Abstract
Starting 90 years ago with a clinical description by Glanzmann of a bleeding disorder associated with a defect in platelet function, technologic advances helped investigators identify the defect as a mutation(s) in the integrin family receptor, αIIbβ3, which has the capacity to bind fibrinogen (and other ligands) and support platelet-platelet interactions (aggregation). The receptor's activation state was found to be under exquisite control, with activators, inhibitors, and elaborate inside-out signaling mechanisms controlling its conformation. Structural biology has produced high-resolution images defining the ligand binding site at the atomic level. Research on αIIbβ3 has been bidirectional, with basic insights resulting in improved Glanzmann thrombasthenia carrier detection and prenatal diagnosis, assays to identify single nucleotide polymorphisms responsible for alloimmune neonatal thrombocytopenia, and the development of αIIbβ3 antagonists, the first rationally designed antiplatelet agents, to prevent and treat thrombotic cardiovascular disease. The future looks equally bright, with the potential for improved drugs and the application of gene therapy and stem cell biology to address the genetic abnormalities. The αIIbβ3 saga serves as a paradigm of rigorous science growing out of careful clinical observations of a rare disorder yielding both important new scientific information and improved diagnosis, therapy, and prevention of other disorders.
Introduction
“Thus blood, for all its raw physicality, its heat, color and smell, remains first and foremost a powerfully symbolic substance—capable of representing the most primeval forces of life, and of death.”1
“… for the blood is life …” Deuteronomy 12:23
To celebrate the 50th anniversary of Blood, we offer an historical account of research on our favorite receptor on the platelet surface, GPIIb/IIIa, or integrin αIIbβ3. This receptor plays an important role in hemostasis and thrombosis, and in accord with the quotations above, both processes have profound effects on life and health. The origin of the English word blood is uncertain. It may derive from a postulated Indo-European root bhel, “bloom” or “sprout,” and it has been speculated that “ancient people looked upon the effusion from incised skin as a sort of blooming,”2 an image well known to practitioners of the bleeding time.
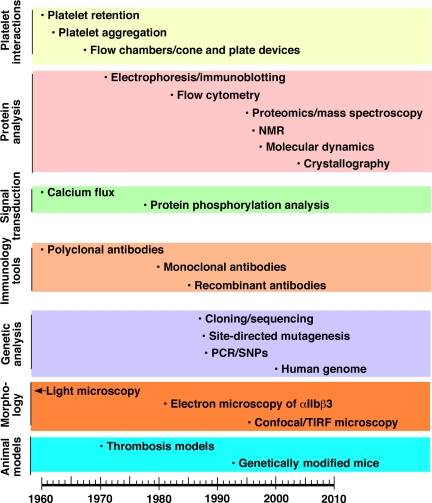
The dominant theme in this review is how improved understanding of the structure and function of αIIbβ3 has led to opportunities to translate that knowledge into biomedical advances, including the development of αIIbβ3 antagonists, the first class of rationally designed antiplatelet agents. The subtheme is how advances in scientific technology deriving from discoveries in other fields have been crucial to improving our understanding of αIIbβ3. Figure 1 is a timeline depicting, by category, approximately when different technologies were introduced into the investigation of blood platelets and/or αIIbβ3. Thus, as with a musical fugue, we will try to tell 2 stories simultaneously, namely the scientific progress in understanding the importance of αIIbβ3 in biology and medicine and the technologic advances that enabled this progress. Because of space constraints, many key observations cannot be cited. Thus, we have chosen to cite a mixture of early work and recent review articles relevant to specific aspects of the αIIbβ3 story. The interested reader may wish to consult several books that contain more detailed information.3–8
Figure 1.
Timeline of application of new technologies to the study of platelets and/or αIIbβ3.
Glanzmann thrombasthenia and the molecular analysis of αIIbβ3
Early platelet discoveries, clinical observations, and laboratory studies
Advances in microscopy and intravital technology paved the way for Bizzozero's landmark description in 1881 of blood platelets and their roles in thrombosis and hemostasis (reviewed in Robb-Smith9). Hayem made many important contributions, including confirming the relationship between hemorrhage and a low platelet count (1890) and describing the importance of platelets to the retraction of blood clots (1878), the latter providing the first in vitro assay of platelet function. Duke, in his landmark paper in 1910, described the ear lobe bleeding time as an in vivo assay that was prolonged in individuals with thrombocytopenia, and corrected when platelet counts increased after transfusion or disease remission.10 These early studies set the stage for Swiss pediatrician Eduard Glanzmann to describe in 1918 a series of patients with an inherited bleeding disorder characterized by mucocutaneous hemorrhage in which the platelet count was normal, but platelet function, as measured by clot retraction, was impaired. He termed the disorder hereditary hemorrhagic thrombasthenia (“weak platelet”), introducing the concept of a qualitative platelet disorder.11 Subsequently, Forio reported that patients with thrombasthenia had prolonged bleeding times, while others observed that thrombasthenic platelets failed to clump or to spread when visualized on blood smears (reviewed in Caen et al12).
Discoveries during the 1950s and early 1960s laid the groundwork for further characterization of the platelet abnormality in Glanzmann thrombasthenia, including observations that normal platelets adhere to connective tissue collagen and aggregate in response to adenosine diphosphate (ADP; reviewed in Marcus and Zucker13). In addition, platelets were found by electron microscopy to be surrounded by an electron-dense “glycocalyx” that is rich in fibrinogen,14 a plasma protein that was later discovered to enjoy an intimate relationship with αIIbβ3 (reviewed in Marcus and Zucker,13 Peerschke,15 and Bennett16).
A major technical advance was the invention of the platelet aggregometer in 1962, which provided a quantitative optical turbidometric method to measure platelet-platelet interactions in a plasma environment.17,18 Using this methodology, several groups reported that thrombasthenic platelets failed to aggregate in response to all known physiologic agonists, including ADP, collagen, epinephrine, serotonin, and thrombin.12,19,20 In parallel with this discovery was the finding that thrombasthenic platelets were deficient in fibrinogen.20–22
Application of electrophoretic techniques
The development of polyacrylamide gel electrophoresis, which permitted high-resolution separation of platelet proteins, provided new opportunities to define the defect in thrombasthenic platelets. By the mid-1970s it was reported that patients' platelets were deficient in 2 glycoproteins, one in the second carbohydrate-staining region and one in the third region. With improved gel resolution these broad carbohydrate-staining regions were subdivided and the proteins deficient or abnormal in Glanzmann thrombasthenia were named glycoproteins IIb (GPIIb) and IIIa (GPIIIa)23,24 (reviewed in Nurden25). A number of modifications of the technique, including carbohydrate-specific staining, labeling of platelet surface proteins and carbohydrates, and separation of proteins in 2 dimensions, provided more detailed information (reviewed in George et al3,26). These studies established that GPIIb and GPIIIa contain carbohydrate residues and that GPIIb (Mr 140 kDa) is composed of a heavy chain and a light chain (Mr 120 and 20 kDa, respectively) held together by disulfide bonds. GPIIIa was found to undergo a paradoxical decrease in electrophoretic mobility upon reduction, suggesting that disulfide bonds in the native protein give it a compact structure. These techniques demonstrated that most, but not all, Glanzmann thrombasthenia patients had dramatic decreases in both GPIIb and GPIIIa. Immunoelectrophoretic techniques were also applied to characterize the abnormalities; they had the advantage of not requiring protein denaturation and were thus able to provide strong evidence that GPIIb and GPIIIa exist as a calcium-dependent heterodimer.27–29
Application of fibrinogen-binding technology
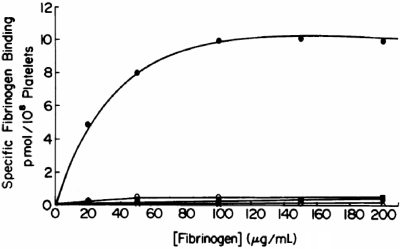
The significance of the relationship between GPIIb/IIIa and fibrinogen, and of the prolonged bleeding time in patients with afibrinogenemia, became clearer in the late 1970s as investigators showed that radiolabeled fibrinogen binding to platelets required platelet activation, and that platelet aggregation required fibrinogen binding30,31 (reviewed in Peerschke15 and Bennett16). The key observations that thrombasthenic platelets fail to bind fibrinogen and that this is not due to a defect in “exposing” the receptor31,32 defined the pathophysiology of Glanzmann thrombasthenia as an inherited deficiency and/or abnormality of the platelet membrane fibrinogen receptor (Figure 2). Confirmation came from later studies showing that fibrinogen could bind to purified GPIIb/IIIa in several different systems.33–35
Figure 2.
Platelet fibrinogen binding studies demonstrate that platelets from patients with Glanzmann thrombasthenia cannot bind fibrinogen in response to ADP stimulation. The upper curve is of platelets from a healthy subject and the 3 lower ones are from 3 different patients. Reprinted with permission from Bennett JS, Vilaire G. J Clin Invest. 1979;64:1393-1401.31
Application of pulse-chase labeling
Until the recent advent of growth-factor cocktails to expand various hematopoietic cells in culture, megakaryocytes were difficult to isolate and study because they represent less than 1% of bone marrow cells. As a result, initial studies in the late 1980s to assess the biosynthesis of GPIIb/IIIa relied on model cell lines with megakaryocyte-like features, including the human erythroleukemia (HEL) cell line.36–38 Nonetheless, cell-free and pulse-chase analysis demonstrated that GPIIb and GPIIIa are made separately in the endoplasmic reticulum, where they form a complex that is then transported to the Golgi for further processing, including carbohydrate modifications and cleavage of the GPIIb precursor molecule into the heavy and light chains.39,40 The need for GPIIb and GPIIIa to complex in order to be expressed on the cell surface41 offered an explanation for the enigma that Glanzmann thrombasthenia patients had marked deficiencies in 2 different proteins, since loss of either one would presumably prevent the other from reaching the surface.
Application of monoclonal antibody and flow cytometry technology
The development of the technique for preparing monoclonal antibodies (mAbs) and its application to platelets provided a valuable new tool to study Glanzmann thrombasthenia. In fact, the first reported mAb to platelets was directed at GPIIb,42 and mAbs to GPIIb and GPIIIa were commonly produced when mice were immunized with human platelets. Some of the mAbs reacted with GPIIb, others with GPIIIa, and still others reacted only with the GPIIb/IIIa complex. A subset of mAbs could block the binding of fibrinogen to GPIIb/IIIa and incubating these mAbs with normal platelets could recapitulate the platelet aggregation defect found in patients with Glanzmann thrombasthenia.43,44 Quantitative studies using mAbs eventually established that each platelet expresses approximately 80 000 GPIIb/IIIa receptors on its surface,45 with an additional internal pool of smaller size that can be recruited to the surface with activation, particularly by so-called strong agonists such as thrombin.46,47 When normalized for the platelet's surface area, the surface density of GPIIb/IIIa was estimated to be truly extraordinary, with receptors less than 200 Å apart on average, making it one of the densest adhesion/aggregation receptors in all of biology. The mAbs also aided in the purification of the receptor, allowing more detailed biochemical characterization and the identification of other proteins that interact with the receptor. Conformation-specific mAbs were also extremely valuable in beginning to unravel the mystery of how GPIIb/IIIa activation results in ligand binding. Thus, using appropriate screening assays, investigators were able to make antibodies that preferentially bound to activated receptors48,49 or ligand-bound receptors.50,51 Some mAbs had the reverse property, losing their binding ability with receptor activation. As the epitopes for these antibodies were identified, it became clear that receptor activation produces conformational changes in multiple regions of both glycoproteins.
The mAbs that blocked fibrinogen binding to GPIIb/IIIa were also valuable in establishing that the GPIIb/IIIa receptor was promiscuous. Ligand-binding studies had established that fibronectin, von Willebrand factor, vitronectin, and thrombospondin could all bind to platelets after appropriate stimulation, but a number of other receptors had been implicated in mediating their binding. The ability of GPIIb/IIIa-specific mAbs to inhibit, at least in part, the binding of all these ligands established that GPIIb/IIIa could also serve as a receptor for these adhesive glycoproteins.52
The mAbs facilitated clinical diagnosis of Glanzmann thrombasthenia since the number of mAb molecules that bound to platelets could readily differentiate patients who lacked the receptor from healthy subjects.53 They also established that the platelets of carriers of Glanzmann thrombasthenia, who do not have a hemorrhagic diathesis, have approximately 50% to 60% of the normal number of surface receptors.54 There was, however, overlap in mAb-binding values between healthy subjects and carriers, and this limited the usefulness of mAb binding to diagnose carriers. The mAbs proved to be extremely useful in prenatal diagnosis because there was a sharp distinction between normal and carrier fetuses on the one hand, and affected fetuses with very low levels of GPIIb/IIIa expression on the other.55 Combining mAbs with electrophorectic techniques and immunoblotting provided even more detailed characterization of the molecular defects in different patients.56,57 These data demonstrated that small amounts of residual GPIIb and/or GPIIIa could be detected in the platelets of nearly all Glanzmann thrombasthenia patients and that the patterns were consistent within kinship groups. They also provided evidence that there was a disproportionate amount of the single-chain precursor form of GPIIb in the platelets of some patients, suggesting a defect in protein maturation.57,58
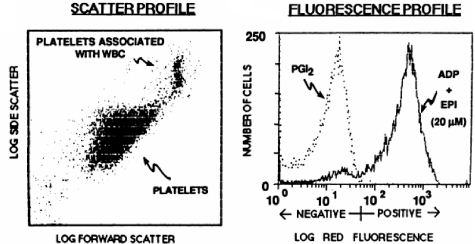
Having mAbs specific for the activated and/or ligand-bound form of the receptor made it theoretically possible to detect activated platelets in the circulation of healthy individuals and patients and to identify individuals with a bleeding diathesis due to defective agonist-induced GPIIb/IIIa activation. The introduction of flow cytometry and the development of methods to study small volumes of whole blood (5 μL) made such studies much easier because they eliminated the need for radioactive materials and they provided both whole population data and data on individual platelets59,60 (Figure 3).
Figure 3.
Application of flow cytometry and the activation-dependent monoclonal antibody PAC-1 to the study of αIIbβ3 conformational changes and ligand binding. (A) Platelets were identified and differentiated from red and white blood cells by their characteristic forward and side-angle light scatter profiles. (B) Platelets were stimulated with ADP and epinephrine or incubated with PGI2 to block activation. The fluorescence histogram depicts biotin-PAC-1 binding to the platelets detected by phycoerythrin-streptavidin. Reprinted from Shattil et al. Blood. 1987;70:307.59
Application of molecular biology
The development of molecular biologic techniques opened yet another exciting era of inquiry, culminating in the cloning and sequencing of the cDNAs for GPIIb and GPIIIa in 1987.61,62 Analysis of genomic DNA established that the proteins are derived from separate genes and chromosomal localization studies found that the genes are relatively near each other on chromosome 17, but not closely linked.63,64 The primary sequences provided major new insights, establishing both subunits as transmembrane proteins. GPIIb was found to have 4 calcium binding motifs, and as had been anticipated from the nonreduced/reduced sodium dodecyl sulfate–polyacrylamide gel data, GPIIIa contained a multitude of disulfide-linked extracellular cysteine residues (56!). Most importantly, these studies established that GPIIb/IIIa is a member of an extended family of heterodimeric adhesion receptors called integrins, each made up of an α subunit (GPIIb) and a β subunit (GPIIIa; reviewed in Pytela et al65 and Hynes66). Moreover, GPIIb/IIIa had a hemi-identical twin, αVβ3,67 which shared the same β subunit (GPIIIa)62 and had an α subunit (αV) that shared 40% homology with αIIb.61,68 Since αVβ3 is widely expressed in different cells, including osteoclasts, endothelial cells, smooth muscle cells and platelets (reviewed in Byzova et al69), some investigators speculated that Glanzmann thrombasthenia would be due exclusively to defects in GPIIb, presuming that loss of αVβ3 might be incompatible with life. That speculation ended when defects in either GPIIb or GPIIIa were identified in Glanzmann thrombasthenia patients70 (reviewed in French and Seligsohn71).
In recognition of GPIIb/IIIa's newly discovered parentage and familial relationships, it adopted a new name, αIIbβ3, that merged the old platelet glycoprotein nomenclature with the agreed-upon conventions for integrin receptors. We will use this designation for the remainder of this review. It is difficult to convey the excitement those in the field felt during the late 1980s and early 1990s, as each day brought profound new insights into the relationships among the integrins. One memorable moment epitomizing the underlying similarities of biologic phenomena occurred at an early conference on integrin receptors when an investigator studying developmental biology showed a polyacrylamide gel of a Drosophila melanogaster integrin receptor mutation (“lethal myospheroid”72) that looked similar to the gels obtained with the platelets of patients with Glanzmann thrombasthenia. It was soon discovered that platelets contain 4 other integrins. In contrast to αIIbβ3, however, these receptors were expressed at low levels, with approximately 1000 copies per platelet of α2β1, α5β1, and α6β1, and only 50 to 100 copies of αVβ3.73–76 The tiny amount of αVβ3, however, was very precious because its presence or absence provided a hint as to whether a Glanzmann thrombasthenic patient's molecular defect was in αIIb or β3, respectively.77
Insights from studies of other integrin receptors began to provide important information about the process of ligand binding to αIIbβ3. Thus, the discovery that the Arg-Gly-Asp (RGD) sequence in fibronectin mediates its interaction to α5β1 (reviewed in Ruoslahti78) rapidly led to the recognition that small peptides and snake venoms containing the RGD sequence could inhibit fibrinogen binding to αIIbβ3 (reviewed in Gould et al79 and Ojima et al80). Moreover, these studies provided the missing link to understanding how von Willebrand factor, fibronectin, vitronectin, and thrombospondin could all bind to αIIbβ3, since as each of these was cloned and their amino acid sequences deduced, they all were found to contain RGD sequences in the regions mediating binding to αIIbβ3. Paradoxically, although fibrinogen contains 2 pairs of RGD sequences, the primary binding sites for αIIbβ3 necessary for platelet aggregation are at the C-termini of the 2 fibrinogen γ-chains, where a KQAGDV sequence provides a motif that can also bind to αIIbβ3.81,82
Application of the polymerase chain reaction
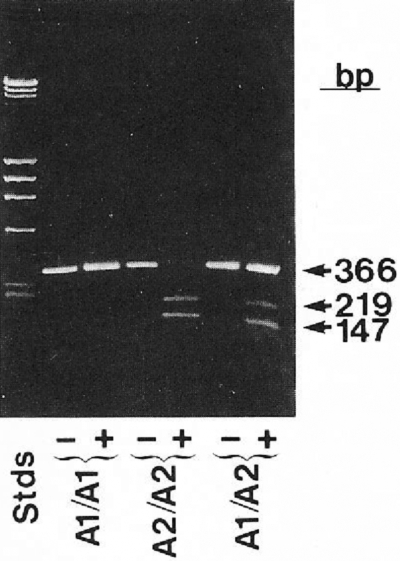
Platelets contain only small amounts of mRNA, and this was a serious limitation in obtaining enough cDNA to study platelet-specific proteins. Thus, the fastidious application of the technique of reverse transcriptase polymerase chain reaction (PCR), which greatly amplifies mRNA signals, to platelets in the late 1980s added an extraordinarily powerful method to identify αIIbβ3 polymorphisms and mutations.83 The most important platelet polymorphism, termed P1A1 or HPA-1, was found to be due to a β3 Leu33Pro polymorphism84 and forms the antigenic epitope responsible for a sizable fraction of patients with neonatal alloimmune thrombocytopenia due to maternal alloimmunization and for most adults with posttransfusion purpura (Figure 4). Additional polymorphisms on αIIb or β3 implicated in causing neonatal alloimmune thrombocytopenia were also identified (reviewed in Valentin and Newman85). These discoveries provided vital information for families at risk of having an affected child. They also permitted embryo selection based on preimplantation diagnosis in cases where the mother is heterozygous for the polymorphism. Functional differences have been ascribed to some of these polymorphisms, but the true extent to which they impart hemorrhagic or thrombotic risk remains to be determined, and is in the purview of the burgeoning field of association studies attempting to link variations in platelet genes, including single nucleotide polymorphisms (SNPs) to variations in platelet function (reviewed in Bray86).
Figure 4.
Application of reverse transcription and the polymerase chain reaction to identify the PlA1 polymorphism as due to a nucleotide mutation leading to a Leu33Pro substitution in the integrin β3 subunit. Bases 56-408 of integrin β3 were enzymatically amplified from individuals who were homozygous PlA2 or heterozygous PlA1/PlA2 and analyzed on agarose tells. The enzyme is sensitive to the T→C change in the sequence at base 196 associated with the PlA2 polymorphism. Reprinted with permission from Newman et al. J Clin Invest. 1989;83:1778-1781.84
The application of PCR to establishing the molecular basis of Glanzmann thrombasthenia in different kindreds has provided a wealth of information about the relationship between αIIbβ3 structure and function. PCR has also permitted rapid and unequivocal carrier determination and DNA-based prenatal diagnosis (by direct gene analysis and by linkage) using amniotic fluid or chorionic villus samples87 (reviewed in Wautier and Gruel88). Since the latter can be obtained at approximately 11 weeks of gestation, the information can be provided to families much earlier than it previously could using mAb-based and functional studies of blood obtained by percutaneous umbilical cord blood sampling at approximately 20 weeks of gestation.
Studies of patients whose platelets express at least 50% of the normal amount of αIIbβ3 but fail to bind fibrinogen, termed variant Glanzmann thrombasthenia, have been particularly instructive in identifying residues involved in ligand binding. For example, the β3 Asp119Tyr and Arg214Gln mutations established the importance of both of these regions of β3 for ligand binding.89,90 Similarly, αIIb mutations of Leu183 and Pro145 affected ligand binding disproportionately to their effects on receptor expression.91,92 Still other mutations in the cytoplasmic domains of patients with variant Glanzmann thrombasthenia provided valuable clues to the mechanisms of inside-out activation of αIIbβ3 by platelet agonists and outside-in signaling initiated by ligand engagement.93,94 Some Glanzmann thrombasthenia mutations paradoxically produced constitutively active receptors, including ones affecting cysteine residues in the thiol-rich regions of β3.95
Application of technology to produce genetically modified mice
Gene targeting has produced a mouse null for β3, resulting in a hemorrhagic diathesis and platelet function abnormalities similar to those observed in patients with Glanzmann thrombasthenia.96 These mice are protected from developing acute thrombosis using a variety of models,97 but are not protected from developing intimal hyperplasia after vascular injury.98 They are also providing valuable new information about the role of αVβ3 in endothelial cells, osteoclasts,99 and wound healing.100 Mice lacking αIIb have also been produced and they, too, have a hemorrhagic diathesis101,102; they have also provided insights into the expression of αIIb during early hematopoiesis. In recent years, the use of genetic technology has been expanded to study the function of mouse platelets that contain mutations of the β3 subunit, thereby shedding light on the role of specific amino acid residues in the β3 cytoplasmic domain.103–105
Application of electron microscopy, X-ray crystallography, nuclear magnetic resonance, and computational chemistry
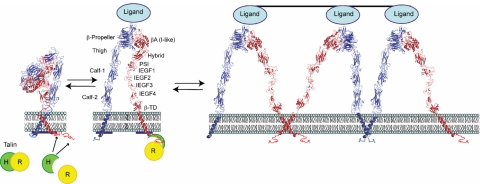
One of the primary goals of biologic science is to visualize the 3-dimensional structures of molecules under different conditions and observe how they interact with other molecules. Coarse insights about αIIbβ3 structure were inferred from the primary sequences of αIIb and β3 and the impact of natural and site-directed mutations.106,107 Electron microscopy added additional structural information, suggesting that both subunits have “head” and “tail” regions; that each “tail” inserts into the plasma membrane; that the subunits make contact in their “head” regions; that fibrinogen binds to the region comprised of the 2 “heads”; and that fibrinogen binding results in long-range conformational changes in the “tail” region of the receptor.108–110 Landmark X-ray crystallographic studies in 2001 and 2002 on the extracellular domains of the αVβ3 receptor, alone and in the presence of the RGD peptide cilengitide, provided the first high resolution structures of an integrin and identified a number of different structural domains that are also common to αIIbβ3 (Figure 5).111,112 The ligand binding pocket was found to span the 2 subunits in the head region, and, most surprising, the receptor was found to adopt a bent conformation.
Figure 5.
Model of αIIbβ3 based on αVβ3 crystal structure and depiction of switchblade model of αIIbβ3 conformational changes associated with activation and ligand binding. Inside-out signaling ultimately results in the binding of the talin head (H) domain binding to the cytoplasmic domain of the β3 subunit, resulting in subunit separation. This is transmitted through the transmembrane domains to the ectodomain where it results in extension of the α and β subunits and perhaps additional changes in the ligand binding region of β3. Ligand then binds, resulting in a swing-out motion of the β3 hybrid and PSI domains that may initiate outside-in signaling. Additional post-ligand binding events may occur, including homo-oligomerization of integrin transmembrane domains, leading to receptor clustering. The “deadbolt” hypothesis posits that modest changes in the β3 βA (I-like) domain brought about by movement of a nearby β3 β-terminal domain loop results in ligand binding, which is then followed by receptor extension and the swing-out motion. Adapted from Qin et al.143 The molecular models of αIIbβ3 were constructed using MODELLER 8v2 and the PDBs ITY6, IU8C, and IYUK as previously described.241 I-EGF, integrin epidermal growth factor domain; β-TD, β-terminal domain.
In 2004, the first crystal structures of the isolated αIIbβ3 headpiece were reported, including the structures of the headpiece complexed with the drugs eptifibatide or tirofiban.113 These studies provided insights into the αIIbβ3 specificity of certain ligands and αIIbβ3 antagonists based on differences in the αIIb binding pocket compared with the αV binding pocket. The αIIbβ3 crystal structure also differed from the αVβ3 structure in the angle between the β3 domain involved in ligand binding (βA [I-like]) and the adjacent domain (β3 hybrid), suggesting that ligand binding is associated with a dramatic swing-out motion of the receptor in this region (Figure 5).
Taken together, the structural data indicate that the αIIbβ3 receptor can undergo several different conformational changes, but it is still uncertain which ones are necessary and/or sufficient for attaining the high-affinity ligand binding state(s). Two different models of αIIbβ3 activation have been proposed. The deadbolt hypothesis suggests that platelet activation leads to movement of the external domain of the β3 subunit adjacent to the membrane (β-terminal domain [β-TD]), which in turn releases a constraint on a portion of the βA (I-like) domain involved in ligand binding and allows it to undergo a subtle conformational change that results in the receptor adopting a high-affinity ligand binding state.114,115 Extension of the receptor at both the genu of αIIb and the interface between the integrin epidermal growth factor-1 (IEGF-1) and IEGF-2 domains of β3 is proposed to occur after ligand binding in this model. The switchblade hypothesis posits that the αIIbβ3 receptor undergoes extension before ligand binding and that swing-out of the β3 hybrid domain occurs concurrent with or after ligand binding.113,116,117 The swing-out motion has been proposed to participate in the initiation of outside-in signaling induced by ligand binding. Thiol-disulfide exchange has also been implicated in αIIbβ3 activation based on biochemical studies and studies of Glanzmann thrombasthenia patients with Cys mutations that result in constitutively active receptors.95,118–123 Post-ligand binding events have also been implicated in controlling the avidity of αIIbβ3, including receptor clustering124,125 and irreversible ligand binding.126
Molecular dynamic simulations of select groups of atoms in the ligand binding regions of αIIbβ3 and αVβ3 have been performed in an attempt to understand the allosteric pathways leading to activation and the energetics of ligand binding.127–129 These computation-intensive studies have been made possible by advances in the application of biophysical and thermodynamic principles to biologic systems and the availability of more powerful computers. The results have provided models of variations in regional flexibility and interactions with water molecules over time, the force needed to remove ligands from the binding pocket under different conditions, and the allosteric pathways leading from one conformation to another. Other computational programs allow one to dock small molecules into the αIIbβ3 ligand binding pocket.130 Finally, NMR studies of the transmembrane and cytoplasmic domains of αIIbβ3 have defined interactions between the subunits and between the subunits and cytoplasmic proteins important in regulating integrin activation in addition to their heterotypic interactions131–133 (reviewed in Ma et al134). These have been proposed to promote receptor clustering into oligomers after activation releases the heterotypic interactions, although other workers posit that αIIbβ3 oligomerization may be driven largely by the binding of fibrinogen or other multivalent ligands. Defining the conformational changes associated with the transition to high affinity ligand binding remains a high priority, with a variety of advanced biophysical and imaging approaches being brought to bear, but a clear consensus has not emerged.135,136
Biochemical, molecular, and genetic analyses of αIIbβ3 signaling
The presence of some regulated stimulus-response pathway leading to αIIbβ3 activation was implicit in early studies that demonstrated that platelet aggregation can be triggered by platelet agonists.17 As additional information emerged, the process that links platelet agonists to αIIbβ3 activation has come to be known as inside-out signaling. This is in contrast to direct activation of αIIbβ3 by the binding of activating monoclonal antibodies51,137,138 or low-molecular-weight ligand mimetics,139 and to outside-in signals that are sent into the platelet as the result of ligand binding and receptor clustering. Indeed, integrins have been aptly described as “bidirectional, allosteric signaling machines,”140 and studies with αIIbβ3 have played a prominent role in our understanding of the machinery (reviewed in Shattil and Newman,141 Watson et al,142 and Qin et al143). In reality, the 2 phases of integrin signaling are likely to be quite interdependent as evidenced by the identification of some signaling molecules, such as certain phospholipases and protein and lipid kinases that participate in both inside-out and outside-in signaling. Furthermore, some outside-in responses (for example, increases in cytoplasmic free calcium or activation of protein kinase C and cytosolic phospholipase A2) may feed back to enhance inside-out activation of additional αIIbβ3 complexes. To characterize integrin signaling, platelet researchers in the 1980s and 1990s began to take advantage of several emerging technologies, including flow cytometry (reviewed in Michelson and Shattil144), activation-dependent antibodies and antibodies specific for phosphorylated amino acids and proteins,49,145–148 expression of αIIbβ3 and recombinant signaling proteins in heterologous cells,149 and genetically modified mice.96,150,151 More recently, knockdown of proteins in embryonic stem cell–derived megakaryocytes by RNA interference has provided additional valuable information.152
Inside-out signaling
Inside-out αIIbβ3 signaling can be considered in terms of (1) stimulators and inhibitors of integrin activation and the platelet receptors with which they interact; (2) intracellular protein-protein interactions and biochemical reactions that couple agonist/antagonist receptor occupancy to the final events that directly regulate αIIbβ3 affinity; and (3) the final regulatory events.
As might be expected for a reaction so critical to hemostasis, there is redundancy in the process of αIIbβ3 activation in the form of regulation by multiple agonists that are either immobilized at the vascular wound site (eg, von Willebrand factor, collagen), generated within the wound (eg, thrombin), or either stored or generated and then released by platelets (eg, ADP, thromboxane A2).153 Numerous signaling receptors for agonists and antagonists have been identified in platelets. Initial application of pharmacologic approaches in the 1960s and 1970s using dose-response relationships and selective agonists and antagonists provided important insights into the structural and functional classes of these receptors (reviewed in Mustard and Packham154). The introduction of molecular biologic techniques led to the cloning and characterization of many different receptors, with studies of platelets in vitro and in vivo often facilitating the elucidation of receptor families. For example, agonist receptors include the G protein–coupled protease-activated thrombin receptors PAR1 and PAR4 in human platelets and PAR3 and PAR4 in murine platelets155–157; the purinergic receptors for ADP (P2Y1, P2Y12 and P2X1)158,159; and the thromboxane A2 receptor.160 Agonist receptors not directly coupled to G proteins include primary adhesion receptors for collagen (GPVI/FcRγ and integrin α2β1) and von Willebrand factor (GP Ib-V-IX; reviewed in Watson et al142 and Varga-Szabo et al161). αIIbβ3 is also considered a stimulatory receptor in the sense that it undergoes conformational changes and clustering to trigger outside-in signals in response to fibrinogen binding147 (reviewed in Shattil and Newman141 and Watson et al142). In circulating platelets, αIIbβ3 activation is ordinarily prevented by several activities derived from endothelial cells, including a cell surface ADPase (CD39) that removes stimulatory ADP,162,163 prostacyclin, which binds to Gs-coupled platelet receptors to activate adenylate cyclase (reviewed in Brass153 and Samuelsson164), and nitric oxide, which activates guanylate cyclase and can also be generated within platelets.165,166
Occupancy of some agonist receptors (eg, PAR1, PAR4, GP Ib-V-IX, GP VI/FcR γ) is usually sufficient to activate αIIbβ3. Other receptors appear to function primarily as mediators of primary platelet adhesion (α2β1; reviewed in Varga-Szabo et al161) or shape change (P2X1 ATP receptors; reviewed in Kunapuli et al167) or promote αIIbβ3 activation in concert with other stimulatory receptors. For example, P2Y1 and P2Y12 function together to induce full fibrinogen binding and platelet aggregation in response to ADP (reviewed in Kunapuli et al167), and PAR3 functions in mouse platelets as a cofactor for the authentic signaling thrombin receptor PAR4.156 The physiologic importance of specific agonist receptors has been established by basic scientists studying mutant mice and by the careful observations by clinicians of patients with bleeding disorders who manifest defective αIIbβ3 activation or platelet aggregation. The introduction of gene targeting technologies to knock out genes has been particularly important in defining the roles of platelet receptors (reviewed in Lee and Blajchman168); however, extrapolation of mouse data to human platelets must be done with caution because, despite many similarities, there can be importance species differences, as exemplified by the PAR receptors (reviewed in Sambrano et al,157 Tsakiris et al,169 and Jirouskova et al170).
The intracellular reactions that couple receptor occupancy to αIIbβ3 activation are complex. Significant progress has been made in establishing which G protein-coupled receptors couple to which G proteins, and how each G protein can initiate downstream signaling (reviewed in Brass153). Some initial biochemical responses to agonist occupancy of non-G protein–linked receptors have also been defined (reviewed in Watson et al142 and Varga-Szabo et al161). Specific second messengers and signaling proteins involved in inside-out signaling have been identified by biochemical studies of normal human platelets and megakaryocytes (reviewed in Shattil and Newman141), genetic approaches in mice (reviewed in Lee and Blajchman168), and studies of humans with rare inherited forms of platelet dysfunction (reviewed in Rao et al171). Messengers include products of phospholipase C (IP3, which increases cytoplasmic free Ca2+; diacylglycerol, which activates several protein kinase C isoforms in platelets as well as CalDAG-GEFI, a Rap1 GTPase exchange factor) and products of phosphatidylinositol 3-kinase (phosphatidylinositol 3,4 bisphosphate and phosphatidylinositol 3,4,5 trisphosphate, which recruit proteins with pleckstrin homology domains to membranes; reviewed in Brass153). An ongoing challenge is to understand how these and other mediators are integrated to regulate αIIbβ3 activation.
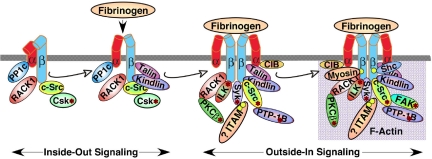
Talin is an actin-binding protein of approximately 250 kDa with a globular head and a rod-like tail that is highly expressed in platelets and can promote integrin activation, primarily by interactions of the talin head's FERM domain with integrin β cytoplasmic domains.172 Strong evidence in support of a necessary role for the protein talin in αIIbβ3 activation has been obtained by means of platelet-specific knockout of the protein in mice.173,174 During platelet activation, talin is recruited from the cytoplasm to αIIbβ3 in a process that is regulated, in part, by the Rap1 GTPase175 and RIAM, a molecular adapter and Rap1 effector176 (Figure 6). A pathway from agonist receptors to Rap1 is suggested by recent findings that platelet activation through G protein–coupled receptors or GP VI leads to Rap1 activation.177,178 Activation of Rap1 may be mediated by CalDAG-GEF1 since platelets from CalDAG-GEF1–deficient mice and humans exhibit defective αIIbβ3 activation and a bleeding diathesis.179,180 Because Rap1 depletion does not result in complete loss of platelet aggregation, pathways to αIIbβ3 activation that are independent of Rap1 must also exist.
Figure 6.
Protein interactions with the cytoplasmic domains of αIIb or β3 regulate integrin signaling. Shown are some, but not all, of the proteins reported to associate with the αIIbβ3 cytoplasmic domains, many in a dynamic fashion. Some associate with αIIbβ3 in resting platelets, while others are recruited to, or dissociate from, the integrin during inside-out or outside-in signaling, leading to F-actin assembly. In addition, several proteins with enzymatic function become activated (asterisk) after fibrinogen binding to αIIbβ3. It is difficult to imagine that all of these proteins can interact with a single αIIbβ3 heterodimer in platelets; however, they might interact with and further regulate oligomers of αIIbβ3 that form in response to fibrinogen binding. Not shown are the many additional adapter molecules, enzymes and substrates that may become recruited through more indirect interactions during the various phases of integrin signaling. Abbreviations: PP1c, protein phosphatase 1c; RACK1, receptor for activated C kinase 1; Csk, c-Src tyrosine kinase; PKCβ, protein kinase Cβ; ILK, integrin-linked kinase; ITAM, a yet-to-be-identified protein with one or more immunoreceptor tyrosine activation motifs; CIB, calcium and integrin-binding 1; Syk, spleen tyrosine kinase.
The mechanism by which talin activates αIIbβ3 has been illuminated by recent nuclear magnetic resonance (NMR) and crystallographic studies of purified components and mutational studies of talin and αIIbβ3 in a heterologous expression system.172 Thus, the F2-3 subdomains of talin's FERM domain may first gain a foothold within a membrane-distal region of the β3 cytoplasmic domain centered at Asn-Pro-Leu-Tyr (NPXY). Then a second interaction with a membrane-proximal region of the β3 cytoplasmic domain may flip the activation switch by disrupting a salt bridge between αIIb and β3, resulting in separation of the αIIb and β3 subunits. Other αIIbβ3-binding proteins, for example members of the FERM-domain–containing kindlin family,181,182 may also regulate αIIbβ3 activation. Whether they do so in concert with talin or independent of it remains an area of active investigation. Similarly, the mechanism(s) linking intracytoplasmic αIIb and β3 subunit separation to the conformational changes in the ectodomains responsible for high-affinity ligand binding still need to be established.
Outside-in signaling
Outside-in signaling by integrins provides a means by which these receptors mediate anchorage-dependent cellular responses. In nucleated cells, outside-in signals regulate cell adhesion, motility, and gene expression programs (reviewed in Hynes140). The anucleate platelet is bereft of DNA synthetic machinery, but it does possess translational machinery and can splice RNA in response to signals both from thrombin receptors and ligand binding to αIIbβ3 (reviewed in Zimmerman and Weyrich183). This provides a potential mechanism for activated platelets to participate, via both release of stored substances and new synthesis of substances, in processes beyond hemostasis and thrombosis, including immunity, inflammation, promotion of tumor metastasis, and angiogenesis184–186 (reviewed in Zarbock et al187). It is intriguing to speculate that outside-in αIIbβ3 signaling might also affect nuclear programs in both megakaryocytes and early definitive hematopoietic stem cells.188,189
In platelets, outside-in αIIbβ3 signaling promotes actin polymerization and cytoskeletal reorganization. Accordingly, it is important for platelet spreading on extracellular matrices under conditions of shear flow, for platelet aggregate stability, and for other post-ligand binding responses, such as clot retraction. Our current knowledge of outside-in signaling in platelets is derived from careful morphologic and biochemical analyses of the cytoskeletons in resting and thrombin-activated platelets190–192; identification of integrin-binding proteins, including various molecular adapters as well as protein and lipid kinases and phosphatases193; studies of platelet responses after interaction with fibrinogen and other αIIbβ3 ligands147; studies of mutant mice; and investigations of platelets from patients with variant Glanzmann thrombasthenia.194 The network of signaling reactions triggered primarily and secondarily by fibrinogen binding to αIIbβ3 is truly impressive, the equal of integrin signaling patterns observed in the much larger blood leukocytes. Many unforeseen parallels are apparent between outside-in αIIbβ3 signaling and signaling triggered by immunoreceptors in platelets and leukocytes, including dependence on the Src family and Syk protein tyrosine kinases (reviewed in Shattil and Newman141 and Watson et al142) and apparent involvement of proteins containing ITAM motifs (reviewed in Abtahian et al195 and Jakus et al196). An emerging picture of αIIbβ3 signaling posits a dynamic interplay among integrin-binding proteins, with the cytoplasmic domains of αIIb and β3 serving a scaffolding function.
αIIbβ3 as a therapeutic target
A potential role of platelets in ischemic vascular disease was inferred from data derived from human pathologic specimens, animal models of thrombus formation, and biochemical studies of patients with acute ischemic events (reviewed in Coller197). There was, however, considerable uncertainty about whether platelets played a sufficiently important role to make antiplatelet therapy an effective intervention. Although an association between aspirin ingestion and bleeding, particularly gastrointestinal bleeding, was recognized by the 1940s, the antiplatelet effects of aspirin were first described in the mid-1960s, greatly facilitated by bleeding time measurements and the introduction of platelet aggregometry (reviewed in Weiss,198 Mustard,199 and Quick200). Subsequent biochemical studies established the dramatic effect of aspirin in suppressing thromboxane A2 production via irreversible acetylation of the enzyme cyclooxygenase-1 (reviewed in Marcus201 and Vane 202). However, early clinical studies of the effects of aspirin on ischemic vascular disease were equivocal, reflecting in part difficulties in designing large clinical trials.203 It was only with the publication of the landmark ISIS-2 study in 1988,204 in which aspirin alone decreased the mortality of acute myocardial infarction by almost 25% and adding aspirin to streptokinase further reduced mortality by almost 25%, that the potential of antiplatelet therapy was clearly demonstrated. The thienopyridine compound ticlopidine, which demonstrated greater inhibition of platelet aggregation than aspirin, entered the U.S. market in the early 1990s based on its greater efficacy than aspirin in the secondary prevention of stroke (reviewed in Savi and Herbert205). Its mechanism of action was later identified as irreversible blockade of the P2Y12 ADP receptor.205
With 2 effective antiplatelet agents available, one of which was very inexpensive and had a long history of acceptable toxicity, it wasn't clear that additional agents would be worth developing. The rationale for trying to develop αIIbβ3 antagonists as therapeutic agents rested on a number of considerations (reviewed in Coller197,206): (1) neither aspirin nor ticlopidine was completely effective in preventing ischemic events; (2) αIIbβ3 antagonists could more completely inhibit platelet aggregation in vitro than either aspirin or ticlopidine; (3) experimental data demonstrated that vaso-occlusion leading to ischemia resulted from platelet-platelet interactions, the primary target of αIIbβ3 antagonists; and (4) the lack of effect of αIIbβ3 antagonists on other platelet adhesion receptors might theoretically allow a single layer of platelets to contribute to hemostasis, whereas the anti-αIIbβ3 effect would greatly diminish platelet thrombus formation. Equally important was the clinical observation that despite suffering variably severe mucocutaneous hemorrhage, patients with Glanzmann thrombasthenia rarely have spontaneous central nervous system bleeding—the most feared hemorrhagic complication of fibrinolytic therapy.207
The mAb 7E3 was selected for in vivo antithrombotic studies based on its ability to bind to dog, primate, and human platelets (reviewed in Coller197,206). Fragments of 7E3 lacking the Fc region were used, rather than the intact antibody, to avoid platelet clearance by Fc receptor-bearing cells of the monocyte-macrophage system that recognize antibody-coated platelets. Administering the F(ab′)2 fragment of 7E3 to dogs was able to produce greater inhibition of ADP-induced platelet aggregation than could be achieved with aspirin and it offered greater pro-tection than aspirin from platelet-mediated thrombosis in dog and primate models of unstable angina or myocardial infarction.208,209 Similar results in these and other animal models were obtained with low molecular weight αIIbβ3 antagonists, including eptifibatide, a cyclic heptapeptide patterned on a KGD motif that conferred selectivity for αIIbβ3,210 and the nonpeptide RGD-mimetic tirofiban.211
For in vivo studies in humans, a chimeric 7E3 Fab molecule designated abciximab was developed containing murine variable regions and human IgG1 constant regions.212 Based on the 2099-patient EPIC study, abciximab was approved by the Food and Drug Administration in 1994 as adjunctive therapy to prevent ischemic complications of coronary artery angioplasty in high-risk patients (reviewed in Topol et al213). Based on additional studies, it was approved for use in percutaneous coronary interventions (PCI) involving stents and in patients with unstable angina who are expected to undergo PCI. Eptifibatide and tirofiban were subsequently approved for human use in patients with unstable angina and those undergoing PCI.214,215
In the EPIC study, abciximab increased the absolute risk of major bleeding by approximately 7%, but by reducing the dose of heparin used in combination with abciximab in subsequent studies, the absolute increase in risk was reduced to approximately 2% or less.216 Eptifibatide and tirofiban confer similar or lesser increase in risk of bleeding. Thrombocytopenia has been reported with all of the αIIbβ3 antagonists, although it is more common with abciximab (reviewed in Aster et al217 and Aster and Bougie218). A number of mechanisms have been proposed to explain the thrombocytopenia, including the presence of antibodies to the murine component of abciximab and antibodies to neoepitopes on αIIbβ3 exposed and/or created by the binding of the drugs to the receptor.
In a large number of randomized, placebo-controlled studies of patients undergoing PCI and/or treatment for unstable angina conducted in the 1990s, these agents demonstrated benefits in reducing the risk of myocardial infarction and the need for urgent reinterventions to treat threatened occlusions (reviewed in Topol et al,213 Curran and Keating,214 Manozzi et al,215 and De et al219). Long-term mortality advantages have also been demonstrated, with most of the benefit paradoxically occurring long after the antiplatelet effects of the drug wore off (reviewed in Topol et al213). Recently, a number of factors have narrowed the indications for these agents in treating cardiovascular disease, including the availability of clopidogrel, a thienopyridine related to ticlopidine that has a better toxicity profile and can achieve rapid inhibition of the P2Y12 receptor with a loading dose (reviewed in in Savi and Herbert205 and Plosker and Lyseng-Williamson220), and bivalirudin, a direct thrombin inhibitor that acts as both an anticoagulant and antiplatelet agent.221 Remaining questions include whether the αIIbβ3 antagonists will prove uniquely beneficial when given very early after the onset of symptoms,222,223 when given by the intracoronary route224,225 (reviewed in Gibson et al226), or when used in combination with a thrombus aspiration device227 or reduced-dose thrombolytic agents when PCI is not immediately available.223,228 The use of αIIbβ3 antagonists to treat acute stroke appeared promising in case reports and early phase studies, but a phase 3 study with abciximab failed to show a benefit; whether modifications in patient selection or dosing would improve the results remains an area of investigation.229
In contrast to the intravenous αIIbβ3 antagonists, oral small molecule αIIbβ3 antagonists have not demonstrated clinical efficacy when administered for secondary prophylaxis and, in fact, have been associated with increased mortality, increased bleeding, and occasionally severe thrombocytopenia230 (reviewed in Quinn et al231). Although it is not understood why these agents failed to have a beneficial effect, it has been speculated that their binding to platelets may induce conformational changes in αIIbβ3 that can both activate the receptor, resulting in paradoxical thrombosis, and expose neoepitopes recognized by some patients' preformed antibodies, resulting in thrombocytopenia.139,230–232
The future
The scientific advances in understanding the structure and function of αIIbβ3 are impressive, but the therapy of Glanzmann thrombasthenia remains unsatisfactory. Thus, while there have been advances in platelet transfusion therapy (including better storage methods, HL-A matching, and leukoreduction), and the introduction of recombinant factor VIIa therapy has provided a blood-free alternative that is frequently effective, though costly (reviewed in Poon233), patients commonly lead lives compromised by variably severe, and sometimes continuous, mucocutaneous hemorrhage, seriously increased risks of surgery and childbirth, and occasional life-threatening gastrointestinal bleeding or trauma-related cerebral hemorrhage.
Bone marrow transplantation and stem cell reconstitution can cure the disorder,234,235 but the risks of these procedures remain substantial, even with newer conditioning regimens, and thus they are still not desirable options for most patients (reviewed in Flood et al236). Proof of concept for gene therapy of Glanzmann thrombasthenia has been provided in animal models237 (reviewed in Wilcox and White238), but much more remains to be done, including how to deal with the immune response to the newly expressed αIIbβ3. Select patients with appropriate nonsense mutations may be candidates for drug therapy to promote DNA “read-through,” but this technology is still in its early phase of development.239 Stem cell biology also offers promise if autologous hematopoietic precursors can be genetically corrected and used to populate the patient's bone marrow or produce platelets in vitro, but the immunologic barriers will likely remain.
Because αIIbβ3 is a proven therapeutic target for antithrombotic therapy, there is reason to speculate on potential ways to improve on the currently available drugs. Drugs that inhibit the receptor without altering its conformation may have several advantages, including a reduction in both thrombocytopenia and receptor activation. Because engagement of the β3 MIDAS motif may be important in initiating the swing-out motion of the hybrid domain, agents that selectively bind to αIIb without effects on β3 may be advantageous.130 Similarly, it may be useful to identify agents that do not bind to the ligand binding site at all but can selectively inhibit inside-out or outside-in signaling.105 In this regard, the currently available high-resolution structures of the β3 integrins, enlightening as they are, represent but snapshots of partial fragments of the whole transmembrane heterodimer. A more complete understanding of the biologically relevant conformational changes in the intact receptor remains an exciting goal for future investigators. In addition, the application of high-throughput screening,130 molecular docking,130 and rational drug design offers hope for identifying novel compounds. Finally, it is possible that monitoring the antiplatelet effects of existing αIIbβ3 antagonists may improve their safety and/or efficacy (reviewed in Harrison et al240). Controlled studies will be needed, however, to test whether dose adjustment based on such monitoring improves clinical outcome.
Conclusions
It is remarkable that studies of αIIbβ3 during the 9 decades since Glanzmann reported patients with the disease that bears his name, and the 5 decades of the American Society of Hematology's existence have gone from intact individual humans to individual atoms at a resolution of 2.8 Å, representing in effect, a span of 27 logs in mass. It is gratifying that the molecular advances have provided patients with Glanzmann thrombasthenia and their families improved diagnosis, carrier detection, and prenatal diagnosis, but it is frustrating that therapeutic advances have lagged. It is also gratifying that understanding the role of αIIbβ3 in platelet thrombus formation has led to the first rationally designed antiplatelet therapeutics and the first anti-integrin therapeutics. The current agents, however, have significant limitations, and the failure of oral αIIbβ3 antagonists was a major disappointment. It is also gratifying that studies of αIIbβ3 have led the way across a wide range of fundamental biologic phenomena related to cell activation, signal transduction, and cytoskeletal rearrangements, as well as genetic and molecular biologic phenomena, including SNPs and the phenomenon of activation-dependent mRNA translation. Given the remarkable trajectory of biomedical science, we anticipate that many more conceptual and practical breakthroughs in this area of hematology await members of the Society and their patients in the next 50 years.
Acknowledgments
We wish to thank Mark Ginsberg, Deborah French, and Uri Seligsohn for their careful review of the manuscript, Dr Marta Murcia for the molecular modeling and images depicted in Figure 6, Suzanne Rivera for outstanding secretarial assistance, and our many colleagues in the field of αIIbβ3 biology who have made important contributions to this remarkable story. We regret that space and format limitations did not allow for more extensive recognition of all of their achievements.
This work was supported in part by grants HL19278, HL56595, HL57900, and HL78784 from the National Heart, Lung, and Blood Institute, grant MH083257 from the National Institute of Mental Health, a Clinical and Translational Science Award (UL1 RR024143) from the National Center for Research Resources, and funds from Stony Brook University (NY).
Biographies
I was drawn to hematology as a fourth-year medical student when I cared for a patient with a severe thromboembolic stroke from an artificial heart valve. I read about the new use of the drug dipyridamole in combination with warfarin to reduce the stroke risk and wrote to Dr Richard Gorlin, one of the authors, for additional information. He wrote back that we knew little about platelet adhesion and so the drug's mechanism of action was uncertain. Dr Marjorie Zucker, one of the giants in platelet physiology, agreed to let me perform an elective in her lab to study the platelet adhesiveness (retention) test, and that is where I fell in love with platelets. I was enormously fortunate then to work for 4 years with Dr Harvey Gralnick at the National Institutes of Health studying the interaction of platelets and von Willebrand factor. Yale Nemerson recruited me in 1976 to the new Division of Hematology at Stony Brook, where I had the great pleasure of helping to build the clinical, educational, and research programs of the division. Dr Arnold Levine built a core hybridoma facility at Stony Brook with New York State support and allowed me to try to produce antibodies to platelets. That truly changed my career. I also benefited greatly from the critical advice and support of some of the elders in the New York “clot club,” including Aaron Marcus, Ralph Nachman, Hymie Nossel, Ted Spaet, and Harvey Weiss.
In 1993 I moved to Mount Sinai as Chair of Medicine, a move made poignant because Dr Gorlin was still there after serving as Chair of Medicine. Finally, in 2001 I moved to Rockefeller University as Physician-in-Chief, where I lead our training and research programs in clinical and translational science. I retain my love of hematology, as evidenced by my continuing to conduct periodic “smear rounds” with the internal medicine residents at Mount Sinai, where I have to guess the entire clinical history of a resident's patient just by looking at the peripheral smear!
Although I did not know it at the time, my introduction to hematology and to a career as a physician-scientist occurred precisely 40 years ago in the decrepit Peabody Building of the Boston City Hospital, a building better suited to the medicine of the early 1900s than the late 1960s. It is there that I began an internship on the Harvard Medical Service, an organization with an illustrious legacy at the interface of basic science and clinical medicine. As a Chicago boy, I had just received a solid medical education at the University of Illinois College of Medicine in Chicago and had moved to Boston at the prescient urging of my wife, who opined that a change in scenery would do us both good. After a few days at the Peabody, I was sure that I was the dumbest intern in my class, the rest having migrated to Boston with track records as valedictorians and such from leading medical schools. My feeling of inadequacy was exacerbated by the formidable intellectual and clinical skills of the supervising residents and attending physicians, and by my inability to heal the infirmities of some of the sickest patients I have ever encountered.
After a year or so, I thought that a career in cardiology was in my future, until a chance conversation with an attending physician, Dr Richard “Buzz” Cooper, Chief of Hematology at the Thorndike Memorial Laboratory, the research and fellowship training arm of the Harvard Medical Service at The City. He invited me to consider a fellowship in hematology, a subject about which I knew little. In those days, hematology was of prime importance at The City, given a steady flow of seminal investigative accomplishments there over the preceding decades by Francis W. Peabody, William B. Castle, Thomas Hale Ham, James H. Jandl, Richard Aster, and others. Never one with long-term plans, I gratefully accepted Dr. Cooper's invitation and received hematology training and mentorship at the Thorndike from the likes of Cooper, Neil Abramson, Bernie Babior, Frank Bunn, and Herman Godwin. As if this were not enough, our weekly research and clinical conferences were attended by other giants in the field, including Bill Castle, Jane DesForges, and Jim Jandl. Making presentations to this group was both intimidating and exhilarating, providing valuable lessons in how accomplished physician-scientists think and execute their work. Within this environment I went from the dumbest intern to the dumbest hematology fellow on the block.
I owe my career as a scientist largely to Buzz Cooper, in whose laboratory I learned more than the basics of red cell membrane biology and biochemistry. It was there I learned the importance of asking the right questions, developing critical thinking and writing skills, performing the right controls, interpreting data rigorously, and generously sharing ideas and reagents with others. My interest in platelet membranes and platelet activation was spawned serendipitously in the mid-1970s as an Assistant Professor at the University of Pennsylvania through collaborations with Cooper, Robert Colman, and Joel Bennett. My interest in integrin biology starting in the 1980s was also an accident of sorts. While screening for monoclonal antibodies to activated platelets, Mike Cunningham, Skip Brass, Jim Hoxie, and I discovered the PAC-1 antibody to GP IIb-IIIa. Little did I know then that this area of inquiry would lead me in subsequent decades to study integrin biology in organisms ranging from zebrafish to mice and humans. The moral of this accidental hematologist's story is that it's possible to try your hand as a physician-scientist without a grand plan ahead of time, provided that you are fortunate enough to have world-class mentors, talented colleagues and collaborators, and a selfless family.
Authorship
Contribution: B.S.C. and S.J.S. wrote the paper.
Conflict-of-interest disclosure: In accord with federal law and the policies of the Research Foundation of the State University of New York, B.S.C. has a royalty interest in abciximab (Centocor), and in accord with federal law and the policies of the Mount Sinai School of Medicine, B.S.C. has a royalty interest in the VerifyNow assay system (Accumetrics). In addition, B.S.C. is a consultant to Accumetrics and Novo-Nordisk and is an inventor of an αIIbβ3 antagonist compound identified by high-throughput screening. S.J.S. declares no competing financial interests.
Correspondence: Dr Barry S. Coller, Laboratory of Blood and Vascular Biology, The Rockefeller University, 1230 York Avenue, Box 309, New York, NY 10065; e-mail: collerb@rockefeller.edu.
References
- 1.Gabriele M. Blood and magic in classical antiquity. In: Bradburne JM, editor. Blood: Art, Power, Politics and Pathology. Munich: Prestel; 2002. pp. 33–39. [Google Scholar]
- 2.Medical Meanings: A Glossary of Word Origins. Philadelphia: American College of Physicians; 1997. [Google Scholar]
- 3.George JN, Nurden AT, Phillips DR, editors. Platelet membrane glycoproteins. New York: Plenum; 1985. [DOI] [PubMed] [Google Scholar]
- 4.Michelson AD, editor. Platelets. Burlington, MA: Academic Press; 2007. [Google Scholar]
- 5.Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Hematology. New York: McGraw-Hill; 2006. [Google Scholar]
- 6.Hoffman R, Benz EJ, Shattil S, Furie B, Cohen HJ, Silberstein LE, McGlave P, editors. Hematology: Basic Principles and Practice. Philadelphia: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- 7.Colman RW, Marder VJ, Clowes AW, George JN, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 8.Kunick TJ, George JG, editors. Platelet Immunobiology: Molecular and Clinical Aspects. Philadelphia: Lippincott Williams & Wilkins; 1989. [Google Scholar]
- 9.Robb-Smith AH. Why the platelets were discovered [review]. Br J Haematol. 1967;13:618–637. doi: 10.1111/j.1365-2141.1967.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 10.Duke WW. The relation of blood platelets to hemorrhagic disease. Description of a method for determining the bleeding time and the coagulation time and report of three cases of hemorrhagic disease relieved by transfusion. JAMA. 1910;55:1185–1192. [Google Scholar]
- 11.Glanzmann E. Hereditäre hämmorhagische Thrombasthenie. Ein Beitrag zur Pathologie der Blutplättchen. Jahrbuch fur Kinderheilkunde und physiche Erziehung. 1918;88:113–141. [Google Scholar]
- 12.Caen JP, Castaldi PA, Leclerc JC, et al. Congenital bleeding disorders with long bleeding time and normal platelet count. I. Glanzmann's thrombasthenia. Am J Med. 1966;41:4–26. [Google Scholar]
- 13.Marcus AJ, Zucker MB. The Physiology of Blood Platelets. New York: Gurne and Stratton; 1965. [Google Scholar]
- 14.Behnke O. Electron microscopical observations on the surface coating of human blood platelets. J Ultrastruct Res. 1968;24:51–69. doi: 10.1016/s0022-5320(68)80016-4. [DOI] [PubMed] [Google Scholar]
- 15.Peerschke EI. The platelet fibrinogen receptor [review]. Semin Hematol. 1985;22:241–259. [PubMed] [Google Scholar]
- 16.Bennett JS. The platelet-fibrinogen interaction. In: George JN, Nurden AT, Phillips DR, editors. Platelet Membrane Glycoproteins. New York: Plenum; 1985. pp. 51–85. [Google Scholar]
- 17.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien J. Platelet aggregation. II. Some results from a new method of study. J Clin Pathol. 1962;15:452–481. doi: 10.1136/jcp.15.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardisty RM, Dormandy KM, Hutton RA. Thrombasthenia: studies on three cases. Br J Haematol. 1964;10:371–383. doi: 10.1111/j.1365-2141.1964.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 20.Zucker MB, Pert JH, Hilgartner MW. Platelet function in a patient with thrombasthenia. Blood. 1966;28:524–534. [PubMed] [Google Scholar]
- 21.Jackson DP, Morse EE, Zieve PD, Conley CL. Thrombocytopathic purpura associated with defective clot retraction and absence of platelet fibrinogen [abstract]. Blood. 1963;22:827. [Google Scholar]
- 22.Nachman RL, Marcus AJ. Immunological studies of proteins associated with the subcellular fractions of thrombasthenic and afibrinogenaemic platelets. Br J Haematol. 1968;15:181–189. doi: 10.1111/j.1365-2141.1968.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 23.Nurden AT, Caen JP. An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol. 1974;28:253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- 24.Phillips DR, Agin PP. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977;60:535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurden AT. Platelet membrane glycoproteins: a look back into the past and a view to the future [review]. Thromb Haemost. 2007;98:49–54. [PubMed] [Google Scholar]
- 26.George JN, Nurden AT, Phillips DR. Molecular defects in interactions of platelets with the vessel wall [review]. N Engl J Med. 1984;311:1084–1098. doi: 10.1056/NEJM198410253111705. [DOI] [PubMed] [Google Scholar]
- 27.Hagen I, Nurden AT, Bjerrum OJ, Solum NO, Caen JP. Immunochemical evidence for protein abnormalities from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Clin Invest. 1980;65:722–731. doi: 10.1172/JCI109719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunicki TJ, Pidard D, Rosa JP, Nurden AT. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981;58:268–278. [PubMed] [Google Scholar]
- 29.Howard L, Shulman S, Sadanandan S, Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. The major antigen consists of a complex of glycoproteins, GPIIb and GPIIIa, held together by Ca2+ and missing in Glanzmann's thrombasthenia. J Biol Chem. 1982;257:8331–8336. [PubMed] [Google Scholar]
- 30.Mustard JF, Packham MA, Kinlough-Rathbone RL, Perry DW, Regoeczi E. Fibrinogen and ADP-induced platelet aggregation. Blood. 1978;52:453–466. [PubMed] [Google Scholar]
- 31.Bennett JS, Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979;64:1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marguerie GA, Plow EF, Edgington TS. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979;254:5357–5363. [PubMed] [Google Scholar]
- 33.Nachman RL, Leung LL. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982;69:263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldassare JJ, Kahn RA, Knipp MA, Newman PJ. Reconstruction of platelet proteins into phospholipid vesicles. Functional proteoliposomes. J Clin Invest. 1988;75:35–39. doi: 10.1172/JCI111693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parise LV, Phillips DR. Reconstitution of the purified platelet fibrinogen receptor. Fibrinogen binding properties of the glycoprotein IIb-IIIa complex. J Biol Chem. 1985;260:10698–10707. [PubMed] [Google Scholar]
- 36.Tabilio A, Rosa JP, Testa U, et al. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984;3:453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bray PF, Rosa JP, Johnston GI, et al. Platelet glycoprotein IIb. Chromosomal localization and tissue expression. J Clin Invest. 1987;80:1812–1817. doi: 10.1172/JCI113277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiagarajan P, Shapiro SS, Sweterlitsch L, McCord S. A human erythroleukemia cell line synthesizes a functionally active glycoprotein IIb-IIIa complex capable of binding fibrinogen. Biochim Biophys Acta. 1987;924:127–134. doi: 10.1016/0304-4165(87)90079-1. [DOI] [PubMed] [Google Scholar]
- 39.Bray PF, Rosa JP, Lingappa VR, et al. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A. 1986;83:1480–1484. doi: 10.1073/pnas.83.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duperray A, Troesch A, Berthier R, et al. Biosynthesis and assembly of platelet GPIIb-IIIa in human megakaryocytes: evidence that assembly between pro-GPIIb and GPIIIa is a prerequisite for expression of the complex on the cell surface. Blood. 1989;74:1603–1611. [PubMed] [Google Scholar]
- 41.O'Toole TE, Loftus JC, Plow EF, et al. Efficient surface expression of platelet GPIIb-IIIa requires both subunits. Blood. 1989;74:14–18. [PubMed] [Google Scholar]
- 42.McEver RP, Baenziger NL, Majerus PW. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980;66:1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett JS, Hoxie JA, Leitman SF, Vilaire G, Cines DB. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983;80:2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coller BS, Peerschke EI, Scudder LE, Sullivan CA. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983;72:325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner CL, Mascelli MA, Neblock DS, et al. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88:907–914. [PubMed] [Google Scholar]
- 46.Woods VL, Jr, Wolff LE, Keller DM. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complexes which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986;261:15242–15251. [PubMed] [Google Scholar]
- 47.Wencel-Drake JD, Plow EF, Kunicki TJ, et al. Localization of internal pools of membrane glycoproteins involved in platelet adhesive responses. Am J Pathol. 1986;124:324–334. [PMC free article] [PubMed] [Google Scholar]
- 48.Coller BS. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet GPIIb/IIIa complex. J Clin Invest. 1985;76:101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 50.Frelinger AL, III, Lam SC, Plow EF, et al. Occupancy of an adhesive glycoprotein receptor modulates expression of an antigenic site involved in cell adhesion. J Biol Chem. 1988;263:12397–12402. [PubMed] [Google Scholar]
- 51.Honda S, Tomiyama Y, Pelletier AJ, et al. Topography of ligand-induced binding sites, including a novel cation-sensitive epitope (AP5) at the amino terminus, of the human integrin beta 3 subunit. J Biol Chem. 1995;270:11947–11954. doi: 10.1074/jbc.270.20.11947. [DOI] [PubMed] [Google Scholar]
- 52.Plow EF, McEver RP, Coller BS, et al. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor and thrombospondin on thrombin-stimulated human platelets. Blood. 1985;66:724–727. [PubMed] [Google Scholar]
- 53.Montgomery RR, Kunicki TJ, Taves C, et al. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983;71:385–389. doi: 10.1172/JCI110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coller BS, Seligsohn U, Zivelin A, et al. Immunologic and biochemical characterization of homozygous and heterozygous Glanzmann's thrombasthenia in Iraqi-Jewish and Arab populations of Israel: comparison of techniques for carrier detection. Br J Haematol. 1986;62:723–735. doi: 10.1111/j.1365-2141.1986.tb04096.x. [DOI] [PubMed] [Google Scholar]
- 55.Seligsohn U, Mibashan RS, Rodeck CH, et al. Prenatal diagnosis of Glanzmann's thrombasthenia. Lancet. 1985;2:1419. doi: 10.1016/s0140-6736(85)92579-6. [DOI] [PubMed] [Google Scholar]
- 56.Coller BS, Seligsohn U, Little PA. Type I Glanzmann thrombasthenia patients from the Iraqi-Jewish and Arab populations in Israel can be differentiated by platelet glycoprotein IIIa immunoblot analysis. Blood. 1987;69:1696–1703. [PubMed] [Google Scholar]
- 57.Nurden AT, Didry D, Kieffer N, McEver RP. Residual amounts of glycoproteins IIb and IIIa may be present in the platelets of most patients with Glanzmann's thrombasthenia. Blood. 1985;65:1021–1024. [PubMed] [Google Scholar]
- 58.Seligsohn U, Coller BS, Zivelin A, Plow EF, Ginsberg MH. Immunoblot analysis of platelet GPIIb in patients with Glanzmann thrombasthenia in Israel. Br J Haematol. 1989;72:415–423. doi: 10.1111/j.1365-2141.1989.tb07725.x. [DOI] [PubMed] [Google Scholar]
- 59.Shattil SJ, Cunningham M, Hoxie JA. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987;70:307–315. [PubMed] [Google Scholar]
- 60.Ginsberg MH, Frelinger AL, Lam SC, et al. Analysis of platelet aggregation disorders based on flow cytometric analysis of membrane glycoprotein IIb-IIIa with conformation-specific monoclonal antibodies. Blood. 1990;76:2017–2023. [PubMed] [Google Scholar]
- 61.Poncz M, Eisman R, Heidenreich R, et al. Structure of the platelet membrane glycoprotein IIb: homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987;262:8476–8482. [PubMed] [Google Scholar]
- 62.Fitzgerald LA, Steiner B, Rall SC, Jr, Lo SS, Phillips DR. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to “integrin.”. J Biol Chem. 1987;262:3936–3939. [PubMed] [Google Scholar]
- 63.Thornton MA, Poncz M, Korostishevsky M, et al. The human platelet alphaIIb gene is not closely linked to its integrin partner beta3. Blood. 1999;94:2039–2047. [PubMed] [Google Scholar]
- 64.Bray PF, Barsh G, Rosa JP, et al. Physical linkage of the genes for platelet membrane glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A. 1988;85:8683–8687. doi: 10.1073/pnas.85.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pytela R, Pierschbacher MD, Ginsberg MH, Plow EF, Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp-specific adhesion receptors [review]. Science. 1986;231:1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- 66.Hynes RO. Integrins: a family of cell surface receptors [review]. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 67.Ginsberg MH, Loftus J, Ryckwaert JJ, et al. Immunochemical and amino-terminal sequence comparison of two cytoadhesins indicates they contain similar or identical beta subunits and distinct alpha subunits. J Biol Chem. 1987;262:5437–5440. [PubMed] [Google Scholar]
- 68.Fitzgerald LA, Poncz M, Steiner B, et al. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor α subunits and platelet glycoprotein IIb. Biochemistry. 1987;26:8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- 69.Byzova TV, Rabbani R, D'Souza SE, Plow EF. Role of integrin alpha (v) beta3 in vascular biology [review]. Thromb Haemost. 1998;80:726–734. [PubMed] [Google Scholar]
- 70.Mt Sinai School of Medicine. Glanzmann Thrombasthenia Database. [Accessed September 6, 2008]; http://sinaicentral.mssm.edu/intranet/research/glanzmann/menu.
- 71.French DL, Seligsohn U. Platelet glycoprotein IIb/IIIa receptors and Glanzmann's thrombasthenia [review]. Arterioscler Thromb Vasc Biol. 2000;20:607–610. doi: 10.1161/01.atv.20.3.607. [DOI] [PubMed] [Google Scholar]
- 72.MacKrell AJ, Blumberg B, Haynes SR, Fessler JH. The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci U S A. 1988;85:2633–2637. doi: 10.1073/pnas.85.8.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kunicki TJ, Nugent DJ, Staats SJ, et al. The human fibroblast class II extracellular matrix receptor mediates platelet adhesion to collagen and is identical to the platelet glycoprotein Ia-IIa complex. J Biol Chem. 1988;263:4516–4519. [PubMed] [Google Scholar]
- 74.Wayner EA, Carter WG, Piotrowicz RS, Kunicki TJ. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: Preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion of fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988;107:1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336:487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- 76.Coller BS, Cheresh DA, Asch E, Seligsohn U. Platelet vitronectin receptor expression differentiates Iraqi-Jewish from Arab patients with Glanzmann thrombasthenia in Israel. Blood. 1991;77:75–83. [PubMed] [Google Scholar]
- 77.Newman PJ, Seligsohn U, Lyman S, Coller BS. The molecular genetic basis of Glanzmann thrombasthenia in the Iraqi-Jewish and Arab populations in Israel. Proc Natl Acad Sci U S A. 1991;88:3160–3164. doi: 10.1073/pnas.88.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruoslahti E. Fibronectin and its receptors [review]. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- 79.Gould RJ, Polokoff MA, Friedman PA, et al. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med [review] 1990;195:168–171. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- 80.Ojima I, Chakravarty S, Dong Q. Antithrombotic agents: from RGD to peptide mimetics [review]. Bioorg Med Chem. 1995;3:337–360. doi: 10.1016/0968-0896(95)00036-g. [DOI] [PubMed] [Google Scholar]
- 81.Kloczewiak M, Timmons S, Bednarek MA, Sakon M, Hawiger J. Platelet receptor recognition domain on the gamma chain of human fibrinogen and its synthetic peptide analogues. Biochemistry. 1989;28:2915–2919. doi: 10.1021/bi00433a025. [DOI] [PubMed] [Google Scholar]
- 82.Farrell DH, Thiagarajan P, Chung DW, Davie EW. Role of fibrinogen α and γ chain sites in platelet aggregation. Proc Natl Acad Sci U S A. 1992;89:10729–10732. doi: 10.1073/pnas.89.22.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newman PJ, Gorski J, White GC, et al. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988;82:739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newman PJ, Derbes RS, Aster RH. The human platelet alloantigens, PlA1 and PlA2, are associated with a leucine33/proline33 amino acid polymorphism in membrane glycoprotein IIIa, and are distinguishable by DNA typing. J Clin Invest. 1989;83:1778–1781. doi: 10.1172/JCI114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valentin N, Newman PJ. Human platelet alloantigens [review]. Curr Opin Hematol. 1994;1:381–387. [PubMed] [Google Scholar]
- 86.Bray PF. Integrin polymorphisms as risk factors for thrombosis [review]. Thromb Haemost. 1999;82:337–344. [PubMed] [Google Scholar]
- 87.French DL, Coller BS, Berkowitz R, et al. Prenatal diagnosis of Glanzmann thrombasthenia using the polymorphic markers BRCA 1 and THRA 1 on chromosome 17. Br J Haematol. 1998;102:582–587. doi: 10.1046/j.1365-2141.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 88.Wautier JL, Gruel Y. Prenatal diagnosis of platelet disorders [review]. Baillieres Clin Haematol. 1989;2:569–583. doi: 10.1016/s0950-3536(89)80034-4. [DOI] [PubMed] [Google Scholar]
- 89.Loftus JC, O'Toole TE, Plow EF, et al. A β3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990;249:915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- 90.Lanza F, Stierle A, Fournier D, et al. A new variant of Glanzmann's thrombasthenia (Strasbourg I).. Platelets with functionally defective glycoprotein IIb-IIIa complexes and a glycoprotein IIIa Arg214Trp mutation. J Clin Invest. 1992;89:1995–2004. doi: 10.1172/JCI115808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grimaldi CM, Chen F, Wu C, et al. Glycoprotein IIb Leu214Pro mutation produces Glanzmann thrombasthenia with both quantitative and qualitative abnormalities in GPIIb/IIIa. Blood. 1998;91:1562–1568. [PubMed] [Google Scholar]
- 92.Basani RB, French DL, Vilaire G, et al. A naturally-occurring mutation near the amino terminus of αIIb defines a new region involved in ligand binding to αIIbβ3. Blood. 2000;95:180–188. [PubMed] [Google Scholar]
- 93.Chen Y-P, Djaffar I, Pidard E. Ser752Pro mutation in the cytoplasmic domain of integrin β3 subunit and defective activation of platelet integrin αIIbβ3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc Natl Acad Sci U S A. 1992;89:10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang R, Shattil SJ, Ambruso DR, Newman PJ. Truncation of the cytoplasmic domain of β3 in a variant form of Glanzmann thrombasthenia abrogates signaling through the integrin αIIbβ3 complex. J Clin Invest. 1997;100:2393–2403. doi: 10.1172/JCI119780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz C, Liu CY, Sun QH, et al. A point mutation in the cysteine-rich domain of glycoprotein (GP) IIIa results in the expression of a GPIIb-IIIa (alphaIIbbeta3) integrin receptor locked in a high-affinity state and a Glanzmann thrombasthenia-like phenotype. Blood. 2001;98:2432–2441. doi: 10.1182/blood.v98.8.2432. [DOI] [PubMed] [Google Scholar]
- 96.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smyth SS, Reis ED, Vaananen H, Zhang W, Coller BS. Variable protection of β3-integrin-deficient mice from thrombosis initiated by different mechanisms. Blood. 2001;98:1055–1062. doi: 10.1182/blood.v98.4.1055. [DOI] [PubMed] [Google Scholar]
- 98.Smyth SS, Reis ED, Zhang W, et al. β3-integrin-deficient mice, but not P-selectin-deficient mice, develop intimal hyperplasia after vascular injury: correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation. 2001;103:2501–2507. doi: 10.1161/01.cir.103.20.2501. [DOI] [PubMed] [Google Scholar]
- 99.McHugh KP, Hodivala-Dilke K, Zheng MH, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reynolds LE, Conti FJ, Lucas M, et al. Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 101.Tronik-Le RD, Roullot V, Poujol C, et al. Thrombasthenic mice generated by replacement of the integrin alpha(IIb) gene: demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood. 2000;96:1399–1408. [PubMed] [Google Scholar]
- 102.Emamboleus NR, Frampton J. The glycoprotein IIb molecule is expressed on early murine hematopoietic progenitors and regulates their numbers in sites of hematopoiesis. Immunity. 2003;19:33–45. doi: 10.1016/s1074-7613(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 103.Law DA, DeGuzmann FR, Heiser P, Ministri-Madrid K, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 104.Zou Z, Chen H, Schmaier AA, Hynes RO, Kahn ML. Structure-function analysis reveals discrete beta3 integrin inside-out and outside-in signaling pathways in platelets. Blood. 2007;109:3284–3290. doi: 10.1182/blood-2006-10-051664. [DOI] [PubMed] [Google Scholar]
- 105.Petrich BG, Fogelstrand P, Partridge AW, et al. The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117:2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collins Tozer EC, Liddington RC, Sutcliffe MJ, Smeeton AH, Loftus JC. Ligand binding to integrin αIIbβ3 is dependent on a MIDAS-like domain in the beta3 subunit. J Biol Chem. 1996;271:21978–21984. doi: 10.1074/jbc.271.36.21978. [DOI] [PubMed] [Google Scholar]
- 107.Bajt ML, Loftus JC. Mutation of a ligand binding domain of beta 3 integrin: integral role of oxygenated residues in alpha IIb beta 3 (GPIIb-IIIa) receptor function. J Biol Chem. 1994;269:20913–20919. [PubMed] [Google Scholar]
- 108.Carrell NA, Fitzgerald LA, Steiner B, Erickson HP, Phillips DR. Structure of human platelet membrane glycoproteins IIb and IIIa as determined by electron microscopy. J Biol Chem. 1985;260:1743–1749. [PubMed] [Google Scholar]
- 109.Weisel JW, Nagaswami C, Vilaire G, Bennett JS. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem. 1992;267:16637–16643. [PubMed] [Google Scholar]
- 110.Du X, Gu M, Weisel JW, et al. Long range propagation of conformational changes in integrin alpha IIb beta 3. J Biol Chem. 1993;268:23087–92. [PubMed] [Google Scholar]
- 111.Xiong JP, Stehle T, Diefenbach B, et al. Crystal structure of the extracellular segment of integrin alphaVbeta3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiong JP, Stehle T, Zhang R, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 113.Xiao T, Takagi J, Coller BS, Wang J, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adair BD, Xiong JP, Maddock C, et al. Three-dimensional EM structure of the ectodomain of integrin (alpha)V(beta)3 in a complex with fibronectin. J Cell Biol. 2005;168:1109–1118. doi: 10.1083/jcb.200410068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu J, Boylan B, Luo BH, Newman PJ, Springer TA. Tests of the extension and deadbolt models of integrin activation. J Biol Chem. 2007;282:11914–11920. doi: 10.1074/jbc.M700249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mor-Cohen R, Rosenberg N, Peretz H, et al. Disulfide bond disruption by a beta 3-Cys549Arg mutation in six Jordanian families with Glanzmann thrombasthenia causes diminished production of constitutively active alpha IIb beta 3. Thromb Haemost. 2007;98:1257–1265. [PubMed] [Google Scholar]
- 119.Zucker MB, Masiello NC. Platelet aggregation caused by dithiothreitol. Thromb Haemost. 1984;51:119–124. [PubMed] [Google Scholar]
- 120.Yan B, Smith JW. A redox site involved in integrin activation. J Biol Chem. 2000;275:39964–39972. doi: 10.1074/jbc.M007041200. [DOI] [PubMed] [Google Scholar]
- 121.Essex DW, Li M. Redox control of platelet aggregation. Biochemistry. 2003;42:129–136. doi: 10.1021/bi0205045. [DOI] [PubMed] [Google Scholar]
- 122.Lahav J, Gofer-Dadosh N, Luboshitz J, Hess O, Shaklai M. Protein disulfide isomerase mediates integrin-dependent adhesion. FEBS Lett. 2000;475:89–92. doi: 10.1016/s0014-5793(00)01630-6. [DOI] [PubMed] [Google Scholar]
- 123.Walsh GM, Sheehan D, Kinsella A, Moran N, O'Neill S. Redox modulation of integrin [correction of integin] alpha IIb beta 3 involves a novel allosteric regulation of its thiol isomerase activity. Biochemistry. 2004;43:473–480. doi: 10.1021/bi0354536. [DOI] [PubMed] [Google Scholar]
- 124.Li R, Babu CR, Lear JD, et al. Oligomerization of the integrin alphaIIbbeta3: roles of the transmembrane and cytoplasmic domains. Proc Natl Acad Sci U S A. 2001;98:12462–12467. doi: 10.1073/pnas.221463098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J Cell Biol. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peerschke EI. Reversible and irreversible binding of fibrinogen to platelets. Platelets. 1997;8:311–317. doi: 10.1080/09537109777177. [DOI] [PubMed] [Google Scholar]
- 127.Haas TA, Plow EF. Development of a structural model for the cytoplasmic domain of an integrin. Protein Eng. 1997;10:1395–1405. doi: 10.1093/protein/10.12.1395. [DOI] [PubMed] [Google Scholar]
- 128.Puklin-Faucher E, Gao M, Schulten K, Vogel V. How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J Cell Biol. 2006;175:349–360. doi: 10.1083/jcb.200602071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murcia M, Jirouskova M, Li J, Coller BS, Filizola M. Functional and computational studies of the ligand-associated metal binding site of beta3 integrins. Proteins. 2008;71:1779–1791. doi: 10.1002/prot.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blue R, Murcia M, Karan C, Jirouskova M, Coller BS. Application of high throughput screening to identify a novel αIIb-specific small molecule inhibitor of αIIbβ3-mediated platelet Interaction with fibrinogen. Blood. 2008;111:1248–1256. doi: 10.1182/blood-2007-08-105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ulmer TS, Calderwood DA, Ginsberg MH, Campbell ID. Domain-specific interactions of talin with the membrane-proximal region of the integrin beta3 subunit. Biochemistry. 2003;42:8307–8312. doi: 10.1021/bi034384s. [DOI] [PubMed] [Google Scholar]
- 132.Lau TL, Partridge AW, Ginsberg MH, Ulmer TS. Structure of the integrin beta3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry. 2008;47:4008–4016. doi: 10.1021/bi800107a. [DOI] [PubMed] [Google Scholar]
- 133.Haas TA, Plow EF. The cytoplasmic domain of alphaIIb beta3. A ternary complex of the integrin alpha and beta subunits and a divalent cation. J Biol Chem. 1996;271:6017–6026. doi: 10.1074/jbc.271.11.6017. [DOI] [PubMed] [Google Scholar]
- 134.Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb)beta(3): activation mechanisms [review]. J Thromb Haemost. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 135.Rocco M, Rosano C, Weisel JW, Horita DA, Hantgan RR. Integrin conformational regulation: uncoupling extension/tail separation from changes in the head region by a multiresolution approach. Structure. 2008;16:954–964. doi: 10.1016/j.str.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ye F, Liu J, Winkler H, Taylor KA. Integrin alpha IIb beta 3 in a membrane environment remains the same height after Mn2+ activation when observed by cryoelectron tomography. J Mol Biol. 2008;378:976–986. doi: 10.1016/j.jmb.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tokuhira M, Handa M, Kamata T, et al. A novel regulatory epitope defined by a murine monoclonal antibody to the platelet GPIIb-IIIa complex (alpha IIb beta 3 integrin). Thromb Haemost. 1996;76:1038–1046. [PubMed] [Google Scholar]
- 138.Kouns WC, Jennings LK. Activation-independent exposure of the GPIIb-IIIa fibrinogen receptor. Thromb Res. 1991;63:343–354. doi: 10.1016/0049-3848(91)90137-l. [DOI] [PubMed] [Google Scholar]
- 139.Du XP, Plow EF, Frelinger AL, III, et al. Ligands “activate” integrin alpha IIb beta 3 (platelet GPIIb-IIIa). Cell. 1991;65:409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- 140.Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 141.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets [review]. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 142.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets [review]. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 143.Qin J, Vinogradova O, Plow EF. Integrin bidirectional signaling: a molecular view [review]. PLoS Biol. 2004;2:726–729. doi: 10.1371/journal.pbio.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Michelson AD, Shattil SJ. The use of flow cytometry to study platelet activation. In: Watson SP, Authi KS, editors. Platelets: A Practical Approach. Oxford: Oxford University Press; 1996. pp. 111–129. [Google Scholar]
- 145.McEver RP, Martin MN. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984;259:9799–9804. [PubMed] [Google Scholar]
- 146.Hsu-Lin S, Berman CL, Furie BC, August D, Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984;259:9121–9126. [PubMed] [Google Scholar]
- 147.Ferrell JE, Jr, Martin GS. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci U S A. 1989;86:2234–2238. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smolenski A, Bachmann C, Reinhard K, et al. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 149.O'Toole TE, Mandelman D, Forsyth J, et al. Modulation of the affinity of integrin αIIbβ3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991;254:845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- 150.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 151.Langenbach R, Morham SG, Tiano HF, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 152.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 153.Brass LF. Molecular basis for platelet activation. In: Hoffman R, Benz E, Shattil S, editors. Hematology Basic Principles and Practice. Philadelphia: Elsevier Churchill-Livingstone; 2005. pp. 1899–1914. [Google Scholar]
- 154.Mustard JF, Packham MA. Factors influencing platelet function: adhesion, release, and aggregation [review]. Pharmacol Rev. 1970;22:97–187. [PubMed] [Google Scholar]
- 155.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 156.Kahn ML, Zheng YW, Huang W, et al. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 157.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis [review]. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 158.Fabre JE, Nguyen M, Latour A, et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- 159.Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 160.Thomas DW, Mannon RB, Mannon PJ, et al. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets [review]. Arterioscler Thromb Vasc Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 162.Kaczmarek E, Koziak K, Sevigny J, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 163.Marcus AJ, Broekman MJ, Drosopoulos JH, et al. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Samuelsson B. Prostaglandins and thromboxanes. Recent Prog Horm Res. 1978;34:239–258. doi: 10.1016/b978-0-12-571134-0.50010-7. [DOI] [PubMed] [Google Scholar]
- 165.Moncada S, Radomski MW, Palmer RM. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988;37:2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 166.Naseem KM, Riba R. Unresolved roles of platelet nitric oxide synthase. J Thromb Haemost. 2008;6:10–19. doi: 10.1111/j.1538-7836.2007.02802.x. [DOI] [PubMed] [Google Scholar]
- 167.Kunapuli SP, Dorsam RT, Kim S, Quinton TM. Platelet purinergic receptors [review]. Curr Opin Pharmacol. 2003;3:175–180. doi: 10.1016/s1471-4892(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 168.Lee DH, Blajchman MA. Animal models. In: Michelson AD, editor. Platelets. San Diego: Academic Press; 2007. pp. 587–610. [Google Scholar]
- 169.Tsakiris DA, Scudder L, Hodivala-Dilke KM, Hynes RO, Coller BS. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost. 1999;81:177–188. [PubMed] [Google Scholar]
- 170.Jirouskova M, Shet AS, Johnson GJ. A guide to murine platelet structure, function, assays, and genetic alterations [review]. J Thromb Haemost. 2007;5:661–669. doi: 10.1111/j.1538-7836.2007.02407.x. [DOI] [PubMed] [Google Scholar]
- 171.Rao AK, Jalagadugula G, Sun L. Inherited defects in platelet signaling mechanisms [review]. Semin Thromb Hemost. 2004;30:525–535. doi: 10.1055/s-2004-835673. [DOI] [PubMed] [Google Scholar]
- 172.Wegener KL, Partridge AW, Han J, et al. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 173.Nieswandt B, Moser M, Pleines I, et al. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007;204:3113–3118. doi: 10.1084/jem.20071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Petrich BG, Marchese P, Ruggeri ZM, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Han J, Lim CJ, Watanabe N, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3 [review]. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 177.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by Gi family members in platelets. J Biol Chem. 2002;277:23382–23390. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 178.Larson MK, Chen H, Kahn ML, et al. Identification of P2Y12-dependent and -independent mechanisms of glycoprotein VI-mediated Rap1 activation in platelets. Blood. 2003;101:1409–1415. doi: 10.1182/blood-2002-05-1533. [DOI] [PubMed] [Google Scholar]
- 179.Crittenden JR, Bergmeier W, Zhang Y, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 180.Pasvolsky R, Feigelson SW, Kilic SS, et al. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–1582. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 182.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function [review]. Arterioscler Thromb Vasc Biol. 2008;28:s17–s24. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Evangelista V, Manarini S, Sideri R, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- 186.Italiano JE, Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation [review]. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 188.Emambokus NR, Frampton J. The glycoprotein IIb molecule is expressed on early murine hematopoietic progenitors and regulates their numbers in sites of hematopoiesis. Immunity. 2003;19:33–45. doi: 10.1016/s1074-7613(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 189.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 190.Jennings LK, Fox JE, Edwards HH, Phillips DR. Changes in the cytoskeletal structure of human platelets following thrombin activation. J Biol Chem. 1981;256:6927. [PubMed] [Google Scholar]
- 191.Hartwig JH, DeSisto M. The cytoskeleton of the resting human blood platelet: structure of the membrane skeleton and its attachment to actin filaments. J Cell Biol. 1991;112:407–425. doi: 10.1083/jcb.112.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Leisner TM, Yuan W, DeNofrio JC, Liu J, Parise LV. Tickling the tails: cytoplasmic domain proteins that regulate integrin alphaIIbbeta3 activation [review]. Curr Opin Hematol. 2007;14:255–261. doi: 10.1097/MOH.0b013e3280dce543. [DOI] [PubMed] [Google Scholar]
- 194.Chen YP, O'Toole TE, Ylanne J, Rosa JP, Ginsberg MH. A point mutation in the integrin beta 3 cytoplasmic domain (S752–>P) impairs bidirectional signaling through alpha IIb beta 3 (platelet glycoprotein IIb-IIIa). Blood. 1994;84:1857–1865. [PubMed] [Google Scholar]
- 195.Abtahian F, Bezman N, Clemens R, et al. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–6949. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jakus Z, Fodor S, Abram CL, Lowell CA, Mocsai A. Immunoreceptor-like signaling by beta 2 and beta 3 integrins [review]. Trends Cell Biol. 2007;17:493–501. doi: 10.1016/j.tcb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 197.Coller BS. Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics [review]. J Clin Invest. 1997;99:1467–1471. doi: 10.1172/JCI119307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Weiss HJ. The discovery of the antiplatelet effect of aspirin: a personal reminiscence [review]. J Thromb Haemost. 2003;1:1869–1875. doi: 10.1046/j.1538-7836.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 199.Mustard JF. Aspirin [review]. J Thromb Haemost. 2004;2:336–337. doi: 10.1111/j.1538-7933.2004.0615b.x. [DOI] [PubMed] [Google Scholar]
- 200.Quick AJ. Bleeding time after aspirin ingestion. Lancet. 1968;1:50. doi: 10.1016/s0140-6736(68)90046-9. [DOI] [PubMed] [Google Scholar]
- 201.Marcus AJ. The role of prostaglandins in platelet function [review]. Prog Hematol. 1979;11:147–171. [PubMed] [Google Scholar]
- 202.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 203.Mann CC, Plummer ML. The Asprin Wars: Money, Medicine and 100 Years of Rampant Competition. Boston: Harvard Business School Press; 1993. [Google Scholar]
- 204.Collabroative Group, ISIS-2 (Second International Study of Infarct Survival) Randomised trial of intravenous streptokinase, oral apsirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 205.Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis [review]. Semin Thromb Hemost. 2005;31:174–183. doi: 10.1055/s-2005-869523. [DOI] [PubMed] [Google Scholar]
- 206.Coller BS. Blockade of platelet GPIIb/IIIa receptors as an antithrombotic strategy [review]. Circulation. 1995;92:2373–2380. doi: 10.1161/01.cir.92.9.2373. [DOI] [PubMed] [Google Scholar]
- 207.George JN, Caen JP, Nurden AT. Glanzmann's thrombasthenia: the spectrum of clinical disease [review]. Blood. 1990;75:1383–1395. [PubMed] [Google Scholar]
- 208.Coller BS, Folts JD, Smith SR, Scudder LE, Jordan R. Abolition of in vivo platelet thrombus formation in primates with monoclonal antibodies to the platelet GPIIb/IIIa receptor: correlation with bleeding time, platelet aggregation and blockade of GPIIb/IIIa receptors. Circulation. 1989;80:1766–1774. doi: 10.1161/01.cir.80.6.1766. [DOI] [PubMed] [Google Scholar]
- 209.Gold HK, Coller BS, Yasuda T, et al. Rapid and sustained coronary artery recanalization with combined bolus injection of recombinant tissue-type plasminogen activator and monoclonal anti-platelet GPIIb/IIIa antibody in a dog model. Circulation. 1988;77:670–677. doi: 10.1161/01.cir.77.3.670. [DOI] [PubMed] [Google Scholar]
- 210.Phillips DR, Scarborough RM. Clinical pharmacology of eptifibatide. Am J Cardiol. 1997;80:11B–20B. doi: 10.1016/s0002-9149(97)00572-9. [DOI] [PubMed] [Google Scholar]
- 211.Barrett JS, Murphy G, Peerlinck K, et al. Pharmacokinetics and pharmacodynamics of MK-383, a selective non-peptide platelet glycoprotein-IIb/IIIa receptor antagonist, in healthy men. Clin Pharmacol Ther. 1994;56:377–388. doi: 10.1038/clpt.1994.152. [DOI] [PubMed] [Google Scholar]
- 212.Coller BS, Scudder LE, Beer J, et al. Monoclonal antibodies to platelet GPIIb/IIIa as antithrombotic agents [review]. Ann NY Acad Sci. 1991;614:193–213. doi: 10.1111/j.1749-6632.1991.tb43703.x. [DOI] [PubMed] [Google Scholar]
- 213.Topol EJ, Lincoff AM, Kereiakes DJ, et al. Multi-year follow-up of abciximab therapy in three randomized, placebo-controlled trials of percutaneous coronary revascularization. Am J Med. 2002;113:1–6. doi: 10.1016/s0002-9343(02)01145-2. [DOI] [PubMed] [Google Scholar]
- 214.Curran MP, Keating GM. Eptifibatide: a review of its use in patients with acute coronary syndromes and/or undergoing percutaneous coronary intervention [review]. Drugs. 2005;65:2009–2035. doi: 10.2165/00003495-200565140-00007. [DOI] [PubMed] [Google Scholar]
- 215.Menozzi A, Merlini PA, Ardissino D. Tirofiban in acute coronary syndromes [review]. Expert Rev Cardiovasc Ther. 2005;3:193–206. doi: 10.1586/14779072.3.2.193. [DOI] [PubMed] [Google Scholar]
- 216.The EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997;336:1689–1696. doi: 10.1056/NEJM199706123362401. [DOI] [PubMed] [Google Scholar]
- 217.Aster RH, Curtis BR, Bougie DW, et al. Thrombocytopenia associated with the use of GPIIb/IIIa inhibitors: position paper of the ISTH working group on thrombocytopenia and GPIIb/IIIa inhibitors. J Thromb Haemost. 2006;4:678–679. doi: 10.1111/j.1538-7836.2006.01829.x. [DOI] [PubMed] [Google Scholar]
- 218.Aster RH, Bougie DW. Drug-induced immune thrombocytopenia [review]. N Engl J Med. 2007;357:580–587. doi: 10.1056/NEJMra066469. [DOI] [PubMed] [Google Scholar]
- 219.De LG, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials [review]. JAMA. 2005;293:1759–1765. doi: 10.1001/jama.293.14.1759. [DOI] [PubMed] [Google Scholar]
- 220.Plosker GL, Lyseng-Williamson KA. Clopidogrel: a review of its use in the prevention of thrombosis [review]. Drugs. 2007;67:613–646. doi: 10.2165/00003495-200767040-00013. [DOI] [PubMed] [Google Scholar]
- 221.Ahrens I, Smith BK, Bode C, Peter K. Direct thrombin inhibition with bivalirudin as an antithrombotic strategy in general and interventional cardiology [review]. Expert Opin Drug Metab Toxicol. 2007;3:609–620. doi: 10.1517/17425225.3.4.609. [DOI] [PubMed] [Google Scholar]
- 222.Van't Hof AW, Ten Berg J, Heestermans T, et al. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME2): a multicentre, double-blind, randomized controlled trial. Lancet. 372:537–546. doi: 10.1016/S0140-6736(08)61235-0. [DOI] [PubMed] [Google Scholar]
- 223.Ellis SG, Tendera M, de Belder MA, et al. Facilitated PCI in patients with ST elevation myocardial infarction. N Eng J Med. 358:2205–2217. doi: 10.1056/NEJMoa0706816. [DOI] [PubMed] [Google Scholar]
- 224.Thiele H, Schindler K, Friedenberger J, et al. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation. 2008;118:49–57. doi: 10.1161/CIRCULATIONAHA.107.747642. [DOI] [PubMed] [Google Scholar]
- 225.Wohrle J, Grebe OC, Nusser T, et al. Reduction of major adverse cardiac events with intracoronary compared with intravenous bolus application of abciximab in patients with acute myocardial infarction or unstable angina undergoing coronary angioplasty. Circulation. 2003;107:1840–1843. doi: 10.1161/01.CIR.0000066852.98038.D1. [DOI] [PubMed] [Google Scholar]
- 226.Gibson CM, Zorkun C, Kunadian V. Intracoronary administration of abciximab in ST-elevation myocardial infarction. Circulation. 2008;118:6–8. doi: 10.1161/CIRCULATIONAHA.108.780718. [DOI] [PubMed] [Google Scholar]
- 227.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 228.Di MC, Dudek D, Piscione F, et al. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet. 2008;371:559–568. doi: 10.1016/S0140-6736(08)60268-8. [DOI] [PubMed] [Google Scholar]
- 229.Mandava P, Thiagarajan P, Kent TA. Glycoprotein IIb/IIIa antagonists in acute ischaemic stroke: current status and future directions [review]. Drugs. 2008;68:1019–1028. doi: 10.2165/00003495-200868080-00001. [DOI] [PubMed] [Google Scholar]
- 230.Cox D. Oral GPIIb/IIIa antagonists: what went wrong [review]? Curr Pharm Des. 2004;10:1587–1596. doi: 10.2174/1381612043384673. [DOI] [PubMed] [Google Scholar]
- 231.Quinn MJ, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa inhibitors: recognition of a two-edged sword [review]? Circulation. 2002;106:379–385. doi: 10.1161/01.cir.0000019581.22812.b2. [DOI] [PubMed] [Google Scholar]
- 232.Hantgan RR, Stahle MC, Connor JH, Connor RF, Mousa SA. AlphaIIbbeta3 priming and clustering by orally active and intravenous integrin antagonists. J Thromb Haemost. 2007;5:542–550. doi: 10.1111/j.1538-7836.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 233.Poon MC. Clinical use of recombinant human activated factor VII (rFVIIa) in the prevention and treatment of bleeding episodes in patients with Glanzmann's thrombasthenia [review]. Vasc Health Risk Manag. 2007;3:655–664. [PMC free article] [PubMed] [Google Scholar]
- 234.Bellucci S, Devergie A, Gluckman E, et al. Complete correction of Glanzmann's thrombasthenia by allogeneic bone marrow transplantation. Br J Haematol. 1985;59:635–641. doi: 10.1111/j.1365-2141.1985.tb07358.x. [DOI] [PubMed] [Google Scholar]
- 235.Connor P, Khair K, Liesner R, et al. Stem cell transplantation for children with Glanzmann thrombasthenia. Br J Haematol. 2008;140:568–571. doi: 10.1111/j.1365-2141.2007.06890.x. [DOI] [PubMed] [Google Scholar]
- 236.Flood VH, Johnson FL, Boshkov LK, et al. Sustained engraftment post bone marrow transplant despite anti-platelet antibodies in Glanzmann thrombasthenia. Pediatr Blood Cancer. 2005;45:971–975. doi: 10.1002/pbc.20365. [DOI] [PubMed] [Google Scholar]
- 237.Fang J, Hodivala-Dilke K, Johnson BD, et al. Therapeutic expression of the platelet-specific integrin, αIIbβ3, in a murine model for Glanzmann thrombasthenia. Blood. 2005;106:2671–2679. doi: 10.1182/blood-2004-12-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wilcox DA, White GC. Gene therapy for platelet disorders: studies with Glanzmann's thrombasthenia [review]. J Thromb Haemost. 2003;1:2300–2311. doi: 10.1046/j.1538-7836.2003.00476.x. [DOI] [PubMed] [Google Scholar]
- 239.Zingman LV, Park S, Olson TM, Alekseev AE, Terzic A. Aminoglycoside-induced translational read-through in disease: overcoming nonsense mutations by pharmacogenetic therapy [review]. Clin Pharmacol Ther. 2007;81:99–103. doi: 10.1038/sj.clpt.6100012. [DOI] [PubMed] [Google Scholar]
- 240.Harrison P, Frelinger AL, III, Furman MI, Michelson AD. Measuring antiplatelet drug effects in the laboratory [review]. Thromb Res. 2007;120:323–336. doi: 10.1016/j.thromres.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 241.Mitchell WB, Li J, Murcia M, et al. Mapping early conformational changes in alphaIIb and beta3 during biogenesis reveals a potential mechanism for alphaIIbbeta3 adopting its bent conformation. Blood. 2007;109:3725–3732. doi: 10.1182/blood-2006-11-058420. [DOI] [PMC free article] [PubMed] [Google Scholar]