Abstract
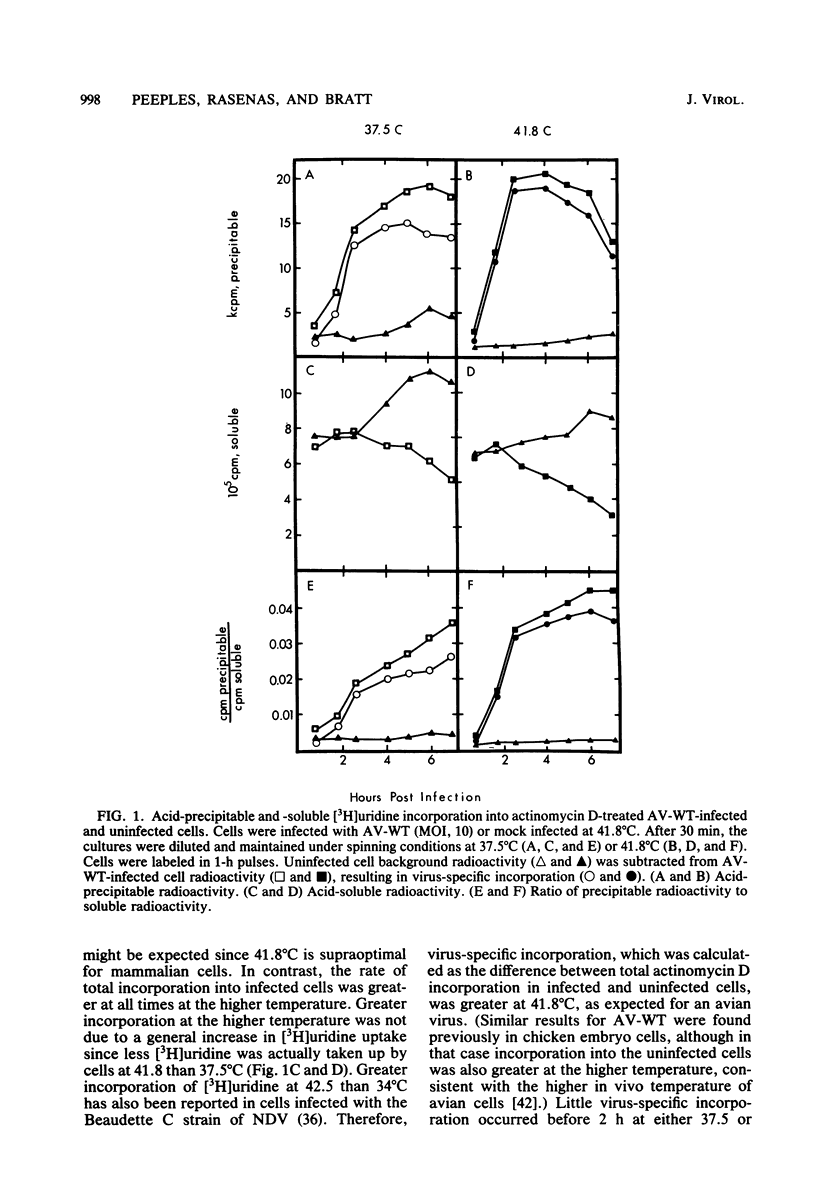
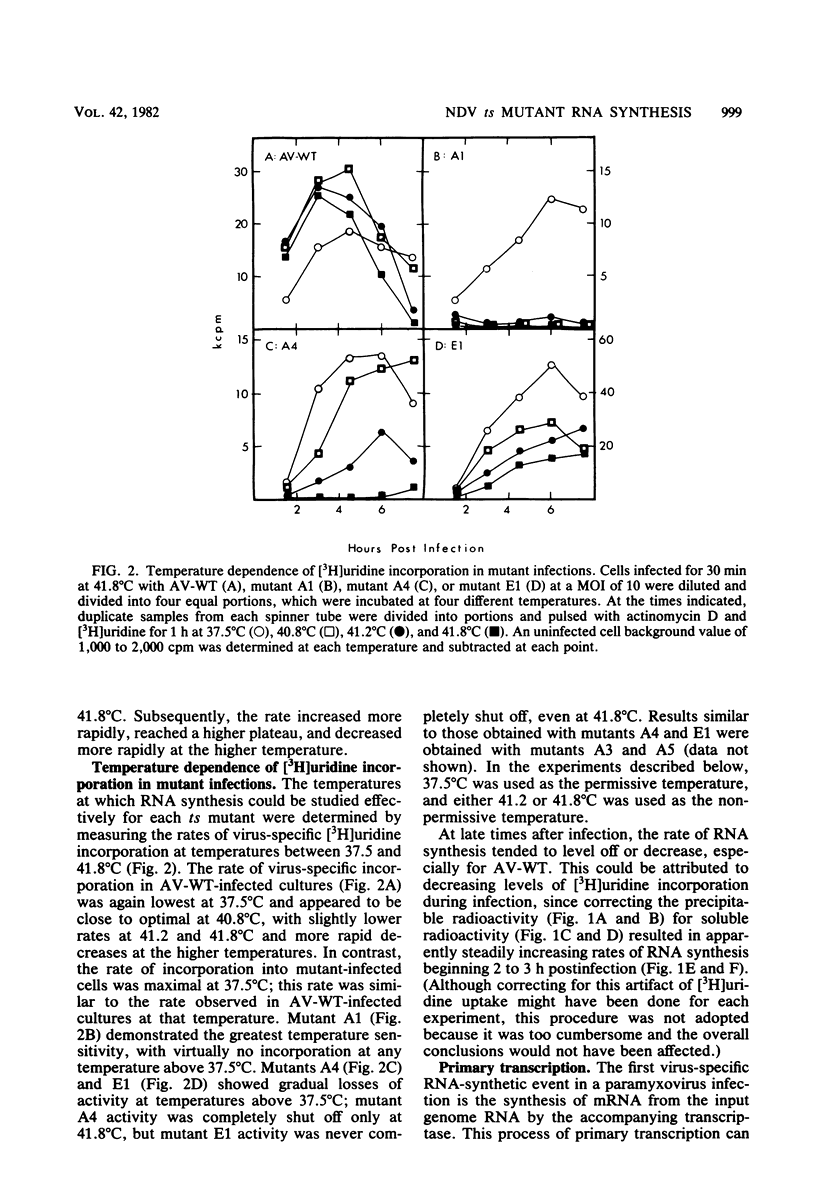
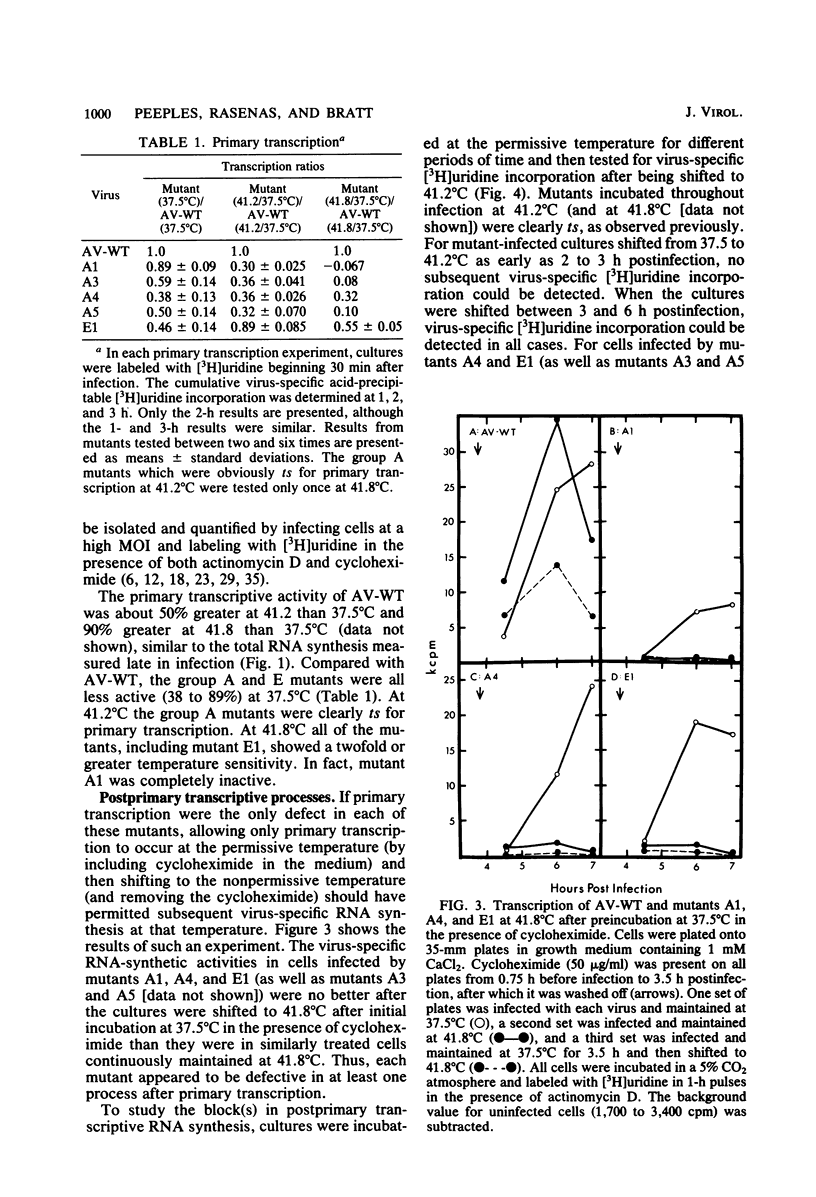
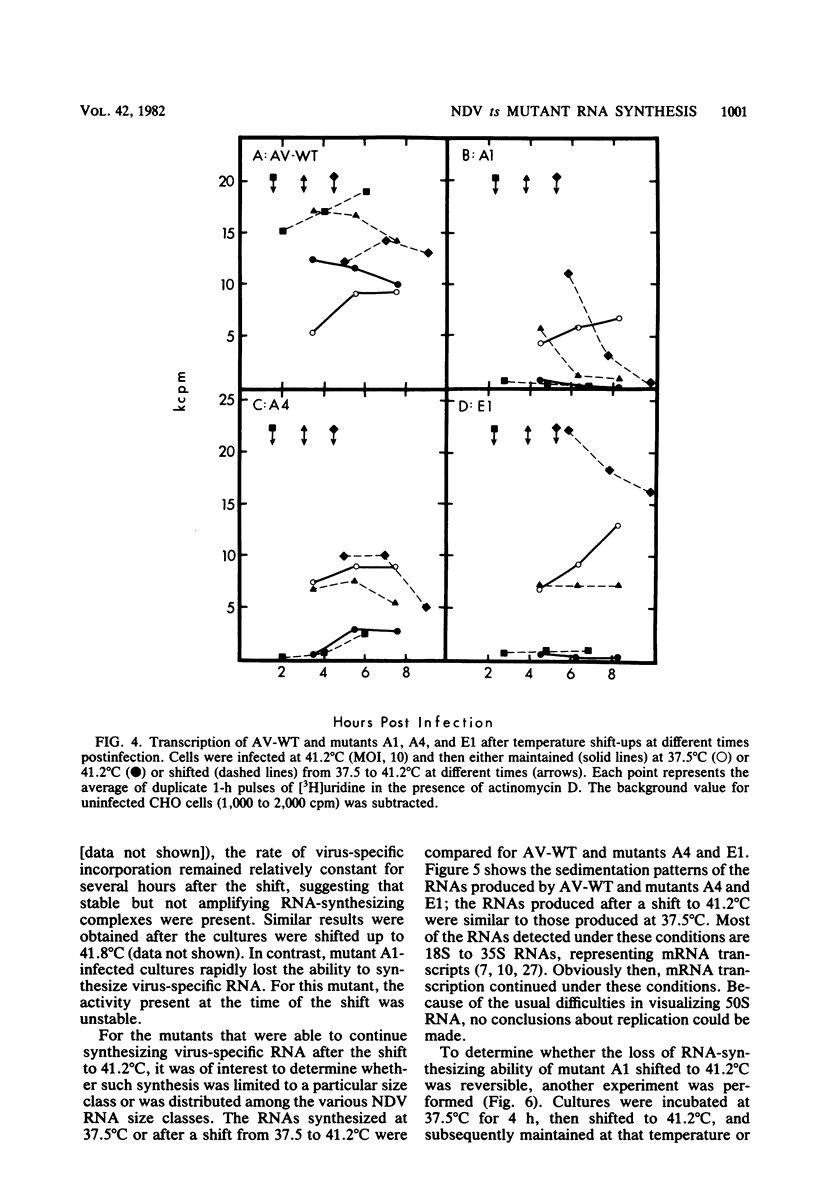
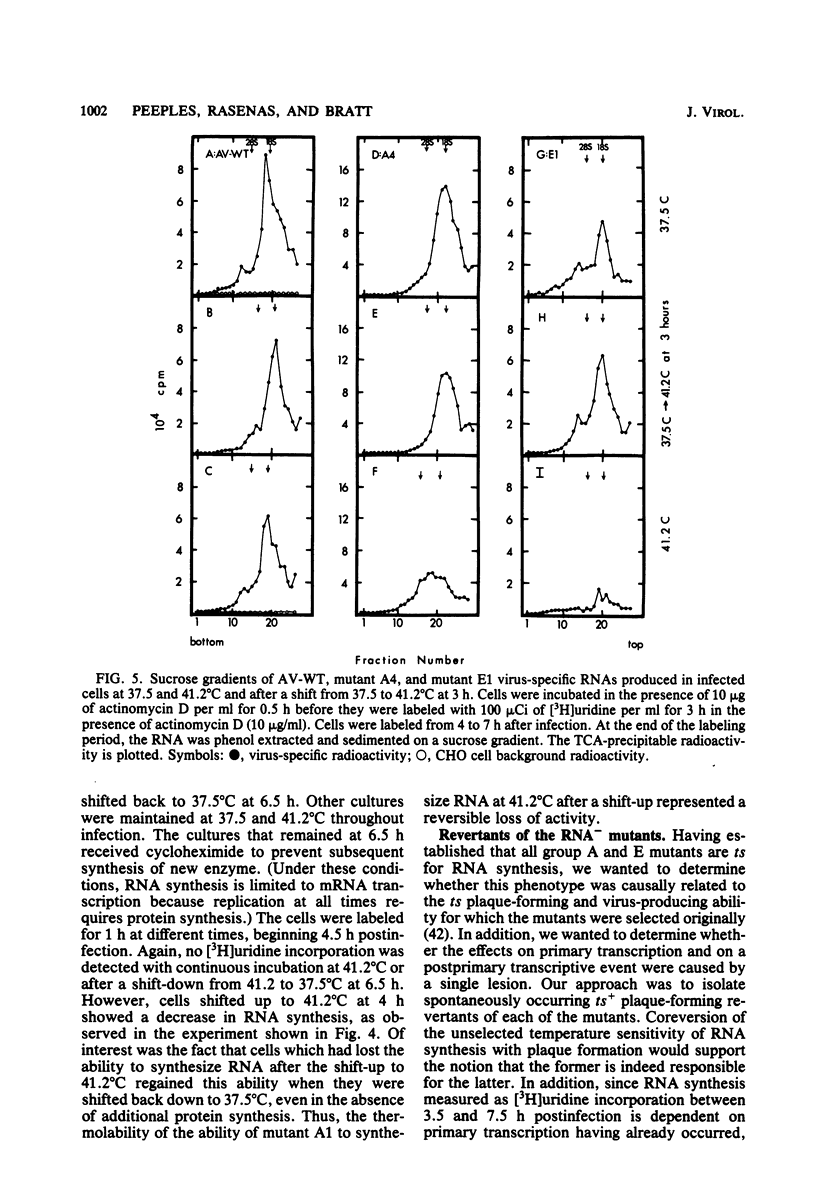
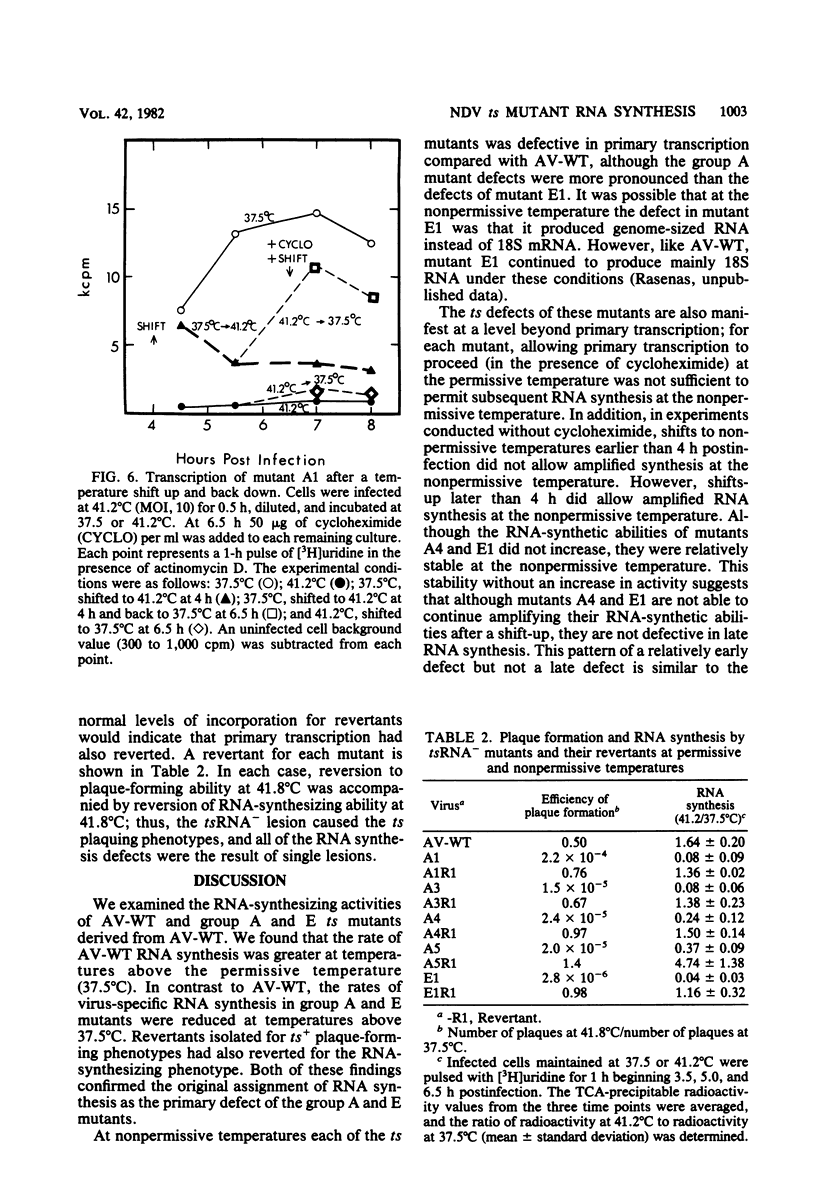
The temperature-sensitive RNA-negative mutants of Newcastle disease virus comprise two complementation groups, group A (seven members) and group E (one member). The RNA-synthesizing activities of four representative members of group A and the single member of group E were compared with the activity of the wild type. These mutants were defective to varying extents in primary transcription at the nonpermissive temperature, ranging from mutant A1, which had no activity, to mutant E1, which lost only 50% of its activity. All of the mutants were also defective in a postprimary transcriptive process since after preincubation at the permissive temperature in the presence of cycloheximide, there was no subsequent RNA synthesis at the nonpermissive temperature upon removal of the cycloheximide. Similarly, in experiments in which cycloheximide was not used, shifts from the permissive temperature to the nonpermissive temperature before 3 h postinfection did not result in RNA synthesis. However, later shifts to the nonpermissive temperature did allow RNA synthesis. With the exception of mutant A1, all of the mutants maintained this RNA-synthetic ability for at least 3 h, suggesting that RNA synthesis from progeny genomes was not the major postprimary transcriptive defect in these mutants. In contrast, the RNA-synthetic ability of mutant A1 rapidly decayed at the nonpermissive temperature, suggesting that the A gene product is involved in RNA synthesis from progeny genomes. The postprimary transcriptive defect(s) of the other mutants may be in the processing or stability of a protein, in the processing of mRNA, or in replication. Plaque-forming revertants (ts+) of all of the mutants coreverted for RNA synthesis. This finding strengthens the relationship between temperature sensitivity for plaquing and both the primary and postprimary RNA-negative phenotypes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Kanda T., Shibuta H. Isolation and characterization of temperature-sensitive mutants of Sendai virus. Microbiol Immunol. 1980;24(11):1053–1068. doi: 10.1111/j.1348-0421.1980.tb02911.x. [DOI] [PubMed] [Google Scholar]
- Bergholz C. M., Kiley M. P., Payne F. E. Isolation and characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Jul;16(1):192–202. doi: 10.1128/jvi.16.1.192-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschkin A. M., Rapp F., Payne F. E. Complementation analysis of measles virus temperature-sensitive mutants. J Virol. 1977 Jan;21(1):439–441. doi: 10.1128/jvi.21.1.439-441.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Relationship between the ribonucleic acid synthesizing capacity of ultraviolet-irradiated Newcastle disease virus and its ability to induce interferon. J Virol. 1971 Oct;8(4):500–508. doi: 10.1128/jvi.8.4.500-508.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinkscales C. W., Bratt M. A., Morrison T. G. Synthesis of Newcastle disease virus polypeptides in a wheat germ cell-free system. J Virol. 1977 Apr;22(1):97–101. doi: 10.1128/jvi.22.1.97-101.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcriptional map for Newcastle disease virus. J Virol. 1980 Sep;35(3):682–693. doi: 10.1128/jvi.35.3.682-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Stone H. O. Isolation of a transcriptive complex from Newcastle disease virions. J Virol. 1976 Sep;19(3):1080–1089. doi: 10.1128/jvi.19.3.1080-1089.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East J. L., Kingsbury D. W. Mumps virus replication in chick embryo lung cells: properties of ribonucleic acids in virions and infected cells. J Virol. 1971 Aug;8(2):161–173. doi: 10.1128/jvi.8.2.161-173.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G. P., Shirodaria P. V., Follett E. A., Pringle C. R. Respiratory syncytial virus ts mutants and nuclear immunofluorescence. J Virol. 1976 Nov;20(2):487–500. doi: 10.1128/jvi.20.2.487-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure M. A., Wright P. F., Chanock R. M. Temperature-sensitive mutants of respiratory syncytial virus. J Virol. 1969 Apr;3(4):414–421. doi: 10.1128/jvi.3.4.414-421.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Isolation and preliminary characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Oct;16(4):1000–1009. doi: 10.1128/jvi.16.4.1000-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Emerson S. U., Wagner R. R. RNA- temperature-sensitive mutants of vesicular stomatitis virus: L-protein thermosensitivity accounts for transcriptase restriction of group I mutants. J Virol. 1976 May;18(2):596–603. doi: 10.1128/jvi.18.2.596-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Kolakofsky D. Effect of defective interfering particles on plus- and minus- strand leader RNAs in vesicular stomatitis virus-infected cells. J Virol. 1980 Sep;35(3):704–709. doi: 10.1128/jvi.35.3.704-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Lesnaw J. A., Reichmann M. E. RNA synthesis by temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. Virology. 1975 Feb;63(2):492–504. doi: 10.1016/0042-6822(75)90322-0. [DOI] [PubMed] [Google Scholar]
- Madansky C. H., Bratt M. A. Noncytopathic mutants of Newcastle disease virus are defective in virus-specific RNA synthesis. J Virol. 1981 Jan;37(1):317–327. doi: 10.1128/jvi.37.1.317-327.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madansky C. H., Bratt M. A. Relationships among virus spread, cytopathogenicity, and virulence as revealed by the noncytopathic mutants of Newcastle disease virus. J Virol. 1981 Dec;40(3):691–702. doi: 10.1128/jvi.40.3.691-702.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Simpson D. Synthesis, stability, and cleavage of Newcastle disease virus glycoproteins in the absence of glycosylation. J Virol. 1980 Oct;36(1):171–180. doi: 10.1128/jvi.36.1.171-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Peeples M. E., Bratt M. A. UV irradiation analysis of complementation between, and replication of, RNA-negative temperature-sensitive mutants of Newcastle disease virus. J Virol. 1982 Mar;41(3):965–973. doi: 10.1128/jvi.41.3.965-973.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S. M., Huang A. S. RNA synthesis of vesicular stomatitis virus. V. Interactions between transcription and replication. J Virol. 1973 Dec;12(6):1395–1400. doi: 10.1128/jvi.12.6.1395-1400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Identification of transcriptive and replicative intermediates in Sendai virus-infected cells. Virology. 1972 Mar;47(3):711–725. doi: 10.1016/0042-6822(72)90561-2. [DOI] [PubMed] [Google Scholar]
- Portner A., Marx P. A., Kingsbury D. W. Isolation and characterization of Sendai virus temperature-sensitive mutants. J Virol. 1974 Feb;13(2):298–304. doi: 10.1128/jvi.13.2.298-304.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive defect of mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1973 Sep;12(3):472–480. doi: 10.1128/jvi.12.3.472-480.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. Sendai virus RNA synthesis and nucleocapsid formation in the presence of cycloheximide. Virology. 1971 Jun;44(3):494–502. doi: 10.1016/0042-6822(71)90362-x. [DOI] [PubMed] [Google Scholar]
- Samson A. C., Chambers P., Lee C. M., Simon E. Temperature-sensitive mutant of Newcastle disease virus which has an altered nucleocapsid-associated protein. J Gen Virol. 1981 May;54(Pt 1):197–201. doi: 10.1099/0022-1317-54-1-197. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Hightower L. E. Identification of the P proteins and other disulfide-linked and phosphorylated proteins of Newcastle disease virus. J Virol. 1981 Jan;37(1):256–267. doi: 10.1128/jvi.37.1.256-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutation on activity of the RNA transcriptase of vesicular stomatitis virus New Jersey. J Virol. 1979 Jun;30(3):692–700. doi: 10.1128/jvi.30.3.692-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutations on the virion-associated RNA transcriptase of vesicular stomatitis virus. J Mol Biol. 1972 Nov 14;71(2):281–291. doi: 10.1016/0022-2836(72)90351-8. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. In vitro synthesis of the full-length complement of the negative-strand genome RNA of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Jan;77(1):294–298. doi: 10.1073/pnas.77.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipis J. E., Bratt M. A. Isolation and preliminary characterization of temperature-sensitive mutants of Newcastle disease virus. J Virol. 1976 Jun;18(3):848–855. doi: 10.1128/jvi.18.3.848-855.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


