Abstract
The patency of prosthetic vascular grafts is impaired by intimal hyper-plasia (IH) near the anastomotic regions. The absence of a functional endothelial monolayer on the prosthetic grafts is an important stimulus for IH. To improve the outcome of synthetic vascular bypass surgery, cell seeding is a promising concept that has been extensively investigated and is still evolving. In the present paper, the concept of prosthetic graft cell seeding is discussed, with emphasis on its newest era: seeding with endothelial progenitor cells. Although experimental studies on prosthetic graft seeding using endothelial progenitor cells have shown excellent results on graft endothelialization, none of these studies reported favourable effects on the more clinically relevant end points such as IH or graft patency.
Keywords: Endothelial progenitor cells, Intimal hyperplasia, Prosthetic graft seeding
Abstract
La perméabilité des greffes vasculaires prosthétiques est perturbée par l’hyperplasie intimale (HI) près des zones anastomosées. L’absence de monocouche endothéliale fonctionnelle sur les greffes prosthétiques est un important stimulus d’HI. Pour améliorer l’issue des pontages vasculaires synthétiques, l’implantation de cellules est un concept prometteur qui a fait l’objet de recherches poussées et qui est toujours en évolution. Dans le présent article, le concept d’implantation de greffes de cellules prosthétiques est abordé, surtout axé sur son domaine le plus novateur : l’implantation de cellules souches endothéliales. Bien que les études expérimentales sur l’implantation de cellules prosthétiques au moyen de cellules souches endothéliales aient obtenu d’excellents résultats sur l’endothélialisation des greffes, aucune n’a d’effets favorables sur les éléments décisifs plus pertinents d’un point de vue clinique, comme l’HI et la perméabilité.
The patency of prosthetic vascular grafts is impaired by intimal hyperplasia (IH) near the anastomotic regions. The absence of a functional endothelial monolayer on the luminal side of prosthetic grafts is an important stimulus for IH. Indeed, the endothelium acts as a first line of defense against vascular disturbances. To fulfill this role successfully, endothelial cells (ECs) have been shown to produce a wide array of auto-, para-and endocrine substances with vasodilatory, antithrombotic and antiproliferative effects (1). Consequently, EC seeding at the luminal surface of prosthetic vascular grafts is a valuable strategy to improve graft patency. Current research on the potency of bone marrow-derived endothelial progenitor cells (EPCs) has shown these cells to be a promising source for graft seeding. In the present review, we will address the clinical problem of prosthetic graft failure and the rationale for graft seeding. Subsequently, we provide an overview of previous experimental studies on graft seeding with ECs, and new insights in the applicability and the risks of EPCs for seeding of prosthetic vascular grafts.
PROSTHETIC VASCULAR GRAFT FAILURE
In patients requiring peripheral arterial bypass grafting or vascular access for chronic hemodialysis, autologous veins are currently the conduit of choice in view of their superior patency rates. For peripheral arterial bypass grafting, the five-year primary patency rate of venous bypasses is 69%, compared with 49% for prosthetic bypass grafts (2). In addition, the one-year primary patency rates of native arteriovenous (AV) fistulas used as vascular access for hemodialysis is approximately 75%, which is superior to the 50% one-year primary patency rate of prosthetic AV grafts (3). However, many patients receive prosthetic grafts when a suitable vein is unavailable, which is more often the case in the aging and diabetic populations. Failure of prosthetic hemodialysis access grafts is predominantly due to a progressive intimal hyperplastic response near the venous anastomosis, which ultimately leads to graft thrombosis. Currently, AV shunt-related morbidity accounts for 20% of all hospitalizations in end-stage renal disease patients, amounting to more than one billion dollars per year being spent on AV shunt-related care in the United States alone (4).
RATIONALE FOR GRAFT SEEDING
To date, there is no effective intervention available to improve graft patency. Successful development of new strategies requires closer insight into the pathogenesis of IH. The latter is thought to reflect a cumulation of several, separate, pathogenic entities, comprising inflammatory, coagulatory and hemodynamic factors (5).
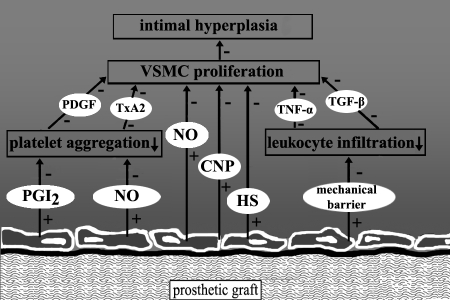
A crucial factor in the activation of coagulation and inflammation is the lack of a functional endothelial monolayer on the prosthetic graft, because the endothelium constitutes the first-line homeostatic defence mechanism by exerting anticoagulatory and anti-inflammatory effects (Figure 1). With regard to the latter, healthy ECs produce nitric oxide, heparan sulphate and C-type natriuretic peptide, which exert potent antiproliferative and antimigratory effects on vascular smooth muscle cells (VSMCs). In addition, implantation of expanded polytetrafluoroethylene material is associated with a ‘foreign body’ response. This inflammatory response includes infiltration of leukocytes into the graft. These inflammatory cells secrete cytokines such as tumour necrosis factor-alpha that have clear proliferative effects. Therefore, graft seeding may suppress this inflammatory response.
Figure 1.
Potential mechanisms of protective effects of seeded endothelial cells (ECs) on intimal hyperplasia in vascular prosthesis. First, the production of nitric oxide (NO), C-type natriuretic peptide (CNP) and heparan sulphate (HS) by ECs leads to inhibiton of profileration and migration of vascular smooth muscle cells (VSMCs). Second, the production of NO and prostacyclin (PGI2) by ECs inhibits platelet aggregation, with subsequent reduction in release of platelet-derived growth factor (PDGF) and thromboxane A2 (TxA2) by platelets. Third, the mechanical barrier formed by ECs prevents infiltration of leukocytes, thereby reducing the production of growth-stimulation cytokines including tumour necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β). + Stimulatory effect;− Inhibitory effect
In humans, complete prosthetic graft endothelialization does occur rarely and usually does not extend beyond 1 cm to 2 cm of the graft edges. Subsequently, the bare prosthetic graft provides a continuous adhesive surface for activated platelets that release thromboxane A2, serotonin and platelet-derived growth factor, all known promoters of VSMC proliferation. The concept of EC seeding of grafts is based on the assumption that a functional endothelial layer attenuates activation of leukocytes and platelets passing through the graft, and potentially contributes to paracrine mediators conveying anti-inflammatory and antiproliferative effects downstream at the outflow tract.
PREVIOUS STUDIES ON IN VITRO GRAFT SEEDING WITH MATURE ECs
Various studies have evaluated the efficacy of in vitro graft seeding using mature ECs. Herring et al (6) were the first to show the benefits of seeding ECs on a polyethylene prosthesis in a canine in vivo model. Since then, different sources and types of cells, isolation methods, graft materials, graft sizes, coatings of grafts, animal models, antithrombotics and methods of follow-up have been used. In subsequent randomized clinical trials, EC seeding of polytetrafluoroethylene grafts resulted in increased patency rates of arterial bypass-grafts after three-and nine-year follow-up, respectively (7). However, a broader clinical implementation of in vitro EC seeding techniques is hampered by the laborious procedures for harvesting, expansion and application of ECs obtained from autologous veins or adipose tissue. These procedures preclude application of in vitro graft seeding techniques for acute interventions. In addition, ECs that are seeded onto the luminal surface of prosthetic grafts before surgery do not retain completely after implantation in vivo due to a lack of adhesive strength. Moreover, seeded microvascular ECs on macrovascular prosthetic grafts may require phenotypic modulation for optimal functioning, because ECs from diverse tissues and vascular beds are heterogeneous with respect to their surface phenotype and protein expression (1).
EPCs: A NEW SOURCE OF ECs FOR GRAFT SEEDING
Bone marrow-derived EPCs have emerged as a promising alternative source of autologous ECs. EPCs are a subset of CD34(+) cells with the potential to proliferate and differentiate into mature ECs (8). Previous studies on in vitro seeding of prosthetic vascular grafts using CD34(+) progenitor cells revealed marked enhancement of graft endothelialization in animal models. Bhattacharya et al (9) were the first to demonstrate that CD34(+) cells, isolated from bone marrow, led to increased endothelialization of vascular grafts in dogs. However, the procedure was laborious because only 120 mL of bone marrow were aspirated, and the CD34(+) cells therefore needed to be enriched using an immunomagnetic bead technique.
Griese et al (10) described a method for the isolation and expansion of circulating EPCs from peripheral blood, and evaluated their therapeutic potential for autologous cell-based therapy of injured blood vessels and prosthetic grafts. The cells needed to be expanded in vitro to yield sufficient numbers for therapeutic applications. These cells were successfully transplanted into balloon-injured carotid arteries and into bioprosthetic grafts in rabbits. This technique also led to rapid endothelialization of denuded vessels and graft segments.
Both studies describe a promising concept, but laborious procedures are still required to harvest and expand ECs before graft seeding. Moreover, neither of these studies reported on beneficial effects on IH in the anastomotic region of prosthetic grafts.
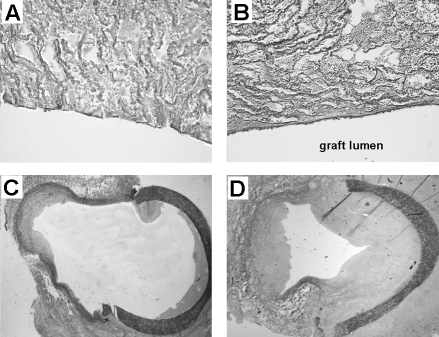
In an effort to circumvent the limitation of cell culture before graft seeding, we recently evaluated the efficacy of anti-CD34 antibody-coated prosthetic grafts, which are thought to bind bone marrow-derived CD34(+) EPCs in vivo (11). By promoting adherence of these endogenous circulating EPCs to the prosthetic graft, the need for in vitro seeding procedures can be avoided. This study was conducted in a validated model of prosthetic AV graft failure in pigs (12). In this model, AV grafts were created bilaterally between the carotid artery and the jugular vein. This approach provides a potent model for investigating therapeutic approaches, because one graft can be experimentally manipulated while the other graft is used as a control. Histological and electron-microscopical analysis of anti-CD34 antibody-coated prosthetic grafts showed almost complete coverage of the graft within three days after implantation. However, in spite of cellular coverage of the luminal surface, the intimal hyper-plastic response at the venous anastomosis of anti-CD34 antibody-coated grafts dramatically increased after 28 days of follow-up (Figure 2). These findings show that anti-CD34 antibody coating is successful in promoting cellular coverage of grafts with endothelial-like cells, whereas these trapped cells failed to exert protective effects for IH formation at the venous outflow tract. Although the exact mechanism of this adverse effect is still unclear, release of platelet-derived growth factor and basic fibroblast growth factor by adhered ECs may have contributed to the observed increase in VSMC proliferation. Alternative explanations for this proliferative response relate to the capacity of CD34(+) pluripotent progenitor cells to differentiate into various cell types. The CD34 epitope is not a specific marker for lineage-committed EPCs. Besides ECs, these cells include platelets, macrophages, granulocytes and VSMCs (13). The latter cell types have been identified as active participants in the process in neointima formation. Furthermore, adequate maturation of captured CD34(+) cells may be hampered by the binding of the immobilized antibody to the CD34 epitope, the turbulent flow pattern at the anastomotic region or the lack of a required microenvironment for optimal functioning.
Figure 2.
Representative sections obtained at four weeks after arteri-ovenous graft implantation in pigs. Enhanced endothelialization in the anti-CD34-coated grafts coincided with profound increase in intimal hyperplasia at the venous anastomosis. Lectin-stained sections of bare (A) and CD34-coated grafts (B) obtained from the centre of the graft. Lectin is a marker for endothelial cells. Elastin von Gieson-stained sections of the venous anastomosis of bare grafts (C) and CD34-coated grafts (D). Reproduced from reference 11 with permission
FUTURE DIRECTIONS FOR EPC SEEDING OF PROSTHETIC GRAFTS
As described in the previous paragraph, various animal studies (9–11) on graft seeding using EPCs revealed accelerated graft endothelialization after in vitro or in vivo EPC seeding. However, none of these studies observed a beneficial effect on IH or graft patency. Thus far, the exact mechanism of this lack of effect is unclear. The process of progenitor cell differentiation is poorly understood. Various studies revealed that function and amount of EPCs depend on several internal and external entities. Risk factors for coronary artery disease, such as diabetes, smoking and chronic renal failure, but also hyper-cholesterolemia and hypertension, are associated with impaired number and function of EPCs (14). On the other hand, several therapeutic modalities have been developed to counteract the reduction of EPC numbers and the decreased functional activity in patients with coronary artery disease. Recent studies showed that vascular endothelial growth factor, the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) promote differentiation of CD34(+) cells into mature ECs (15). Therefore, adaptation of the matrix composition of the prosthetic grafts using these molecules may ameliorate the vasculoprotective function of seeded EPCs. In addition, accumulating data suggest that an adequate balance between CD34(+) and CD14(+)CD34(−) cells is required for optimal EC differentiation and function of EPCs (16). Therefore, a mixture of CD14(+) and CD34(+) cells may result in EC coverage with a high potential to improve vascular homeostasis.
Finding the right balance in atheroprotective factors, thereby improving EPC number and function, may theoretically ameliorate vascular graft patency when EPC-coated grafts are used. However, this hypothesis needs careful consideration because animal experiments are executed in an environment lacking risk factors that influence EPC number and function.
CONCLUSION
EPCs have emerged as a promising source for prosthetic graft seeding. The expanding knowledge of EPC function and differentiation should stimulate further research on the therapeutic application of EPCs to improve the poor patency rates of prosthetic grafts.
REFERENCES
- 1.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- 2.Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–62. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Schwab SJ, Harrington JT, Singh A, et al. Vascular access for hemodialysis. Kidney Int. 1999;55:2078–90. doi: 10.1046/j.1523-1755.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- 4.USRDS 2002. Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2002. [Google Scholar]
- 5.Rotmans JI, Pasterkamp G, Verhagen HJ, Pattynama PM, Blankestijn PJ, Stroes ES. Hemodialysis access graft failure: Time to revisit an unmet clinical need? J Nephrol. 2005;18:9–20. [PubMed] [Google Scholar]
- 6.Herring M, Gardner A, Glover J. A single-staged technique for seeding vascular grafts with autogenous endothelium. Surgery. 1978;84:498–504. [PubMed] [Google Scholar]
- 7.Deutsch M, Meinhart J, Fischlein T, Preiss P, Zilla P. Clinical autologous in vitro endothelialization of infrainguinal ePTFE grafts in 100 patients: A 9-year experience. Surgery. 1999;126:847–55. [PubMed] [Google Scholar]
- 8.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya V, McSweeney PA, Shi Q, et al. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood. 2000;95:581–5. [PubMed] [Google Scholar]
- 10.Griese DP, Ehsan A, Melo LG, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: Implications for cell-based vascular therapy. Circulation. 2003;108:2710–5. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 11.Rotmans JI, Heyligers JM, Verhagen HJ, et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 2005;112:12–8. doi: 10.1161/CIRCULATIONAHA.104.504407. [DOI] [PubMed] [Google Scholar]
- 12.Rotmans JI, Velema E, Verhagen HJ, et al. Rapid, arteriovenous graft failure due to intimal hyperplasia: A porcine, bilateral, carotid arteriovenous graft model. J Surg Res. 2003;113:161–71. doi: 10.1016/s0022-4804(03)00228-2. [DOI] [PubMed] [Google Scholar]
- 13.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 15.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: A novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 16.Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. CD34- blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–12. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]