Abstract
An early (i.e., 15 min) single systemic administration of the 5-HT1A receptor agonist 8-OH-DPAT enhances behavioral recovery after experimental traumatic brain injury (TBI). However, acute administration of pharmacotherapies after TBI may be clinically challenging and thus the present study sought to investigate the potential efficacy of a delayed and chronic 8-OH-DPAT treatment regimen. Forty-eight isoflurane-anesthetized adult male rats received either a controlled cortical impact or sham injury and beginning 24 hrs later were administered 8-OH-DPAT (0.1 or 0.5 mg/kg) or saline vehicle (1.0 mL/kg) intraperitoneally once daily until all behavioral assessments were completed. Neurobehavior was assessed by motor and cognitive tests on post-operative days 1–5 and 14–19, respectively. The lower dose of 8-OH-DPAT (0.1 mg/kg) enhanced motor performance, acquisition of spatial learning, and memory retention vs. both the higher dose (0.5 mg/kg) and vehicle treatment (p < 0.05). These data replicate previous findings from our laboratory showing that 8-OH-DPAT improves neurobehavior after TBI, and extend those results by demonstrating that the benefits can be achieved even when treatment is withheld for 24 hrs. A delayed and chronic treatment regimen may be more clinically feasible.
Keywords: beam-walking, controlled cortical impact, functional recovery, learning and memory, Morris water maze, neurobehavior, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) affects 1.5 to 2 million individuals in the United States each year. Approximately 100,000 severe-TBI survivors endure long-term memory and/or physical impairments that require rigorous and costly rehabilitative therapy [18,41,58]. Treatment options for brain injury are limited and typically consist of augmenting or restoring dysfunctional neurotransmitter systems. Preclinical evaluation of various pharmacological agents has yielded several potential treatment candidates. For example, both agonists and antagonists affecting acetylcholine (ACh), dopamine (DA), and glutamate neurotransmission have shown marked benefits in the laboratory [10,12, 14,15,29,30,33,39]. Unfortunately, with the exception of a few small clinical studies [42,47,49,61], translating from bench to bedside has not yielded the same beneficial effects observed in the laboratory. This realization suggests that additional therapies or other neurotransmitter systems should be evaluated.
In part because of its widespread modulation of the major neurotransmitters ACh, DA, and glutamate, the serotonin (5-HT) system, and in particular the 5-HT1A receptor, is considered a significant pharmacological target for the treatment of various central nervous system (CNS) diseases [63]. While a plethora of reports exist showing benefits of 5-HT1A receptor stimulation in the treatment of anxiety and depression [2,44,45], there is a paucity of studies evaluating the role of this 5-HT receptor subtype on CNS trauma.
The few studies that do exist indicate that 5-HT1A receptor agonists exert beneficial effects. Administration of 5-HT1A receptor agonists before or after focal cerebral ischemia provides neuroprotection as evidenced by decreased histopathology [8,55,59]. A significant reduction in cortical lesion volume has also been reported following treatment with the 5-HT1A receptor agonist BAY x 3702 after subdural hematoma [1]. Studies from our laboratory using the controlled cortical impact (CCI) injury model, which produces many of the characteristics of human TBI [27], have demonstrated that an early and continuous infusion of the 5-HT1A receptor agonist repinotan HCL or a single administration of 8-OH-DPAT enhances cognitive recovery in a water maze task, decreases cortical lesion volume, and confers hippocampal neuron survival [5,28,32,34,35]. Taken together, these findings suggest that 5-HT1A receptor agonists are beneficial in a variety of brain injury models. However, while the benefits of this early therapeutic paradigm are compelling, the potential efficacy of delayed and chronic 8-OH-DPAT treatments after TBI is unknown. This issue is paramount given the secondary sequelae that are prevalent hours to days after TBI that affect the recovery process.
2. Materials and methods
2.1. Subjects
Forty-eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were housed in standard steel-wire mesh cages and maintained in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with free access to food and water. After one week of acclimatization the rats underwent beam-walk training and then were randomly assigned to one of the following group conditions: TBI + 8-OH-DPAT (0.1 mg/kg; n=12), TBI + 8-OH-DPAT (0.5 mg/kg; n=12), TBI + Vehicle (1 mL/kg; n=12), Sham + 8-OH-DPAT (0.1 mg/kg; n=4), Sham + 8-OH-DPAT (0.5 mg/kg; n=4), or Sham + Vehicle (1 mL/kg; n=4).
2.2. Surgery
A surgical level of anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane, respectively, in 2:1 N2O:O2 in a vented anesthesia chamber. Following endotracheal intubation the rats were secured in a stereotaxic frame and mechanically ventilated. Utilizing aseptic techniques a 6-mm craniectomy was made in the right hemisphere between bregma and lambda and from midline to the coronal ridge. A controlled cortical impact (CCI) of 2.8 mm tissue deformation at 4 m/sec produced an injury of moderate severity as previously described [9,26,32,34]. Sham control rats underwent all anesthetic and surgical manipulations except the impact. Anesthesia was discontinued immediately after CCI or sham injury and the incision was promptly closed with nylon sutures. The rats were subsequently extubated and acute neurological evaluations were performed. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996). Every attempt was made to limit the number of subjects used and to minimize suffering.
2.3. Acute neurological evaluation
Hind limb reflexive ability was assessed following the cessation of anesthesia by gently squeezing the rats’ paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to prone position.
2.4. Drug administration
8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) was purchased from Sigma-Aldrich (St. Louis, MO) and was prepared daily by dissolving in sterile physiological saline. 8-OH-DPAT (0.1 mg/kg or 0.5 mg/kg) or a comparable volume of vehicle (1 mL/kg saline) was administered intraperitoneally beginning 24 hr after CCI or sham injury and once daily until all behavioral evaluations were completed (i.e., post-operative day 19). On the days when behavioral assessments were conducted, treatments were administered 1 hr prior to testing by an experimenter unaware of group conditions. The doses of 8-OH-DPAT and route of administration were selected based on previous studies from our laboratory [5,28,35] showing this regimen to confer neuroprotection and promote behavioral recovery after TBI.
2.5. Motor performance
Beam-balance and beam-walk performances were assessed with well-established tests [5,10,26,28–35]. The beam-balance task consists of placing the rat on an elevated (90 cm) narrow wood beam (1.5 cm wide) and recording the time it remains on for a maximum of 60 sec. The beam-walk task, originally devised by Feeney and colleagues [13], consists of training/assessing rats using a negative-reinforcement paradigm to escape ambient light and white noise by traversing an elevated narrow wood beam (2.5 × 100 cm) and entering a darkened goal box situated at the opposite end. When the rat enters the goal box the adverse stimuli (i.e., light and noise) are terminated and thus serve as reinforcement for completing the task. Performance was assessed by recording both the elapsed time to traverse the beam as well as the distance traveled. The scoring criteria for distance traveled is based on a rating scale from 0 to 5, where 0 indicates an inability to ambulate beyond the start location, 1–4 corresponds to distal segments of 20, 40, 60, or 80 cm from the start point, respectively, and 5 corresponds with traversing the entire length of the beam (100 cm) and entering the goal box. Rats were tested for beam-balance and beam-walk performance on post-operative days 1–5 and were provided three trials (60 sec allotted time) per day on each task. The average daily scores for each subject were used in the statistical analyses.
2.6. Cognitive function
2.6.1. Acquisition of spatial learning
Spatial learning was assessed in a water maze task demonstrated to be sensitive to cognitive function/dysfunction after TBI [20,32,51,57] The maze was a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Acquisition of spatial learning was initiated on post-operative day 14 and continued until day 18. The paradigm consisted of providing a block of four daily trials for five consecutive days to locate the platform when it was submerged 2 cm below the water surface (i.e., invisible to the rat). For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials (4-min inter-trial interval). The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
One day after the final acquisition training session (Day 19), all rats were given a single probe trial to measure retention. Briefly, the platform was removed from the pool and the rats were placed in the maze from the location point most distal to the quadrant where the platform was previously situated (i.e., “target quadrant”) and allowed to freely explore the pool for 30 sec. Typically, rats that have learned the specific location of the escape platform exhibit a spatial bias and spend significantly more time in the target quadrant. The percent time spent in the target quadrant was used in the statistical analysis. Following the probe assessment, the rats were provided four additional trials to locate the platform when it was raised 2 cm above the water surface (i.e., visible to the rat). While this task has been used to test for non-hippocampal damage [3], its use in the present study was as a control procedure to determine the contributions of non-spatial factors (e.g., sensory-motor performance, motivation, and visual acuity) on water maze outcome.
The data were obtained using a spontaneous motor activity recording & tracking (SMART) system (San Diego Instruments, San Diego, CA).
2.7. Data analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statview 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). The acute neurological, probe trial, and swim speed data were analyzed by one-factor ANOVAs. When the overall ANOVA revealed a significant effect, the data were further analyzed with the Bonferroni/Dunn post-hoc test to determine specific group differences. The data are presented as the mean ± standard error (SE) and are considered significant when corresponding p values are ≤ 0.05 or as determined by the Bonferroni/Dunn statistic after adjusting for multiple comparisons.
3. Results
One sham control rat received an inadvertent dura mater tear during the craniectomy and was omitted from the study. Hence, the statistical analyses are based on 47 rats.
3.1. Acute neurological function
No significant differences were observed among the TBI groups in hind limb withdrawal latency in response to a brief paw pinch [range 163.0 ± 6.3 sec to 177.2 ± 4.9 sec, p > 0.05] or for return of righting ability [range 369.1 ± 14.6 sec to 403.5 ± 14.8 sec, p > 0.05] after the cessation of anesthesia. The lack of significant group differences in these acute neurological indices suggests that all rats experienced an equivalent level of brain injury and anesthesia.
3.2. Motor function
3.2.1 Beam-balance
No pre-surgical differences were observed among groups as all rats were capable of balancing on the beam for the allotted 60 sec (Fig. 1). However, following TBI, a repeated measures ANOVA revealed significant Group [F 5, 41 = 6.320, p = 0.002] and Day [F 5, 205 = 5.735, p < 0.0001] differences, as well as a significant Group x Day interaction [F 25, 205 = 1.635, p = 0.034]. No significant group difference was revealed between the TBI + 8-OH-DPAT (0.1 mg/kg) and TBI + Vehicle [p = 0.085], even though the latter had not reached baseline by the end of testing. However, both TBI + 8-OH-DPAT (0.1 mg/kg) and TBI + Vehicle were markedly improved vs. TBI + 8-OH-DPAT (0.5 mg/kg) [p < 0.0001 and p = 0.0016, respectively]. No differences were observed among the sham groups, regardless of treatment, as all were able to maintain their balance for the full 60 sec [p > 0.05]. Additionally, the shams differed from both the TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg) groups [p < 0.05], but not the TBI + 8-OH-DPAT (0.1 mg/kg) group [p > 0.05].
Fig. 1.
Mean (± SE) time (sec) balancing on an elevated narrow beam prior to, and after, TBI or Sham injury. *p < 0.05 vs. TBI + 8-OH-DPAT (0.5 mg/kg). **p < 0.05 vs. TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg), but not TBI + 8-OH-DPAT (0.1 mg/kg).
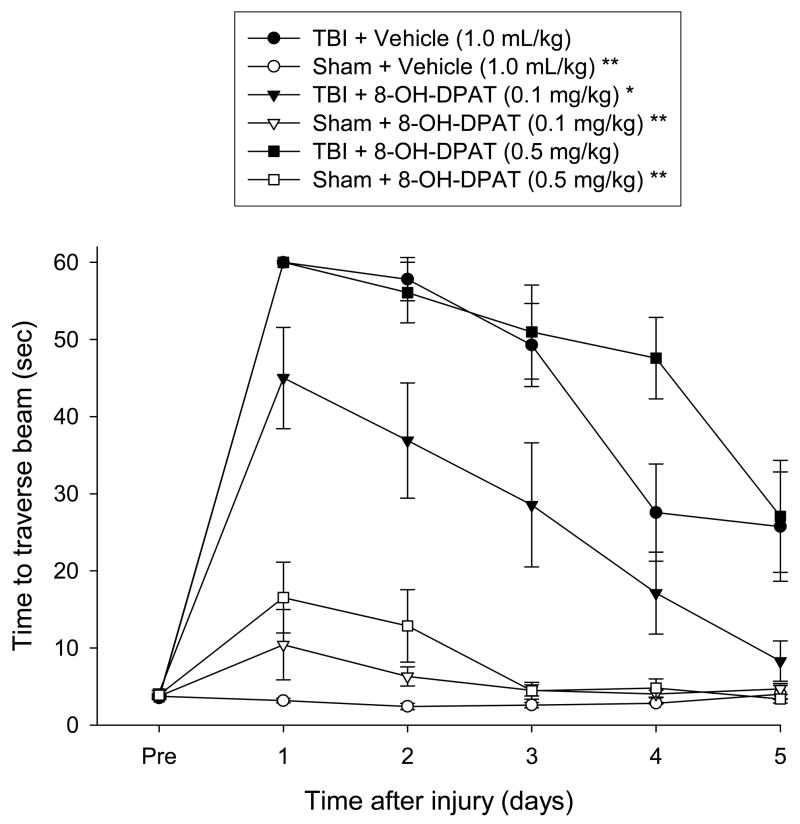
3.2.2 Beam-walk (time to traverse)
Similar to beam-balance there were no pre-surgical differences in time to traverse the beam among groups as all rats were proficient and reached the goal box in approximately 5 sec (Fig. 2). The ANOVA revealed a significant Group [F 5, 41 = 18.400, p < 0.0001] and Day [F 5, 205 = 21.339, p < 0.0001] differences, as well as a significant Group x Day interaction [F 25, 205 = 4.143, p < 0.0001]. The TBI + 8-OH-DPAT (0.1 mg/kg) group was able to traverse the beam significantly quicker (i.e., demonstrating a facilitation of motor recovery) than both the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups [p < 0.0001 and p < 0.0002, respectively], neither of which differed from one another [P = 0.331]. While there were no statistically significant differences among the Sham groups [p > 0.05], the Sham + 8-OH-DPAT (0.5 mg/kg), and to a lesser extent the Sham + 8-OH-DPAT (0.1 mg/kg), had slightly longer traversal times on post-operative days 1 and 2 vs. Sham + Vehicle (Fig. 2). However, despite the slight differences, all Sham groups differed from all TBI groups [p < 0.05].
Fig. 2.
Mean (± SE) time (sec) to traverse an elevated narrow beam prior to, and after, TBI or Sham injury. *p < 0.05 vs. TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg). **p < 0.05 vs. all TBI groups.
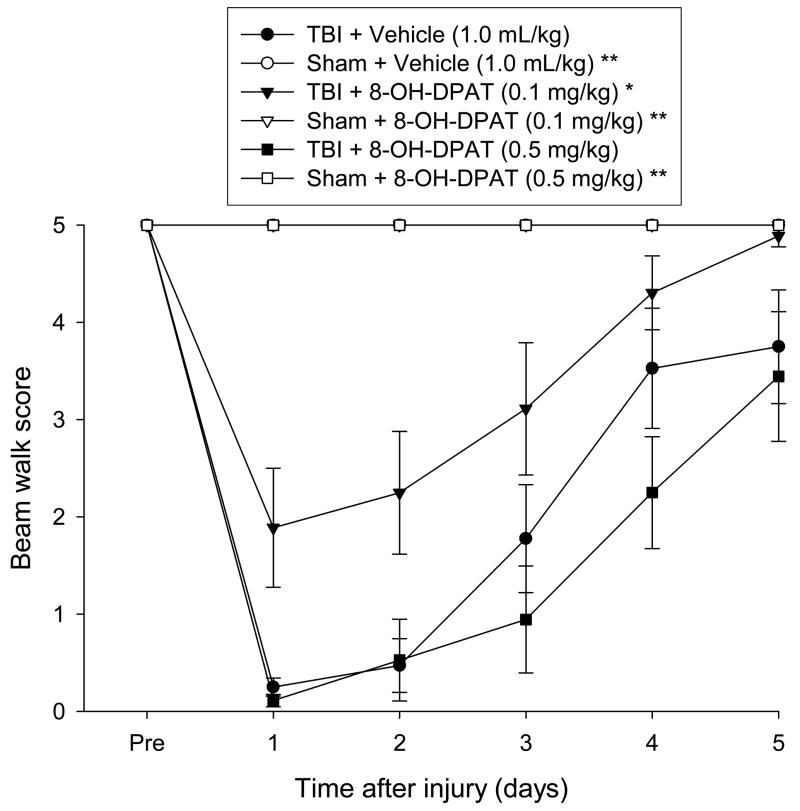
3.2.3 Beam-walk (distance traveled score)
No pre-surgical differences were observed among groups as all rats were capable of traversing the entire length of the beam for a score of 5 (Fig. 3). However, a repeated measures ANOVA performed on the data after the CCI injury revealed significant Group [F 5, 41 = 15.506, p = 0.0001] and Day [F 5, 205 = 16.011, p < 0.0001] differences, as well as a significant Group × Day interaction [F 25, 205 = 3.955, p < 0.0001]. The TBI + 8-OH-DPAT (0.1 mg/kg) group traversed greater distances on the beam (i.e., demonstrating a facilitation of motor recovery) vs. both the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups [p < 0.0001 and p < 0.0007, respectively]. Specifically, by the last day of testing, the TBI + 8-OH-DPAT (0.1 mg/kg) group had returned to baseline performance, while the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups were only capable of traversing about 60–80 cm down the beam for scores of 3–4 before the allotted time expired. There was no difference between the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups [p = 0.196]. All Sham groups differed from the TBI groups [p < 0.05], but not from one another [p > 0.05].
Fig. 3.
Mean (± SE) distance traveled along an elevated narrow beam prior to, and after, TBI or Sham injury. *p < 0.05 vs. TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg). **p < 0.05 vs. all TBI groups.
3.3. Cognitive function
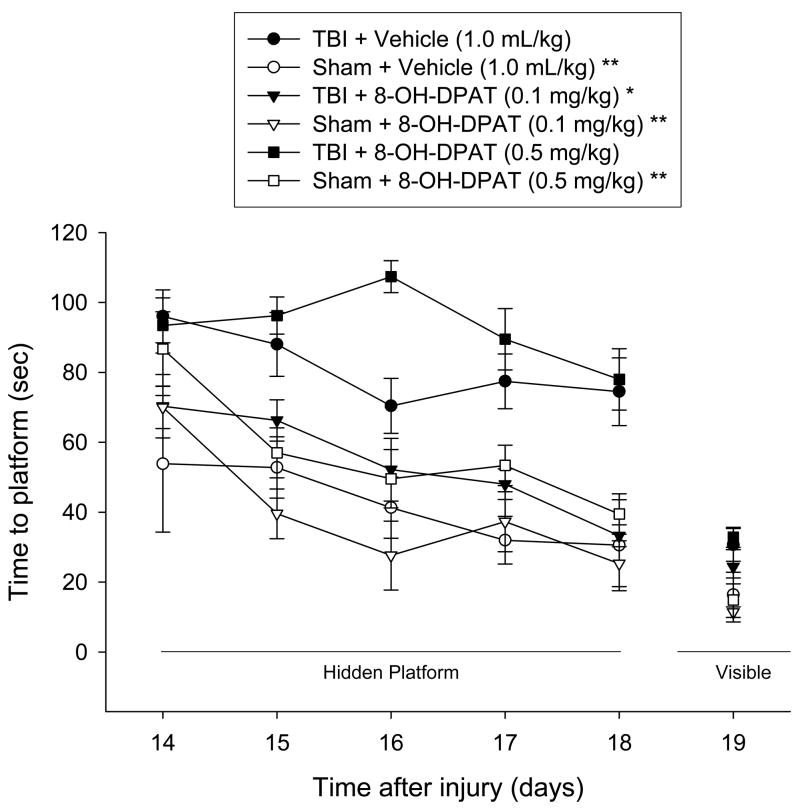
3.3.1. Acquisition of spatial learning
Analysis of the post-CCI injury water maze data revealed significant Group [F 5, 41 = 12.438, p < 0.0001] and Day [F 4, 164 = 12.325, p < 0.0001] differences. Post-hoc analysis indicated that the TBI + 8-OH-DPAT (0.1 mg/kg) group was able to locate the hidden platform significantly quicker over time vs. both the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups [p < 0.0001]. While no statistical difference was revealed overall between the TBI+8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups [p = 0.017; adjusted for multiple comparisons], a single day ANOVA revealed a marked difference on the third day of training (i.e., post-operative day 16) with the TBI + 8-OH-DPAT (0.5 mg/kg) group performing worse than the TBI + Vehicle group [p = 0.0008]. As depicted in Fig. 4, there were no statistically significant differences among the Sham groups [p > 0.05], which differed from both the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups [p < 0.05], but not the TBI + 8-OH-DPAT (0.1 mg/kg) [p > 0.05].
Fig. 4.
Mean (± SE) time (sec) to locate either a hidden (submerged) or visible (raised) platform in a water maze. *p < 0.05 vs. TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg). **p < 0.05 vs. TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg), but not TBI + 8-OH-DPAT (0.1 mg/kg). No significant differences were revealed for time to locate the visible platform.
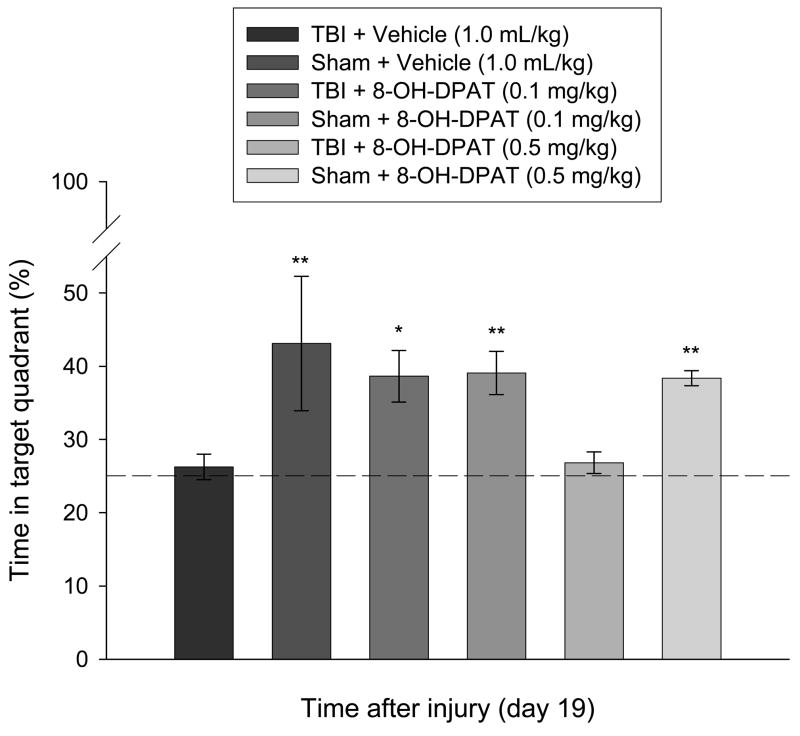
3.3.2. Probe trial (i.e., memory retention)
Analysis of memory retention revealed a significant difference among groups [p = 0.0006], which was attributed to the three Sham groups and the TBI + 8-OH-DPAT (0.1 mg/kg) group spending significantly more time in the target quadrant vs. both the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups. Specifically, as depicted in Fig. 5, the TBI + 8-OH-DPAT (0.5 mg/kg) and TBI + Vehicle groups spent 26.8 ± 1.4 % and 26.2 ± 1.7 % of the time, respectively, in the target quadrant, which is at the level of random chance performance and is in marked contrast to the 38.6 ± 3.5 % time devoted by the TBI + 8-OH-DPAT (0.1mg/kg) group. The Sham + Vehicle, Sham + 8-OH-DPAT (0.1mg/kg), and Sham + 8-OH-DPAT (0.5mg/kg) groups had times of 43.1 ± 9.1, 39.0 ± 2.9, and 38.3 ± 1.0 %, respectively, which did not differ from one another or with the TBI + 8-OH-DPAT (0.1 mg/kg) group [p > 0.05].
Fig. 5.
Mean (± SE) percentage of time spent in the target quadrant (i.e., where platform was previously located) following a single probe trial 19 days after cortical impact or sham injury. The dotted line represents performance at the chance level (25%). *p < 0.05 vs. TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg). **p < 0.05 vs. TBI + Vehicle and TBI + 8-OH-DPAT (0.5 mg/kg), but not TBI + 8-OH-DPAT (0.1 mg/kg).
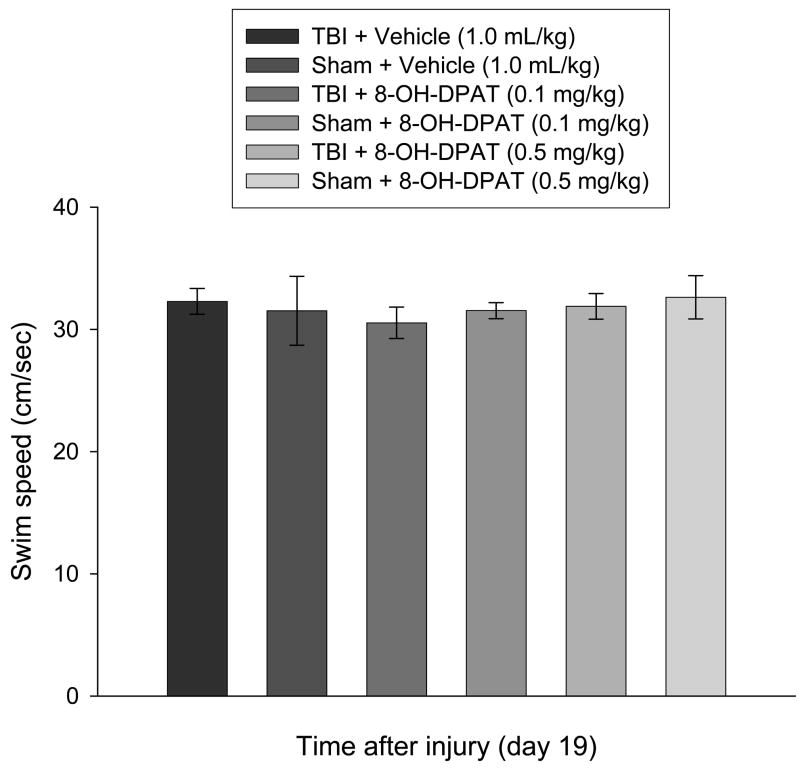
3.3.3. Swim speed and visible platform performance
No significant differences in swim speed (range = 30.5 ± 1.3 cm/sec to 32.9 ± 2.5 cm/sec; Fig. 6) or visible platform acquisition (Fig. 4) were observed among the groups, suggesting that neither motor impairments nor visual disparities influenced the assessment of place learning.
Fig. 6.
Mean (± SE) swim speed (cm/sec). No differences were observed among groups, regardless of injury assignment or drug treatment, suggesting that spatial learning was not influenced by motor deficits.
4. Discussion
Previous studies from our laboratory have shown that a single and early (i.e., 15 min post-TBI) systemic administration of the 5-HT1A receptor agonist 8-OH-DPAT (0.5 mg/kg) enhances behavioral recovery, decreases lesion volume, and increases hippocampal CA3 survival [5,28,32,35]. While this experimental paradigm has produced consistent results, the clinical relevance of very early treatment is not feasible. A recent study designed to elucidate the therapeutic window after a single administration of 8-OH-DPAT found that delaying treatment by even one hr after TBI proved to be ineffective as no differences were observed in any of the behavioral outcome measures vs. saline-treated controls [5]. Those data suggest that an early and narrow critical period exists for the behavioral recovery afforded by a single 8-OH-DPAT treatment paradigm. Hence, the goal of the current study was to provide 8-OH-DPAT (0.1 mg/kg and 0.5 mg/kg) in a delayed and chronic design. The rationale was that if one or both of the doses of 8-OH-DPAT exhibit enhancement of recovery after TBI, like that seen after a single administration, then the possibility for a novel clinical pharmacotherapy might exist.
The data revealed that 8-OH-DPAT enhanced beam-walking, acquisition of spatial learning, and memory retention relative to vehicle-treated controls. Interestingly, only the lower dose (0.1 mg/kg) of 8-OH-DPAT afforded the benefits. The higher dose (0.5 mg/kg) group was similar to the vehicle-treated group in all behavioral tasks, with the exception of beam-balance where it performed worse. The lack of an effect with the 0.5 mg/kg dose is surprising because it was this dose that provided the optimal benefit when administered acutely after CCI injury, whereas the 0.1 mg/kg dose was ineffective [35]. The differences in dose response in mediating behavioral performance after TBI may be due to alterations in the number of 5-HT1A receptors, receptor sensitivity [36], or effects at presynaptic vs. post-synaptic receptors [2,38,45]. Furthermore, while 8-OH-DPAT is selective for the 5-HT1A receptor, it also binds to the 5-HT7 receptor. Studies have shown that depending on the dose, either one or both of these receptors can be activated, which would produce different effects on functional outcome [44,45].
Despite a markedly different treatment paradigm, the findings of the current study are consistent with our previously published data. However, while we have replicated several studies showing that 8-OH-DPAT enhances cognitive performance, there are numerous other reports suggesting that 8-OH-DPAT impairs cognition [4,16,22,24,25,44–46,48,54]. Salient differences between our studies and those of others include the animal models used (i.e., TBI vs. non-injured), behavioral tasks (i.e., traditional water maze evaluations vs. “novel” water maze assessments, object recognition, Pavlovian learning paradigms, and radial arm maze), doses, time of administration relative to behavioral testing, and the number of administrations [4,16,22,24,25,44–46,48,54]. Any of these differences alone could contribute to the discrepancy in findings from study to study, but when two or more of these are combined it becomes considerably more difficult to explain. The differences between CNS injured and normal animals is a plausible explanation especially because after other CNS injuries, such as ischemia or subdural hematoma, 5-HT1A receptor agonists exert neuroprotection [1,6–8]. While the sham animals were not significantly impaired by the 8-OH-DPAT treatment, the higher dose (0.5 mg/kg) slowed beam-walking and water maze performance on the first two days of testing. Similar dose response findings have been reported by Meneses and colleagues who showed that both short-term and long-term memory were impaired by higher vs. lower doses of 8-OH-DPAT (0.5 mg/kg vs. 0.06 mg/kg), respectively, in non-injured animals [45]. Taken together these data suggest that dose, timing, and the number of administrations may be important considerations when providing 5-HT1A receptor agonists to intact animals.
Potential mechanisms contributing to the beneficial effects observed with chronic 8-OH-DPAT in our TBI paradigm may be via restoration of dopamine (DA) neurotransmission, which is altered after TBI [12,40,43,60,64,65]. Administration of the 5-HT1A receptor agonists MKC-242, 8-OH-DPAT, and buspirone increase DA levels in the prefrontal cortex and hippocampus [2,50], which are critical regions for cognitive processing. Data supporting the notion that DA neurotransmission is important for spatial learning and memory comes from studies showing that amantadine, bromocriptine, and methylphenidate (all D2 receptor agonists) improve functional outcome and/or preserve hippocampal CA3 cell survival after TBI [12,29,30,33], and that D2 receptor antagonists, such as haloperidol impair recovery [13,17,23,31,62]. Furthermore, several lines of evidence indicate that TBI in rats produces hippocampal and medial septal cell loss, as well as disturbances in cognitive function that may be related to chronic decreases in ACh neurotransmission [11,53]. A time-dependent loss of choline acetyltransferase (ChAT) enzymatic activity, the enzyme responsible for ACh synthesis, and ChAT immunohistochemical staining is also reported after TBI [19,37,52,56]. The reduction in ChAT activity after TBI decreases ACh neurotransmission and may be mediating the cognitive impairments observed after brain injury. Data from our laboratory support the notion that 8-OH-DPAT may mediate the benefits seen after TBI by protecting against TBI-induced ChAT cell loss (in review). Furthermore, another 5-HT1A receptor agonist, repinotan HCL, has been reported to decrease AChE and increase ChAT activity [21].
In conclusion, these data replicate previous findings from our laboratory showing that 8-OH-DPAT improves neurobehavior after TBI, and extend those results by demonstrating that the benefits can be achieved even when treatment is delayed by 24 hrs and administered chronically. A delayed and chronic treatment regimen may be more clinically feasible. Although 5-HT1A receptor agonists are novel to TBI, they are used routinely in treating neuropsychiatric disorders in humans and thus may be an alternative and promising therapeutic approach for clinical TBI. Indeed, a randomized, double-blind, placebo controlled preliminary study evaluating the efficacy of the 5-HT1A receptor agonist repinotan HCL reported that the proportion of patients having good outcome or moderate disability (Glasgow Outcome Scale) was somewhat greater in repinotan-treated patients (60%) than in placebo (50%). Moreover, the 5-HT1A receptor agonist was shown to have a favorable safety and tolerability profile [49]. Continued studies evaluating the potential efficacy of delayed and chronic 8-OH-DPAT after experimental TBI are warranted. Ongoing studies in our laboratory are focused on determining the potential additive effect of combining 5-HT1A receptor agonists with other therapies, such as environmental enrichment, just as we have done after a single administration [32], as well as elucidating potential mechanisms for the observed effects.
Acknowledgments
This work was supported, in part, by National Institutes of Health grants HD043851 and HD046700 awarded to AEK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alessandri B, Tsuchida E, Bullock RM. The neuroprotective effect of a new serotonin receptor agonist, BAY x3702, upon focal ischemic brain damage caused by acute subdural hematoma in the rat. Brain Res. 1999;845:232–35. doi: 10.1016/s0006-8993(99)01948-4. [DOI] [PubMed] [Google Scholar]
- 2.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 3.Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 4.Carli M, Luschi R, Garofalo P, Samanin R. 8-OH-DPAT impairs spatial but not visual learning in a water maze by stimulating 5HT1A receptors in the hippocampus. Behav Brain Res. 1995;67:67–74. doi: 10.1016/0166-4328(94)00105-o. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JP, Aslam HA, Hoffman AN, Zafonte RD, Kline AE. The neurobehavioral benefit conferred by a single systemic administration of 8-OH-DPAT after brain trauma is confined to a narrow therapeutic window. Neurosci Lett. 2007;416:165–8. doi: 10.1016/j.neulet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vry J, Dietrich H, Glaser T, Heine HG, Horvath E, Jork R, Maertins T, Mauler F, Optiz W, Scherling D, Schohe-Loop R, Schwarz T. BAY X 3702. Drugs of the Future. 1997;22:341–49. [Google Scholar]
- 7.De Vry J, Jentzsch KR. Discriminative stimulus properties of the 5-HT1A receptor agonist BAY x 3702 in the rat. Eur J Pharmacol. 1998;357:1–8. doi: 10.1016/s0014-2999(98)00503-2. [DOI] [PubMed] [Google Scholar]
- 8.De Vry J, Schohe-Loop R, Heine H-G, Greuel JM, Mauler F, Schmidt B, Sommermeyer H, Glaser T. Characterization of the aminomethylchroman derivative BAY x 3702 as a highlypotent 5-hydroxytryptamine 1A receptor agonist. J Pharmacol Exp Ther. 1998;284:1082–94. [PubMed] [Google Scholar]
- 9.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 10.Dixon CE, Flinn P, Bao J, Venya R, Hayes RL. Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp Neurol. 1997;146:479–90. doi: 10.1006/exnr.1997.6557. [DOI] [PubMed] [Google Scholar]
- 11.Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith R, Baum E, Marion DW, DeKosky ST. One-year study of spatial memory performance, brain morphology and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16:109–22. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- 12.Dixon CE, Kraus MF, Kline AE, Ma X, Yan HQ, Griffith RG, Wolfson BM, Marion DW. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor Neurol Neurosci. 1999;14:285–94. [PubMed] [Google Scholar]
- 13.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–57. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 14.Feeney DM, Sutton RL. Pharmacotherapy for recovery of function after brain injury. Crit Rev Neurobiol. 1987;3:135–97. [PubMed] [Google Scholar]
- 15.Feeney DM, Weisend MP, Kline AE. Noradrenergic pharmacotherapy, intracerebral infusion and adrenal transplantation promote functional recovery after cortical damage. J Neur Transplant Plast. 1993;4:199–213. doi: 10.1155/NP.1993.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galeotti N, Ghelardini C, Bartolini A. Role of 5HT1A receptors in a mouse passive avoidance paradigm. Jpn J Pharmacol. 2000;84:418–24. doi: 10.1254/jjp.84.418. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Bullman S. Differential effects of haloperidol and clozapine on motor recovery after sensorimotor cortex injury in rats. Neurorehabil Neural Repair. 2002;16:321–25. doi: 10.1177/154596830201600402. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein M. Traumatic brain injury: a silent epidemic. Ann Neurol. 1990;27:327. doi: 10.1002/ana.410270315. [DOI] [PubMed] [Google Scholar]
- 19.Gorman LK, Fu K, Hovda DA, Murray M, Traystman RJ. Effects of traumatic brain injury on the cholinergic system in the rat. J Neurotrauma. 1996;13:457–63. doi: 10.1089/neu.1996.13.457. [DOI] [PubMed] [Google Scholar]
- 20.Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 21.Harkany T, Mulder J, Horvath KM, Keijser J, van der Meeberg EK, Nyakas C, Luiten PG. Oral post-lesion administration of 5-HT(1A) receptor agonist repinotan hydrochloride (BAY x 3702) attenuates NMDA-induced delayed neuronal death in rat magnocellular nucleus basalis. Neuroscience. 2001;108:629–42. doi: 10.1016/s0306-4522(01)00444-4. [DOI] [PubMed] [Google Scholar]
- 22.Helsley S, Siegel TL, Fiorella D, Rabin RA, Winter JC. WAY100635 reverses 8-OH-DPAT induced performance impaiment in the radial maze. Prog Neuro-Psychopharmacol & Biol Psychiat. 1998;22:1179–84. doi: 10.1016/s0278-5846(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman AN, Cheng JP, Zafonte RD, Kline AE. Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 2008 doi: 10.1016/j.lfs.2008.08.007. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kant GJ, Meininger GR, Maughan KR, Wright WL, Robinson TN, 3rd, Neely TM. Effects of the serotonin receptor agonists 8-OH-DPAT and TFMPP on learning as assessed using a novel water maze. Pharmacol Biochem Behav. 1996;53:385–90. doi: 10.1016/0091-3057(95)02038-1. [DOI] [PubMed] [Google Scholar]
- 25.Kant GJ, Wylie RM, Chu K, Ghosh S. Effects of the serotonin agonists 8-OH-DPAT, buspirone, and DOI on water maze performance. Pharmacol Biochem Behav. 1998;59:729–35. doi: 10.1016/s0091-3057(97)00553-4. [DOI] [PubMed] [Google Scholar]
- 26.Kline AE, Bolinger BD, Kochanek PM, Carlos TM, Yan HQ, Jenkins LW, Marion DW, Dixon CE. Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res. 2002;937:22–31. doi: 10.1016/s0006-8993(02)02458-7. [DOI] [PubMed] [Google Scholar]
- 27.Kline AE, Dixon CE. Contemporary in vivo models of brain trauma and a comparison of injury responses. In: Miller LP, Hayes RL, editors. Head Trauma: Basic, Preclinical and Clinical Directions. John Wiley & Sons; NY: 2001. pp. 65–84. [Google Scholar]
- 28.Kline AE, Massucci JL, Dixon CE, Zafonte RD, Bolinger BD. The therapeutic efficacy conferred by the 5-HT1A receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J Neurotrauma. 2004;21:175–85. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- 29.Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J Neurotrauma. 2004;21:1712–22. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- 30.Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–25. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 31.Kline AE, Massucci JL, Zafonte RD, Dixon CE, DeFeo JR, Rogers EH. Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental traumatic brain trauma. Crit Care Med. 2007;35:919–924. doi: 10.1097/01.CCM.0000256722.88854.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav Brain Res. 2007;177:186–94. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280:163–66. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- 34.Kline AE, Yu J, Horvath E, Marion DW, Dixon CE. The selective 5-HT1A receptor agonist repinotan HCL attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–55. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 35.Kline AE, Yu J, Massucci JL, Zafonte RD, Dixon CE. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci Lett. 2002;333:179–82. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- 36.Kreiss DS, Lucki I. Desensitization of 5-HT1A autoreceptors by chronic administration of 8-OH-DPAT. Neuropharmacology. 1992;10:1073–76. doi: 10.1016/0028-3908(92)90110-b. [DOI] [PubMed] [Google Scholar]
- 37.Leonard JR, Maris DO, Grady MS. Fluid percussion injury causes loss of forebrain choline acetyltransferase and nerve growth factor receptor immunoreactive cells in the rat. J Neurotrauma. 1994;11:379–92. doi: 10.1089/neu.1994.11.379. [DOI] [PubMed] [Google Scholar]
- 38.Lüttgen M, Elvander E, Madjid N, Ögren SO. Analysis of the role of 5-HT1A receptors in spatial and aversive learning in the rat. Neuropharmacology. 2005;48:830–52. doi: 10.1016/j.neuropharm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Lyeth BG, Liu S, Hamm RJ. Combined scopolamine and morphine treatment of traumatic brain injury in the rat. Brain Res. 1993;617:69–75. doi: 10.1016/0006-8993(93)90614-s. [DOI] [PubMed] [Google Scholar]
- 40.Massucci JL, Kline AE, Ma X, Zafonte RD, Dixon CE. Time dependent alterations in dopamine tissue levels and metabolism after experimental traumatic brain injury in rats. Neurosci Lett. 2004;372:127–31. doi: 10.1016/j.neulet.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 41.Max W, Mackenzie EJ, Rice DP. Head injuries: costs and consequences. J Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- 42.McDowell S, Whyte J, D’Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121:1155–64. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- 43.McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63:1426–33. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- 44.Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–25. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 45.Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behav Brain Res. 2007;184:81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Misane I, Ögren SO. Selective 5HT1A antagonists WAY100635 and NAD-299 attenuate the impairment of passive avoidance caused by scopolamine in the rat. Neuropsychopharmacology. 2003;28:253–64. doi: 10.1038/sj.npp.1300024. [DOI] [PubMed] [Google Scholar]
- 47.Ohman J, Braakman R, Legout V Traumatic Brain Injury Study Group. Repinotan (BAY x 3702): a 5HT1A agonist in traumatically brain injured patients. J Neurotrauma. 2001;18:1313–21. doi: 10.1089/08977150152725614. [DOI] [PubMed] [Google Scholar]
- 48.Pitsikas N, Rigamonti AE, Cella SG, Muller EE. The 5HT1A receptor antagonist WAY100635 improves rats performance in different models of amnesia evaluated by the object recognition task. Brain Res. 2003;983:215–222. doi: 10.1016/s0006-8993(03)03091-9. [DOI] [PubMed] [Google Scholar]
- 49.Plenger PM, Dixon CE, Castillo RM, Frankowski RF, Yablon SA, Levin HS. Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch Phys Med Rehab. 1996;77:536–40. doi: 10.1016/s0003-9993(96)90291-9. [DOI] [PubMed] [Google Scholar]
- 50.Sakaue M, Somboonthum P, Nishihara B, Koyama Y, Hashimoto H, Baba A, Matsuda T. Postsynaptic 5-hydroxytryptamine (1A) receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br J Pharmacol. 2000;129:1029–34. doi: 10.1038/sj.bjp.0703139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14:615–27. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt RH, Grady MS. Loss of forebrain cholinergic neurons following fluid-percussion injury: implications for cognitive impairment in closed head injury. J Neurosurg. 1995;3:496–502. doi: 10.3171/jns.1995.83.3.0496. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt RH, Scholten KJ, Maughan PH. Cognitive impairment and synaptosomal choline uptake in rats following impact acceleration injury. J Neurotrauma. 2000;17:1129–39. doi: 10.1089/neu.2000.17.1129. [DOI] [PubMed] [Google Scholar]
- 54.Schneider AM, Wilkins E, Firestone A, Everbach EC, Naylor JC, Simson PE. Enhanced retention in the passive-avoidance task by 5-HT1A receptor blockade is not associated with increased activity of the central nucleus of the amygdala. Learning and Memory. 2003;10:394–400. doi: 10.1101/lm.54903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semkova I, Wolz P, Krieglstein J. Neuroprotective effect of 5-HT1A receptor agonist, Bay x 3702, demonstrated in vitro and in vivo. Eur J Pharmacol. 1998;359:251–60. doi: 10.1016/s0014-2999(98)00634-7. [DOI] [PubMed] [Google Scholar]
- 56.Sinson G, Perri BR, Trojanowski JQ, Flamm ES, Mcintosh TK. Improvement of cognitive deficits and decreased cholinergic neuronal cell loss and apoptotic cell death following neurotrophin infusion after experimental traumatic brain injury. J Neurosurg. 1997;86:511–18. doi: 10.3171/jns.1997.86.3.0511. [DOI] [PubMed] [Google Scholar]
- 57.Smith DH, Okiyama K, Thomas MJ, Claussen B, Mcintosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–69. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 58.Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–57. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 59.Torup L, Møller A, Sager TN, Diemer NH. Neuroprotective effect of 8-OH-DPAT in global cerebral ischemia assessed by stereological cell counting. Eur J Pharmacol. 2000;395:137–41. doi: 10.1016/s0014-2999(00)00175-8. [DOI] [PubMed] [Google Scholar]
- 60.Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95:457–65. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- 61.Whyte J, Hart T, Schuster K, Fleming M, Polansky M, Coslett HB. Effects of methylphenidate on attentional function after traumatic brain injury a randomized, placebo-controlled trial. Am J Phys Med Rehabil. 1997;76:440–50. doi: 10.1097/00002060-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Wilson MS, Gibson CJ, Hamm RJ. Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am J Phys Med Rehabil. 2003;82:871–79. doi: 10.1097/01.PHM.0000091982.33232.CB. [DOI] [PubMed] [Google Scholar]
- 63.Wilson MS, Hamm RJ. Effects of fluoxetine on the 5-HT1A receptor and recovery of cognitive function after traumatic brain injury in rats. Am J Phys Med Rehabil. 2002;8:364–72. doi: 10.1097/00002060-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Yan HQ, Kline AE, Ma X, Hooghe-Peters EL, Marion DW, Dixon CE. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. NeuroReport. 2001;12:2323–27. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- 65.Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. NeuroReport. 2002;13:1899–1901. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]