Abstract
INTRODUCTION
Secondary prevention is needed following coronary artery bypass graft (CABG) surgery to reduce the subsequent risk of unstable angina, myocardial infarction and death. However, little research exists on the use of cardiovascular medical therapy in CABG surgery patients. The objective of the present study is to describe the use of cardiovascular medical therapy among patients discharged after CABG surgery.
METHODS
The use of acetylsalicylic acid, clopidogrel, warfarin, antilipid agents, beta-blockers, calcium channel blockers, nitrates and angiotensin-converting enzyme (ACE) inhibitors was examined among 320 patients enrolled in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry. Logistic regression identified the determinants of medication use at 12 months following CABG surgery.
RESULTS
Most patients were male, hyperlipidemic and underwent CABG surgery for relief of angina symptoms. At admission, discharge and at 12 months, acetylsalicylic acid was used in 71%, 92% and 87% of cases, respectively, and some form of antiplatelet agent was used in 74%, 94% and 89% of cases, respectively. The use of antilipid agents remained constant, from 55% at admission to 57% at discharge. However, 24% of patients were not receiving antilipid agents at 12 months. The use of beta-blockers was 57% at admission, 71% at discharge and 64% at 12 months. The use of calcium channel blockers and nitrates decreased modestly from admission to discharge and remained stable at approximately 20% and 22%, respectively, at 12 months. ACE inhibitor use remained stable, from 33% at admission to 38% at 12-months. Hyperlipidemia, hypertension, obesity and pre-CABG surgery left ventricular ejection fraction less than 40% were all found to be important determinants of 12-month medication use. Importantly, the use at discharge was an important determinant of 12-month use of for each medication examined in the present study.
CONCLUSIONS
The use of antilipid agents, beta-blockers and ACE inhibitors was found to be too low among post-CABG surgery patients, who are known to benefit from their use, and the use of nitrates was too high. Discharge from hospital provides a unique opportunity for physicians to modify the use of cardiovascular medical therapy among patients undergoing CABG surgery.
Keywords: Cardiovascular medical therapy, Coronary artery bypass graft, Discharge, Secondary prevention
Abstract
INTRODUCTION
La prévention secondaire s’impose après une chirurgie pour pontage coronarien afin de réduire le risque de récidive de l’angine instable et de l’infarctus du myocarde et le risque de mortalité. Par contre, peu de recherches ont porté sur la pharmacothérapie cardiovasculaire chez les patients opérés pour pontage. L’objectif de la présente étude est de décrire l’utilisation de ce type de traitement chez les patients qui ont obtenu leur congé après une chirurgie pour pontage coronarien.
MÉTHODE
L’utilisation de l’acide acétylsalicylique, du clopidogrel, de la warfarine, des hypolipidémiants, des bêta-bloquants, des anticalciques, des dérivés nitrés et des inhibiteurs de l’enzyme de conversion de l’angiotensine (IECA) a été analysée chez 320 patients inscrits au registre ROSETTA-CABG (pour Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery). L’analyse de régression logistique a permis d’identifier les facteurs qui ont influé sur le recours à ces médicaments au 12e mois suivant une chirurgie pour pontage.
RÉSULTATS
La majorité des patients étaient de sexe masculin; ils souffraient d’hyperlipémie et avaient subi leur pontage pour corriger des symptômes d’angine. À l’admission, au moment du congé, puis après 12 mois, l’acide acétylsalicylique était utilisé chez 71 %, 92 % et 87 % et un antiplaquettaire, chez 74 %, 94 % et 89 % des sujets, respectivement. L’utilisation d’hypolipidémiants est restée constante, soit de 55 % à l’admission à 57 % au moment du congé. Par contre, 24 % des patients ne recevaient aucun hypolipidémiant au 12e mois. L’utilisation des bêta-bloquants était de 57 % à l’admission, de 71 % au moment du congé et de 64 % au 12e mois. L’utilisation des anticalciques et des dérivés nitrés a diminué modestement entre le moment de l’admission et celui du congé et est restée stable à environ 20 % et 22 %, respectivement au 12e mois. L’emploi des IECA est resté stable, de 33 % qu’il était à l’admission, à 38 % au 12e mois. L’hyperlipidémie, l’hypertension, l’obésité et une fraction d’éjection ventriculaire gauche pré-pontage inférieure à 40 % ont toutes été d’importants facteurs déterminants pour ce qui est de l’utilisation des médicaments au 12e mois. À noter, l’utilisation de chacun des médicaments analysés dans le cadre de la présente étude au moment du congé a été un important facteur déterminant de leur utilisation au 12e mois.
CONCLUSION
L’emploi des hypolipidémiants, des bêta-bloquants et des IECA s’est révélé insuffisant chez les patients ayant subi un pontage coronarien car on sait qu’ils peuvent leur être bénéfiques; en revanche, l’utilisation des dérivés nitrés s’est révélée excessive. Le moment où le patient obtient son congé de l’hôpital après une chirurgie pour pontage coronarien est une occasion idéale pour le médecin de modifier sa pharmacothérapie cardiovasculaire.
Coronary artery bypass graft (CABG) surgery relieves angina symptoms and decreases morbidity and mortality in patients suffering from coronary artery disease (CAD) (1,2). Despite the benefits of CABG surgery, 15% to 25% of patients develop graft closure within one year following the procedure (3–6). Cardiovascular medical therapy following CABG surgery is therefore warranted to prevent graft closure and subsequent cardiac events. Very few studies have examined the benefits of cardiovascular medical therapy specifically among post-CABG surgery patients (7–10). However, many factors that place a post-CABG surgery patient at increased risk for cardiac events are comorbidities for which targeted medical therapy already exists (11–19). In spite of this evidence, a number of researchers have documented a lack of optimal therapy among patients with a history of myocardial infarction (MI), congestive heart failure (CHF) or prior percutaneous coronary intervention (PCI) (20–25). To our knowledge, very little research has examined patterns of the use of cardiovascular medical therapy in post-CABG surgery patients. For this reason, we examined the use of cardiovascular medical therapy among patients undergoing CABG surgery in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry. The objectives of the present study were to examine the patterns of use of medical therapy in post-CABG surgery patients and among subgroups of post-CABG surgery patients, and to identify the determinants of the 12-month use of cardiac medications.
METHODS
The ROSETTA-CABG Registry
The ROSETTA-CABG Registry is a prospective, multicentre study examining the use of functional stress testing after CABG surgery in 16 centres across Belgium, Canada, France, Pakistan, the United Kingdom and the United States. As part of the protocol, data on medical therapy were prospectively collected at admission, discharge and at 12 months following CABG surgery. Twelve-month follow-up data were available for 341 patients. The Research and Ethics Committee of each institution involved approved the study, and written informed consent was obtained before patients were enrolled in the study.
To be included in the present study, patients were to have undergone a first successful CABG surgery (during which all ischemic areas were considered to be revascularized and the patient had no major in-hospital events), and undergone only isolated CABG surgery (ie, no valve surgery, aortic reconstruction, etc). Patients with the following criteria were excluded: those who were participating in conflicting studies; those who had contraindications to any repeat cardiac procedures (ie, cardiac catheterization, PCI, repeat CABG surgery); those who had contraindications or were unable to undergo follow-up functional testing; those who were pregnant or likely to become pregnant; those who had a medical condition with a prognosis of survival of less than one year; or those who were likely to be unavailable for a 12-month follow-up.
Study population and data collection
Of the 341 patients enrolled, 11 patients were lost to follow-up, eight patients died and two patients had missing 12-month medication data. Thus, cardiovascular medical therapy data among 320 patients from 13 centres in six countries were examined. Very few baseline differences existed between patients who were included and excluded from the analyses. Compared with patients included in the analysis, those excluded had less hypertension and hyperlipidemia, but more history of CHF and prior MI. None of these differences were statistically significant. Data were specifically collected on the use of acetylsalicylic acid, clopidogrel, warfarin, antilipid agents, beta-blockers, nitrates, calcium channel blockers (CCBs) and angiotensin-converting enzyme (ACE) inhibitors at admission, discharge and at 12 months. The use of nitrates included only oral and patch preparations, and data on contraindications and allergies to these medications were also collected. Fewer than 1% of patients had contraindications or allergies to medications from admission to 12 months following CABG surgery; thus, these patients were not excluded from the analyses.
In addition to data on medical therapy, data on demographic, clinical and procedural characteristics at baseline and discharge following CABG surgery were collected. These include data on baseline characteristics such as CHF, Canadian Cardiovascular Society (CCS) angina class, diabetes, hyperlipidemia, hypertension, left ventricular function and prior MI.
Statistical analysis
Descriptive analyses were performed to determine the frequency of the use of each cardiac medication at baseline, discharge and at 12 months. In addition to examining the overall proportions of these medications, the use of combination anti-ischemic therapy (defined as the use of two or more of beta-blockers, CCBs or nitrates) and the use of these medications in patients with various comorbidities were also examined. Continuous data are presented as the mean ± SD, and dichotomous data are presented as percentages. Univariate and multivariate analyses were used to identify determinants of the use of cardiac medications at 12 months post-CABG surgery. All variables with P<0.10 in univariate analyses were entered into the multivariate model. χ2 tests were used for the univariate analyses, and logistic regression was used for multivariate analyses.
RESULTS
Most patients were men older than 60 years of age with multiple risk factors for CAD (Table 1). More than 50% of patients had hyperlipidemia and hypertension, and more than 25% were diabetic and had previously suffered an MI. Most patients underwent CABG surgery for the relief of angina symptoms. The vast majority of patients had at least three vessels bypassed and virtually all had the left internal mammary artery used as a bypass conduit. Over one-half of patients had saphenous vein grafts used, and only 15% of patients had the radial artery used as a bypass conduit.
TABLE 1.
Baseline clinical and procedural characteristics among 320 patients enrolled in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry
| Characteristic | |
|---|---|
| Mean age ± SD, years | 63±10 |
| Male sex, % | 81 |
| Mean left ventricular ejection fraction ± SD | 54±14 |
| Canadian Cardiovascular Society angina class III–IV, % | 40 |
| Hyperlipidemia, % | 75 |
| Hypertension, % | 65 |
| Diabetes mellitus, % | 28 |
| History of congestive heart failure, % | 8 |
| History of angina, % | 73 |
| Prior myocardial infarction, % | 34 |
| Prior percutaneous coronary intervention, % | 18 |
| Prior coronary artery bypass graft surgery, % | 3 |
| Peripheral vascular disease, % | 7 |
| Main reason for coronary artery bypass graft surgery, % | |
| Angina symptoms | 69 |
| Positive functional test | 14 |
| Recent myocardial infarction | 12 |
| Other | 5 |
| Number of grafts bypassed, % | |
| 1–2 | 19 |
| 3–4 | 64 |
| ≥5 | 18 |
| Bypass conduits, % | |
| Left interior mammary artery | 97 |
| Radial artery | 15 |
| Saphenous vein | 62 |
ANTIPLATELET AND ANTICOAGULANT THERAPY
Patterns of use
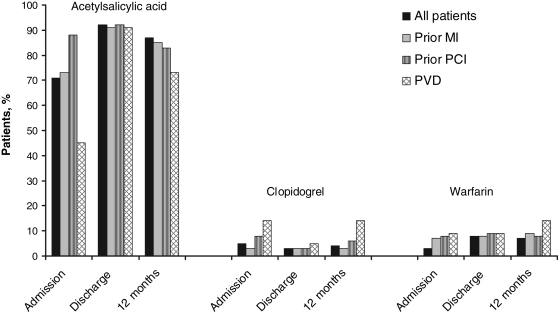
The use of acetylsalicylic acid increased from admission to discharge, with 13% of patients not receiving acetylsalicylic acid at 12 months (Figure 1). Among the nonusers of acetylsalicylic acid, only 6% were receiving other agents, such as clopidogrel and/or warfarin, at 12 months. Seven per cent of patients were not receiving any antiplatelet or anticoagulant therapy at 12 months. Moreover, all 7% had been discharged with some form of antiplatelet or anticoagulant agent. The use of acetylsalicylic acid was similar at discharge among different patient subgroups (Figure 1). However, among each patient subgroup, fewer than 90% were receiving acetylsalicylic acid at 12 months.
Figure 1.
Use of antiplatelet and anticoagulant therapy among 320 patients undergoing coronary artery bypass graft surgery in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry. MI Myocardial infarction; PCI Percutaneous coronary intervention; PVD Peripheral vascular disease
Determinants of use
Only two variables were significant determinants of acetylsalicylic acid use at 12 months in the multivariate model (Table 2). Both unstable angina and the use of acetylsalicylic acid at discharge were found to be important determinants of 12-month use. Lastly, patients who had been treated with clopidogrel or warfarin at 12 months were less likely to have been treated with acetylsalicylic acid at 12 months.
TABLE 2.
Results of multivariate analyses of cardiovascular medical therapy at 12 months among 320 patients in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry
| OR | 95% CI | P | |
|---|---|---|---|
| Acetylsalicylic acid | |||
| Reason for CABG surgery: unstable angina | 0.2 | 0.1–0.5 | 0.0003 |
| Use at discharge | 4.2 | 1.4–12.6 | 0.0098 |
| Warfarin at 12 months | 0.12 | 0.02–0.19 | <0.0001 |
| Clopidogrel at 12 months | 0.04 | 0.01–0.18 | <0.0001 |
| Antilipid agents | |||
| Hyperlipidemia | 3.3 | 1.8–6.0 | 0.0002 |
| Reason for CABG surgery: unstable angina | 0.5 | 0.3–0.9 | 0.0300 |
| Use at discharge | 5.1 | 2.7–9.6 | <0.0001 |
| Beta-blockers* | |||
| Use at discharge | 10.0 | 5.7–17.6 | <0.0001 |
| Calcium channel blockers | |||
| Hypertension | 3.3 | 1.5–7.2 | 0.0030 |
| Obesity | 2.2 | 1.1–4.2 | 0.0252 |
| Use at discharge | 3.5 | 1.8–6.8 | 0.0002 |
| Nitrates* | |||
| Pre-CABG surgery LVEF <40% | 5.0 | 1.8–13.8 | 0.0020 |
| Use at discharge | 16.5 | 5.7–47.9 | <0.0001 |
| Angiotensin-converting enzyme inhibitors | |||
| Male sex | 0.4 | 0.2–0.8 | 0.0060 |
| Pre-CABG surgery LVEF <40% | 2.7 | 1.3–5.7 | 0.0095 |
| Hypertension | 2.2 | 1.2–4.0 | 0.0121 |
| Use of angiotensin-II receptor blockers | 0.14 | 0.04–0.55 | 0.0050 |
| Use at discharge | 6.0 | 3.2–11.1 | <0.0001 |
In the multivariate analyses of use of beta-blockers and nitrates at 12 months, other baseline comorbidities were found to be important determinants. These variables had important associations with 12-month use, but their effects could not be distinguished in the multivariate model from that of their use at discharge, and hence, they are not presented in this table. CABG Coronary artery bypass graft; LVEF Left ventricular ejection fraction
ANTILIPID AGENTS
Patterns of use
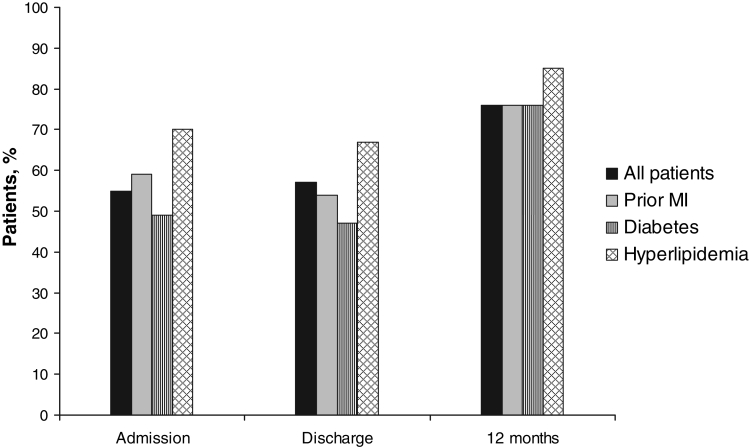
The use of antilipid agents did not change from admission to discharge, but it did increase substantially postdischarge (Figure 2). However, 24% of patients were still not receiving antilipid agents at 12 months. Patterns of the use of antilipid agents were similar among patients with prior MI and diabetes. Although patients with hyperlipidemia were more likely to receive antilipid agents at admission, discharge and 12 months, 15% of patients with hyperlipidemia were still not receiving antilipid agents at 12 months.
Figure 2.
Use of antilipid agents among 320 patients undergoing coronary artery bypass graft surgery in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry. MI Myocardial infarction
Determinants of use
In the multivariate model, hyperlipidemia, unstable angina as the reason for CABG surgery, and use of antilipid agents at discharge were all found to be determinants of the use of antilipid agents at 12 months (Table 2). Patients with hyperlipidemia were more likely to receive antilipid agents at 12 months than those without hyperlipidemia. In contrast, patients who had CABG surgery due to unstable angina were 50% less likely to receive antilipid agents at 12 months. Lastly, patients who were receiving antilipid agents at discharge were more likely to receive antilipid agents at 12 months. Contrary to expectations, prior MI, diabetes and obesity were not found to be significant determinants of 12-month use of antilipid agents.
ANTI-ISCHEMIC THERAPY
Patterns of use
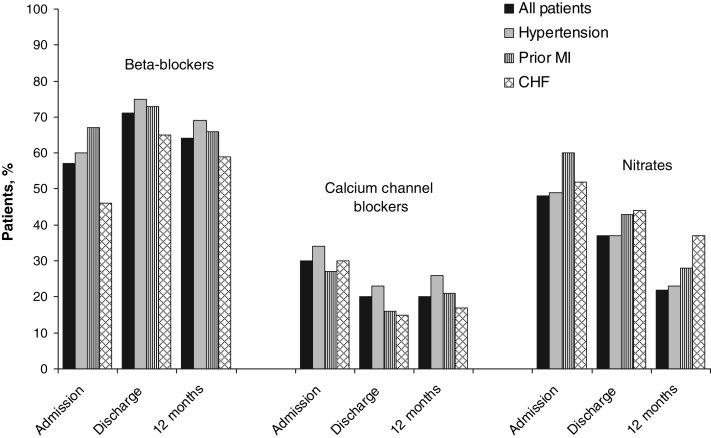
The use of individual anti-ischemic therapy can be found in Figure 3. Importantly, approximately 60% of patients were prescribed beta-blockers at discharge and an additional 25% were prescribed these agents at 12 months. In contrast, 22% of patients were taken off these agents by discharge and an additional 21% were taken off by 12 months. Fifteen per cent of patients who were not receiving CCBs at discharge were receiving them at 12 months, and 60% were no longer receiving them at that time. Similar trends were found for the use of nitrates; 11% of patients were prescribed these agents and 60% were taken off the agents in the 12 months following discharge from the hospital. There were no substantive differences among patient subgroups for any of the three anti-ischemic medications. It is noteworthy, however, that patients with hypertension were more likely to receive beta-blockers and CCBs than other patient subgroups. Moreover, patients with prior MI and CHF were more likely to receive nitrates and less likely to receive CCBs than other patient subgroups.
Figure 3.
Use of anti-ischemic therapy among 320 patients undergoing coronary artery bypass graft surgery in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry. CHF Congestive heart failure; MI Myocardial infarction
A large proportion of patients were still receiving anti-ischemic agents at discharge and at 12 months. More than 50% of all patients were receiving beta-blockers at 12 months, and approximately 20% were receiving CCBs and nitrates at this time. At hospital admission, 45% were receiving combination anti-ischemic therapy (two or more of beta-blockers, CCBs or nitrates) (Table 3). Although double therapy decreased from discharge to 12 months, only 23% of patients were receiving no form of anti-ischemic agent at 12 months. Most importantly, among patients whose main reason for CABG surgery was relief of angina symptoms, more than one-half were still receiving anti-ischemics at discharge and at 12 months. Among patients with no CHF, hypertension or prior MI, for example, beta-blocker use was 48% at admission, 62% at discharge and 56% at 12 months.
TABLE 3.
Anti-ischemic medical therapy* among 320 patients in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry
| Admission, % | Discharge, % | 12 months, % | |
|---|---|---|---|
| Triple therapy | 13 | 1 | 3 |
| Double therapy | 32 | 36 | 22 |
| Monotherapy | 34 | 52 | 51 |
| No anti-ischemic medications | 22 | 11 | 23 |
Anti-ischemic medical therapy is defined as the use of one or more of beta-blockers, calcium channel blockers or nitrates
Determinants of use
Only one variable was found to be a significant determinant of the 12-month use of beta-blockers in the multivariate analyses (Table 2). Patients who received beta-blockers at discharge were more likely to receive them at 12 months than those who did not receive them at discharge. Hypertension was also found to increase the odds of receiving beta-blockers at 12 months. However, when including both variables in the same multivariate model, only beta-blocker use at discharge remained the significant determinant.
Three variables were found to be determinants for the use of CCBs at 12 months in the multivariate analyses (Table 2). Patients with both hypertension and obesity were more likely to receive CCBs at 12 months. The use of CCBs at admission and discharge were also found to be important determinants of 12-month use. Surprisingly, the use of the radial artery as a bypass graft or CCS angina class before CABG surgery or at 12 months were not found to be associated with the 12-month use of CCBs.
Two variables were found to be determinants of the 12-month use of nitrates (Table 2). Patients with a pre-CABG surgery left ventricular ejection fraction less than 40% were more likely to receive nitrates at 12 months. As with the use of beta-blockers, the use of nitrates at discharge was also found to be highly correlated with baseline determinants. Women were 77% more likely to receive nitrates at 12 months, and patients with a history of CHF were also more likely to receive nitrates at 12 months. However, after controlling for use at discharge, female sex and a history of CHF were no longer found to be significant determinants of 12-month use.
ACE INHIBITORS
Patterns of use
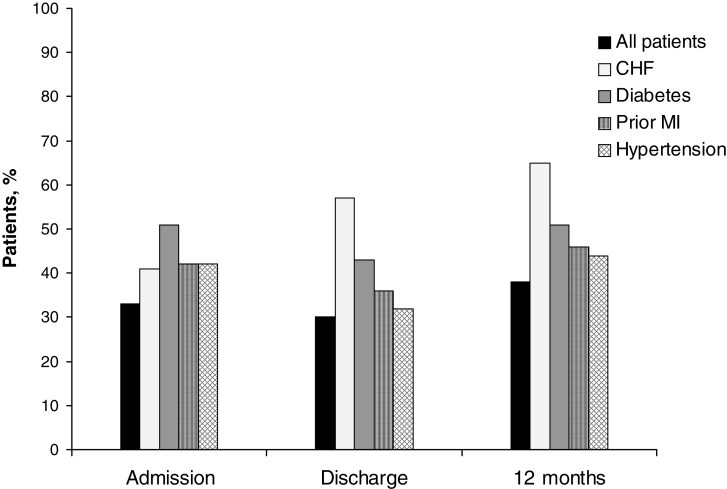
The use of ACE inhibitors was low at admission, discharge and at 12 months (Figure 4). Patients with CHF and diabetes were much more likely to receive ACE inhibitors at discharge and at 12 months when compared with other patient subgroups (Figure 3). In contrast, only 65% of those with a history of CHF were receiving ACE inhibitors at 12 months. Patients with prior MI or hypertension were only slightly more likely to receive ACE inhibitors at discharge and at 12 months than other patient subgroups.
Figure 4.
Use of angiotensin-converting enzyme inhibitors among 320 patients undergoing coronary artery bypass graft surgery in the Routine versus Selective Exercise Treadmill Testing After Coronary Artery Bypass Graft Surgery (ROSETTA-CABG) Registry. CHF Congestive heart failure; MI Myocardial infarction
Determinants of use
In the multivariate model, four variables were found to be significant determinants of use of ACE inhibitors at 12 months (Table 2). Patients with a pre-CABG surgery left ventricular ejection fraction less than 40% and patients with hypertension were 2.7 times and 2.2 times, respectively, more likely to receive ACE inhibitors at 12 months. In contrast, men were 60% less likely to receive ACE inhibitors at 12 months compared with women. Interestingly, diabetes, prior MI and a history of CHF were not found to be significant determinants of the 12-month ACE inhibitor use in the multivariate model. The use of ACE inhibitors at discharge was found to be an important determinant of the use of ACE inhibitors at 12 months. Lastly, the use of angiotensin-II receptor blockers at 12 months was also found to be inversely associated with the use of ACE inhibitors at 12 months.
DISCUSSION
The present study was designed to examine the patterns of the use of cardiovascular medical therapy among patients undergoing CABG surgery. Although previous studies have shown a lack of optimal therapy among patients with CAD, CHF, prior MI and prior PCI, no study has, to our knowledge, examined the use of cardiovascular medical therapy among CABG surgery patients. In general, we found little variation from discharge to 12-months post-CABG surgery in the use of acetylsalicylic acid, clopidogrel, warfarin, beta-blockers, CCBs or ACE inhibitors. However, the use of antilipid agents increased substantially postdischarge, whereas the use of nitrates decreased modestly.
Although many patients had comorbidities, suggesting that they would benefit from specific medical therapy, these subgroups were not always more likely to receive a specific medication than other patients. For example, the use of beta-blockers among patients with prior MI is well known to decrease mortality (15,16). However, patients with prior MI were found to be no more likely to receive beta-blockers than other patient subgroups at discharge and at 12 months following CABG surgery. Similarly, patients with diabetes or prior MI were only slightly more likely to receive ACE inhibitors at 12 months than patients without these comorbidities. These differences were not statistically significant, nor were these comorbidities found to be significant determinants of 12-month use.
Past research has demonstrated the efficacy of acetylsalicylic acid and antilipid agents at reducing the risk of subsequent cardiovascular events among CAD patients with multiple comorbidities, including CABG surgery patients (8,13–15). Thus, we expected to find fewer differences among patient subgroups. However, very little research exists to support the use of beta-blockers, CCBs or nitrates specifically among CABG surgery patients. Therefore, we would have expected only those patients with comorbidities, who are known to benefit from these medications, to have significantly higher frequencies of use than patients without these comorbidities. We would not have expected the use of beta-blockers to be zero due to their use in the treatment of hypertension and in post-MI and CHF patients. However, patients with these comorbidities were no more likely to receive these medications than patients without them. In contrast, nitrates are principally indicated for the treatment of angina. In the present study, patients were to have been completely revascularized at the time of the index CABG surgery to be eligible for enrolment. Thus, a new prescription of nitrates would have been for symptoms that could be due to graft occlusion/stenosis or the progression of native coronary disease. Because only 4% of patients were hospitalized for unstable angina in the 12 months following CABG surgery, and because patients undergoing CABG surgery have very low rates of angina post-CABG surgery, the necessity for nitrate use should have been quite low in this group. Despite these facts, more than 15% of patients were prescribed nitrates between discharge and 12 months following CABG surgery. Although the use of CCBs was comparable with that of nitrates, it is more difficult to judge whether CCB use at 12 months is excessive or insufficient because of their use for indications other than angina therapy.
When examining the determinants of the use of cardiovascular medical therapy at 12 months, the most important determinants found were baseline comorbidities and the use of the that medication at discharge. However, some of these baseline comorbidities were so highly correlated with the use of the medication at discharge that after accounting for its use at discharge in the model, they were no longer significant determinants of 12-month use. This finding suggests that the most important time when a patient is being prescribed appropriate therapy may occur in-hospital, and not postdischarge.
Comparison with other studies
To our knowledge, very few researchers have examined patterns of the use of cardiovascular medical therapy specifically among patients undergoing CABG surgery. However, when comparing the results found in the present study with those of previous studies that examined the use of cardiovascular medical therapy among other patient subgroups, similar trends were observed. Previous studies have demonstrated a lack of optimal therapy among CAD patients, patients with CHF and those undergoing PCI (20–25). Spencer et al (20) found lipid-lowering agents to be underused post-MI, with fewer than 25% of patients discharged from the hospital on this therapy. Allen et al (22) found that only 47% of patients eligible for lipid-lowering agents were receiving them at admission and 58% were receiving them at discharge. Likewise, Hasdai et al (24) found that although patients had been successfully revascularized following PCI, the use of anti-ischemic agents at six months was over-prescribed. Thirty-nine per cent of patients were receiving beta-blockers, 57% were receiving CCBs and 36% were receiving nitrates, despite the lack of angina in 69% of patients. This trend of the overuse of anti-ischemic agents persisted at 12 months, when only 23% of patients did not receive either of the anti-ischemic medications. Similarly, in the present study, we found the use of antilipid agents to be too low at 12 months. Although hyperlipidemia was a significant determinant of 12-month use, 15% of patients with this comorbidity were not receiving antilipid agents. Moreover, the use of anti-ischemic agents at 12 months following CABG surgery among fully revascularized patients was also unexpectedly high.
The results of the present study demonstrate the need for the implementation of strategies to help physicians adhere to guidelines on cardiovascular medical therapy following CABG surgery. Several researchers (26–28) have explored approaches to improving the treatment gap in cardiovascular care and prevention. Audit with feedback on performance may play an important role in achieving this goal among post-CABG surgery patients and should be explored further.
Limitations
Several potential limitations of the present study should be mentioned. Dosages were not collected, nor were the specific medications within classes of drugs. It is also possible that even though patients were prescribed the appropriate medications, they were not compliant in their medication use. Clinical event rates were low during the 12-month follow-up period, and consequently, the association between medication use and clinical outcomes could not be explored. Lastly, it is possible that there were unknown confounders that were not controlled for in our analyses, such as study centre. Because this was a multicentre study involving 13 centres in six countries, it is possible that there were differences in the use of cardiovascular medical therapy among study centres. However, when including the study centre in the multivariate models, it was not found to greatly affect the beta coefficients of baseline clinical determinants already in the model. This variation could have been explored further with the use of random effects modelling and other correlated data analytical methods. It is important to point out, however, that such analysis methods typically yield similar point estimates of effect, but with wider confidence intervals. Lastly, it is also possible that centres involved in the ROSETTA-CABG Registry may have patterns of care not representative of those of other centres outside the registry.
CONCLUSIONS
The present study was designed to examine the use of cardiovascular medical therapy in patients undergoing CABG surgery. Patients with hyperlipidemia were more likely to receive antilipid agents, and patients with CHF were more likely to receive ACE inhibitors at 12 months than other patient subgroups. Little variation was found among patient subgroups in the use of all other cardiac medications studied. We also expected the use of antilipid agents, ACE inhibitors and beta-blockers to be higher among patient subgroups with comorbidities who are known to benefit from these medications. Moreover, the use of nitrates among patients who were fully revascularized and had little indication for their use appeared inappropriately high. Methods to help physicians better modify the use of cardiovascular medical therapy post-CABG surgery are greatly needed. The time of discharge from hospital provides a unique opportunity for physicians to modify the use of cardiovascular medical therapy appropriately among CABG surgery patients.
ACKNOWLEDGEMENTS
Dr Eisenberg is a senior physician-scientist at the Fonds de la recherche en santé du Québec. Dr Pilote is a physician-scientist at the Canadian Institute of Health Research. This study was funded by the Heart and Stroke Foundation of Canada and the Fonds de la recherche en santé du Québec.
REFERENCES
- 1.Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. N Engl J Med. 1984;311:1333–9. doi: 10.1056/NEJM198411223112102. [DOI] [PubMed] [Google Scholar]
- 2.Hultgren HN, Peduzzi P, Detre K, Takaro T. The 5 year effect of bypass surgery on relief of angina and exercise performance. Circulation. 1985;72:V79–83. [PubMed] [Google Scholar]
- 3.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: Overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists’ Collaboration. Lancet. 1994;344:563–70. doi: 10.1016/s0140-6736(94)91963-1. (Erratum in 1994;344:1446) [DOI] [PubMed] [Google Scholar]
- 4.Weintraub WS, Clements SD, Jr, Crisco LV, et al. Twenty-year survival after coronary artery surgery: An institutional perspective from Emory University. Circulation. 2003;107:1271–7. doi: 10.1161/01.cir.0000053642.34528.d9. [DOI] [PubMed] [Google Scholar]
- 5.Bourassa MG, Campeau L, Lesperance J. Effects of bypass surgery on the coronary circulation: Incidence and effects of vein graft occlusion. Cardiovasc Clin. 1977;8:107–18. [PubMed] [Google Scholar]
- 6.Kloster FE, Kremkau EL, Ritzmann LW, Rahimtoola SH, Rosch J, Kanarek PH. Coronary bypass for stable angina: A prospective randomized study. N Engl J Med. 1979;300:149–57. doi: 10.1056/NEJM197901253000401. [DOI] [PubMed] [Google Scholar]
- 7.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. (Erratum in 2002;324:141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborative overview of randomised trials of antiplatelet therapy –I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81–106. (Erratum in 1994;308:1540) [PMC free article] [PubMed] [Google Scholar]
- 9.Mangano DT Multicenter Study of Perioperative Ischemia Research Group. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;347:1309–17. doi: 10.1056/NEJMoa020798. [DOI] [PubMed] [Google Scholar]
- 10.The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336:153–62. doi: 10.1056/NEJM199701163360301. (Erratum in 1997;337:1859) [DOI] [PubMed] [Google Scholar]
- 11.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1991 guidelines for coronary artery bypass graft surgery). American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;34:1262–347. doi: 10.1016/s0735-1097(99)00389-7. [DOI] [PubMed] [Google Scholar]
- 12.Ryan TJ, Antman EM, Brooks NH, et al. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction: Executive summary and recommendations: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee on management of acute myocardial infarction) Circulation. 1999;100:1016–30. doi: 10.1161/01.cir.100.9.1016. [DOI] [PubMed] [Google Scholar]
- 13.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 14.Gavaghan TP, Gebski V, Baron DW. Immediate postoperative aspirin improves vein graft patency early and late after coronary artery bypass graft surgery. A placebo-controlled, randomized study. Circulation. 1991;83:1526–33. doi: 10.1161/01.cir.83.5.1526. [DOI] [PubMed] [Google Scholar]
- 15.White CW, Gobel FL, Campeau L, et al. Post Coronary Artery Bypass Graft Trial Investigators. Effect of an aggressive lipid-lowering strategy on progression of atherosclerosis in the left main coronary artery from patients in the Post Coronary Artery Bypass Graft Trial. Circulation. 2001;104:2660–5. doi: 10.1161/hc4701.099730. [DOI] [PubMed] [Google Scholar]
- 16.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 17.Hjalmarson A, Elmfeldt D, Herlitz J, et al. Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet. 1981;2:823–7. doi: 10.1016/s0140-6736(81)91101-6. [DOI] [PubMed] [Google Scholar]
- 18.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–14. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. (Erratum in 2000;342:1376, 2000;342:748) [DOI] [PubMed] [Google Scholar]
- 20.Spencer F, Scleparis G, Goldberg RJ, Yarzebski J, Lessard D, Gore JM. Decade-long trends (1986 to 1997) in the medical treatment of patients with acute myocardial infarction: A community-wide perspective. Am Heart J. 2001;142:594–603. doi: 10.1067/mhj.2001.117776. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg MJ, Califf RM, Cohen EA, Adelman AG, Mark DB, Topol EJ. Use of evidence-based medical therapy in patients undergoing percutaneous coronary revascularization in the United States, Europe, and Canada. Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT-I) and Canadian Coronary Atherectomy Trial (CCAT) investigators. Am J Cardiol. 1997;79:867–72. doi: 10.1016/s0002-9149(97)00005-2. [DOI] [PubMed] [Google Scholar]
- 22.Allen JK, Blumenthal RS, Margolis S, Young DR. Status of secondary prevention in patients undergoing coronary revascularization. Am J Cardiol. 2001;87:1203–6. A7. doi: 10.1016/s0002-9149(01)01496-5. [DOI] [PubMed] [Google Scholar]
- 23.Rogers WJ, Bowlby LJ, Chandra NC, et al. Treatment of myocardial infarction in the United States (1990 to 1993). Observations from the National Registry of Myocardial Infarction. Circulation. 1994;90:2103–14. doi: 10.1161/01.cir.90.4.2103. [DOI] [PubMed] [Google Scholar]
- 24.Hasdai D, Lerman A, Grill DE, Rihal CS, Homes DR., Jr Medical therapy after successful percutaneous coronary revascularization. Ann Intern Med. 1999;130:108–15. doi: 10.7326/0003-4819-130-2-199901190-00004. [DOI] [PubMed] [Google Scholar]
- 25.Pearson TA, Peters TD. The treatment gap in coronary artery disease and heart failure: Community standards and the post-discharge patient. Am J Cardiol. 1997;80(8B):45H–52H. doi: 10.1016/s0002-9149(97)00820-5. [DOI] [PubMed] [Google Scholar]
- 26.Montague T, Cox J, Kramer S, et al. Improving cardiovascular outcomes in Nova Scotia (ICONS): A successful public-private partnership in primary healthcare. Hosp Q. 2003;6:32–8. doi: 10.12927/hcq..16498. [DOI] [PubMed] [Google Scholar]
- 27.McAlister FA, Taylor L, Teo KK, et al. The treatment and prevention of coronary heart disease in Canada: Do older patients receive efficacious therapies? The Clinical Quality Improvement Network (CQIN) Investigators. J Am Geriatr Soc. 1999;47:811–8. doi: 10.1111/j.1532-5415.1999.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 28.Montague T, Taylor L, Martin S, et al. Can practice patterns and outcomes be successfully altered? Examples from cardiovascular medicine. The Clinical Quality Improvement Network (CQIN) Investigators. Can J Cardiol. 1995;11:487–92. [PubMed] [Google Scholar]