Abstract
We have identified at least 2 highly promiscuous major histocompatibility complex class II T-cell epitopes in the Fc fragment of IgG that are capable of specifically activating CD4+CD25HiFoxP3+ natural regulatory T cells (nTRegs). Coincubation of these regulatory T-cell epitopes or “Tregitopes” and antigens with peripheral blood mononuclear cells led to a suppression of effector cytokine secretion, reduced proliferation of effector T cells, and caused an increase in cell surface markers associated with TRegs such as FoxP3. In vivo administration of the murine homologue of the Fc region Tregitope resulted in suppression of immune response to a known immunogen. These data suggest that one mechanism for the immunosuppressive activity of IgG, such as with IVIG, may be related to the activity of regulatory T cells. In this model, regulatory T-cell epitopes in IgG activate a subset of nTRegs that tips the resulting immune response toward tolerance rather than immunogenicity.
Introduction
Induction of specific tolerance to self- or to foreign antigens is the goal of therapy for autoimmunity, transplant rejection, and allergy; unresponsiveness is also desirable in the context of therapy with potentially immunogenic autologous proteins (such as factor VIII) and nonautologous proteins (such as botulinum toxin). Until recently, therapeutic tolerance induction relied on broad-spectrum interventions that resulted in widespread effects on immunity, rather than on strategies directed toward restoring a balance between effector immune responses and regulatory immune responses to a specific protein.
Natural means of controlling autoimmune responses (natural tolerance) and of inducing tolerance (adaptive tolerance) are known to exist. For example, suppression of inflammation by CD4+CD25HiFoxP3+ natural regulatory T cells (nTRegs) is an important mechanism of effector T-cell regulation, and may represent one of the critical forms of autoregulatory response to self-antigens. Upon antigen-specific activation through their TCR, nTRegs are able to suppress bystander effector T-cell responses to unrelated antigens by contact-dependent and -independent mechanisms. Adaptive TReg (aTReg) induction is one outcome of a T-regulatory immune response, and sustained tolerance (to grafts, to allergens, and to autologous proteins) probably requires the existence of aTRegs with the same antigen specificity as the self-reactive T cells.1–3 Adaptive TRegs are also known as induced TRegs (iTRegs). However, despite extensive efforts and with few exceptions,4,5 the antigen specificity of nTRegs is still unknown.
Natural TRegs may also control immune responses to autologous proteins to which central tolerance may not exist. For example, it has been suggested that T cells need to be rendered tolerant to the variable regions of antibodies that have undergone somatic hypermutation.6 To date, no natural TRegs that respond to IgG epitopes have been identified nor have adaptive TRegs to hypervariable IgG regions been identified.
We scanned the Fc region of IgG for natural TReg epitopes that may explain (1) tolerance to antibody variable regions and (2) the induction of tolerance to selected antigens after administration of therapeutic immunoglobulins or Ig fusion proteins.7,8 Using peripheral blood mononuclear cells (PBMCs) from individuals allergic to either house dust mite Dermatophagoides pteronyssinus (HDM) or to the major birch tree allergen, Bet v 1141-155, we evaluated the effect of these IgG TReg epitopes (“Tregitopes”) in a standard 2-step “bystander suppression” assay. We explored whether the Tregitopes induced aTReg to Bet v 1141-155 using HLA DR*1501 tetramers to the Bet v 1141-155 epitope. We also coadministered HDM lysate and Tregitopes to HLA transgenic mice and observed suppression of immune response to HDM as measured by whole-antibody enzyme-linked immunosorbent assay (ELISA) and IL-4 enzyme-linked immunosorbent spot (ELISpot). Further studies need to be performed, but these Tregitopes may provide an explanation for the limited immunogenicity of Fc fusion proteins, the expansion of CD4+CD25Hi regulatory T cells after administration of therapeutic IVIG,8 and the observed effect of immunoglobulin therapy on autoimmune diseases and other medical conditions.
Methods
Computational epitope mapping
To determine whether TReg epitopes exist in immunoglobulin G, we used the EpiMatrix and ClustiMer epitope-mapping algorithms (EpiVax) to scan the complete amino acid sequence of human IgG sequences derived from the human IgG germ-line heavy and light chain sequences (GenBank accession J00228 and J00241, respectively9). The EpiMatrix system is a suite of epitope-mapping tools (including EpiMatrix, ClustiMer, and BlastiMer) that has been validated over the course of more than a decade, both in vitro and in vivo (for example, see De Groot et al10 and Koita et al11). For this evaluation of IgG sequences, we used EpiMatrix to identify 9-mer peptides likely to bind to at least 1 of 8 common class II alleles (DRB1*0101, *0301, *0401, *0701, *0801, *1101, *1301, and *1501).10 Then, using the ClustiMer algorithm, we mapped the EpiMatrix motif matches (for these 8 alleles) along the length of IgG and calculated the density of motifs for the panel of 8 HLA alleles. Epitope-dense regions, or clusters of HLA-binding potential, are usually highly immunogenic in vitro and in vivo.12,13
Peptide synthesis
Peptides were synthesized using solid-phase Fmoc chemistry. Automated production was carried out on Gilson, Applied Biosystems, and Advanced Chemtech peptide synthesizers, initially at SynPep (Dublin, CA) and subsequently at New England Peptide (Gardner, MA). Tregitope 289 was amidated on the C-terminus. Peptides were purified via reverse phase high-performance liquid chromatography (HPLC) to more than 80% purity and characterized by mass spectrometry.
HLA-binding assay
Soluble MHC-binding assays were performed as previously described.11,14 Nonbiotinylated test peptide was suspended in a 96-well polypropylene plate for binding measurements at concentrations ranging from 0.1 μM to 400 μM in triplicate wells. Purified recombinant HLA class II monomers (Benaroya Research Institute, Seattle, WA), in a solution containing 1 mM PefaBloc, 0.75% n-Octyl-B-D-glucopyranoside in 150 mM citrate-phosphate buffer (pH 5.4), were then added to a final concentration of 200 ng/well. The 96-well plates were incubated at 37°C in 5% CO2 for 45 minutes. Biotinylated influenza hemagglutinin peptide 307-319 (a known HLA DRB*0101 binder) was added to a final concentration of 0.1 μM per well and incubated at 37°C for 20 hours. The contents of each well were then added to a 96-well high-binding ELISA plate previously coated with the antihuman HLA-DR L243 capture antibody (Becton Dickinson, San Jose, CA) and incubated at 4°C for 20 hours. The plate was then developed by addition of 100 μL europium-labeled streptavidin at 10 μg/mL (Perkin-Elmer, Waltham, MA) and 100 μL enhancement buffer (Perkin-Elmer) to each well. After incubation in the dark at room temperature for 15 to 30 minutes, fluorescence was measured on a Wallac Victor 3-V time-resolved fluourometer (Turku, Finland). Binding curves were fitted by nonlinear regression analysis and concentration that inhibits binding by 50% (IC50) values calculated (SigmaPlot, San Jose, CA). Binding strength was categorized based on comparisons with known peptides; an IC50 of 250 μM or more is indicative of a weak or nonbinding interaction.
PBMC isolation
Peripheral blood samples were obtained from 1 of 3 sources for this study. Normal donor blood was purchased by EpiVax from the Rhode Island Blood Center in Providence, RI. Citrated peripheral blood was obtained from birch pollen–allergic patients recruited at Hospital Béclère (Clamart, France), after informed consent was obtained according to the Declaration of Helsinki and under the approval of the Comité Consultatif pour la Protection des Patients dans la Recherche Biomédicale (CCPPRB, Béclère). Protocols for blood draws observed US federal and French guidelines and were approved by the respective institutional review boards (CCPPRB and Independent Review Consulting [IRC], Corte Madera, CA). PBMCs were isolated by Ficoll density gradient centrifugation, according to the Accuspin protocol (Sigma-Aldrich, St Louis, MO). Cryopreserved PBMCs isolated from dust mite–allergic individuals were purchased from Cellular Technologies (Cleveland, OH).
TReg depletion
To evaluate the role of CD4+CD25Hi cells in the indirect suppression assay, cells were surface stained with anti-CD4 and anti-CD25 fluorescence-labeled antibodies (BD Biosciences, San Jose, CA) and run on a 3-laser fluorescence-activated cell sorting (FACS) Aria high-speed cell sorter (BD Biosciences). CD4+ cells with a CD25 mean fluorescence intensity greater than 100 were sorted and discarded. Cells from all remaining gates were combined and used in the assay.
Suppression assays
HDM lysate.
To test for direct (adaptive) suppression of immune response, sorted and unsorted PBMCs were plated at 5 × 106 cells/well in a 12-well tissue culture dish (Corning, Corning, NY) and stimulated with 2 μg/mL D pteronyssinus (HDM) lysate (Greer Labs, Lenoir, NC), HDM lysate with 10 μg/mL Tregitopes, or no peptide and placed in a 37°C incubator with 5% CO2. After 24 hours, 10 U/mL IL-2 and 20 ng/mL IL-7 (R&D Systems, Minneapolis, MN) were added to each of the wells. The cells were fed every 2 days by half media replacement containing the same concentration of cytokines. On day 8, all the PBMCs were collected, washed, and restimulated with 2 μg/mL HDM lysate. On day 8, all the PBMCs were collected, washed, and restimulated with 2 μg/mL HDM lysate in IFN-γ or IL-4 ELISpot kits (Mabtech, Nacka Strand, Sweden) according to manufacturer's instructions. Culture supernatants were analyzed by Searchlight multiplex ELISA assay (Pierce, Woburn, MA).
C3d peptides.
Suppression was also tested with a second antigenic system, C3d. The complement component C3d has recently been established as an autologous T-helper cell target.15 Human PBMCs were cultured for 8 days as described for HDM lysate with 3 different sets of stimuli: 1 control and 2 experimental. The control was a pool of immunogenic C3d peptides alone. The 2 stimulation groups were (1) a pool of immunogenic C3d peptides with hTregitope 167, and (2) a pool of immunogenic C3d peptides with hTregitope 134. Cells were harvested and washed with PBS, and 2 × 105 cells/well were plated into a 96-well plate and restimulated with the immunogenic peptide pool alone, the immunogenic peptide pool and Tregitopes, or no peptide (negative control) for 65 hours. Supernatants were analyzed by multiplexed ELISA analysis.
Tetramer studies.
PBMCs from 3 different HLA-DRB1*1501 birch pollen–allergic donors were stimulated weekly with autologous PBMCs pulsed with Bet v 1141-155 peptide (10 μg/mL) with or without Tregitopes (10 μg/mL). After the third stimulation, cells were stained with the HLA-DRB1*1501 Bet v 1141-155 tetramer for 45 minutes. The cytokine secretion profile of CD4+ Bet v 1141-155 tetramer–positive cells was subsequently determined using a cytokine surface-capture assay.
To establish the phenotypic profile of Bet v 1141-155–specific T cells, PBMCs from an HLA-DRB1*1501 birch pollen–allergic donor were stimulated weekly with autologous PBMCs pulsed with Bet v 1141-155 peptide (10 μg/mL) with or without Tregitopes at 10 μg/mL. After the second stimulation, cells were stained with the HLA-DRB1*1501 Bet v 1141-155 tetramer and with a combination of antibodies directed against the indicated surface markers. For control purposes, a subset of cells was also stained with irrelevant tetramers. PBMCs (106) were stained with Fl-labeled antibodies as described16 according to manufacturers' protocols (eBioscience [San Diego, CA] and BD Biosciences). All stained cells were run on a FACSCalibur (BD Biosciences), and data were analyzed using FlowJo software (TreeStar, Eugene, OR). In addition, cytokine secretion was measured by ELISA using PC-conjugated anti–IL-5 and anti–IL-10 antibodies (Cytokine Secretion Assay kit from Miltenyi Biotech, Auburn, CA).
Coadministration in HLA transgenic mice
We used HLA-DRB1*0401 transgenic mice produced by Dr Chella David (Mayo Clinic, Rochester, MN)17 for the studies described here. The lines were generated by coinjection of DR alpha and DRB1*0401 beta gene fragments into mouse embryos to produce knockin transgenes. The DR4 genes were subsequently introduced individually into class II–negative H2Abo mice to produce strains expressing class II HLA and no mouse class II MHC, as determined by FACS analysis.
HLA DR4 transgenic mice (4- to 6-week-old females) were injected weekly 3 times subcutaneously (scruff of the neck) with (1) 50 μg HDM alone, (2) 50 μg HDM and 50 μg murine homologue of Tregitope 289, or (3) PBS sham control. In a fourth arm, mice were first sensitized to HDM through 3 weekly injections of 50 μg HDM and then treated with coinjections of HDM (50 μg) and Tregitope 289 (50 μg). One week after the final injections, mice were killed, and splenocytes harvested and plated in murine IL-4 ELISpot plates as described in “HDM lysate.” The following were added to the plated cells (in triplicate): PBS (no-stimulus control), HDM lysate, purified HDM antigen DerP2, or PHA.
Serum was obtained by cardiac puncture. Quantification of IgG antibody to HDM antigen was determined by antibody-capture ELISA. HDM antigen DerP2 (10 μg/mL) was placed into a 96-well microtiter plate overnight at 4°C. The plates were then washed with phosphate-buffered saline containing 0.05% Tween 20 (PBST) and blocked for 3 hours at room temperature with 5% fetal bovine serum (FBS; Gibco, Carlsbad, CA) in PBS. Serial dilutions of sera in 0.5% FBS/PBS were added to the plates and incubated at room temperature for 2 hours. The microtiter plates were then washed with PBST and 100 μL goat anti–mouse IgG (gamma-chain– specific) conjugated to horseradish peroxidase (Southern Biotechnology Associates, Birmingham, AL) diluted 1:10 000 in 0.5% FBS/PBS was added to each well. Microtiter plates were washed in PBST and then developed with 3,3′,5,5′-tetramethylbenzidine (TMB; Moss, Pasadena, MD). Absorbances were read at a wavelength of 450 nm measured on a Wallac Victor 3-V time-resolved fluourometer. Correction for optical imperfections in the plate was made by subtraction of intensities at 540 nm from the 450-nm values. Response to positive control PHA was robust after both immunization conditions and both assay readouts (data not shown). The animal studies protocols were approved by Biomedical Research Models (BRM docket 07-11) and were carried out under Office of Laboratory Animal Welfare assurance number A-4234-01 to BRM.
Results
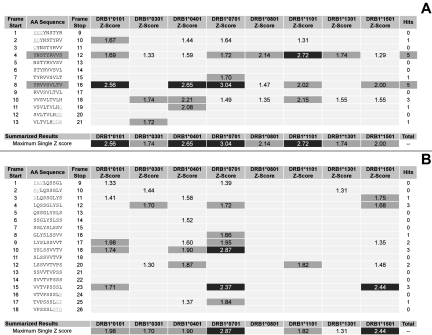
Using EpiMatrix and ClustiMer, we analyzed human Fc and defined 2 clusters of MHC-binding motifs. Human Tregitope 289 (hTregitope 289, Figure 1A) located in the Fc sequence of IgG, contains 2 frames with EpiBars, features characteristic of promiscuous epitopes. Interestingly, the predicted epitope is located in the region known to be involved in FcR binding and is conserved in all IgG allotypes but is not conserved between isotypes. Human Tregitope 167 (hTregitope 167, Figure 1B) does not contain a classic EpiBar; however, it does contain a high number of T-cell epitopes compared with expectation based on similar protein sequences.
Figure 1.
EpiMatrix analysis of human Tregitopes. EpiMatrix Z scores for eight common HLA alleles are shown, for each of the overlapping 9 mer frames for hTregitope 289 (A) and 167 (B). The EpiMatrix Z score indicates the potential of a 9-mer frame to bind to a given HLA allele; the strength of the score is indicated by the shading. The top 5% of scores are shaded medium and the top 1% of scores are shaded darkest. All scores in the Top 5% (Z-Score ≥ 1.64) are considered “Hits.” Scores in the top 10% (shown but not highlighted) are considered elevated; other scores are masked for simplicity. Frames containing four or more alleles scoring above 1.64 are referred to as EpiBars and are highlighted. These frames have an increased likelihood of binding to HLA. Flanking amino acids, added to stabilize the cluster during in vitro testing, are underlined.
hTregitope 289 and hTregitope 167 were synthesized and evaluated in soluble HLA-binding assays for their ability to compete with high-affinity HLA-specific biotinylated peptides. IC50 values were calculated by nonlinear regression analysis using the SigmaPlot analysis program. As predicted, the peptides bound to multiple HLA molecules with high affinity (Figure 2); Tregitope 167 bound and with high affinity to all 5 HLA class II alleles, and hTregitope 289 bound with moderate affinity to HLA DRB1*0101 and DRB1*0301, and with high affinity to HLA DRB1*0401 and 0701, but did not bind to HLA DRB1*1501.
Figure 2.
HLA binding results. The IC50 value (μM) and affinity interpretation for each of the epitopes is shown. Based on comparisons with known peptides IC50 scores lower than 25 μM indicate high binding, while an IC50 above 400 μM is indicative of a weak or nonbinding interaction.
Activation of nTRegs
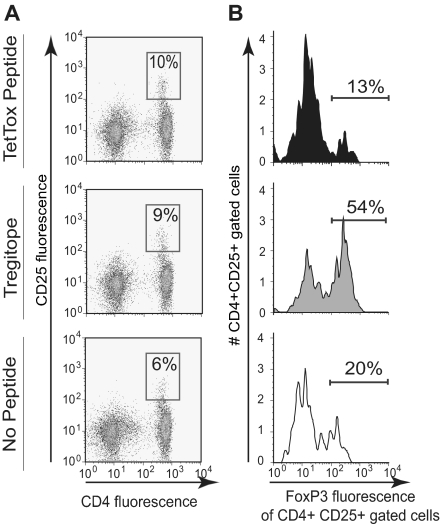
To determine whether the predicted peptides could activate a subset of nTRegs, we added the hTregitopes (167 and 289), tetanus toxin peptide, or solvent control (no peptide) to a culture of freshly isolated human PBMCs in vitro. After 4 days, the cells were collected and analyzed by flow cytometry. We found similar increases in CD25 expression in both the tetanus- and hTregitope-stimulated samples, indicating T-cell activation by both peptides (Figure 3A; results shown for hTregitope 289). However, expression of FoxP3 within the CD4+CD25Hi subset differed significantly depending on the stimulus used. Tetanus stimulation led to an expected decrease in expression of FoxP3, suggesting that the CD4+CD25Hi subset was primarily T effector cells (Teffs), whereas Tregitope stimulation led to a more than 2-fold increase in expression of FoxP3, suggesting activation of nTRegs (Figure 3B), thereby confirming the expected activity of the Tregitopes.
Figure 3.
Activation of natural Tregs in the presence of Tregitope. Human PBMC were stimulated directly in vitro for 4 days in the presence of tetanus toxin peptide (TT830-844), Tregitope, or no stimulus. A. Cells were stained extracellularly with anti-CD4 and anti-CD25 and intracellularly with FoxP3 and analyzed by flow cytometry. Incubation with Tregitope increased the percentage of CD4+CD25+FoxP3+ T cells (54%) compared to TT830-844 (13%) or no stimulus (20%). Numbers in boxes are the percentage of cells in that gate. B. From top to bottom, FoxP3 expression is shown in PBMC from the same subject following incubation with Tetanus toxin epitope (13%); hTregitope 289 (54%) and no stimulus (20%).
Induction of aTRegs
In nonallergic individuals, aTRegs suppress immune responses that lead to the generation of IL-5 and allergen-specific IgE. We therefore evaluated whether coincubation of hTregitopes and HDM lysate could suppress cytokine secretion in PBMCs from HDM-allergic individuals. PBMCs from HDM-allergic individuals were stimulated with HDM lysate with or without Tregitopes for 8 days, washed, and restimulated with HDM lysate alone. Supernatants were collected on day 10 and cytokine secretion was analyzed by ELISA (Figure 4). Expansion of aTRegs was measured by FACS.
Figure 4.
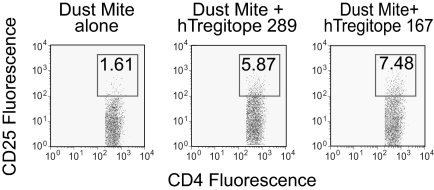
Expansion of TReg (CD4/CD25Hi cells) following coincubation with Tregitope. The expansion of CD4+CD25Hi T cells among PBMC restimulated with dust mite lysate following primary incubation of PBMC with either dust mite lysate alone or dust mite lysate and hTregitope 289, or dust mite lysate and hTregitope 167. FoxP3 was not assessed. Numbers in boxes are the percentage of cells in that gate.
As shown in Figure 4, coincubation of PBMCs with DM antigen and Tregitopes leads to expansion of CD4+CD25Hi cells; increases of 3.7-fold and 4.7-fold (over DM antigen alone) were observed after hTregitope 167 coincubation and hTregitope 289 coincubation, respectively. Suppression of immune response to a coincubated antigen, in the presence of an expanded population of CD4+CD25Hi T regulatory cells, suggests that aTRegs were induced during the coincubation.
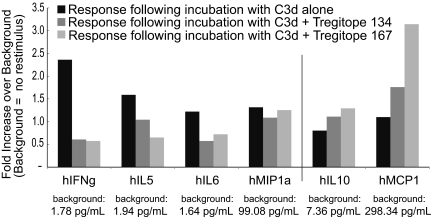
Furthermore, as shown in Figure 5, coincubation of PBMCs with a pool of immunogenic peptides derived from C3d and hTregitopes leads to suppression of IFN-γ, IL-5, and IL-6 (T effector cytokines) and induction of IL-10 and MCP-1 (cytokines associated with TReg function) compared with antigen alone. Thus we have documented the following for both hTregitope 289 and hTregitope 167: (1) the expansion of antigen-specific CD4+CD25Hi aTRegs after initial incubation with Tregitopes and DM antigen and (2) associated up-regulation of TReg cytokine secretion and down-regulation of T effector cytokine secretion.
Figure 5.
Response to immunogenic peptides in the presence of Tregitope 167. Responses to antigen restimulation following initial stimulation with a pool of immunogenic peptides derived from C3d protein (■); C3d peptides plus Tregitope 134 ( ); and C3d peptides plus Tregitope 167 (
); and C3d peptides plus Tregitope 167 ( ). Responses are shown as fold increase over background, which was no stimulus (control) in the secondary incubation. The respective baseline (background) values in pg/ml are indicated within the x-axis labels. There was no difference in levels of IL-4, TNFα, or TGFβ1 (data not shown).
). Responses are shown as fold increase over background, which was no stimulus (control) in the secondary incubation. The respective baseline (background) values in pg/ml are indicated within the x-axis labels. There was no difference in levels of IL-4, TNFα, or TGFβ1 (data not shown).
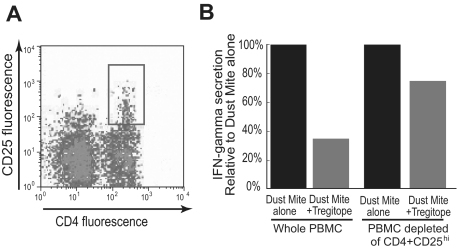
TReg depletion experiment.
As shown in the previous 2 assays, costimulation of PBMCs with HDM lysate and hTregitope leads to a significant reduction of effector cytokine secretion in a bystander suppression assay. Depleting CD4+CD25Hi cells by flow sorting before the coincubation step results in a reduction in the amount of bystander suppression, indicating that CD4+CD25Hi cells (TRegs) are essential for Tregitope-mediated suppression. (Figures 6A,B).
Figure 6.
Depletion of CD4+CD25Hi Treg population reduces suppression of Teff response. Tregitope suppression is dependent on CD4+CD25Hi T cells. A. PBMC from allergic individuals were stained with anti-CD4 and anti-CD25 antibodies and analyzed by flow cytometry. The CD4+CD25Hi subset (gate 100) was sorted and discarded. B. CD4+CD25Hi depleted and nondepleted PBMC were costimulated with HDM lysate with or without hTregitope 289. CD4+CD25Hi-depleted PBMC were less able to suppress IFN-γ than were nondepleted PBMC. The levels of IFN-γ were reduced from 33.5 pg/mL to 11.8 pg/mL in the whole PBMC and from 16.5 pg/mL to 12.4 pg/mL in the CD4+CD25Hi-depleted subset.
Phenotypic markers of aTReg expansion.
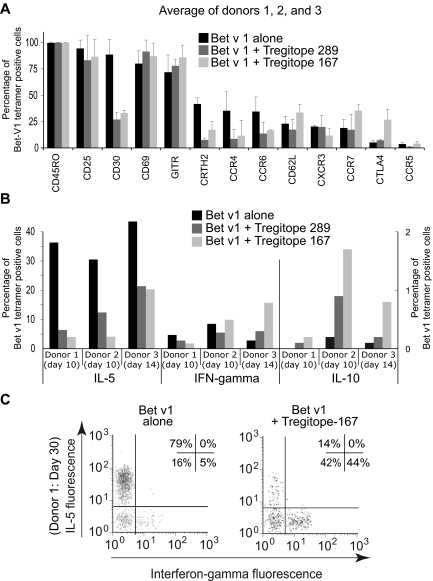
To further characterize modulation of cytokine responses by allergen-specific CD4+ T cells after hTregitope costimulation, we investigated the phenotype of the cells responding to coincubated antigens by combining MHC class II tetramer staining with a cytokine surface capture assay. PBMCs were isolated from 3 individuals with severe birch tree allergies and stimulated with an HLA DR1*1501–restricted immunodominant peptide (aa's 1141-1155) from the major birch tree allergen, Bet v 1. Representative results are shown in Figure 7; results are also summarized in Table 1.
Figure 7.
Birch pollen epitope-specific immune responses in the presence of Tregitopes. Coculture of PBMC with Bet v 1 allergen with either hTregitope 289 or hTregitope 167 leads to a Th2 to Th1/TReg shift. PBMC from three birch tree-pollen-allergic subjects were costimulated with Bet v 1141-155 peptide with or without hTregitope. A. Ten to 14 day Tregitope costimulation led Bet v 1141-155 tetramer positive CD4+ cells to decrease Th2-associated surface markers and to increase regulatory-associated surface markers GITR and CTLA4 (average of donors I, II, and III). B. Coincubation also led to a decrease of Bet v 1141-155 tetramer–positive CD4+ cells secreting IL-5 (left axis) and a modest increase in Bet v 1141-155 tetramer–positive CD4+ cells secreting IL-10 (right axis). C. PBMC from Donor I were tested under prolonged culture for 30 days; under these conditions, coculture with Tregitope 167 led to a reduction in the number of Bet v 1141-155–specific cells secreting IL-5 and to an increase in Bet v 1141-155–specific cells that were Th0 (neither IL-5– nor IFN-γ–secreting, 42%) or Th1 (IFN-γ–secreting, 44%). Numbers in boxes are the percentage of cells in the respective quadrant.
Table 1.
Summary of results for 3 HLA DR1*1501-positive birch pollen–allergic subjects
| Tregitope | Highest-scoring motif match, z-score | Sum of EpiMatrix scores to DRB1*1501 | % Suppression of IL-5 response to Bet v 1141-155 |
||
|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | |||
| hTregitope 289 | 2.0 (allele) | 2.0 | 82 | 59 | 50 |
| hTregitope 167 | 2.4 (allele) | 5.9 | 89 | 86 | 53 |
To understand the effect of Tregitope costimulation in allergy, we separated Bet v 1141-155–specific CD4+ T cells into 2 populations, one stimulated with Bet v 1141-155 peptide alone and one costimulated with Bet v 1141-155 peptide and hTregitopes 167 and 289 (coincubation with Tregitope 289 induced similar modification of the cell surface markers; data are available for only one subject, not shown). Cell surface flow cytometric analysis of each population was performed after 2 weeks of culture. As shown in Figure 7A, most Bet v 1141-155–specific CD4+ T cells were contained within the CD45RO+CD25+C62L−CCR7− (activated effector memory) T-cell population. After coincubation with hTregitope 167, a slight increase of tetramer-stained CTLA-4+ T cells was observed (Figure 7A), suggesting a possible conversion from Th2 to aTRegs in this mixed population of tetramer-stained cells. For the 2 subjects (donor 1 and donor 2) who had detailed cell surface marker phenotypes performed, an increase in CTLA-4 expression of 3- and 5-fold was observed (from 6.2% to 19.7%, and from 6.6% to 33.8% of Bet v 1141-155 CD4+ T cells, respectively, in the presence of hTregitope 167, compared with stimulation performed with Bet v 1141-155 peptide alone). Tregitope coincubation led to a relative increase in CCR7-expressing cells, suggesting that the Bet v 1141-155–specific CD4+ TRegs were central memory TRegs.18 Tregitope coincubation also led to a significant decrease in expression of CCR4, CD30, CRTH2, and CCR6, which have been shown to be associated with Th2 responses.19,20
Most Bet v 1141-155–specific CD4+ T cells from birch pollen–allergic donors are “Th2-committed” and produce high levels of IL-5 after Bet v 1141-155 stimulation, as shown in Figure 7B. In contrast, IL-5 secretion was significantly reduced when Bet v 1141-155–specific CD4+ T cells were coincubated with Bet v 1141-155 peptide and hTregitope 167 and hTregitope 289 (Table 1).
We subsequently evaluated the modulation of cytokine responses by allergen-specific CD4+ T cells after extended Tregitope costimulation. After 30 days in culture, Tregitope costimulation contributed to the development of a mixed populations of cells, some of which were no longer IFN-γ expressing or expressing IL-5 (42% of cells; Figure 7C bottom left quadrant), and some of which (44%) demonstrated a shift from a Th2 to Th1/Th0 response by Bet v 1141-155–specific CD4+ T cells (Figure 7C). This response is significant in the context of allergic subjects who have recently been shown to have possible deficits in TReg number and function.
Of note, for these HLA DR*1501–positive patients, the effect of hTregitope 167 was more pronounced (5-fold increase in aTReg induction) relative to the effect of hTregitope 289 (3-fold increase (data not shown). For donor 1, after 10 days in vitro stimulation, compared with Bet v 1141-155 peptide stimulation alone, hTregitope 167 costimulation decreased the proportion of IFN-γ–producing CD4 T cells from 4.7% to 1.8% (2.5-fold), and IL-5–producing CD4 T cells from 36.4% to 4% (9-fold). Costimulation with hTregitope 289 decreased IFN-γ–producing CD4 T cells from 4.7% to 2.8% (2-fold), and IL-5–producing CD4 T cells from 36.4% to 6.4% (6-fold). Because the composition of the responding T cells may be mixed (they were sorted for tetramer staining, not CD4+ and CD25Hi), we could observe only a slight increase in IL-10 production, which may reflect aTReg activity. This is also suggested by the change in cell surface phenotype.
The study subjects were selected to be HLA DR1*1501 because tetramers with a high affinity for the Bet v 1141-155 epitope were available.16 The presence of other class II HLA DR alleles was not evaluated in detail. hTregitope 289 did not bind to DRB1*1501 in soluble HLA-binding assays. The results of the study suggest that the effect of hTregitope 289 was diminished in these HLA DRB1*1501 subjects due to failure to bind to at least 1 of their 2 HLA class II DRB1 molecules (Table 1). In contrast, hTregitope 167 binds avidly to HLA DRB1*1501 (IC50 8.4 μM; 87% inhibition of binding at 50 μM) and was more effective at suppressing IL-5 responses in these subjects.
In vivo studies
Dust mites cause significant allergic responses in humans, and the mouse model using house dust mite (HDM) lysate is accepted as a model that is similar to humans.21 We sought to determine whether the Tregitopes could suppress the effector (IL-4 associated) immune response to HDM in vivo. As shown in Figure 8, HDM immunization (black bars) provokes a robust response by both IL-4 (Figure 8A) and anti-HDM antigen antibody titers (Figure 8B). Both of these responses are reduced (gray bars) when HDM lysate is instead coadministered with the murine homologue of hTregitope 289. Responses to HDM in sham-immunized animals were negative as expected. Humoral responses to HDM antigen (Figure 8B) and IL-4 ELISpot to HDM lysate (Figure 8A) are correlated. Figure 8A shows 38% suppression of ELISpot response in DM naive mice (P = .001) and 84% in the case of presensitized mice (P ≤ .001). Similarly, ELISA responses to HDM antigen were suppressed by 61.4% (P = .138). Whereas differences as measured by ELISpot were highly significant, the difference in antibody response did not reach statistical significance due to a single mouse outlier; were this value to be excluded, the suppression would be 87% (P = .013). This study confirmed the ability of the murine equivalents of human Tregitopes to suppress both antibody and T-cell responses to coadministered DM antigen in vivo, an effect that is amplified when more antigen-specific effector T cells are circulating, as was observed for the HDM-presensitized group of mice.
Figure 8.
In vivo studies with mTregitopes. IL-4 and antibody responses to HDM lysate and dust mite antigen are shown. Purified HDM antigen DerP2 is a component of HDM lysate. HLA DR4 transgenic mice were immunized 3 times with (1) HDM lysate alone, (2) HDM lysate plus Tregitope 289, or (3) sham control. In an additional arm, mice were first pre-sensitized to HDM lysate by 3 weekly injections followed by 3 additional weekly injections of an equal mixture of HDM lysate plus Tregitope 289. HDM lystate immunization (■) provokes a robust response by as assessed by IL-4 ELISpot (A) and anti HDM Antigen antibody titers (B). These responses are both significantly reduced (▩) when HDM lysate is coadministered with the murine homologue of Tregitope 289. Responses to HDM lysate in sham-immunized animals are negative, as expected. Antibody response (B) and IL-4 ELISpot (A) are correlated. Panel A shows 38% suppression of ELISpot response in HDM-naive mice (P < .001) and 84% in the case of HDM presensitized mice (P < .001). Panel B shows 61% suppression of HDM antigen antibody response in HDM-naive mice (P = .138); ELISA was not performed for HDM-presensitized mice. Error bars indicate SD.
Discussion
Given the many associations among tolerance, the Fc region of IgG, and the expansion of CD4+CD25Hi T cells after IVIG therapy,8 we searched for regulatory T-cell epitopes in the Fc fragment of IgG. We scanned the complete amino acid sequence of IgG using the EpiMatrix and ClustiMer epitope-mapping algorithms. Two strong epitope clusters were identified in the Fc; one was found in the CH2 domain of Fc. The hTregitope 289 cluster contained a pattern that we have observed to be common to many promiscuous and immunodominant epitopes: a characteristic bandlike pattern representing multiple HLA-binding motifs in a single 9-mer frame. Sequences that contain this bandlike pattern, now termed an “EpiBar,” include tetanus toxin825-850, influenza HA307-319, and GAD65557-567. Interestingly, the predicted hTregitope 289 is located in a region known to be involved in FcR binding, and that is conserved in all IgG allotypes but is not conserved between isotypes. hTregitope 167, which contains a cluster of putative HLA-binding motifs, is near the hinge region, and is also highly conserved among IgG sequences.
The presence of regulatory T-cell epitopes in the Fc region may explain earlier studies of tolerance demonstrating that immunization with antigens fused to the IgG or its Fc region could tolerize against the antigen. Immunoglobulin and the Fc region of IgG have been shown to be extremely effective as carriers of therapeutic moieties for nearly 3 decades. The efficacy of these proteins was originally thought to be due to their long half-life in vivo, as well as their ability to bind to Fc receptors. For example, Borel first demonstrated22 that coupling of haptens to murine IgG isotypes led to both T- and B-cell tolerance to the coupled epitopes. Baxevanis et al extended this work by examining the effect of coupling an antigen with human Fc (hFc). They found that hFc could induce tolerance in mice, but the truncated Fc domain (CH3) could not induce tolerance. They concluded that tolerogenic epitopes may exist in CH2 domain.23 Zambidis and Scott24 eventually replicated these studies, and Phillips et al7 showed that fusion of an IgG heavy chain to antigen, or administration of the Fc region in conjunction with the antigen, could induce tolerance.7,24 Further studies demonstrated that MHC class II molecules were required for induction of tolerance,25 that the IgG carrier region plays an important role in immune suppression by IgG fusion26 but FcR binding is not required,25,27 and that CD25 regulatory T cells were required for both the induction and maintenance of tolerance.28,29
The most recent example of a successful effector-Fc fusion is etanercept, a fusion protein between soluble TNF receptor type II and the Fc region of human IgG1 that has been exploited for the control of a wide range of chronic inflammatory diseases. Several additional chimeric proteins composed of immunoglobulin constant regions linked to a protein of interest, or fragment thereof, have been described.30 These molecules usually possess both the biologic activity associated with the linked molecule of interest as well as the effector function, and some other desired characteristic associated with the immunoglobulin constant region (eg, biologic stability, cellular secretion). We propose that their efficacy may also be due to the presence of regulatory epitopes in the IgG.
Thus, the tolerizing effect of immunoglobulin therapy (such as with IVIG), IgG, and Fc could be explained by the occurrence of natural T epitopes (Tregitopes) in the Fc domain of IgG. We have also shown that coadministration of the Tregitopes derived from the Fc region suppresses immune response to an antigen, and that this response is dependent, at least in part, on the presence of regulatory T cells (as defined by the cell surface markers CD4 and CD25Hi). In addition, we have shown that the binding and activity of these peptides is restricted by HLA, and that the effect is independent of Fc binding (these are synthetic peptides and are not therefore glycosylated; nor is hTregitope 167 located in the FcR-binding region).
According to current explanations of tolerance, T cells recognizing self-antigens with high affinity are deleted but autoreactive T cells with moderate affinity may escape deletion and be converted to function as “natural” regulatory T cells (nTRegs).31 These nTRegs are exported to the periphery and suppress autoimmunity. A second form of tolerance occurs in the periphery where mature T cells are converted to an “adaptive” TReg phenotype upon activation via their T-cell receptor in the presence of IL-10 and TGF-β. The role of these adaptive TRegs (aTRegs) may be to dampen effector immune responses (after the primary, vigorous immune reaction, as a means of controlling inflammation), or possibly to facilitate coexistence with beneficial symbiotic bacteria and viruses. Although we cannot at present directly investigate hTregitope 289– and hTregitope 167–specific T cells, we demonstrated (1) expansion of cells that have the phenotypic and cytokine characteristics of nTRegs after incubation (3 day) with the hTregitopes; (2) HLA-specific but promiscuous binding; (3) HLA restriction of the suppressive effect of the hTregitopes in coincubation assays; and (4) abrogation of the suppression after removal of the CD4+CD25Hi cells. Tetramers containing these Tregitopes are in development; these tetramers will permit the direct enumeration and cloning of Tregitope-specific cells for future studies.
In addition, we believe that we demonstrated the induction of aTRegs after coincubation of the hTregitopes with several different antigens (HDM, birch pollen). After coincubation for 2 weeks, we demonstrated the presence of epitope-specific cells that exhibited cell surface markers consistent with the induction of aTRegs (CTLA4, GITR). Despite the lack of demonstrated in vitro hTregitope 289 binding to HLA DRB1*1501, hTregitope 289 also suppressed IL-5 secretion by Bet v 1141-155–stained T cells from these HLA DRB1*1501–expressing subjects. This suggested that the initial stimulation of hTregitope 289–specific nTRegs (restricted by the subjects' other DRB1 allele) was followed by the induction of HLA DR1*1501–restricted, Bet v 1141-155–specific aTRegs. And finally, we have shown that coadministration of the murine version of human Tregitopes in a murine model of HDM allergy suppresses immune response to a coadministered protein in vivo. The suppression of immune response to HDM lysate that was observed in vivo has been replicated using other immunogenic proteins (immunogenic peptide from the FPX peptibody, botulinum toxin peptides; data not shown). We note that Tregitope administration in the context of presensitized mice was more effective. We believe that this may be due to the requirement for contact between circulating effector T (and B) cells and the nTRegs activated by nTregitope administration. At the time of Tregitope treatment, DM lysate–presensitized mice would have more antigen-specific T cells in the circulation, and therefore greater likelihood that an nTReg would encounter an activated T effector after subcutaneous injection of the peptide. We will explore this purported mechanism of action.
Proposed model
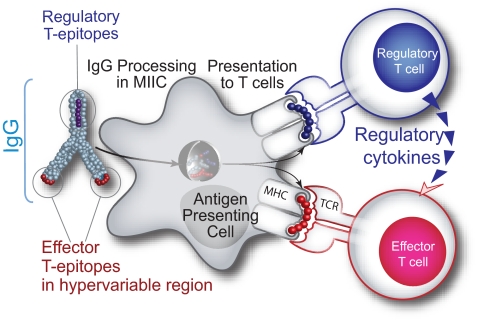
There are at least 2 hTregitopes in human IgG (289 and 167). We have identified at least 6 other putative Tregitopes in IgG (data not shown). We hypothesize that the net effect of positive “effector” epitopes derived from the antigen and negative regulatory epitopes (Tregitopes) presented on the surface of antigen-presenting cells (APCs) may influence the overall immune response to the antigen. Fc fragment of IgG (a dimer) contains 2 epitopes that, in the absence of associated antigen and danger signals such as Toll-like receptor (TLR) ligands, engage cells of a natural regulatory phenotype. When IgG and antigen (either complexed or separately) are internalized into the same APC (and TLR ligands or other “danger signals” are present), the response to effector epitopes may outnumber the Tregitopes, resulting in an initial inflammatory response. As antigen is cleared and the ratio of Ig to antigen increases, the balance tips toward tolerance, diminishing immune response and reducing further tissue damage and adverse systemic effects. (Figure 9).
Figure 9.
Hypothesized tolerizing mechanism of IgG. We have discovered conserved T-cell epitopes in IgG that engage natural regulatory T cells. We hypothesize that antibody-derived Treg epitopes (dark blue epitope) activate regulatory T cells, which leads to suppression of effector T cells that recognize effector epitopes (red epitope), like those of IgG hypervariable regions to which central tolerance does not exist. Whether this suppression is mediated by regulatory cytokines alone or by contact-dependent signaling, or both, has yet to be determined.
This model does not ignore the contribution of Fc receptors to IgG-mediated anti-inflammatory processes. Fc-gamma receptors (FcγRs) are required for rapid uptake of IgG and immune complexes into antigen-presenting cells during the initial inflammatory phase, and the inhibitory Fc receptor, FcγRIIb, increases the threshold for cell activation during the refractory phase of immune response. In our model, Tregitope activation of nTRegs would stimulate the release of cytokines such as IL-4 and IL-10 that are known to shift expression from the activating FcγRI and FcγR IIa to FcγR IIb.32
Tregitopes in the Fc region of IgG may also explain how antibody variable regions escape immune recognition after they have undergone somatic hypermutation.33 In addition, as soluble immune complexes (antibody and antigen) have been shown to traverse the placenta34 and tolerance to allergens has been shown to be transferable from mother to offspring,35 it invites the question of whether the effect of Tregitopes contained in IgG could be involved in the induction of nTRegs and adaptive TRegs in the thymus during neonatal development.
Although not directly investigated in our studies, the discovery of Tregitopes in IgG points to regulatory T-cell induction after IVIG/immunoglobulin therapy, as has been shown in several studies (see for example Ephrem et al8 and Kessel et al36). It should be noted that there are significant differences in the route of administration and the doses given in the referenced murine studies and the subcutaneous doses we administered to dust mite–sensitized mice. In future studies, the relationship between Tregitope administration (intravenous, subcutaneous) and TReg induction will be explored using tetramers, and the effect of the route of administration will be explored. Immunoglobulin therapy has been used in the context of organ transplant and graft-versus-host disease, new-onset type 1 diabetes, multiple sclerosis, and allergy.37–40 Thus, the importance of the discovery of Tregitopes in the Fc region of IgG may extend beyond an explanation of immunoglobulin-related immunosuppression. The discovery of a natural regulatory T-cell epitope in the Fc fragment of IgG may lead to a disease-management paradigm shift, allowing clinicians to safely harness the potential of natural regulatory T cells to regulate immune responses in a wide range of health conditions.
Acknowledgments
The intellectual contributions of EpiVax collaborator Paul Knopf to the development and evolution of the concepts described in this paper are gratefully acknowledged. The following individuals are recognized for their technical contributions: Christine Malboeuf, Si-Han Hai, and Daniel Rivera.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.S.D.G. and W.M. conceived the hypothesis for this research program, designed the experiments, reviewed the data, and wrote the paper; J.A.M. provided project management expertise, reviewed research results in detail, developed all of the figures for this paper, and assisted with the editing of the paper; L.M. assisted with the immunization of the mice and the design and execution of the murine studies; P.M., E.W., and L.V.O. designed and performed the birch pollen assays; and D.W.S. provided expert guidance regarding previous studies and existing literature, reviewed the data, suggested additional experiments, and assisted with the development and editing of the paper.
Conflict-of-interest disclosure: Four of the coauthors are employees of EpiVax (A.S.D.G., J.A.M., L.M., and W.M.) and 2 (A.S.D.G., W.M.) are majority stockholders. D.W.S. is a consultant for EpiVax. E.W., L.V.O., and P.M. are employees of Stallergenes. These authors recognize the presence of a potential conflict of interest and affirm that the descriptions of experiments represented in this paper are original and unbiased observations.
Correspondence: Anne S. De Groot, EpiVax, 146 Clifford Street, Providence, RI 02903; e-mail: annied@epivax.com.
References
- 1.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 2.Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med. 2004;199:1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durinovic-Belló I, Rosinger S, Olson JA, et al. DRB1*0401-restricted human T cell clone specific for the major proinsulin73-90 epitope expresses a down-regulatory T helper 2 phenotype. Proc Natl Acad Sci U S A. 2006;103:11683–11688. doi: 10.1073/pnas.0603682103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumida T, Kato T, Hasunuma T, Maeda T, Nishioka K, Matsumoto I. Regulatory T cell epitope recognized by T cells from labial salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 1997;40:2271–2273. doi: 10.1002/art.1780401225. [DOI] [PubMed] [Google Scholar]
- 6.Eyerman MC, Zhang X, Wysocki LJ. T cell recognition and tolerance of antibody diversity. J Immunol. 1996;157:1037–1046. [PubMed] [Google Scholar]
- 7.Phillips WJ, Smith DJ, Bona CA, Bot A, Zaghouani H. Recombinant immunoglobulin-based epitope delivery: a novel class of autoimmune regulators. Int Rev Immunol. 2005;24:501–517. doi: 10.1080/08830180500379648. [DOI] [PubMed] [Google Scholar]
- 8.Ephrem A, Chamat S, Miquel C, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–722. doi: 10.1182/blood-2007-03-079947. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Biotechnology Information. [Accessed August 30, 2008];GenBank. http://www.ncbi.nlm.nih.gov/Genbank/
- 10.De Groot AS, Bishop EA, Khan B, et al. Engineering immunogenic consensus T helper epitopes for a cross-clade HIV vaccine. Methods. 2004;34:476–487. doi: 10.1016/j.ymeth.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Koita OA, Dabitao D, Mahamadou I, et al. Confirmation of immunogenic consensus sequence HIV-1 T-cell epitopes in Bamako, Mali and Providence, Rhode Island. Hum Vaccin. 2006;2:119–128. doi: 10.4161/hv.2869. [DOI] [PubMed] [Google Scholar]
- 12.McMurry J, Sbai H, Gennaro ML, Carter EJ, Martin W, De Groot AS. Analyzing Mycobacterium tuberculosis proteomes for candidate vaccine epitopes. Tuberculosis (Edinb) 2005;85:95–105. doi: 10.1016/j.tube.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.McMurry JA, Gregory SH, Moise L, Rivera D, Buus S, De Groot AS. Diversity of Francisella tularensis Schu4 antigens recognized by T lymphocytes after natural infections in humans: identification of candidate epitopes for inclusion in a rationally designed tularemia vaccine. Vaccine. 2007;25:3179–3191. doi: 10.1016/j.vaccine.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Reijonen H, Kwok W. Use of HLA class II tetramers in tracking antigen-specific T-cells and mapping T-cell epitopes. Methods. 2003;29:282–288. doi: 10.1016/s1046-2023(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 15.Knopf PM, Rivera DS, Hai SH, McMurry J, Martin W, De Groot AS. Novel function of complement C3d as an autologous helper T-cell target. Immunol Cell Biol. 2008;86:221–225. doi: 10.1038/sj.icb.7100147. [DOI] [PubMed] [Google Scholar]
- 16.Van Overtvelt L, Wambre E, Maillère B, et al. Assessment of Bet v 1-specific CD4+ T cell responses in allergic and nonallergic individuals using MHC class II peptide tetramers. J Immunol. 2008;180:4514–4522. doi: 10.4049/jimmunol.180.7.4514. [DOI] [PubMed] [Google Scholar]
- 17.Pan S, Trejo T, Hansen J, Smart M, David CS. HLA-DR4 (DRB1*0401) transgenic mice expressing an altered CD4-binding site: specificity and magnitude of DR4-restricted T cell response. J Immunol. 1998;161:2925–2929. [PubMed] [Google Scholar]
- 18.Tosello V, Odunsi K, Souleimanian NE, et al. Differential expression of CCR7 defines two distinct subsets of human memory CD4+CD25+ Tregs. Clin Immunol. 2008;126:291–302. doi: 10.1016/j.clim.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- 20.D'Ambrosio D, Iellem A, Bonecchi R. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 21.Zuleger CL, Gao X, Burger MS, Chu Q, Payne LG, Chen D. Peptide induces CD4+CD25+ and IL-10+ T cells and protection in airway allergy models. Vaccine. 2005;23:3181–3186. doi: 10.1016/j.vaccine.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Borel Y. Haptens bound to self IgG induce immunologic tolerance, while when coupled to syngeneic spleen cells they induce immune suppression. Immunol Rev. 1980;50:71–104. doi: 10.1111/j.1600-065x.1980.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 23.Baxevanis CN, Ioannides CD, Reclos GJ, Papamichail M. Evidence for distinct epitopes on human IgG with T cell proliferative and suppressor function. Eur J Immunol. 1986;16:1013–1016. doi: 10.1002/eji.1830160824. [DOI] [PubMed] [Google Scholar]
- 24.Zambidis ET, Scott DW. Epitope-specific tolerance induction with an engineered immunoglobulin. Proc Natl Acad Sci U S A. 1996;93:5019–5024. doi: 10.1073/pnas.93.10.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Amine M, Melo M, Kang Y, Nguyen H, Qian J, Scott DW. Mechanisms of tolerance induction by a gene-transferred peptide-IgG fusion protein expressed in B lineage cells. J Immunol. 2000;165:5631–5636. doi: 10.4049/jimmunol.165.10.5631. [DOI] [PubMed] [Google Scholar]
- 26.Kang Y, Melo M, Deng E, Tisch R, El-Amine M, Scott DW. Induction of hyporesponsiveness to intact multi-determinant foreign protein via retroviral-mediated gene expression: the IgG scaffold is important for induction and maintenance of immunologic hyporesponsiveness. Proc Natl Acad Sci U S A. 1999;96:8609–8614. doi: 10.1073/pnas.96.15.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Amine M, Hinshaw JA, Scott DW. In vivo induction of tolerance by an Ig peptide is not affected by the deletion of FcR or a mutated IgG Fc fragment. Int Immunol. 2002;14:761–766. doi: 10.1093/intimm/dxf049. [DOI] [PubMed] [Google Scholar]
- 28.Lei TC, Scott DW. Induction of tolerance to fVIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B-cells as Ig fusion proteins. Blood. 2005;105:4865–4870. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soukhareva N, Jiang Y, Scott DW. Role of regulatory T cells in B-cell delivered gene therapy for the treatment of diabetes. Cell Immunol. 2006;240:41–46. doi: 10.1016/j.cellimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Watier H. Variability factors in the clinical response to recombinant antibodies and IgG Fc-containing fusion proteins. Expert Opin Biol Ther. 2005;5(suppl 1):S29–S36. doi: 10.1517/14712598.5.1.s29. [DOI] [PubMed] [Google Scholar]
- 31.Bluestone JA, Abbas AK. Nature reviews. Immunology. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 32.Joshi T, Ganesan LP, Cao X, Tridandapani S. Molecular analysis of expression and function of hFc RIIbl and b2 isoforms in myeloid cells. Mol Immunol. 2006;43:839–850. doi: 10.1016/j.molimm.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Reitan SK, Hannestad K. Immunoglobulin heavy chain constant regions regulate immunity and tolerance to idiotypes of antibody variable regions. Proc Natl Acad Sci U S A. 2002;99:7588–7593. doi: 10.1073/pnas.052150899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrahamson DR, Rodewald R. Evidence for the sorting of endocytic vesicle contents during the receptor-mediated transport of IgG across the newborn rat intestine. J Cell Biol. 1981;91:270–280. doi: 10.1083/jcb.91.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Telemo E, Jakobsson I, Westrom BR, Folkesson H. Maternal dietary antigens and the immune response in the offspring of the guinea-pig. Immunology. 1987;62:35–38. [PMC free article] [PubMed] [Google Scholar]
- 36.Kessel A, Ammuri H, Peri R, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–5575. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- 37.Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 2006;6:459–466. doi: 10.1111/j.1600-6143.2005.01214.x. [DOI] [PubMed] [Google Scholar]
- 38.Colagiuri S, Leong GM, Thayer Z, et al. Intravenous immunoglobulin therapy for autoimmune diabetes mellitus. Clin Exp Rheumatol. 1996;14(suppl 15):S93–S97. [PubMed] [Google Scholar]
- 39.Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy. Asthma and Immunology. J Allergy Clin Immunol. 2006;117(4) suppl:S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Soelberg Sorensen P. Intravenous polyclonal human immunoglobulins in multiple sclerosis. Neurodegener Dis. 2008;5:8–15. doi: 10.1159/000109932. [DOI] [PubMed] [Google Scholar]