean score of eIF3f protein expression was significantly decreased in well-differentiated and moderately differentiated pancreatic cancer tissues compared to adjacent normal pancreas (P < 0.05; Figure 5 and Table 3). In poorly differentiated adenocarcinoma, eIF3f expression is lower than normal pancreas, but not statistically significant. In addition, eIF3f appears to localize exclusively in the cytoplasm. Therefore, we were able to confirm that eIF3f protein was significantly decreased in human pancreatic cancer tissues.
Figure 5.
eIF3f protein is decreased in pancreatic cancer. Immunohistochemistry was performed on a pancreatic cancer tissue microarray using eIF3f antibody. Representative immunohistochemistry analysis of eIF3f protein in normal pancreas and different stages of pancreatic cancer tissues were shown. Magnification: 400×. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table 3.
Mean Score of eIF3f Protein Expression in Normal Pancreas and Pancreatic Cancer Tissues
| Tissue type | # of cases | Score | P-valuea |
|---|---|---|---|
| Normal pancreas | 11 | 2.42 ± 0.57 | |
| Well-differentiated adenocarcinoma | 8 | 1.55 ± 0.83 | 0.026 |
| Moderately differentiated adenocarcinoma | 19 | 1.66 ± 0.60 | 0.002 |
| Poorly differentiated adenocarcinoma | 9 | 1.89 ± 0.78 | 0.110 |
Compared to normal pancreas.
DISCUSSION
It has been previously demonstrated that the loss of heterozygosity (LOH) of alleles on chromosome 11p15 is a common event in numerous types of tumors including pancreatic cancers [28–37], which suggests that 11p15 may be involved in tumor development. Recently, one group found 50% allelic loss at 11p15.4 in pancreatic acinar cell carcinoma by LOH [38]. Another group found deletions in 11p15 in focal hyperplasia of islet cells of the pancreas in sporadic persistent hyperinsulinemic hypoglycemia of infancy but not in normal pancreatic cells [35]. Deletions at 11p15.4 were also found by other groups in ductal pancreatic cancer as well as endocrine pancreatic cancer [36,37]. The higher frequency of LOH observed in this region in malignant tissues as compared to benign ones suggests that 11p15.4 chromosome region is the target of genetic alterations. However, which specific gene is responsible for the tumorigenesis is not clear.
We have demonstrated that eIF3f expression is significantly decreased at the mRNA level in many human tumors including pancreatic cancer [17]. However, the molecular mechanism of the decrease expression is unknown. The eIF3f gene is located at chromosome region 11p15.4. We can demonstrate that loss of eIF3f gene allele is associated with the decreased expression of eIF3f in pancreatic cancer. Similarly, loss of heterozygosity of eIF3e (INT6) gene has been reported in breast carcinomas [39]. Human eIF3f gene is 8.0 Mb long and has 8 exons. We did not find any mutation in exon 1 and promoter of eIF3f gene, except a polymorphism deletion of T at position −224. There are reports of polymorphism in the promoter regulate gene transcription [40]. A search for the consensus sequences of transcription factors in the putative eIF3f gene promoter region revealed a possible binding site for REB1 at nt −224 to −217. Therefore, it is possible that the deletion at nt −224 in the promoter region of eIF3f gene may have functional significance. We also can not rule out mutations in exons 2–8. Further studies are needed to clarify this issue.
In the current study, we were able to demonstrate both at the protein and mRNA level that eIF3f expression is decreased in pancreatic cancer tissues. Decrease of eIF3f protein is even observed in the well-differentiated adenocarcinoma specimens. Expression of another eIF3 subunit that was shown to be decreased in human tumors is eIF3e (INT-6) [16]. Enforced expression of eIF3f inhibits translation, cell growth, cell proliferation and induces apoptosis in melanoma and pancreatic cancer cells [17]. Knockdown of eIF3f prevents apoptosis in tumor cells [17]. Recent studies from Zhang et al. [4] also showed decreased cell growth, cell proliferation, colony formation and increased apoptosis in eIF3f-over-expressing NIH3T3 cells. These findings suggest that eIF3f may be an important negative regulator of cell growth and proliferation. The mechanisms by which decreased eIF3f expression contributes to pancreatic cancer development are not clear. One possible explanation is that decreased eIF3f may lead to increased eIF3 complex level and eIF3 activity, which in turn stimulates translation of proteins involved in cell proliferation. eIF3f was found to interact with mTOR and S6K1 [41,42]. Therefore, another possible mechanism is that decreased eIF3f may deregulate the function of mTOR pathway, which in turn leads to increased translation of oncogenic proteins and malignant transformation.
Acknowledgments
We would like to acknowledge the G.I. SPORE program at the Arizona Cancer Center and the SPORE tissue core. We also thank Ray Nagle, Thomas Grogan, Ronald Schifman, Walden Browne, Robert Calaluce, Catherine Rangel, Yvette Frutiger and Roshni Mehta for providing the pancreatic cancer tissue array. This work was supported by G.I. SPORE Grant CA95060 from the National Cancer Institute, ABRC grant (5-102), and grant CA70145 (MN).
Abbreviations
- eIF3f
eukaryotic initiation factor 3 subunit f
- RT-PCR
reverse transcription polymerase chain reaction
- LOH
loss of heterozygosity
- TMA
tissue microarray
- LCM
laser capture micro-dissection
- ISH
in situ hybridization
References
- 1.Cowgill SM, Muscarella P. The genetics of pancreatic cancer. Am J Surg. 2003;186:279–286. doi: 10.1016/s0002-9610(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 2.Akerele CE, Rybalova I, Kaufman HL, et al. Current approaches to novel therapeutics in pancreatic cancer. Invest New Drugs. 2003;21:113–129. doi: 10.1023/a:1022936914328. [DOI] [PubMed] [Google Scholar]
- 3.Gold EB, Goldin SB. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am. 1998;7:67–91. [PubMed] [Google Scholar]
- 4.Zhang L, Pan X, Hershey JW. Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J Biol Chem. 2007;282:5790–5800. doi: 10.1074/jbc.M606284200. [DOI] [PubMed] [Google Scholar]
- 5.Rosenwald IB. The role of translation in neoplastic transformation from a pathologist’s point of view. Oncogene. 2004;23:3230–3247. doi: 10.1038/sj.onc.1207552. [DOI] [PubMed] [Google Scholar]
- 6.Avdulov S, Li S, Michalek V, et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–563. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Mamane Y, Petroulakis E, Rong L, et al. eIF4E—From translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 8.Donze O, Jagus R, Koromilas AE, et al. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pestova TV, Hellen CU. Functions of eukaryotic factors in initiation of translation. Cold Spring Harb Symp Quant Biol. 2001;66:389–396. doi: 10.1101/sqb.2001.66.389. [DOI] [PubMed] [Google Scholar]
- 10.Lin L, Holbro T, Alonso G, et al. Molecular interaction between human tumor marker protein p150, the largest subunit of eIF3, and intermediate filament protein K7. J Cell Biochem. 2001;80:483–490. doi: 10.1002/1097-4644(20010315)80:4<483::aid-jcb1002>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Pincheira R, Chen Q, Zhang JT. Identification of a 170-kDa protein over-expressed in lung cancers. Br J Cancer. 2001;84:1520–1527. doi: 10.1054/bjoc.2001.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothe M, Ko Y, Albers P, et al. Eukaryotic initiation factor 3 p110 mRNA is overexpressed in testicular seminomas. Am J Pathol. 2000;157:1597–1604. doi: 10.1016/S0002-9440(10)64797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nupponen NN, Porkka K, Kakkola L, et al. Amplification and overexpression of p40 subunit of eukaryotic translation initiation factor 3 in breast and prostate cancer. Am J Pathol. 1999;154:1777–1783. doi: 10.1016/S0002-9440(10)65433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayeur GL, Hershey JW. Malignant transformation by the eukaryotic translation initiation factor 3 subunit p48 (eIF3e) FEBS Lett. 2002;514:49–54. doi: 10.1016/s0014-5793(02)02307-4. [DOI] [PubMed] [Google Scholar]
- 15.Marchetti A, Buttitta F, Miyazaki S, et al. Int-6, a highly conserved, widely expressed gene, is mutated by mouse mammary tumor virus in mammary preneoplasia. J Virol. 1995;69:1932–1938. doi: 10.1128/jvi.69.3.1932-1938.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchetti A, Buttitta F, Pellegrini S, et al. Reduced expression of INT-6/eIF3-p48 in human tumors. Int J Oncol. 2001;18:175–179. doi: 10.3892/ijo.18.1.175. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Kahle A, Hershey JW, et al. Decreased expression of eukaryotic initiation factor 3f deregulates translation and apoptosis in tumor cells. Oncogene. 2006;25:4923–4936. doi: 10.1038/sj.onc.1209495. [DOI] [PubMed] [Google Scholar]
- 18.Shi J, Feng Y, Goulet AC, et al. The p34cdc2-related cyclin-dependent kinase 11 interacts with the p47 subunit of eukaryotic initiation factor 3 during apoptosis. J Biol Chem. 2003;278:5062–5071. doi: 10.1074/jbc.M206427200. [DOI] [PubMed] [Google Scholar]
- 19.Trovato M, Ulivieri A, Dominici R, et al. Clinico-pathological significance of cell-type-specific loss of heterozygosity on chromosome 7q21: Analysis of 318 microdissected thyroid lesions. Endocr Relat Cancer. 2004;11:365–376. doi: 10.1677/erc.0.0110365. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Flood SJ, Marmaro J, et al. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto M, Banerjee D, Menon LG, et al. Overexpression of E2F-1 in lung and liver metastases of human colon cancer is associated with gene amplification. Cancer Biol Ther. 2004;3:395–399. [PubMed] [Google Scholar]
- 22.Guo XZ, Friess H, Di Mola FF, et al. KAI1, a new metastasis suppressor gene, is reduced in metastatic hepatocellular carcinoma. Hepatology. 1998;28:1481–1488. doi: 10.1002/hep.510280606. [DOI] [PubMed] [Google Scholar]
- 23.Panoskaltsis-Mortari A, Bucy RP. In situ hybridization with digoxigenin-labeled RNA probes: Facts and artifacts. Bio-techniques. 1995;18:300–307. [PubMed] [Google Scholar]
- 24.Friess H, Lu Z, Graber HU, et al. bax, but not bcl-2, influences the prognosis of human pancreatic cancer. Gut. 1998;43:414–421. doi: 10.1136/gut.43.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.di Mola FF, Friess H, Scheuren A, et al. Transforming growth factor-betas and their signaling receptors are coexpressed in Crohn’s disease. Ann Surg. 1999;229:67–75. doi: 10.1097/00000658-199901000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dundas SR, Lawrie LC, Rooney PH, et al. Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol. 2005;205:74–81. doi: 10.1002/path.1672. [DOI] [PubMed] [Google Scholar]
- 27.Coupry I, Monnet L, Attia AA, et al. Analysis of CBP (CREBBP) gene deletions in Rubinstein-Taybi syndrome patients using real-time quantitative PCR. Hum Mutat. 2004;23:278–284. doi: 10.1002/humu.20001. [DOI] [PubMed] [Google Scholar]
- 28.O’Briant KC, Bepler G. Delineation of the centromeric and telomeric chromosome segment 11p15.5 lung cancer suppressor regions LOH11A and LOH11B Genes Chromosomes. Cancer. 1997;18:111–114. doi: 10.1002/(sici)1098-2264(199702)18:2<111::aid-gcc5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Kahng YS, Lee YS, Kim BK, et al. Loss of heterozygosity of chromosome 8p and 11p in the dysplastic nodule and hepatocellular carcinoma. J Gastroenterol Hepatol. 2003;18:430–436. doi: 10.1046/j.1440-1746.2003.02997.x. [DOI] [PubMed] [Google Scholar]
- 30.Sweetser DA, Chen CS, Blomberg AA, et al. Loss of heterozygosity in childhood de novo acute myelogenous leukemia. Blood. 2001;98:1188–1194. doi: 10.1182/blood.v98.4.1188. [DOI] [PubMed] [Google Scholar]
- 31.Karnik P, Paris M, Williams BR, et al. Two distinct tumor suppressor loci within chromosome 11p15 implicated in breast cancer progression and metastasis. Hum Mol Genet. 1998;7:895–903. doi: 10.1093/hmg/7.5.895. [DOI] [PubMed] [Google Scholar]
- 32.Eccles DM, Gruber L, Stewart M, et al. Allele loss on chromosome 11p is associated with poor rvival in ovarian cancer. Dis Markers. 1992;10:95–99. [PubMed] [Google Scholar]
- 33.Henry I, Grandjouan S, Couillin P, et al. Tumor-specific loss of 11p15.5 alleles in del11p13 Wilms tumor and in familial adrenocortical carcinoma. Proc Natl Acad Sci USA. 1989;86:3247–3251. doi: 10.1073/pnas.86.9.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr NJ, Chun YH, Yun K, et al. Pancreatoblastoma is associated with chromosome 11p loss of heterozygosity and IGF2 overexpression. Med Pediatr Oncol. 2002;39:52–54. doi: 10.1002/mpo.10050. [DOI] [PubMed] [Google Scholar]
- 35.Fournet JC, Mayaud C, de Lonlay P, et al. Unbalanced expression of 11p15 imprinted genes in focal forms of congenital hyperinsulinism: Association with a reduction to homozygosity of a mutation in ABCC8 or KC NJ11. Am J Pathol. 2001;158:2177–2184. doi: 10.1016/S0002-9440(10)64689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson KM, Harris JE. Selected features of nonendocrine pancreatic cancer. Exp Biol Med (Maywood) 2001;226:521–537. doi: 10.1177/153537020122600604. [DOI] [PubMed] [Google Scholar]
- 37.Rigaud G, Missiaglia E, Moore PS, et al. High resolution allelotype of nonfunctional pancreatic endocrine tumors: Identification of two molecular subgroups with clinical implications. Cancer Res. 2001;61:285–292. [PubMed] [Google Scholar]
- 38.Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: Frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953–962. doi: 10.1016/s0002-9440(10)64917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyazaki S, Imatani A, Ballard L, et al. The chromosome location of the human homolog of the mouse mammary tumor-associated gene INT6 and its status in human breast carcinomas. Genomics. 1997;46:155–158. doi: 10.1006/geno.1997.4996. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Harris RB, Rosson D, et al. Functional analysis of human ornithine decarboxylase alleles. Cancer Res. 2000;60:6314–6317. [PubMed] [Google Scholar]
- 41.Harris TE, Chi A, Shabanowitz J, et al. mTOR-dependent stimulation of the association of eIF4G and eIF3 by insulin. EMBO J. 2006;25:1659–1668. doi: 10.1038/sj.emboj.7601047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holz MK, Ballif BA, Gygi SP, et al. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]