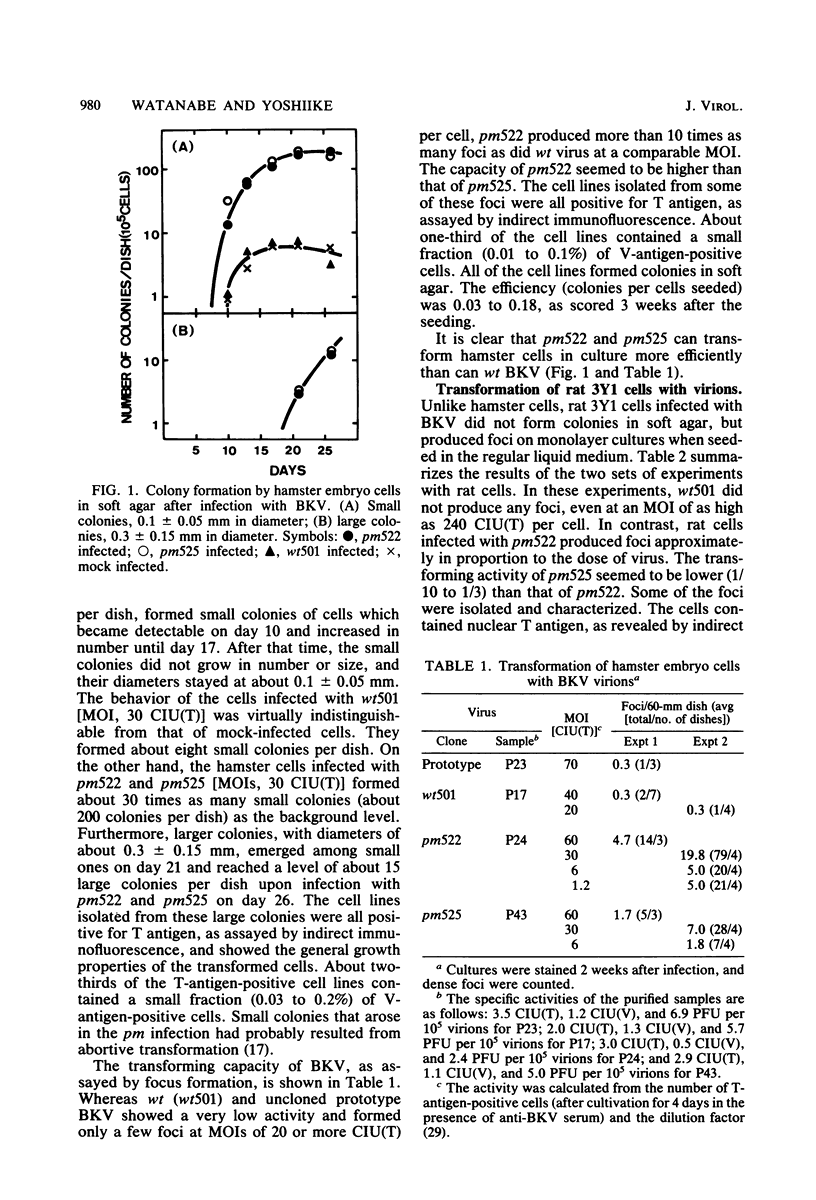
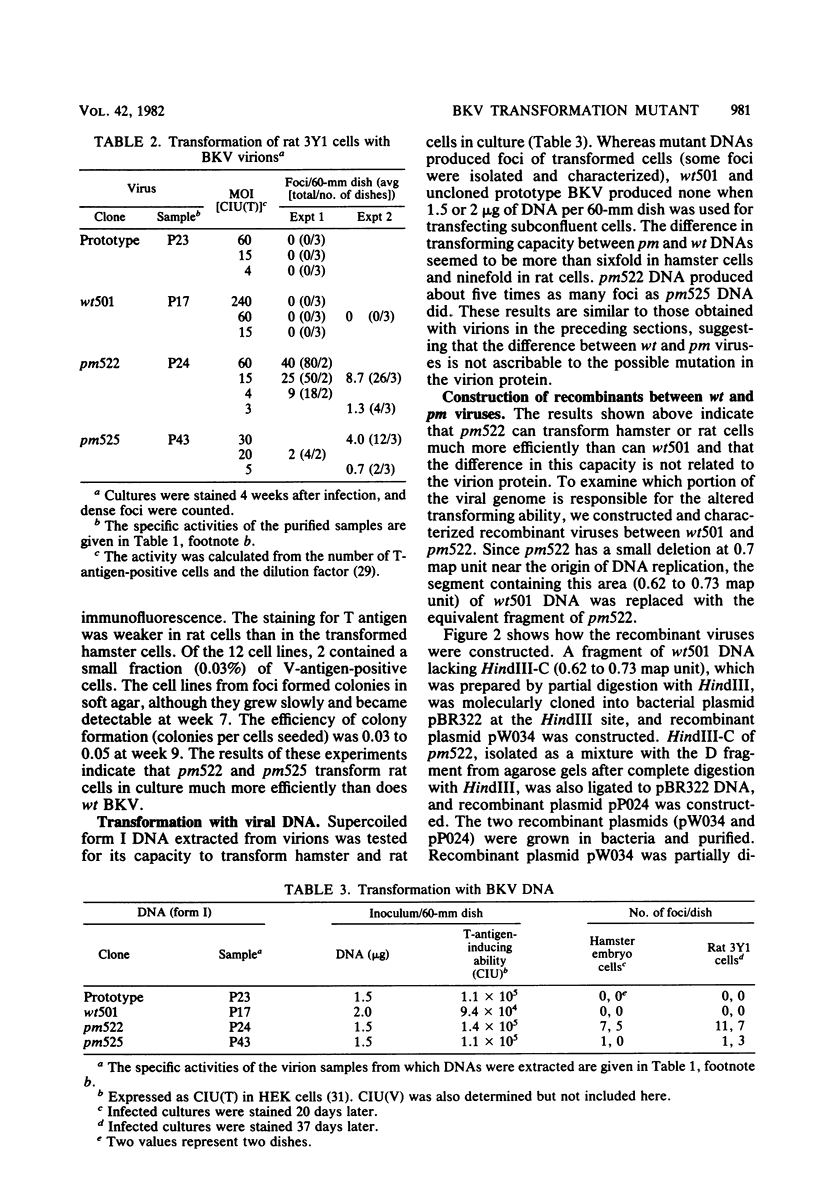
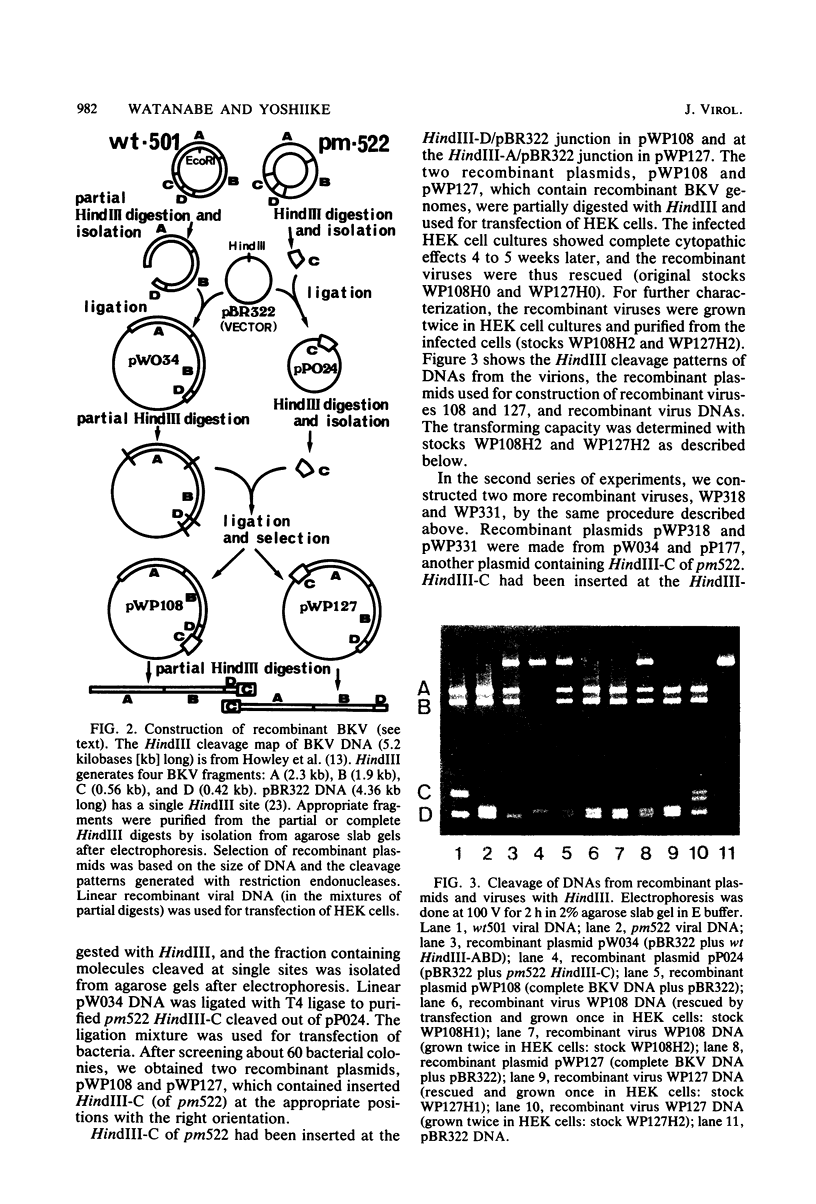
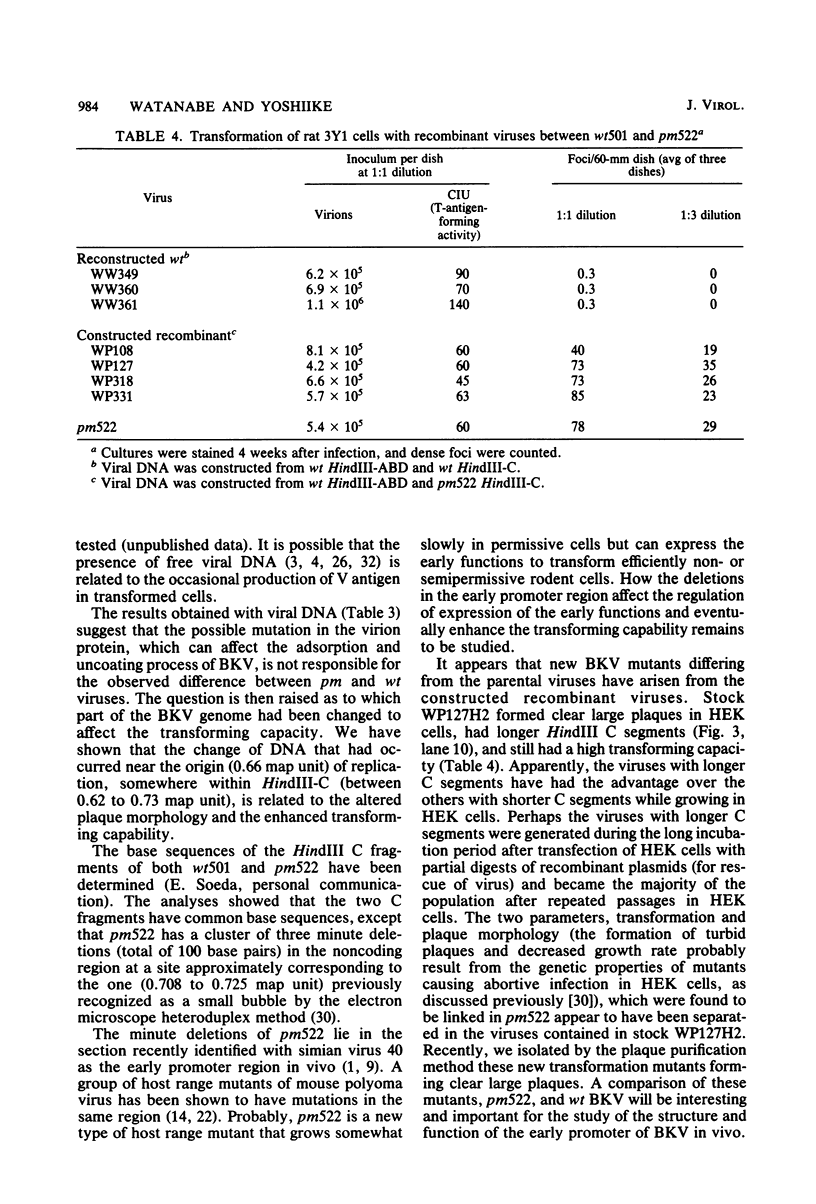
Abstract
A turbid-plaque-forming mutant (pm522) of human papovavirus BK, which has a small deletion at about 0.7 map unit and grows somewhat more slowly in human cells than does wild-type BK virus, transformed hamster and rat cells in culture much more efficiently than did wild-type virus. Another plaque morphology mutant, pm525, forming turbid plaques larger than those of pm522 also had a high transforming capacity. The similar difference in transforming capability between wild-type and plaque morphology viruses was observed with DNAs extracted from virions. Recombinant viruses were constructed from the wild-type DNA fragment lacking HindIII-C (0.62 to 0.73 map unit) and pm522 HindIII-C (including the origin of replication) by the molecular cloning method. Characterization of the recombinants showed that the change near the origin of DNA replication was responsible both for the altered plaque morphology and for the enhanced transforming capacity of the BK virus mutant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chenciner N., Grossi M. P., Meneguzzi G., Corallini A., Manservigi R., Barbanti-Brodano G., Milanesi G. State of viral DNA in BK virus-transformed rabbit cells. Virology. 1980 May;103(1):138–148. doi: 10.1016/0042-6822(80)90132-4. [DOI] [PubMed] [Google Scholar]
- Chenciner N., Meneguzzi G., Corallini A., Grossi M. P., Grassi P., Barbanti-Brodano G., Milanesi G. Integrated and free viral DNA in hamster tumors induced by BK virus. Proc Natl Acad Sci U S A. 1980 Feb;77(2):975–979. doi: 10.1073/pnas.77.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Physical map of the BK virus genome. J Virol. 1975 Oct;16(4):959–973. doi: 10.1128/jvi.16.4.959-973.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Major E. O., Di Mayorca G. Malignant transformation of BHK21 clone 13 cells by BK virus--a human papovavirus. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3210–3212. doi: 10.1073/pnas.70.11.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Portolani M., Barbanti-Brodano G., Placa M. L. Malignant transformation of hamster kidney cells by BK virus. J Virol. 1975 Feb;15(2):420–422. doi: 10.1128/jvi.15.2.420-422.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehafer J., Salmi A., Colter J. S. Isolation and characterization of BK virus-transformed hamster cells. Virology. 1977 Mar;77(1):356–366. doi: 10.1016/0042-6822(77)90432-9. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Martin M. A. Transformation of hamster kidney cells by BK papovavirus DNA. J Virol. 1975 Jan;17(1):247–253. doi: 10.1128/jvi.17.1.247-253.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Koprowski H., Iwasaki Y. Malignant transformation of hamster brain cells in vitro by human papovavirus Bk. J Natl Cancer Inst. 1976 Mar;56(3):671–673. doi: 10.1093/jnci/56.3.671. [DOI] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Aizawa T., Furuno A., Muto T. Polyoncogenicity and insulinoma-inducing ability of BK Virus, a human Papovavirus, in Syrian golden hamsters. J Natl Cancer Inst. 1979 Jul;63(1):119–126. [PubMed] [Google Scholar]
- Watanabe S. Virus DNA synthesizing ability of T antigen-forming defective SV40 produced by successive undiluted passages. J Gen Virol. 1975 Jan;26(1):49–57. doi: 10.1099/0022-1317-26-1-49. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K., Nozawa A., Yuasa Y., Uchida S. Viable deletion mutant of human papovavirus BK that induces insulinomas in hamsters. J Virol. 1979 Dec;32(3):934–942. doi: 10.1128/jvi.32.3.934-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K., Yuasa Y., Uchida S. Natural occurrence of deletion mutant of human papovavirus BK capable of inducing T antigen. J Gen Virol. 1981 Jun;54(Pt 2):431–435. doi: 10.1099/0022-1317-54-2-431. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Furuno A., Watanabe S., Yoshiike K. Occurrence of free, defective viral DNA in a hamster tumor induced by human papovavirus BK. Virology. 1980 May;103(1):241–244. doi: 10.1016/0042-6822(80)90143-9. [DOI] [PubMed] [Google Scholar]
- Yoshike K., Defendi V. Addition of extra DNA sequences to simian virus 40 DNA in vivo. J Virol. 1977 Aug;23(2):323–337. doi: 10.1128/jvi.23.2.323-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Schegget J., Voves J., van Strien A., van der Noordaa J. Free viral DNA in BK virus-induced hamster tumor cells. J Virol. 1980 Aug;35(2):331–339. doi: 10.1128/jvi.35.2.331-339.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Noordaa J. Infectivity, oncogenicity and transforming ability of BK virus and BK virus DNA. J Gen Virol. 1976 Mar;30(3):371–373. doi: 10.1099/0022-1317-30-3-371. [DOI] [PubMed] [Google Scholar]



