Abstract
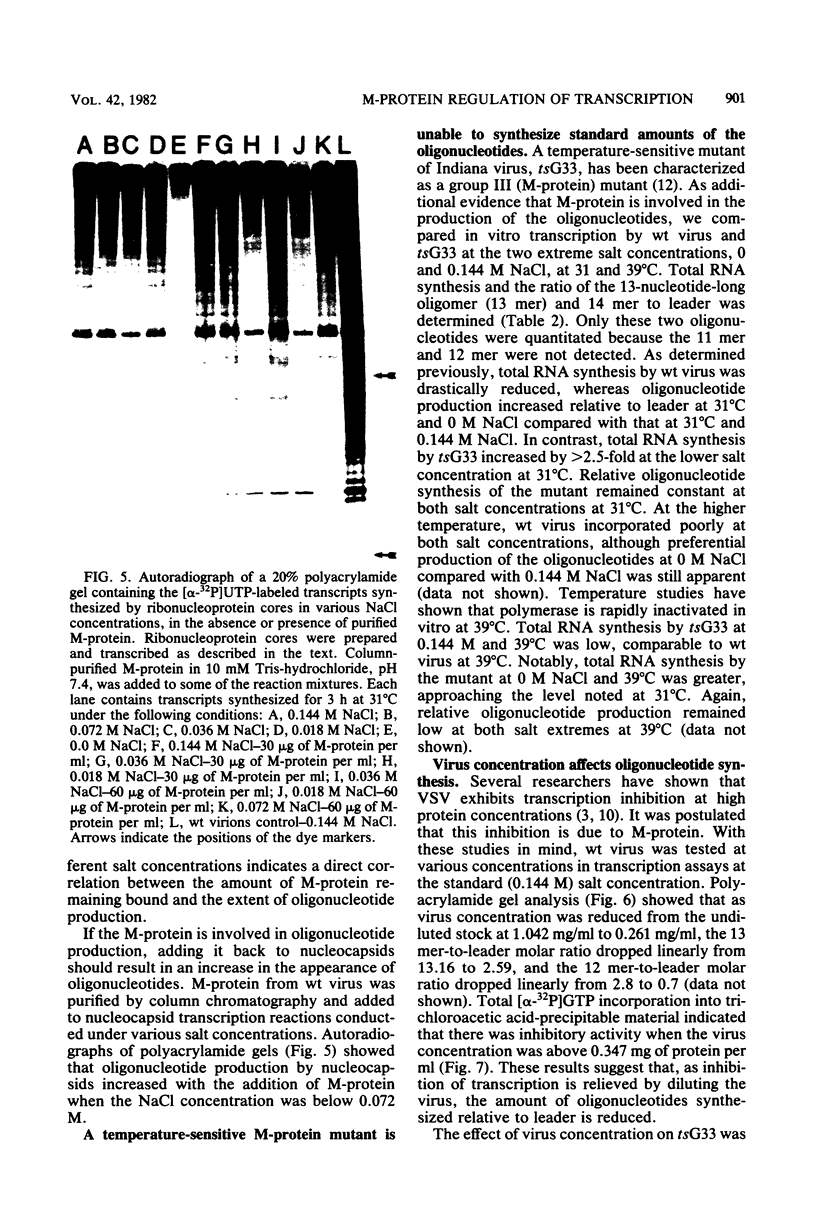
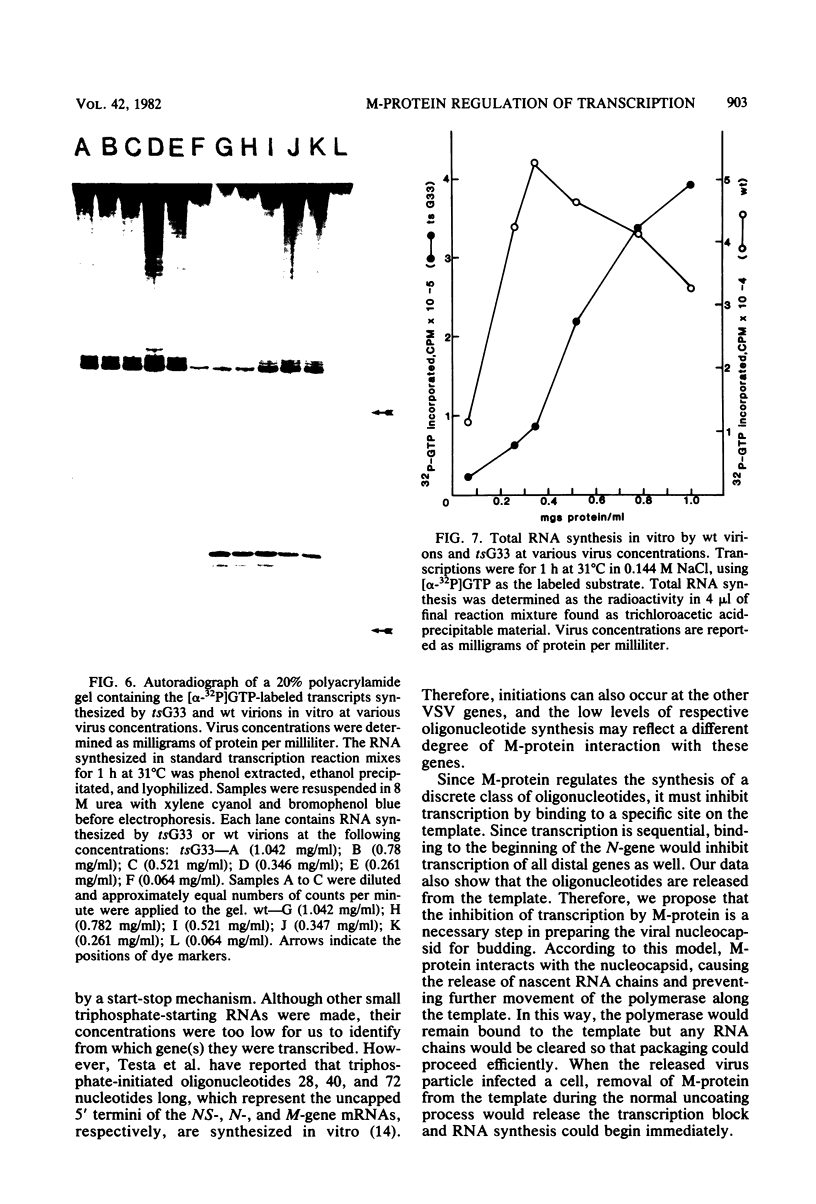
Wild-type Indiana virus transcribed four 11- to 14-nucleotide-long, 5' N-gene mRNA sequences in vitro. The amount of oligonucleotides synthesized relative to leader by wild-type virions varied inversely with the salt concentration of the transcription reaction. Reduced oligonucleotide synthesis by nucleocapsids at all salt concentrations tested and a comparison of the proteins remaining bound to the template of nucleocapsids and virions transcribed in different NaCl concentrations suggested that the matrix (M) protein regulates oligonucleotide synthesis. Examination of the transcription products synthesized in no NaCl and 0.144 M NaCl by an M-protein mutant and an increase in oligonucleotide synthesis by nucleocapsids when purified M-protein was added to transcription reactions confirmed M-protein's role in oligonucleotide synthesis. Wild-type virion mRNA synthesis was inhibited, and oligonucleotide synthesis was greater than leader synthesis at high virus concentrations. As the virus was diluted, inhibition of mRNA synthesis was relieved and oligonucleotide synthesis was reduced. The M-protein mutant tsG33 exhibited neither transcription inhibition at high virus concentrations nor the reciprocal synthesis of mRNA and the oligonucleotides seen with wild-type virions. These results are entirely consistent with the stop-start model of transcription and suggest a model for the control of transcription by M-protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Identification of promoter-proximal oligonucleotides and a unique dinucleotide, pppGpC, from in vitro transcription products of vesicular stomatitis virus. J Virol. 1981 Jul;39(1):93–103. doi: 10.1128/jvi.39.1.93-103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mellon M. G., Emerson S. U. Rebinding of transcriptase components (L and NS proteins) to the nucleocapsid template of vesicular stomatitis virus. J Virol. 1978 Sep;27(3):560–567. doi: 10.1128/jvi.27.3.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Kingsbury D. T. Inhibitor of vesicular stomatitis virus transcriptase in purified virions. Nature. 1974 Mar 1;248(5443):45–47. doi: 10.1038/248045a0. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. Identification and characterization of a group of discrete initiated oligonucleotides transcribed in vitro from the 3' terminus of the N-gene of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):889–896. doi: 10.1128/jvi.42.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]






