Abstract
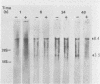
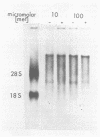
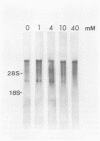
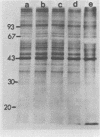
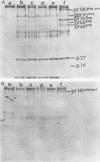
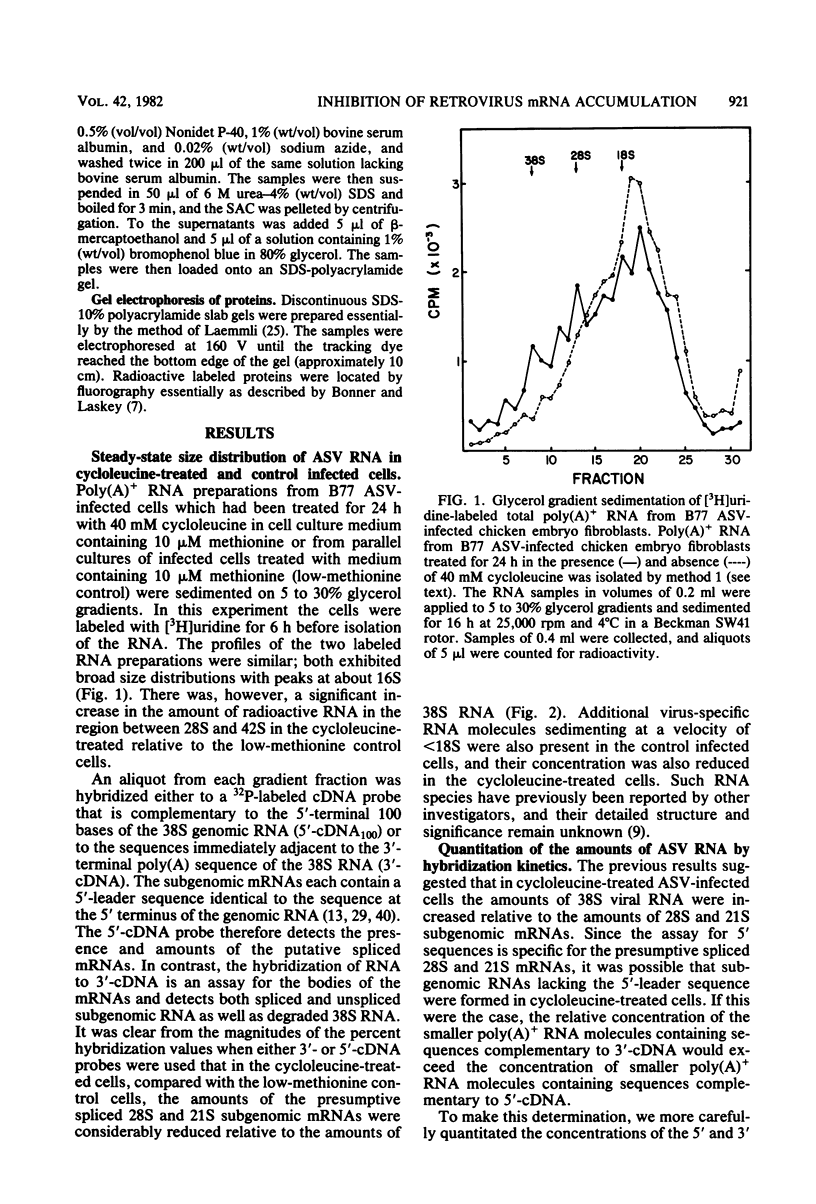
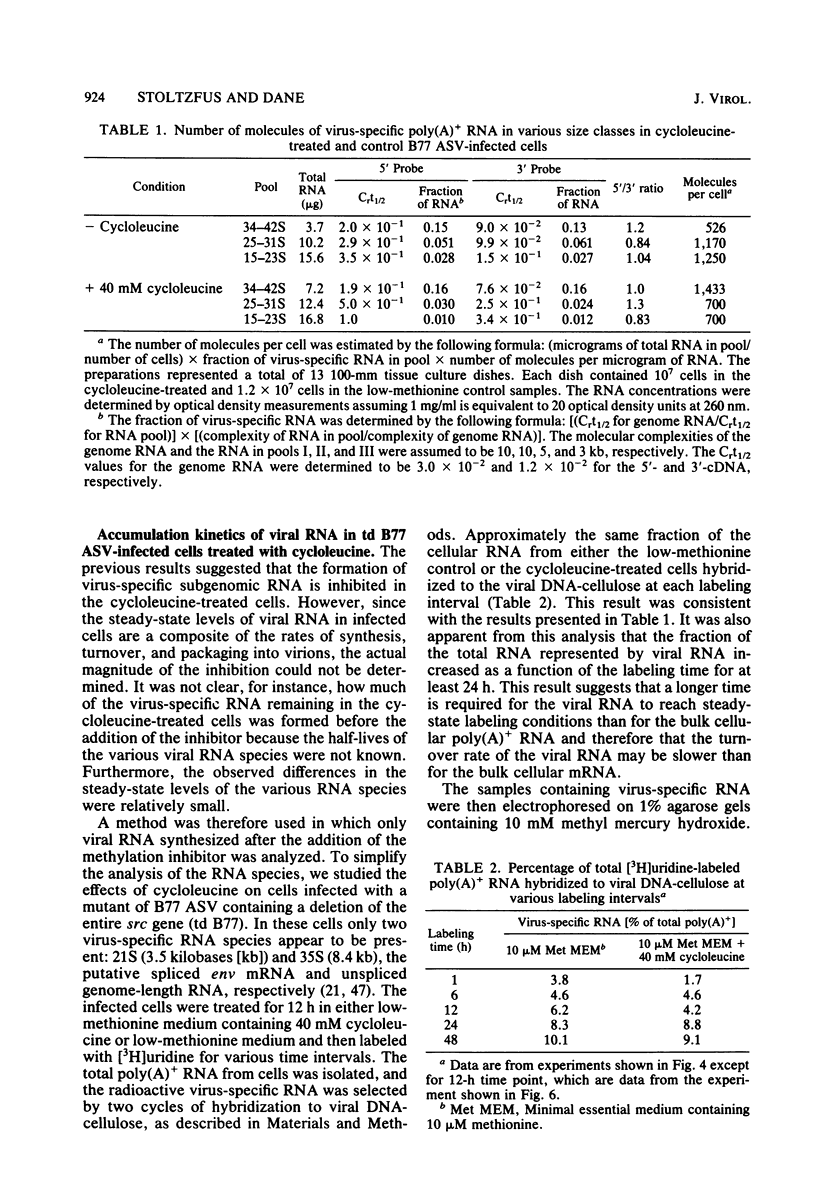
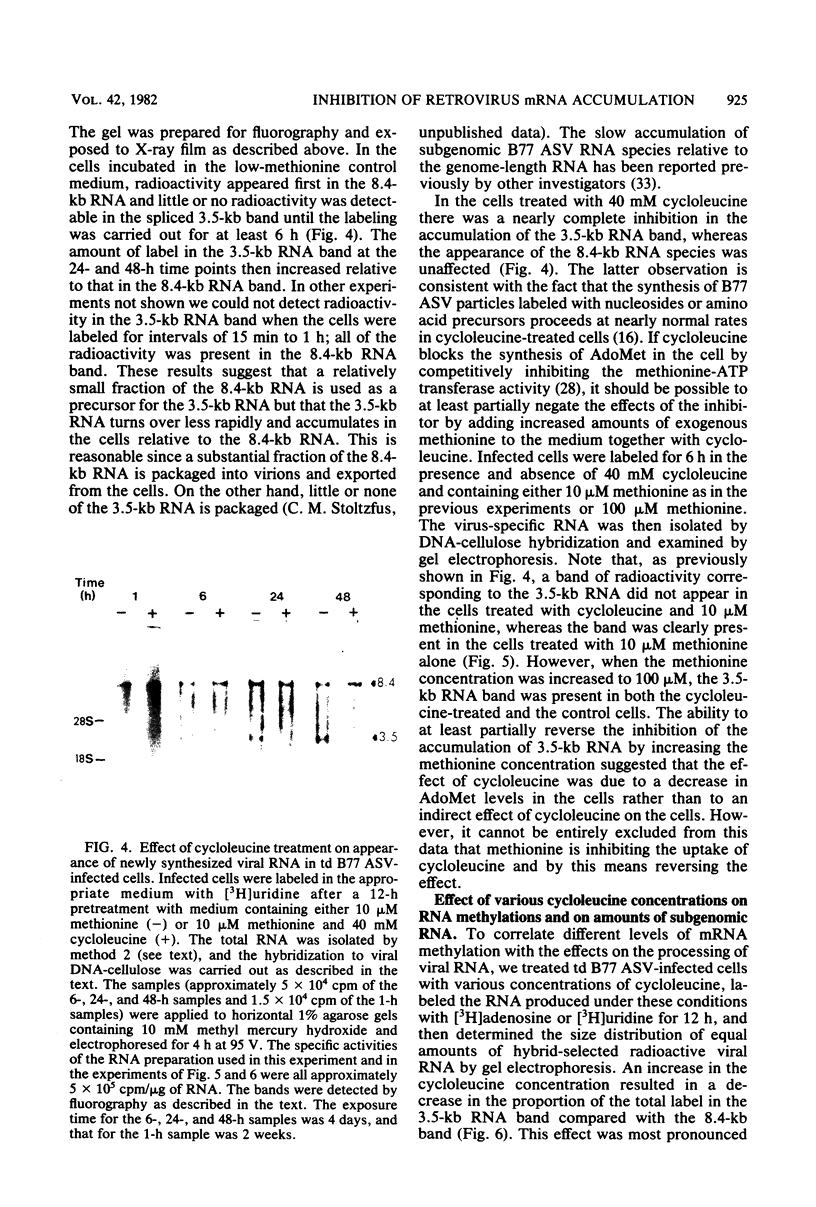
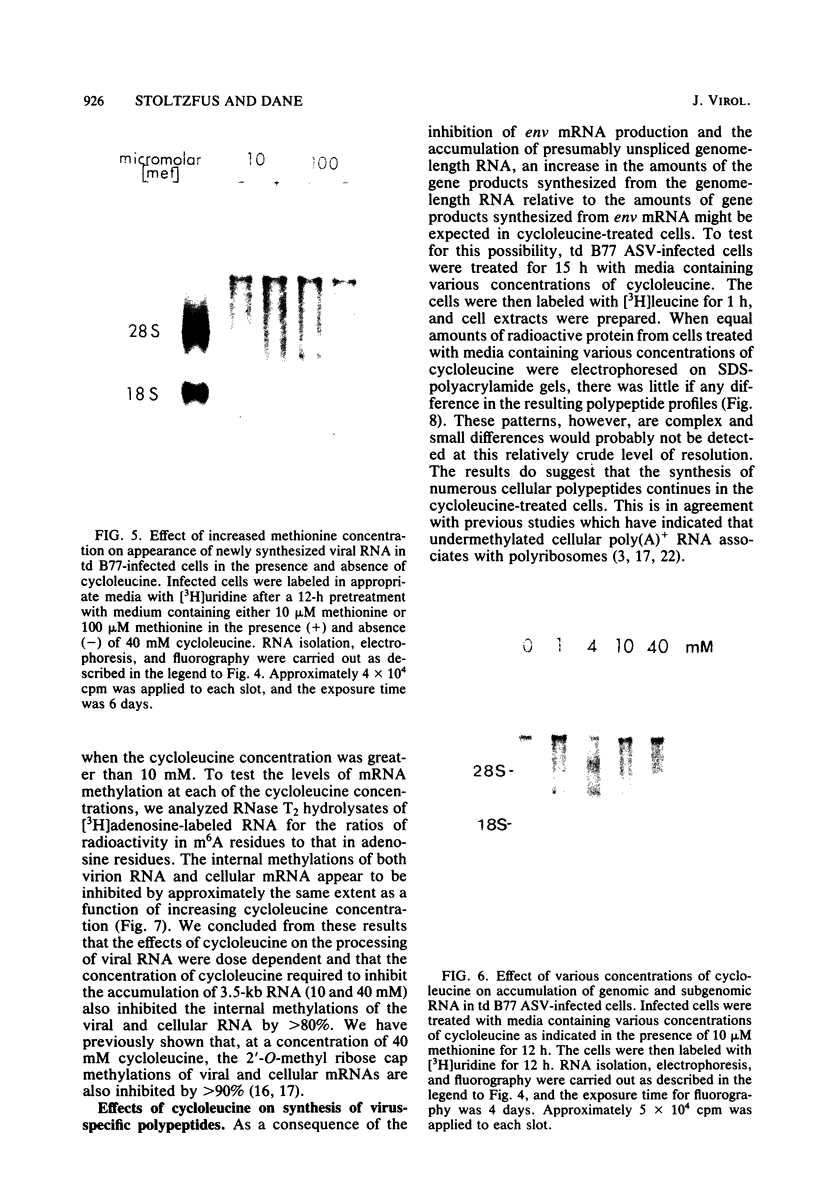
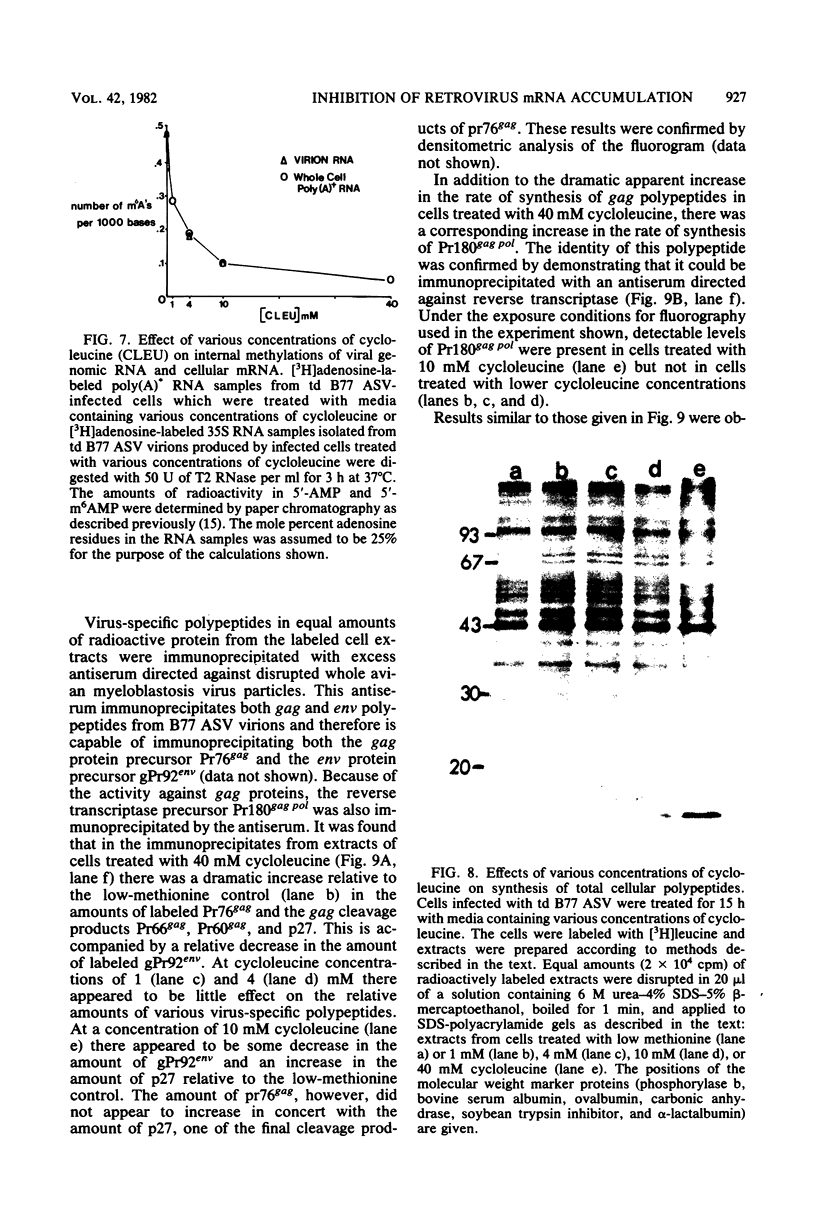
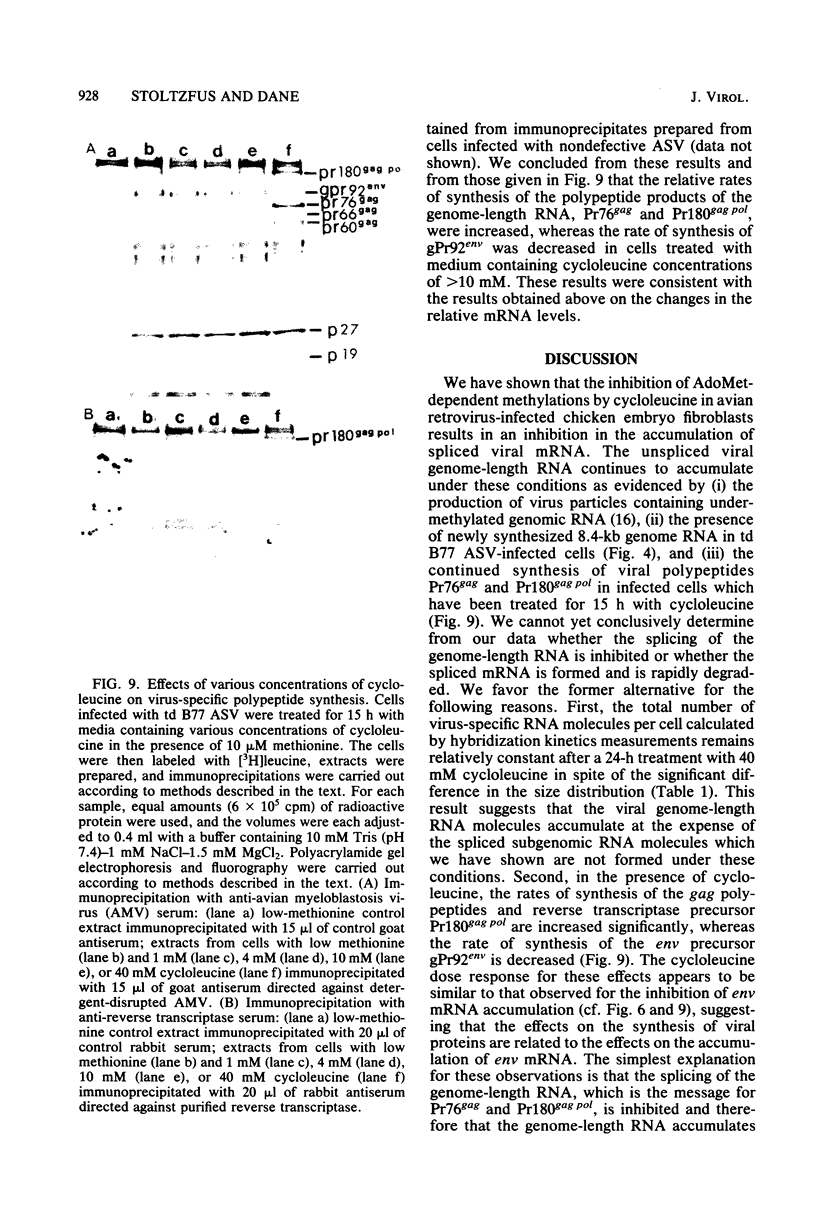
The synthesis and processing of B77 avian sarcoma virus RNA in infected chicken embryo fibroblasts was followed in the presence and absence of cycloleucine, a competitive inhibitor of the synthesis of S-adenosylmethionine and thus an inhibitor of RNA methylations. An increase in the steady-state levels of genome-length RNA and a decrease in the steady-state levels of subgenomic RNA molecules were obtained in the S-adenosylmethionine-depleted avian sarcoma virus-infected cells after 24 h of treatment with the inhibitor. The total number of virus-specific RNA molecules per cell, however, remained relatively constant under either condition. The production of newly synthesized virus-specific RNA in cycloleucine-treated and untreated cells infected with a transformation-defective strain of B77 avian sarcoma virus was followed as a function of [3H]uridine labeling time. The accumulation of radioactive genome-length 8.4-kilobase (kb) RNA continued in cycloleucine-treated cells, and virus particle production proceeded at normal rates as previously shown by incorporation of labeled nucleoside precursors or amino acids. In contrast, newly synthesized 3.5-kb subgenomic mRNA, the putative mRNA for the envelope protein precursor, failed to accumulate in the treated cells. The extent of the inhibition in the appearance of the radioactive 3.5-kb RNA was correlated with the extent of the inhibition of viral genomic and cellular mRNA methylations and was a function of the cycloleucine concentration. Under conditions in which the accumulation of 3.5-kb envelope protein mRNA was blocked by the cycloleucine treatment, there were significant increases in the rate of synthesis of the polypeptide products of the genome-length RNA, the precursors to the non-glycosylated gag proteins (Pr76gag), and the reverse transcriptase (Pr 180gag pol) relative to the rate of synthesis of the envelope protein precursor (gPr 92env). These results suggest that there is an S-adenosylmethionine requirement for the splicing, but not for the synthesis, packaging, or messenger function, of avian retrovirus genome-length RNA. Possible reasons for this requirement are discussed.
Full text
PDF


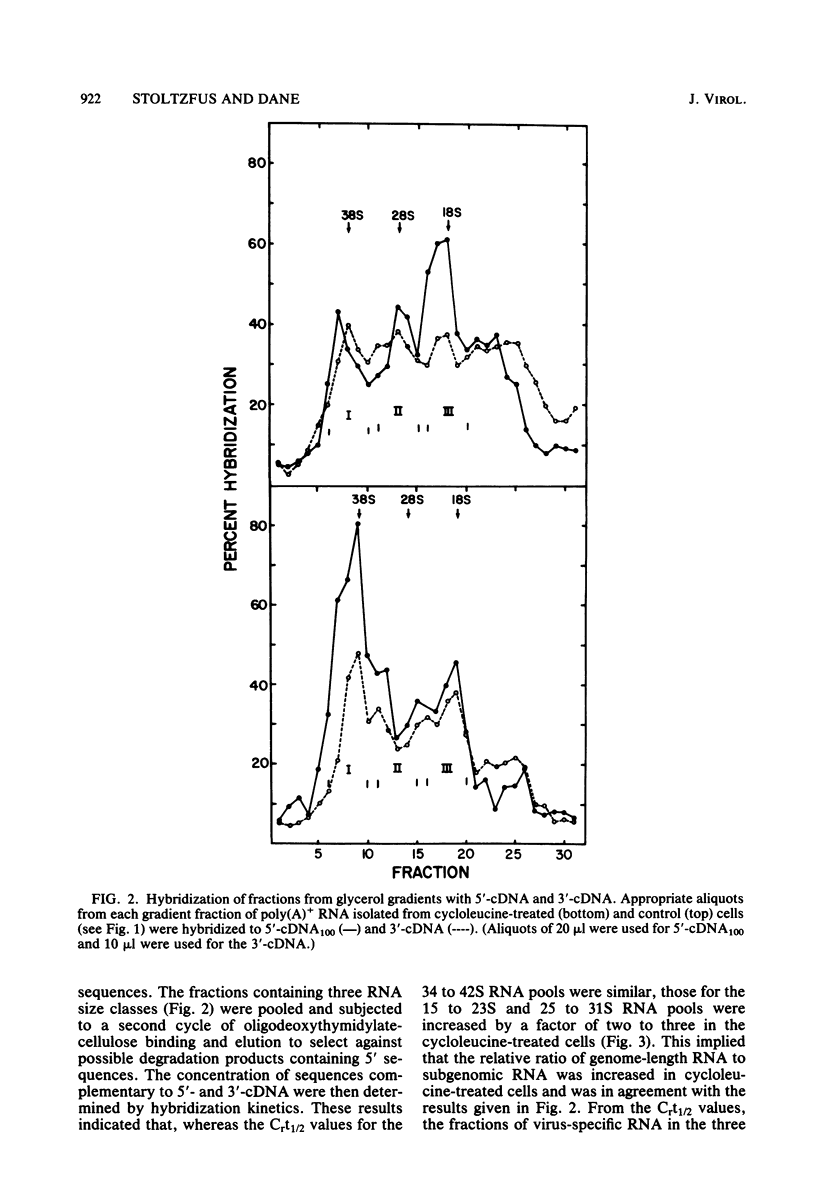
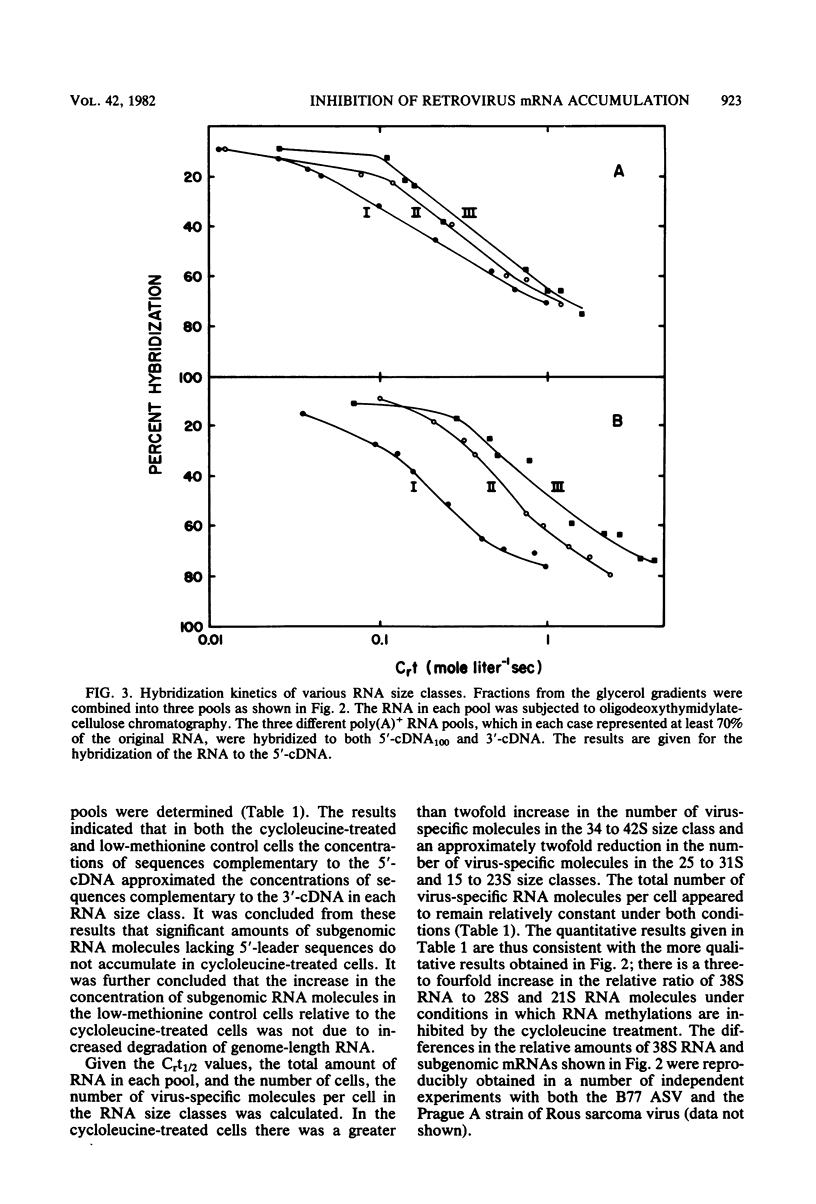



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Dhar R., Khoury G. Methylation of nuclear simian virus 40 RNAs. J Virol. 1979 Oct;32(1):52–60. doi: 10.1128/jvi.32.1.52-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W. Promotion and limitation of genetic exchange. Science. 1979 Jul 27;205(4404):361–365. doi: 10.1126/science.377489. [DOI] [PubMed] [Google Scholar]
- Bachellerie J. P., Amalric F., Caboche M. Biosynthesis and utilization of extensively undermethylated poly(A)+ RNA in CHO cells during a cycloleucine treatment. Nucleic Acids Res. 1978 Aug;5(8):2927–2943. doi: 10.1093/nar/5.8.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Beemon K., Keith J. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J Mol Biol. 1977 Jun 15;113(1):165–179. doi: 10.1016/0022-2836(77)90047-x. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Caboche M. Methionine metabolism in BHK cells: the regulation of methionine adenosyltransferase. J Cell Physiol. 1977 Sep;92(3):407–424. doi: 10.1002/jcp.1040920309. [DOI] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Lavi S., Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979 Jun 25;6(8):2879–2899. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Coulter A. W., Lombardini J. B., Sufrin J. R., Talalay P. Structural and conformational analogues of L-methionine as inhibitors of the enzymatic synthesis of S-adenosyl-l-methionine. 3. Carbocyclic and heterocyclic amino acids. Mol Pharmacol. 1974 Mar;10(2):319–334. [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Processing and function of undermethylated chicken embryo fibroblast mRNA. J Biol Chem. 1979 Jul 10;254(13):5591–5594. [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977 Feb 8;16(3):471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- Dimock K., Stolzfus C. M. Cycloleucine blocks 5'-terminal and internal methylations of avian sarcoma virus genome RNA. Biochemistry. 1978 Aug 22;17(17):3627–3632. doi: 10.1021/bi00610a032. [DOI] [PubMed] [Google Scholar]
- Engel J. D., von Hippel P. H. Effects of methylation on the stability of nucleic acid conformations. Studies at the polymer level. J Biol Chem. 1978 Feb 10;253(3):927–934. [PubMed] [Google Scholar]
- Friedrich R., Kung H. J., Baker B., Varmus H. E., Goodman H. M., Bishop J. M. Characterization of DNA complementary to nucleotide sequences at the 5'-terminus of the avian sarcoma virus genome. Virology. 1977 Jun 1;79(1):198–215. doi: 10.1016/0042-6822(77)90345-2. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J., Stavnezer E., Bishop J. M. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975 Oct 16;257(5527):618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehler M., Coward J., Rottman F. Cytoplasmic location of undermethylated messenger RNA in Novikoff cells. Nucleic Acids Res. 1979 Mar;6(3):1161–1175. doi: 10.1093/nar/6.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith J., Fraenkel-Conrat H. Identification of the 5' end of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3347–3350. doi: 10.1073/pnas.72.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Scheible P., Smith R. E. Correlation of RNA binding affinity of avian oncornavirus p19 proteins with the extent of processing of virus genome RNA in cells. J Virol. 1980 Sep;35(3):722–731. doi: 10.1128/jvi.35.3.722-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardini J. B., Coulter A. W., Talalay P. Analogues of methionine as substrates and inhibitors of the methionine adenosyltransferase reaction. Deductions concerning the conformation of methionine. Mol Pharmacol. 1970 Sep;6(5):481–499. [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Lewis P., Dierks P. Purification of virus-specific RNA from chicken cells infected with avian sarcoma virus: identification of genome-length and subgenome-leghth viral RNAs. J Virol. 1978 Jul;27(1):227–238. doi: 10.1128/jvi.27.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Mellon P., Duesberg P. H., Martin G. S. env Gene of Rous sarcoma virus: identification of the gene product by cell-free translation. J Virol. 1980 Mar;33(3):993–1003. doi: 10.1128/jvi.33.3.993-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Stacey D. W. Messenger activity of virion RNA for avian leukosis viral envelope glycoprotein. J Virol. 1979 Mar;29(3):949–956. doi: 10.1128/jvi.29.3.949-956.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Kuhnert L. K. Evidence for the identity of shared 5'-terminal sequences between genome RNA and subgenomic mRNA's of B77 avian sarcoma virus. J Virol. 1979 Nov;32(2):536–545. doi: 10.1128/jvi.32.2.536-545.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Montgomery J. A. Selective inhibition of avian sarcoma virus protein synthesis in 3-deazaadenosine-treated infected chicken embryo fibroblasts. J Virol. 1981 Apr;38(1):173–183. doi: 10.1128/jvi.38.1.173-183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Snyder P. N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975 Nov;16(5):1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Tal J., Kung H. J., Varmus H. E., Bishop J. M. Characterization of DNA complementary to nucleotide sequences adjacent to poly(A) at the 3'-terminus of the avian sarcoma virus genome. Virology. 1977 Jun 1;79(1):183–197. doi: 10.1016/0042-6822(77)90344-0. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]