Abstract
Although the etiology of eosinophilic myocarditis (EM) is not always apparent, several causes are identified, including hypersensitivity to a drug or substance, with the heart as the target organ. However, symptoms and signs of hypersensitivity are not found in all patients. EM can lead to progressive myocardial damage with destruction of the conduction system and refractory heart failure. The present report describes three cases of biopsy-proven EM with different presentations, including acute coronary syndrome, cardiogenic shock and newly diagnosed heart failure. In one patient, hypersensitivity to sumatriptan was suspected to be the underlying cause. All patients responded well to treatment with steroids, angiotensin-converting enzyme inhibitors and beta-blockers. There was a complete recovery of the ventricular function in all cases.
Keywords: Eosinophilic, Hypersensitivity, Myocarditis, Sumatriptan
Abstract
Bien que l’étiologie de la myocardite à éosinophiles (MÉ) ne soit pas toujours apparente, plusieurs causes sont connues, y compris l’hypersensibilité à un médicament ou à une substance, le cœur étant l’organe cible. Cependant, on ne constate pas les symptômes et les signes d’hypersensibilité chez tous les patients. La MÉ peut entraîner des dommages myocardiques évolutifs accompagnés d'une destruction du système de conduction et d’une insuffisance cardiaque réfractaire. Le présent compte rendu décrit trois cas de MÉ démontrée par biopsie sous trois présentations différentes, soit un syndrome coronarien aigu, un choc cardiogène et une insuffisance cardiaque de novo. Chez un patient, l’hypersensibilité au sumatriptan a été présumée comme la cause sousjacente. Tous les patients ont bien réagi à la corticothérapie, à des inhibiteurs de l’enzyme de conversion de l’angiotensine et à des bétabloquants. Dans tous les cas, la fonction ventriculaire s’est complètement rétablie.
Eosinophilic myocarditis (EM) is a rare, potentially fatal disease if left untreated. The spectrum of clinical presentation is wide. The present report describes three different clinical presentations of EM. It also demonstrates the response to steroid therapy with complete recovery of ventricular function and the disappearance of inflammatory cell infiltrate in a repeat endomyocardial biopsy (EMB). The incidence, etiology, histopathology, clinical manifestations, diagnosis, treatment and prognosis of EM are discussed.
CASE PRESENTATIONS
Case 1
A 40-year-old man presented to the emergency department with a history of flu-like illness, fever, malaise and chills, followed by severe nonpleuritic chest pain and shortness of breath. He had a 13-year history of psoriasis treated with topical steroids, phototherapy and intralesional steroids. He was not asthmatic, had no allergies and did not take any regular medications. There was no significant animal or bird exposure history. He was self-employed as a carpet cleaner.
On arrival, he was in no acute distress, afebrile, with a heart rate of 90 beats/min and a blood pressure of 85/50 mmHg. A general physical examination was unremarkable except for a psoriatic plaque on the right leg without nail or joint involvement. Cardiovascular examination showed no jugular venous distension, gallops, rubs or murmurs.
Blood work revealed only an elevated eosinophil count of 1.1×109/L (normal values less than 0.4×109/L) and troponin I of 46 μg/L (normal values less than 0.1 μg/L); the results of other laboratory tests are shown in Table 1. An electrocardiogram (ECG) revealed T wave inversion in the anterolateral leads, and the chest radiograph was normal. The diagnosis of acute coronary syndrome (ACS) was made and he was referred to a tertiary centre for selective coronary angiogram (SCA), which revealed normal coronary arteries. The echocardiogram showed mildly impaired global left ventricular (LV) systolic function with a visually estimated ejection fraction (EF) of 50%; there were no valvular lesions.
TABLE 1.
Laboratory values
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Hemoglobin, g/L (NV 134–170) | 138 | 126 | 130 |
| White blood cells, ×109/L (NV 4.0–11.0) | 7.8 | 15.1 | 9.5 |
| Neutrophils, ×109/L (NV 2.3–7.7) | 4.5 | 13.4 | 6.7 |
| Eosinophils, ×109/L (NV <0.4) | 1.1 | 0.0 | 0.0 |
| ESR, mm/h (NV 1–10) | 64 | 32 | 12 |
| AST, U/L (NV 15–45) | 69 | 191 | 350 |
| ALT, U/L (NV 20–65) | 56 | 194 | 227 |
| Troponin T, μg/L (NV <0.05) | 0.18 | 1.29 | 3.67 |
| N-terminal probrain natriuretic peptide, pg/mL (NV <95) | 26 | 50 | 102 |
| Mean right atrial pressure, mmHg (NV 0–6) | 14 | 9 | |
| Pulmonary artery pressure, mmHg (NV 15–30/5–13) | 36/21 | 22/13 | |
| Mean pulmonary artery wedge pressure, mmHg (NV 2–12) | 16 | 13 | |
| Cardiac index, L/min/m2 (NV 2.5–4.5) | 4.7 | 1.7 |
ALT Alanine aminotransferase; AST Aspartate aminotransferase; ESR Erythrocyte sedimentation rate; NV Normal value
The EMB showed changes of EM with inflammatory cell infiltrates that appeared to follow the interstitial and perivascular tissue planes and were also localized within the subendocardial tissues. The infiltrates were composed of mononuclear inflammatory cells, as well as eosinophils. In many locations, eosinophils were very prominent. Occasional myocytes showed degeneration or necrosis, but this was not a prominent feature. There was no vasculitis and no microorganisms were seen. Special stains for iron and amyloid were negative.
The patient was started on oral prednisone at 1 mg/kg/day, beta-blockers and angiotensin-converting enzyme (ACE) inhibitors. At one-month follow-up, he had no recurrence of his initial symptoms, and the eosinophil count became normal at 0.3×109/L. A repeat echocardiogram showed normal ventricular function with an EF of 65%. Prednisone was stopped, and he was continued on beta-blockers and ACE inhibitors.
Initially, exposure to chemicals in carpet cleaning products was postulated to be a potential cause of EM, but no relationship could be determined.
Case 2
A 50-year-old man on sumatriptan three times every two weeks presented to the emergency department with a chronic migraine headache and acute shortness of breath associated with nausea, vomiting, diaphoresis and increasing retrosternal chest pain. He noted an increased frequency of his migraine headaches associated with vague retrosternal chest pain and epigastric pain over a period of six months before admission. The dose of sumatriptan was increased to four days a week 10 days before presentation. This was followed after a few days later by fever, chills and sweats associated with increasing chest discomfort, myalgia and malaise. He denied any history of skin or oral ulcers, skin rash, arthritis, visual changes, and focal weakness or numbness. There was no history of asthma, diarrhea, abdominal pains or hematuria. He had an extensive history of travel (including Africa, southeast Asia and Mexico) and a long-standing history of smoking. His drug history included the use of nonsteroidal anti-inflammatory drugs and acetylsalicylic acid for many years and sumatriptan as needed for the past two years.
On examination, the patient’s blood pressure was 85/65 mmHg and his temperature was 38°C. Jugular venous pressure was at 6 cm above the sternal angle with a positive hepatojugular reflux. The apex beat was displaced laterally. There were no heaves or thrills and heart sounds were distant. He had a fourth heart sound and a few bibasilar crackles. He had no peripheral edema. The ECG showed nonspecific T wave and ST changes.
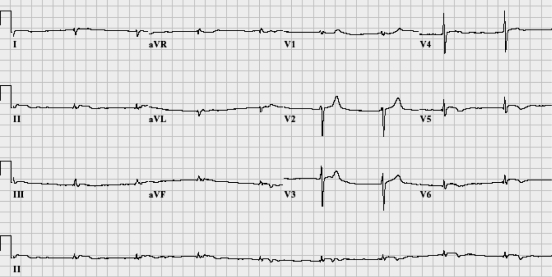
Within 2 h of admission, he had worsening chest pain associated with significant bradycardia (heart rate of 50 beats/min) and hypotension (blood pressure of 60/40 mmHg), requiring treatment with atropine and dopamine. A repeat ECG showed ST elevation in the inferolateral leads (Figure 1), and troponin T was positive at 0.94 μg/L (normal values less than 0.05 μg/L).
Figure 1.
Electrocardiogram showing minor ST elevation in the inferolateral leads associated with T wave inversion
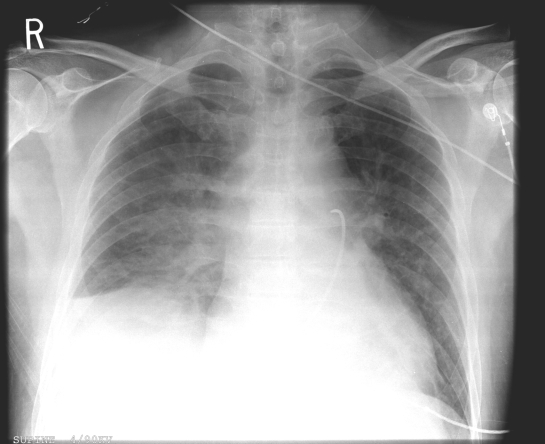
The patient was taken to the coronary catheterization laboratory. The SCA showed normal coronary arteries and LVEF of 54%; the results of right heart catheterization and laboratory tests are shown in Table 1. A chest radiograph showed significant pulmonary edema with a small right-sided pleural effusion (Figure 2). The echocardiogram showed globally depressed ventricular function with an EF of 45% and a very small pericardial effusion.
Figure 2.
Supine chest x-ray showing cardiomegaly, bilateral pulmonary edema and right pleural effusion. A pulmonary artery catheter, inserted via the inferior vena cava, with its tip lying in the pulmonary artery, is seen
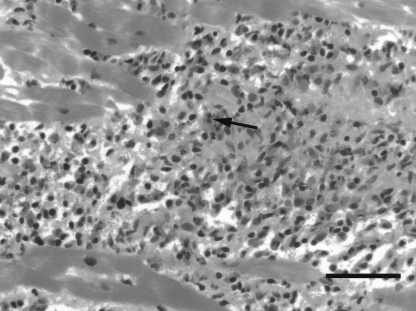
The EMB showed extensive necrotizing myocarditis in association with a mononuclear inflammatory cell infiltrate that also had a large quantity of eosinophils and degree of eosinophil degranulation (Figure 3). No granulomas or microorganisms were seen. Special stains for iron and amyloid were negative. No viral inclusions were identified. The overall histological appearance was considered to be one of a necrotizing EM.
Figure 3.
Representative photomicrograph showing necrotizing eosinophilic myocarditis in the endomyocardial biopsy specimen from case 2. Note the extensive inflammatory cell infiltrate composed of mononuclear inflammatory cells and numerous eosinophils (arrow). Hematoxylin and eosin stain. Bar, 50 μm
Sumatriptan was stopped and the patient was treated initially by prednisone 1 mg/kg/day in addition to beta-blockers, ACE inhibitors and diuretics, after successfully weaning off an inotropic agent. A repeat echocardiogram after two months of therapy showed improvement in LV function, with EF of 60%. Eight months after his presentation, he continued to do well with preserved normal ventricular function. Prednisone and ACE inhibitors were stopped, and he continued on beta-blockers.
Drug hypersensitivity to sumatriptan was suspected as the cause of EM in this case.
Case 3
A previously healthy 26-year-old man was referred by his family physician to St Paul’s Hospital, Vancouver, British Columbia, for management of newly diagnosed congestive heart failure. The diagnosis was based on the symptoms of palpitations, fatigue, vague chest discomfort, and cardiomegaly and pulmonary congestion visible on chest radiograph. He had developed a flu-like illness with low-grade fever, chills, myalgia and headache a week earlier. There had been no preceding cough, hemoptysis, orthopnea, paroxysmal nocturnal dyspnea or ankle edema. His past medical history was not significant.
On examination, he was in no respiratory distress. His heart rate was 80 beats/min and he had a blood pressure of 82/55 mmHg; he was afebrile. Breath sounds were normal throughout, with no adventitious sounds. Heart sounds were slightly distant, with a soft third heart sound. No murmurs or rubs were noted, and there was no lower limb edema. His abdomen was not distended and showed no evidence of hepatosplenomegaly.
The results of laboratory tests and right heart catheterization are shown in Table 1. The ECG showed a right bundle branch block and left anterior hemiblock, and occasional premature ventricular contractions.
The SCA showed normal coronary arteries with markedly depressed LV function, with EF estimated at 20%. An echocardiogram showed normal left and right ventricular sizes, and severely depressed global LV systolic function, with an EF of 20%.
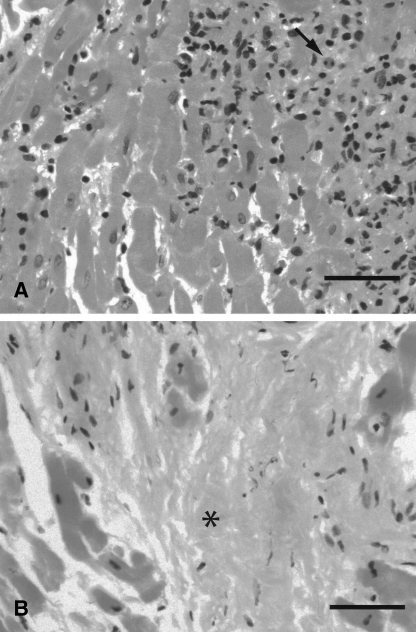
The EMB showed a diffuse interstitial inflammatory process with extensive myocyte destruction (Figure 4A). There were lymphocytes and macrophages with occasional eosinophils seen focally throughout the infiltrate. The infiltrate had a vague granulomatous appearance. There were no giant cells, vasculitis or microorganisms seen. Stains for iron and amyloid were negative.
Figure 4.
Representative photomicrograph of active (A) and healed (B) eosinophilic myocarditis in endomyocardial biopsy specimens from case 3. Active myocarditis (A) shows an inflammatory cell infiltrate containing occasional eosinophils (arrow), while healed myocarditis (B) shows areas of fibrosis (asterisk). Hematoxylin and eosin stain. Bar, 50 μm
The patient was started on anticoagulants because of his low cardiac output; treatment with beta-blockers, ACE inhibitors and diuretics were initiated, and prednisone was started at 1 mg/kg/day.
A follow-up echocardiogram performed four months later showed normal left and right ventricular sizes and functions (visually estimated LVEF of 65%). A repeat EMB showed no evidence of cardiac myocyte or vascular injury, and the absence of eosinophils and granulomatous inflammation (Figure 4B). Areas of patchy subendocardial and interstitial fibrosis, consistent with healing myocarditis, were seen. Anticoagulants and prednisone were stopped. Three months later, beta-blockers were stopped and the patient was maintained on ACE inhibitors only.
One year after his presentation, he continued to do well and had a normal echocardiographic assessment. The cause of EM in this patient was never determined.
DISCUSSION
EM is rarely recognized clinically and is often first discovered at postmortem examination. Studies have reported EM seen in 0.5% of unselected autopsy series, and in more than 20% of explanted hearts from heart transplant recipients. EM in the latter group consisted of mostly the hypersensitivity subtype, because these patients were on variety of medications (1).
Frequently, the cause of the disease remains unknown. However, there are numerous drugs and drug classes that have been implicated in causing the hypersensitivity form of EM (Table 2). The hypersensitivity form may develop early during the drug use or may have a delayed presentation of up to two years. This form of EM has also been seen in patients treated with dobutamine infusion, but it is unclear whether this reaction is the hypersensitivity to the drug itself or to its preservative, sodium bisulfite (2). In our second case, we suspected that EM was due to a hypersensitivity reaction to sumatriptan, which was used for a month before the patient’s presentation. With the support of steroids, ACE inhibitors and beta-blockers, the patient improved after stopping the sumatriptan. To our knowledge, this is the first report to describe this association.
TABLE 2.
Principal drugs capable of causing hypersensitivity myocarditis
| Antibiotic | Anti-inflammatory |
| Amphotericin B | Indomethacin |
| Ampicillin | Oxyphenbutazone |
| Chloramphenicol | Phenylbutazone |
| Penicillin | Diuretic |
| Tetracycline | Acetazolamide |
| Streptomycin | Chlorthalidone |
| Cephalosporin | Hydrochlorothiazide |
| Sulfonamide | Spironolactone |
| Sulfadiazine | Other |
| Sulfisoxazole | Amitriptyline |
| Anticonvulsant | Methyldopa |
| Phenindione | Sulfonylurea |
| Phenytoin | Tetanus toxoid |
| Carbamazepine | Dobutamine |
| Antituberculous | Digoxin |
| Isoniazid | Captopril and enalapril |
| Para-aminosalicylic acid |
Data from reference 13
In addition to hypersensitivity, other diagnoses for eosinophilic infiltration of myocardium are parasitic infestation and idiopathic hypereosinophilic syndrome (3). Cardiac disease occurs in more than 50% of patients with idiopathic hypereosinophilic syndrome (defined as an absolute eosinophil count greater than 1.5×109/L lasting for more than six months in the absence of any known cause of hypereosinophilia and with evidence of organ involvement). The hypereosinophilic syndrome represents a spectrum of diseases, including Davies’ endomyocardial fibrosis and Loffler’s myocarditis. Cardiac disease is the major cause of morbidity and mortality in patients with this syndrome (4).
The characteristic histopathology of EM is a mixed inflammatory cell infiltrate containing a variable amount of eosinophils within the myocardium.
Eosinophilic infiltration ranges from mild localized involvement, with small foci of inflammatory cells containing few eosinophils, to marked multifocal or widespread infiltrates that can easily be observed at low magnification. In a few cases, atrial involvement may be more common than ventricular involvement (5).
Myocardial infiltrates vary in their geographic distribution as being either perivascular or interstitial. The epicardium and endocardium can be involved to a certain extent too. There is no apparent relationship between the extent of the eosinophilic infiltrate in EM and clinical symptoms (2). Additional features that may be seen include myocyte necrosis, fibrosis, granuloma formation and fibrinoid necrosis of collagen. Myocyte necrosis is common in hypereosinophilic syndrome and is associated with extensive endocardial eosinophilic infiltration, resulting in endocardial scarring and restrictive cardiomyopathy.
Myocyte necrosis is not common in the hypersensitivity form of EM; consequently, fibrosis is typically absent or minimal and all inflammatory lesions are approximately at the same stage. A more severe form of hypersensitivity EM is termed acute eosinophilic necrotizing myocarditis. It is characterized by a more severe eosinophilic infiltration, with marked edema and myocyte necrosis, and it usually follows a fulminant course. Myocyte necrosis has been associated with eosinophil degranulation and the deposition of the major basic protein of an eosinophilic granule, and it may result from increased cell membrane permeability and inhibition of mitochondrial respiration (6,7). The second patient presented with this form of EM and responded well to a combination of steroids, ACE inhibitors and beta-blockers.
The spectrum of the presentations of EM is wide, as demonstrated by our case series. The first patient presented with chest pain, which was initially thought to be ACS, and he was referred for an SCA. The second patient presented with what was initially thought to be ACS, but was associated with cardiogenic shock and rapid hemodynamic compromise, necessitating urgent heart catheterization. Newly diagnosed unexplained heart failure following a flu-like illness was the presentation of our third patient. Cardiac arrhythmias and sudden death are possible presentations as well.
The symptoms and signs accompanying EM are nonspecific and include fever, skin rashes, sinus tachycardia, conduction delays and ST-T wave abnormalities. Peripheral blood eosinophilia, which was present in the first case, is not necessarily seen in all cases of EM, and thus, the diagnosis is often not suspected clinically (8). Myocyte death caused by necrosis and apoptosis plays a role in the development of heart failure; furthermore, myocardial fibrosis can occur despite treatment, and puts the patient at high risk for fatal arrhythmias.
The majority of published case reports are based on autopsy diagnosis, while a few reports are based on EMB findings and explanted hearts. EMB is a valuable tool to confirm the diagnosis if positive, but it is not a very sensitive technique because the infiltrates in EM are often focal (estimated sensitivity at 50%) (9). The presence of peripheral blood eosinophilia on admission or the new appearance of eosinophilia during hospitalization should provoke the consideration of a repeat of EMB if initial testing does not lead to a diagnosis. Underlying inflammatory disorders were suspected in our patients based on their acute presentations and normal coronary angiograms, which prompted obtaining EMBs.
Treatment should include withdrawal of possible etiologically relevant drug(s). Although withdrawal of potentially lifesaving inotropic therapy is not recommended, consideration may be given to changing to a different inotrope or to one that does not contain sodium bisulfite. Some patients with EM have documented dramatic responses to steroid therapy, including those with a history of severe LV dysfunction and aborted sudden death (10). In a case report, intravenous methylprednisolone bolus (1 g/day for three days) followed by 1 mg/kg/day oral prednisone, with gradual tapering for one year demonstrated an improvement in symptoms, a reduction of the eosinophil count and increased EF (11). In agreement with these reports, all our patients demonstrated complete recovery with high-dose oral steroids followed by gradual tapering. Steroid use was combined with ACE inhibitors and beta-blockers.
The role of immunosuppressive therapy remains controversial. Immunosuppressive therapy may prevent reappearance of EM (10). Aggarwal et al (12) reported a successful use of a combination of steroids and azathioprine at a dose of 2 mg/kg in a patient diagnosed to have EM who presented with cardiogenic shock. Two weeks later, a repeat EMB showed almost complete resolution of the eosinophilic infiltrate associated with clinical improvement.
REFERENCES
- 1.Winters GL, McManus BM. Myocarditis. In: Silver MD, Gotlieb AI, Schoen FJ, editors. Cardiovascular Pathology. Philadelphia: Churchill Livingstone; 2001. pp. 256–84. [Google Scholar]
- 2.Takkenberg JJ, Czer LS, Fishbein MC, et al. Eosinophilic myocarditis in patients awaiting heart transplantation. Crit Care Med. 2004;32:714–21. doi: 10.1097/01.ccm.0000114818.58877.06. [DOI] [PubMed] [Google Scholar]
- 3.De Alava E, Panizo-Santos A, Fernandez-Gonzalez AL, Pardo-Mindan FJ. Eosinophilic myocarditis in patients waiting for heart transplantation. Cardiovasc Pathol. 1995;4:43–6. doi: 10.1016/1054-8807(94)00021-i. [DOI] [PubMed] [Google Scholar]
- 4.Ommen SR, Seward JB, Tajik AJ. Clinical and echocardiographic features of hypereosinophilic syndromes. Am J Cardiol. 2000;86:110–3. doi: 10.1016/s0002-9149(00)00841-9. [DOI] [PubMed] [Google Scholar]
- 5.Fenoglio JJ, Jr, McAllister HA, Jr, Mullick FG. Drug related myocarditis. I. Hypersensitivity myocarditis. Hum Pathol. 1981;12:900–7. doi: 10.1016/s0046-8177(81)80195-5. [DOI] [PubMed] [Google Scholar]
- 6.Tai PC, Hays DJ, Clark JB, Spry CJ. Toxic effects of human eosinophil products on isolated rat heart cells in vitro. Biochem J. 1982;204:75–80. doi: 10.1042/bj2040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young JD, Peterson CG, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–6. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- 8.Talierco CP, Olney BA, Lie JT. Myocarditis related to drug hypersensitivity. Mayo Clin Proc. 1985;60:463–8. doi: 10.1016/s0025-6196(12)60870-2. [DOI] [PubMed] [Google Scholar]
- 9.Burke AP, Saenger J, Mullick F, Virmani R. Hypersensitivity myocarditis. Arch Pathol Lab Med. 1991;115:764–9. [PubMed] [Google Scholar]
- 10.Galiuto L, Enriquez-Sarano M, Reeder GS, et al. Eosinophilic myocarditis manifesting as myocardial infarction: Early diagnosis and successful treatment. Mayo Clin Proc. 1997;72:603–10. [PubMed] [Google Scholar]
- 11.Corradi D, Vaglio A, Maestri R, et al. Eosinophilic myocarditis in a patient with idiopathic hypereosinophilic syndrome: Insights into mechanisms of myocardial cell death. Hum Pathol. 2004;35:1160–3. doi: 10.1016/j.humpath.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal A, Bergin P, Jessup P, Kaye D. Hypersensitivity myocarditis presenting as cardiogenic shock. J Heart Lung Transplant. 2001;20:1241–4. doi: 10.1016/s1053-2498(01)00313-8. [DOI] [PubMed] [Google Scholar]
- 13.Kounis NG, Zavras GM, Soufras GD, Kitrou MP. Hypersensitivity myocarditis. Ann Allergy. 1989;62:71–4. [PubMed] [Google Scholar]