Abstract
Background
Attention deficit/hyperactivity disorder (ADHD) and lead exposure are high-prevalence conditions among children.
Objective
Our goal was to investigate the association between ADHD and blood lead levels (BLLs) in Chinese children, adjusting for known ADHD risk factors and potential confounding variables.
Methods
We conducted a pair-matching case–control study with 630 ADHD cases and 630 non-ADHD controls 4–12 years of age, matched on the same age, sex, and socioeconomic status. The case and control children were systematically evaluated via structured diagnostic interviews, including caregiver interviews, based on the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., revised criteria (DSM-IV-R). We evaluated the association between BLLs and ADHD using the Pearson chi-square test for categorical variables and the Student t-test for continuous data. We then performed conditional multiple variables logistic regression analyses with backward stepwise selection to predict risk factors for ADHD.
Results
There was a significant difference in BLLs between ADHD cases and controls. ADHD cases were more likely to have been exposed to lead during childhood than the non-ADHD control subjects, with adjustment for other known risk factors [children with BLLs ≥ 10 μg/dL vs. ≤ 5 μg/dL; OR = 6.0; 95% confidence interval (CI) = 4.10–8.77, p < 0.01; 5–10 μg/dL vs.≤ 5 μg/dL, OR = 4.9; 95% CI = 3.47–6.98, p < 0.01]. These results were not modified by age and sex variables.
Conclusions
This was the largest sample size case–control study to date to study the association between BLLs and ADHD in Chinese children. ADHD may be an additional deleterious outcome of lead exposure during childhood, even when BLLs are < 10 μg/dL.
Keywords: attention deficit hyperactivity disorder, blood lead levels, case-control study
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood psychiatric disorders, characterized by developmentally inappropriate levels of inattention, impulsivity, and hyperactivity [American Psychiatric Association (APA) 2000; Biederman and Faraone 2005; Rappley 2005; Remschmidt 2005]. Prevalence of ADHD in children has been reported to be 3–8% worldwide (Froehlich et al. 2007; Leung et al. 1996; Remschmidt 2005). Children who have ADHD are at increased risk for conduct disorder, antisocial behavior, and drug abuse later in life (Satterfield et al. 2007). Moreover, the costs associated with their medical care and education are substantial (Leibson et al. 2001).
Although the causes of ADHD remain unclear, both genetic and environmental factors are thought to influence the etiology of ADHD (Banerjee et al. 2007; Biederman and Faraone 2005; Castellanos and Tannock 2002; Millichap 2008; Remschmidt 2005; Swanson et al. 2007). Family, twin, and adoption studies have demonstrated high heritability, and various polymorphisms of dopamine-related genes have been found to increase susceptibility to ADHD (Castellanos and Tannock 2002; Durston 2003; Khan and Faraone 2006). Furthermore, many environmental risk factors and potential gene–environment interactions have also been shown to increase the risk for the disorder (Banerjee et al. 2007; Biederman and Faraone 2005; Millichap 2008; Thapar et al. 2007). Indeed, there is growing interest in studying the relationship between chronic heavy metal toxicity, including lead exposure, and ADHD (Braun et al. 2006; Konofal and Cortese 2007; Millichap 2008; Nigg et al. 2008).
Lead is one of the well-established environmental poisons, and its general toxic effects, particularly in children, continue to be a major public health issue worldwide [American Academy of Pediatrics (AAP) 2005; Lidsky and Schneider 2003; Needleman 2004]. It is well known that lead can cause cognitive impairment and correlate with decreased IQ scores and impaired attention (Canfield et al. 2003; Koller et al. 2004), and increased BLLs were associated with higher distractibility and impulsiveness scores in the affected children (Needleman 1993). The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) recommended that child blood lead levels (BLLs) not exceed 10 μg/dL (CDC 1991; WHO 1995). As a result of rapid industrialization in China, it is estimated that tens of millions of children 1–18 years of age have BLLs ≥ 10 μg/dL (Huo et al. 2007; Ren et al. 2006; Wang and Zhang 2006). Furthermore, several recent studies have shown that cognitive deficits and behavioral problems in children still exist even with BLLs < 10 μg/dL (Binns et al. 2007; Braun et al. 2006; Canfield et al. 2003; Koller et al. 2004).
Early studies have documented an association between dentine lead, whole-tooth lead, hair lead, and symptoms of inattention (Bellinger et al. 1994a, 1994b; Fergusson et al. 1993; Needleman and Leviton 1979; Needleman et al. 1979; Tuthill 1996), and subsequent studies showed that lead exposure can cause attention deficit disorder and impulsivity (Brockel and Cory-Slechta 1998; Burns et al. 1999; Eppright et al. 1997; Kahn et al. 1995; Minder et al. 1994; Silva et al. 1988; Thomson et al. 1989; Wasserman et al. 1998, 2001). However, these studies may not be conclusive. First, few studies have investigated the effect of lead exposure on ADHD formally diagnosed with the established criteria in International Classification of Diseases, 10th Revision (ICD-10) (WHO 2007) or in Diagnostic and Statistical Manual of Mental Disorders, 4th ed., revised (DSM-IV-R; APA 2000) (Burns et al. 1999; Eppright et al. 1997; Silva et al. 1988). Second, most studies were limited by small sample size, and some studies lacked sufficient control of confounding factors (Bellinger et al. 1994b; Minder et al. 1994). Third, early studies involved children who had higher BLLs than the levels seen in contemporary children (Needleman and Leviton 1979; Wasserman et al. 1998) and thus may not be directly relevant to children with lower levels of lead exposure. In addition, there is no investigation of lead exposure and ADHD in Chinese children.
Therefore, in an effort to evaluate the association between BLLs and the risk for ADHD in Chinese children, we performed a case–control study involving large samples of children 4–12 years of age. We also considered known potential variables in the etiology of ADHD, such as delivery characteristics, perinatal factors, parental psychosocial factors, and prenatal exposure to tobacco and alcohol.
Methods
Subjects
This study was designed as a pair-matching case–control study. ADHD subjects were consecutively recruited from children coming for initial or follow-up assessment from October 2003 to August 2007 in two pediatric clinics at the Anhui Provincial Children’s Hospital and the Institute of Anhui Traditional Chinese Medicine in Anhui Province, People’s Republic of China. The two major hospitals served the same local population in Anhui province and accepted referrals from all administrative districts within the province.
All ADHD (ICD-10 codes F90, 208–210) subjects were children of Chinese Han nationality 4–12 years of age at the time of investigation and had a lifetime medical history that fully met the DSM-IV-R criteria (APA 2000; Su et al. 2001) for ADHD. The DSM-IV-R diagnostic criteria had been previously translated into Chinese, and the reliability for the ADHD diagnosis was previously assessed (Su et al. 2001).
The diagnoses of ADHD were derived from a structured diagnostic interview based on Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-E) (Ambrosini 2000), which was modified to assess DSM-IV-R criteria and incorporate parents’ and teachers’ reports of behavioral symptoms, clinical observation of behavior, the Aberrant Behavior Checklist (Aman and Singh 1986), and tests of attention such as the Conners’ Continuous Performance Test (Homack and Riccio 2006). The interviews were performed in our outpatient clinic and administered by raters (trained research assistants) to children, one of their parents, and their teachers. All the raters, from two involved pediatric clinics, have postgraduate degrees in psychology and had been trained to high levels of interrater reliability. We computed κ coefficients of agreement between all the raters and three experienced board-certified child psychiatrists who listened to audio taped interviews made by the raters. Based on 120 interviews, the median κ was 0.87; κ was 0.95 for ADHD. Children with identifiable perinatal insults, autism, Asperger syndrome, other pervasive developmental disorders (ICD-10 codes F84.0–F84.9, 308.0), and mental retardation (ICD-10 codes F70–F79, 312–315) were excluded.
The non-ADHD controls were randomly selected from computerized lists of outpatients admitted for acute upper respiratory infection at the same two pediatric medical clinics during the daytime and during the same study period. By the pair-matched design, each ADHD case and control set had the same sex, the same age (difference between birthdays within 6 months), and almost the same level of socioeconomic status (SES). The controls were given the same full diagnostic assessment as the ADHD cases and screened only for the absence of ADHD without exclusion of any other diagnosis except for the same exclusion criteria applied to cases. SES is measured by poverty-to-income ratio (PIR), and PIR is the ratio of family income to the poverty threshold for the year of the interview. Low SES was defined as having PIR values < 1 in our analysis and high SES as having PIR > 3. The others were regarded as middle SES. The study was approved by our institutional review boards and complied with all applicable requirements of the United States. The parents of all the children in this study provided written informed consent at enrollment.
Blood sampling and analysis
Blood samples (2 mL/child) were collected in heparinized syringes. Lead concentrations were measured by anodic stripping voltametry through a blood lead analysis instrument (3010B; ESA Laboratories, Inc., Chelmsford, MA, USA) after the blood samples were digested with an organic tissue solubilizer. The limit of detection was 1.0 μg/dL. No detectable values were given values of 0.70 (1.0 divided by the square root of 2). Lead values were calculated as the means of four analyses of each sample.
Covariates
Epidemiologic studies have shown that male sex, low SES, and young age are associated with a raised prevalence of ADHD. Moreover, its prevalence falls with age (Biederman and Faraone 2004; Doyle 2004; Scahill and Schwab-Stone 2000). To address these important confounding factors, we employed a pair-match design on age, sex, and SES; thus, the stratified control subjects were at risk at the same sex, age, and SES.
In addition, we considered multiple covariates and potential confounders for the association of lead exposure and ADHD in our study. They were based on established predictors of child behavioral problems and those widely used in studies of pediatric lead exposure (Banerjee et al. 2007; Biederman and Faraone 2005; Linnet et al. 2003; Mick et al. 2002; Millichap 2008; St Sauver et al. 2004; Swanson et al. 2007; Wasserman et al. 2001). The following variables were used: family history of ADHD (ADHD in parents and siblings, diagnosed by psychiatrists, obtained from clinical reports), household composition (normal: child lives with biological parents; single: child lives with only one parent; or recombined: child lives in remarried family), maternal tobacco use during pregnancy (at least one cigarette per day during the last trimester), maternal drinking during pregnancy (at least two glasses per week during the entire pregnancy), labor complications, cesarean delivery, perinatal distress [low birth weight and admission to a neonatal intensive care unit (NICU) as markers], parents’ age at childbirth, and parents’ education. Because all the cases recruited were of Chinese Han nationality, the variable of ethnic origin could not be used in our analysis. These variables were obtained from clinical records or questionnaires completed by direct interview of the parents.
Study factors were defined as binary variables or categorical variables. For example, maternal smoking and drinking habits during pregnancy were recorded as binary variables—that is, drinker or nondrinker, cigarette smoker or nonsmoker. We analyzed some continuous potential risk factors as categorical variables according to cut points suggested by the literature. For example, low birth weight is typically defined as < 2,500 g (St Sauver et al. 2004). Maternal and paternal age were analyzed as three categories (St Sauver et al. 2004): < 20 years, 20–30 years, and > 30 years of age. Maternal and paternal education were analyzed as ≤ 9 years of compulsory education, high school education (9–12 years), or some college or advanced training (≥ 12 years).
Data analysis
The association between BLLs and ADHD was evaluated using the Pearson chi-square test for categorical variables and the Student t-test for continuous data. We also performed a conditional logistic regression analysis with a binary outcome of ADHD in relation to BLLs, adjusting for other potential confounding factors (Hosmer and Lemeshow 2000). In the regression model, BLLs were analyzed as an ordered categorical variable and recorded as three categories: ≤ 5 μg/dL, 5–10 μg/dL, and ≥ 10 μg/dL. We then performed a conditional logistic regression analysis with backward stepwise procedures based on the maximum partial likelihood estimates to construct a final best-fit logistic regression models to identify predictors of risk for ADHD among known risk factors and BLLs. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for differing levels of exposure. All statistical tests were considered to be significant at an alpha level of 0.05 on a two-tailed test and performed with the statistical software SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
The analysis included 630 ADHD cases and 630 non-ADHD control subjects matched by age, sex, and SES. There were 434 sets of boys and 196 sets of girls. The average ages were 7.9 ± 2.1 years. The mean BLLs were 8.77 ± 3.89 μg/dL in the ADHD group and 5.76 ± 3.39 μg/dL in the control group. The ADHD group had higher BLLs (p < 0.05, see Table 1). There was no significant difference in BLLs between males and females.
Table 1.
Mean values of BLL of ADHD cases and controls.
| BLL (μg/dL) (mean ± SE)
|
|||||
|---|---|---|---|---|---|
| Group | Total (n = 630) | Male (n = 434) | Female (n = 196) | Percent > 5 μg/dL | Percent > 10 μg/dL |
| ADHD | 8.77 ± 3.89 | 8.89 ± 4.0 | 8.67 ± 4.17 | 75.8 | 24.4 |
| Control | 5.76 ± 3.39 | 5.86 ± 3.44 | 5.55 ± 3.21 | 49.8 | 10.1 |
| t-Value, χ2 | t = 14.6 | t = 11.96 | t = 8.30 | χ2 = 237 | χ2 = 116 |
| p-Value | < 0.05 | < 0.05 | < 0.05 | < 0.01 | < 0.01 |
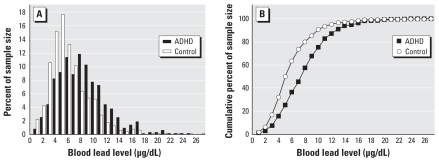
Figure 1 shows BLL distribution and cumulative distribution of ADHD children and controls. Only 10.1% of non-ADHD children had BLLs > 10 μg/dL, whereas this percentage increased significantly to 24.4% in ADHD children. (χ2 = 237, p < 0.01). In addition, 49.8% of non-ADHD children had BLLs > 5 μg/dL, whereas of the ADHD cases, 74.7% had BLLS > 5 μg/dL (χ2 = 116, p < 0.01).
Figure 1.
(A) Blood lead distribution (%) of ADHD cases and controls. (B) Cumulative distribution (%) of blood lead for ADHD cases and controls.
Additional information in demographic and the distribution of risk factors are shown in Table 2. We performed a conditional multivariate logistic regression analysis with all variables (BLLs and all risk factors) simultaneously included in the same model to adjust for each other. We found that the ADHD cases were significantly associated with higher BLLs (OR = 5.19, p < 0.01 for children with BLLs 5–10 μg/dL; OR = 7.15, p < 0.01 for with BLLs ≥ 10 μg/dL, using the sample with BLLs ≤ 5 μg/dL as referent) and family history of ADHD (OR = 4.54, p < 0.01, compared with the sample without familial ADHD history). The risk for ADHD decreased as the mother’s education level increased (OR = 0.69, p = 0.017, using the sample of ≤ 9 years maternal education as referent). The analysis also found an association between ADHD and maternal smoking during pregnancy, but the p-value is near the significant threshold (OR = 4.04, p = 0.047). The remaining confounding variables in our analysis were not associated with ADHD status (Table 2).
Table 2.
Demographic and distribution of risk factors of ADHD cases and controls.
| Characteristic | ADHD (n = 630) | Controls (n = 630) | ORa | p-Valuea |
|---|---|---|---|---|
| Matched factors | ||||
| Age (years) | ||||
| 4–6 | 172 | 172 | — | — |
| 7–9 | 303 | 303 | ||
| 10–12 | 155 | 155 | ||
| Sex | ||||
| Male | 434 | 434 | — | — |
| Female | 196 | 196 | ||
| SES (PIR) | ||||
| ≤ 1 | 58 | 58 | — | — |
| 1–3 | 436 | 436 | ||
| ≥ 3 | 136 | 136 | ||
| Child factors | ||||
| Blood lead (μg/dL) | ||||
| ≤ 5 | 101 | 316 | ||
| 5–10 | 326 | 255 | 5.19 | < 0.01 |
| ≥ 10 | 203 | 59 | 7.15 | < 0.01 |
| Household composition | ||||
| Two parent | Referent | Referent | ||
| Single parent | 27 | 23 | 1.05 | 0.89 |
| Recombined | 15 | 17 | 0.50 | 0.11 |
| Low birth weight (< 2,500 g) | 55 | 64 | 0.68 | 0.13 |
| Twin | 4 | 10 | 0.29 | 0.08 |
| Family history of ADHD | 21 | 4 | 4.54 | 0.02 |
| Pregnancy, labor, or delivery characteristics | ||||
| Labor or delivery complications | 58 | 70 | 0.71 | 0.16 |
| Surgical procedure required | 59 | 67 | 0.63 | 0.06 |
| Premature labor | 27 | 22 | 0.84 | 0.65 |
| NICU required | 39 | 45 | 0.57 | 0.08 |
| Parental factors | ||||
| Age of mother (years) | ||||
| ≤ 20 | 81 | 65 | ||
| 20–30 | 461 | 459 | ||
| ≥30 | 88 | 106 | 0.92 | 0.75 |
| Age of father (years) | ||||
| ≤ 20 | 20 | 16 | ||
| 20–30 | 479 | 493 | ||
| ≥ 30 | 131 | 121 | 1.15 | 0.41 |
| Maternal education (years) | ||||
| ≤ 9 | 154 | 127 | ||
| 9–12 | 393 | 371 | ||
| ≥ 12 | 83 | 132 | 0.69 | 0.017 |
| Paternal education (years) | ||||
| ≤ 9 | 123 | 103 | ||
| 9–12 | 373 | 376 | ||
| ≥12 | 134 | 151 | 1.07 | 0.97 |
| Maternal drinking during pregnancy | 11 | 6 | 1.20 | 0.77 |
| Maternal smoking during pregnancy | 6 | 9 | 4.04 | 0.047 |
—, no data.
Obtained from the multivariate logistic regression model that simultaneously included all the risk factors and the BLLs.
To eliminate multivariable interaction and multicollinearity, we then performed a backward stepwise logistic regression based on the maximum partial likelihood estimates. In the final best-fit model, the association between ADHD and maternal smoking during pregnancy was excluded, but ORs for the associations between ADHD and family history of ADHD, maternal education levels, and BLLs were largely unchanged from estimates obtained in the original model used in Table 2 (Table 3).
Table 3.
Risk factors identified in stepwise logistic regression model.a
| Variables | βb | SE | Wald test | p-Value | ORc (95% CI) |
|---|---|---|---|---|---|
| BLL | |||||
| ≤ 5 | |||||
| 5–10 | 1.59 | 0.18 | 79.86 | < 0.01 | 4.92 (3.47–6.98) |
| ≥10 | 1.79 | 0.19 | 85.79 | < 0.01 | 6.00 (4.11–8.77) |
| Family history of ADHD | 1.73 | 0.63 | 7.51 | < 0.01 | 5.65 (1.64–19.46) |
| Maternal education (years) | |||||
| ≤9 | |||||
| 9–12 | −0.13 | 0.17 | 0.60 | 0.438 | 0.88 (0.63–1.23) |
| ≥ 12 | −0.49 | 0.19 | 6.90 | < 0.01 | 0.62 (0.43–0.88) |
Variable(s) entered on step 1: BLLs, household composition, birth weight, twin, family history of ADHD, labor complications, cesarean, premature labor, NICU, mother’s age, father’s age, maternal education, paternal education, prenatal tobacco exposure and prenatal alcohol exposure.
βvalues are the estimated unstandardized regression coefficients.
OR indicates likelihood of an ADHD.
We also performed a conditional multivariate stepwise logistic regression analysis stratified by sex and age. As with the total sample, ADHD cases were significantly associated with higher BLLs than the lower BLLs in all subdefinitions (Table 4), which indicates that increased risk for ADHD associated with BLLs is not modified by age and sex.
Table 4.
Increased risks for ADHD associated with BLLs in different sample definitions.
| BLL (μg/dL) | OR (95% CI) | p-Value |
|---|---|---|
| Total sample (n = 1,260) | ||
| ≤ 5 | 1 | — |
| 5–10 | 4.92 (3.47–6.98) | < 0.01 |
| ≥ 10 | 6.00 (4.11–8.77) | < 0.01 |
| Male sample (n = 868) | ||
| ≤ 5 | 1 | — |
| 5–10 | 4.49 (2.97–6.80) | < 0.01 |
| ≥ 10 | 6.69 (4.20–10.67) | < 0.01 |
| Female sample (n = 392) | ||
| ≤ 5 | 1 | — |
| 5–10 | 5.62 (2.79–11.0) | < 0.01 |
| ≥ 10 | 7.38 (3.66–14.88) | < 0.01 |
| Age 4–6 years sample (n = 344) | ||
| ≤ 5 | 1 | — |
| 5–10 | 6.86 (3.17–14.86) | < 0.01 |
| ≥ 10 | 13.41 (5.04–35.69) | < 0.01 |
| Age 7–9 years sample (n = 606) | ||
| ≤ 5 | 1 | — |
| 5–10 | 5.78 (3.33–10.00) | < 0.01 |
| ≥ 10 | 8.53 (4.72–15.422) | < 0.01 |
| Age 10–12 years sample (n = 310) | ||
| ≤ 5 | 1 | — |
| 5–10 | 3.89 (1.82–8.32) | < 0.01 |
| ≥ 10 | 4.13 (2.01–8.49) | < 0.01 |
This table is obtained from the multivariate logistic regression model that simultaneously included all the risk factors and the BLL. OR indicates likelihood of ADHD.
Discussion
We examined the effect of BLLs in ADHD cases and non-ADHD controls with adjustment for most other known risk factors in Chinese children 4–12 years of age. We found ADHD cases were more likely to have been exposed to lead. The children with BLLs > 10 μg/dL had 4.1- to 8.7-fold higher risk for ADHD. Even when their BLLs were under the recommended level but > 5 μg/dL, the risk also showed a 3.5- to 7.0-fold increase. Family history of ADHD was another positive high-risk factor. Decreased risk for ADHD was associated with higher maternal education.
Lead may play important roles in the etiology of ADHD. Recently, Braun et al. (2006) analyzed data from a U.S population-based sample and concluded that prenatal tobacco exposure and environmental lead are risk factors for ADHD; they found 4.1-fold increased odds of ADHD with increased BLLs. Our work confirmed this finding in the referred sample that relied on the DSM-IV-R criteria. In addition, Nigg et al. (2008) reported that low-level lead exposure was associated with formal clinical diagnostic ADHD by DSM-IV-R criteria in a community sample in the United States, where regulations have reduced the incidence of lead poisoning and the current population average of lead levels is relatively lower. This is consistent with our finding that the risk of ADHD was associated with low lead exposures (< 10 μg/dL) in Chinese children. Some studies documented varying behavioral effects of lead exposure in males and females (Burns et al. 1999; Ris et al. 2004). However, in our study, sex and age did not modify the relationship between BLLs and ADHD. Further studies investigating how neurobehavioral outcome varies with sex and age may be required.
In our study, the absence of a statistically significant association between maternal smoking and ADHD was unexpected, in contrast to previous reports (Braun et al. 2006; Linnet et al. 2003; Swanson et al. 2007). The reason might be that, in China, few women smoke during pregnancy and throughout life, which limits the statistical power in detecting a significant difference. Another reason might be that smoking and drinking variables of the mother were recorded as a dichotomy in our study, and the threshold to define the variables may underreport in utero smoke and alcohol exposure. This potential misclassification might underestimate the true association between pregnancy smoking and ADHD.
Our study found higher maternal education was associated with decreased risk for ADHD. This result is consistent with previous work, which indicated that low maternal education, low SES, and single parenthood are important adverse factors for ADHD (Biederman and Faraone 2005; Millichap 2008; St Sauver et al. 2004). Apart from environmental risk factors for ADHD, our current report indicated that children with a family history of ADHD were more likely to be diagnosed with ADHD. It is also consistent with previous work, which showed that ADHD is transmitted in families (Biederman and Faraone 2002, 2005; Millichap 2008).
Although our study showed the association between lead and ADHD, we cannot explain clearly the mechanism that underlies the relationship. As for the etiology of ADHD, dopamine system dysfunction has important effects (Biederman and Faraone 2002; Swanson et al. 2007). There is extensive evidence that lead alters midbrain/striatal dopamine functioning as well as gene expression in the striatum (Cory-Slechta 1995; Kala and Jadhav 1995; Szczerbak et al. 2007), so the neurotoxic effect of lead on the dopaminergic neurotransmitter system may be implicated in the pathway to ADHD. Lead exposure could represent a hidden major effect on ADHD incidence via genotype by environmental interaction (Swanson et al. 2007).
Strengths of the study
The strengths of this study are as follows: a) Our sample size was the largest to date in case–control studies to investigate BLLs and ADHD. b) The ADHD diagnosis was made through an extensive clinical evaluation based on the DSM-IV-R diagnostic instrument and performed by child and adolescent psychiatrists. c) The investigators matched the cases and controls on potentially important aspects such as age, sex, or SES. This is important because lead levels are highest among younger children compared with adolescents, and ADHD is of higher prevalence among children of elementary and middle-school age.
Clinical implications
This study suggests that there is a link between ADHD and BLLs, and the results reinforced findings from previous studies (Braun et al. 2006; Nigg et al. 2008). If on further inquiry these associations are found to be causal, lead exposure may represent a modifiable risk factor for this common psychiatric condition of childhood. Considering that ADHD typically starts in early childhood and that lead poisoning is one of the most common and entirely preventable pediatric problems (AAP 2005; CDC 1991), strategies in public health must focus on practicing primary and secondary prevention of lead exposure in children. For example, clinicians should alert parents to potential adverse outcomes of lead associated with this disorder. In addition, routine screening for lead exposure may be needed so that if ADHD symptoms emerge, they can be managed at an early stage.
Moreover, BLLs < 10 μg/dL also indicated a risk factor. This may suggest that the lower standard may effectively protect children from the harmful effects of lead, which is consistent with previous investigations (AAP 2005; Canfield et al. 2003; Surkan et al. 2007).
Some studies (Ruff et al. 1993; Tong et al. 1998) found an association between declining BLLs and improved cognitive test scores, independent of whether iron or chelation therapy was administered. However, other works (AAP 2005; Canfield et al. 2003) indicated that the damage caused by blood lead exposure is irreversible. Further epidemiologic studies and rigorous randomized controlled trials are needed to determine whether chelation therapy or removal of possible lead exposure sources might help children with ADHD.
Limitations
This study has several limitations. First, temporality between high BLLs and ADHD could not be ascertained definitely in this case–control design. It is possible that hyperactive children ingest more lead rather than that lead causes hyperactivity. Therefore, further studies with serial lead measurements, even from the antenatal to the postnatal period, and more continuous measures of ADHD symptoms or of specific underlying neurobehavioral domains may be required to document the temporal relationship between lead exposure and development of ADHD. Second, some might argue that concurrent blood lead tests are not an adequate biomarker of a child’s lifetime exposure. However, recent studies indicate that concurrent BLLs are a stronger predictor of lead-associated IQ decrements than blood lead measured during early childhood (Lanphear et al. 2005). Third, recall bias is one of the problems of the case–control study. In this study, the main variable of interest was the BLLs, which were objectively measured. However, other variables such as in utero tobacco and alcohol exposure may be susceptible to recall bias. Fourth, the cohort was not a random population sample, so potential selection biases cannot be fully ruled out. In addition, because all our sample was Chinese Han, no children living in foster families were included, and none of the sample had health insurance, our results may not generalize to children with different socioeconomic or ethnic backgrounds. Finally, it is interesting that Nigg et al. (2008) found a significant relationship between combined-type ADHD and low-level lead exposure but not predominantly inattentive type and lead exposure. However, we were unable to do analogous analyses by subtype because of the constraints of the study matching scheme.
Conclusions
This hospital-based case–control study suggests a strong relationship exists between ADHD and lead exposure, even low-level lead exposure (< 10 μg/dL BLL), in Chinese children. If this finding is confirmed in future studies, the potential to prevent ADHD by reducing childhood lead exposure should be considered.
Footnotes
H.L.W. and X.T.C. contributed equally to this work.
This work was supported by the National Basic Research Program of China (2002CB512907), the National Nature Science Foundation of China (30630057; 30670554; 30670662; 30672290), Academia Sinica (KZCX3-SW-437), Anhui High Education Natural Science Program (ZD2008010-2), the China Postdoctoral Science Foundation (20060400719), and K.C. Wong Education Foundation of Hong Kong.
References
- AAP (American Academy of Pediatrics) Lead exposure in children: prevention, detection, and management. Pediatrics. 2005;116(4):1036–1046. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]
- Aman MG, Singh NN. Aberrant Behavior Checklist: Manual. New York: Slosson Educational Publications; 1986. [Google Scholar]
- Ambrosini PJ. Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS) J Am Acad Child Adolesc Psychiatry. 2000;39(1):49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic & Statistical Manual for Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-R) [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96(9):1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Hu H, Titlebaum L, Needleman HL. Attentional correlates of dentin and bone lead levels in adolescents. Arch Environ Health. 1994a;49(2):98–105. doi: 10.1080/00039896.1994.9937461. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Allred E, Rabinowitz M. Pre- and postnatal lead exposure and behavior problems in school-aged children. Environ Res. 1994b;66(1):12–30. doi: 10.1006/enrs.1994.1041. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Current concepts on the neurobiology of attention-deficit/hyperactivity disorder. J Atten Disord. 2002;6(suppl 1):S7–S16. doi: 10.1177/070674370200601s03. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. The Massachusetts General Hospital studies of gender influences on attention-deficit/hyperactivity disorder in youth and relatives. Psychiatr Clin North Am. 2004;27(2):225–232. doi: 10.1016/j.psc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Binns HJ, Campbell C, Brown MJ. Interpreting and managing blood lead levels of less than 10 microg/dL in children and reducing childhood exposure to lead: recommendations of the Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Pediatrics. 2007;120(5):e1285–e1298. doi: 10.1542/peds.2005-1770. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockel BJ, Cory-Slechta DA. Lead, attention, and impulsive behavior: changes in a fixed-ratio waiting-for-reward paradigm. Pharmacol Biochem Behav. 1998;60(2):545–552. doi: 10.1016/s0091-3057(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Burns JM, Baghurst PA, Sawyer MG, McMichael AJ, Tong SL. Lifetime low-level exposure to environmental lead and children’s emotional and behavioral development at ages 11–13 years. The Port Pirie Cohort Study. Am J Epidemiol. 1999;149(8):740–749. doi: 10.1093/oxfordjournals.aje.a009883. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348(16):1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endopheno-types. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- CDC. Preventing Lead Poisoning in Young Children. Atlanta, GA: Centers for Disease Control and Prevention; 1991. [Google Scholar]
- Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- Doyle R. The history of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):203–214. doi: 10.1016/j.psc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Ment Retard Dev Disabil Res Rev. 2003;9(3):184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Eppright TD, Vogel SJ, Horwitz E, Tevendale HD. Results of blood lead screening in children referred for behavioral disorders. Mo Med. 1997;94(6):295–297. [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Early dentine lead levels and subsequent cognitive and behavioural development. J Child Psychol Psychiatry. 1993;34(2):215–227. doi: 10.1111/j.1469-7610.1993.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Homack S, Riccio CA. Conners’ Continuous Performance Test (2nd ed.; CCPT-II) J Atten Disord. 2006;9(3):556–558. doi: 10.1177/1087054705283578. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. Hoboken, NJ: John Wiley & Sons, Inc; 2000. [Google Scholar]
- Huo X, Peng L, Xu X, Zheng L, Qiu B, Qi Z, et al. Elevated blood lead levels of children in Guiyu, an electronic waste recycling town in China. Environ Health Perspect. 2007;115:1113–1117. doi: 10.1289/ehp.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn CA, Kelly PC, Walker WO., Jr Lead screening in children with attention deficit hyperactivity disorder and developmental delay. Clin Pediatr (Phila) 1995;34(9):498–501. doi: 10.1177/000992289503400909. [DOI] [PubMed] [Google Scholar]
- Kala SV, Jadhav AL. Low level lead exposure decreases in vivo release of dopamine in the rat nucleus accumbens: a microdialysis study. J Neurochem. 1995;65(4):1631–1635. doi: 10.1046/j.1471-4159.1995.65041631.x. [DOI] [PubMed] [Google Scholar]
- Khan SA, Faraone SV. The genetics of ADHD: a literature review of 2005. Curr Psychiatry Rep. 2006;8(5):393–397. doi: 10.1007/s11920-006-0042-y. [DOI] [PubMed] [Google Scholar]
- Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environ Health Perspect. 2004;112:987–994. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konofal E, Cortese S. Lead and neuroprotection by iron in ADHD [Letter] Environ Health Perspect. 2007;115:A398–A399. doi: 10.1289/ehp.10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O’Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA. 2001;285(1):60–66. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- Leung PW, Luk SL, Ho TP, Taylor E, Mak FL, Bacon-Shone J. The diagnosis and prevalence of hyperactivity in Chinese schoolboys. Br J Psychiatry. 1996;168(4):486–496. doi: 10.1192/bjp.168.4.486. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126(Pt 1):5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160(6):1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41(4):378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Millichap JG. Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(2):e358–e365. doi: 10.1542/peds.2007-1332. [DOI] [PubMed] [Google Scholar]
- Minder B, Das-Smaal EA, Brand EF, Orlebeke JF. Exposure to lead and specific attentional problems in schoolchildren. J Learn Disabil. 1994;27(6):393–399. doi: 10.1177/002221949402700606. [DOI] [PubMed] [Google Scholar]
- Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- Needleman HL. The current status of childhood low-level lead toxicity. Neurotoxicology. 1993;14(2–3):161–166. [PubMed] [Google Scholar]
- Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979;300(13):689–695. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Leviton A. Lead and neurobehavioural deficit in children. Lancet. 1979;2(8133):104. doi: 10.1016/s0140-6736(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Knottnerus GM, Martel MM, Nikolas M, Cavanagh K, Karmaus W, et al. Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol Psychiatry. 2008;63(3):325–331. doi: 10.1016/j.biopsych.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappley MD. Clinical practice. Attention deficit-hyperactivity disorder. N Engl J Med. 2005;352(2):165–173. doi: 10.1056/NEJMcp032387. [DOI] [PubMed] [Google Scholar]
- Remschmidt H. Global consensus on ADHD/HKD. Eur Child Adolesc Psychiatry. 2005;14(3):127–137. doi: 10.1007/s00787-005-0439-x. [DOI] [PubMed] [Google Scholar]
- Ren HM, Wang JD, Zhang XL. Assessment of soil lead exposure in children in Shenyang, China. Environ Pollut. 2006;144(1):327–335. doi: 10.1016/j.envpol.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Ris MD, Dietrich KN, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and neuropsychological outcome in adolescence. J Int Neuropsychol Soc. 2004;10(2):261–270. doi: 10.1017/S1355617704102154. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Bijur PE, Markowitz M, Ma YC, Rosen JF. Declining blood lead levels and cognitive changes in moderately lead-poisoned children. JAMA. 1993;269(13):1641–1646. [PubMed] [Google Scholar]
- Satterfield JH, Faller KJ, Crinella FM, Schell AM, Swanson JM, Homer LD. A 30-year prospective follow-up study of hyperactive boys with conduct problems: adult criminality. J Am Acad Child Adolesc Psychiatry. 2007;46(5):601–610. doi: 10.1097/chi.0b013e318033ff59. [DOI] [PubMed] [Google Scholar]
- Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child Adolesc Psychiatr Clin N Am. 2000;9(3):541–555. vii. [PubMed] [Google Scholar]
- Silva PA, Hughes P, Williams S, Faed JM. Blood lead, intelligence, reading attainment, and behaviour in eleven year old children in Dunedin, New Zealand. J Child Psychol Psychiatry. 1988;29(1):43–52. doi: 10.1111/j.1469-7610.1988.tb00687.x. [DOI] [PubMed] [Google Scholar]
- St Sauver JL, Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Early life risk factors for attention-deficit/hyperactivity disorder: a population-based cohort study. Mayo Clin Proc. 2004;79(9):1124–1131. [PubMed] [Google Scholar]
- Su L, Wan G, Yang Z, Yu S, Li X. The study of diagnostic criteria for attention deficit hyperactivity disorder. Chin J Nerv Ment Dis. 2001;27(3):199–201. [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels < 10 microg/dL. Neurotoxicology. 2007;28(6):1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Szczerbak G, Nowak P, Kostrzewa RM, Brus R. Maternal lead exposure produces long-term enhancement of dopaminergic reactivity in rat offspring. Neurochem Res. 2007;32(10):1791–1798. doi: 10.1007/s11064-007-9306-0. [DOI] [PubMed] [Google Scholar]
- Thapar A, Langley K, Asherson P, Gill M. Gene-environment interplay in attention-deficit hyperactivity disorder and the importance of a developmental perspective. Br J Psychiatry. 2007;190:1–3. doi: 10.1192/bjp.bp.106.027003. [DOI] [PubMed] [Google Scholar]
- Thomson GO, Raab GM, Hepburn WS, Hunter R, Fulton M, Laxen DP. Blood-lead levels and children’s behaviour—results from the Edinburgh Lead Study. J Child Psychol Psychiatry. 1989;30(4):515–528. doi: 10.1111/j.1469-7610.1989.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Tong S, Baghurst PA, Sawyer MG, Burns J, McMichael AJ. Declining blood lead levels and changes in cognitive function during childhood: the Port Pirie Cohort Study. JAMA. 1998;280(22):1915–1919. doi: 10.1001/jama.280.22.1915. [DOI] [PubMed] [Google Scholar]
- Tuthill RW. Hair lead levels related to children’s classroom attention-deficit behavior. Arch Environ Health. 1996;51(3):214–220. doi: 10.1080/00039896.1996.9936018. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang J. Blood lead levels in children, China. Environ Res. 2006;101(3):412–418. doi: 10.1016/j.envres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Pine DS, Graziano JH. Contribution of maternal smoking during pregnancy and lead exposure to early child behavior problems. Neurotoxicol Teratol. 2001;23(1):13–21. doi: 10.1016/s0892-0362(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Staghezza-Jaramillo B, Shrout P, Popovac D, Graziano J. The effect of lead exposure on behavior problems in preschool children. Am J Public Health. 1998;88(3):481–486. doi: 10.2105/ajph.88.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. International Programme on Chemical Safety. Inorganic Lead. Environmental health criteria 165. Geneva: World Health Organization; 1995. [Google Scholar]
- WHO (World Health Organization) International Classification of Diseases. 10. 2007. [[accessed8 Apr 2007]]. Available: http://www.who.int/classifications/apps/icd/icd10online/