Abstract
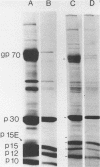
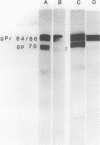
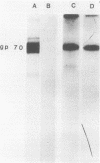
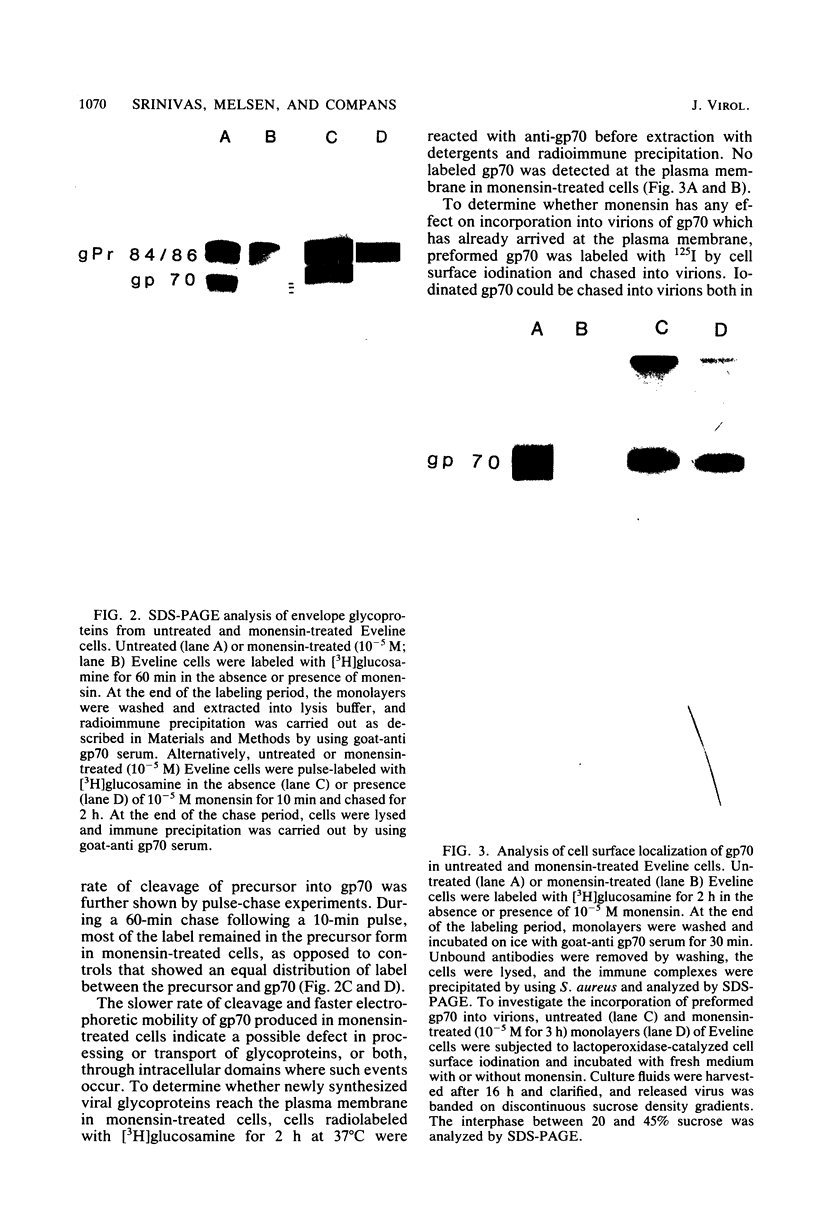
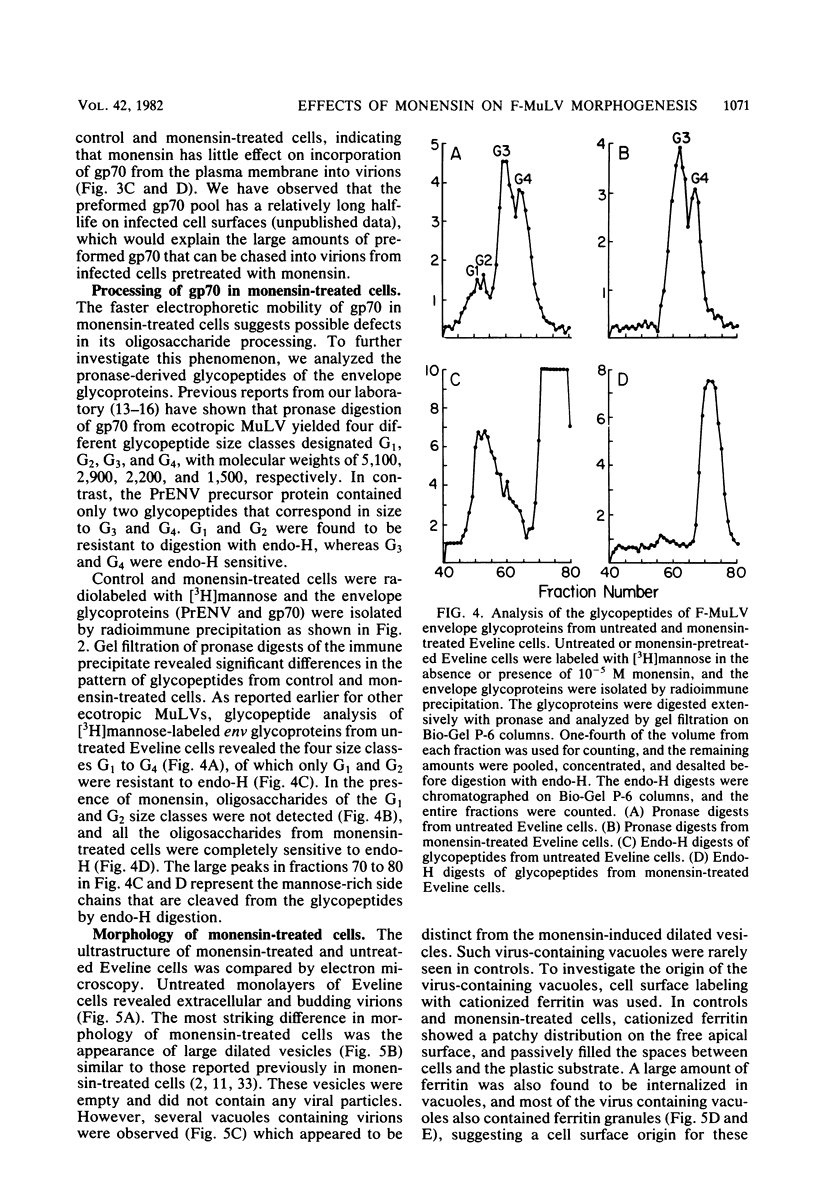
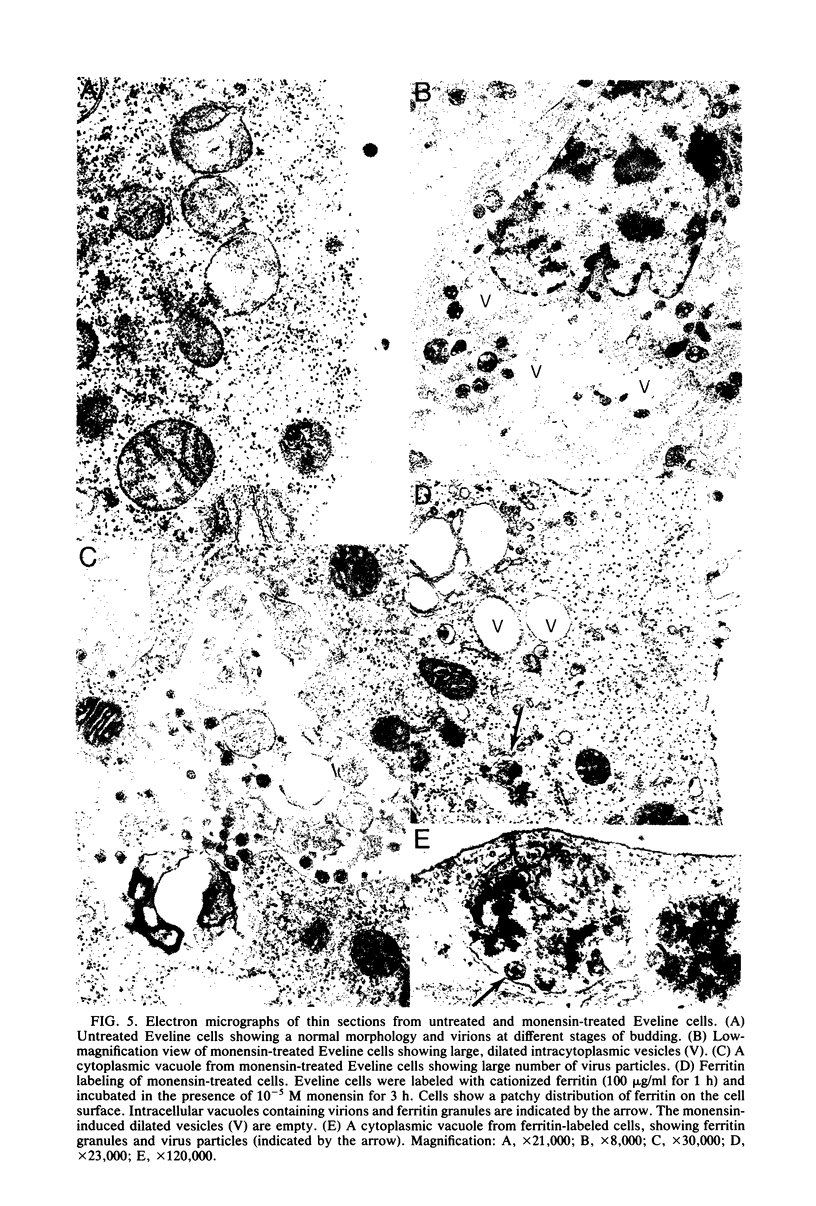
The transport of the gp70 glycoprotein to the cell surface and concomitant release of infectious virus was inhibited by treatment of Friend murine leukemia virus-infected Eveline cells with the sodium ionophore monensin. Virus yields were reduced more than 50-fold by 10(-5) M monensin, whereas particle production was reduced by 50% in monensin-treated cells. The resulting particles failed to incorporate newly synthesized gp70 and p15(E), whereas the other structural proteins, p30, p15, p12, and p10, were incorporated into virions. However, monensin did not inhibit the incorporation into virions of preformed gp70. A reduction in the efficiency of cleavage of the PrENV glycoprotein precursor and a defect in the processing of simple endo-H-sensitive to complex endo-H-resistant oligosaccharides suggest that intracellular transport of gp70 may be blocked before its entry into the Golgi apparatus. Fewer particles were found to bud from the cell surface, but intracellular vacuoles with budding virions were detected. Ferritin labeling and pulse-chase studies suggested a cell surface origin for these vacuoles. These experiments indicate that monensin inhibits the transport of Friend murine leukemia virus glycoproteins at an early stage, with a resultant block in the assembly and release of infectious virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Bassin R. H., Weaver C. Comparison of murine sarcoma viruses in nonproducer and S + L - -transformed cells. J Virol. 1972 Apr;9(4):701–704. doi: 10.1128/jvi.9.4.701-704.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso F. V., Compans R. W. Differential effect of monensin on enveloped viruses that form at distinct plasma membrane domains. J Cell Biol. 1981 Jun;89(3):700–705. doi: 10.1083/jcb.89.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Langlois A. J., Schäfer W. Polypeptides of mammalian oncornaviruses. IV. Structural components of murine leukemia virus released as soluble antigens in cell culture. Virology. 1975 Dec;68(2):550–555. doi: 10.1016/0042-6822(75)90297-4. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Bradac J., Hunter E. Effect of tunicamycin on cell fusion induced by Mason-Pfizer monkey virus. J Virol. 1981 May;38(2):770–776. doi: 10.1128/jvi.38.2.770-776.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., Jelalian K. Cell surface expression of the env gene polyprotein of dual-tropic mink cell focus-forming murine leukemia virus. J Virol. 1979 Jun;30(3):720–728. doi: 10.1128/jvi.30.3.720-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting T., Ruta M., Kabat D. Mutant cells that abnormally process plasma membrane glycoproteins encoded by murine leukemia virus. Cell. 1981 Jun;24(3):847–858. doi: 10.1016/0092-8674(81)90110-0. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Basak S., Compans R. W. Glycopeptides of murine leukemia viruses. I. Comparison of two ecotropic viruses. J Virol. 1979 Jul;31(1):1–7. doi: 10.1128/jvi.31.1.1-7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. C., Famulari N. G., Compans R. W. Glycoproteins of murine leukemia viruses. III. Glycosylation of env precursor glycoproteins. J Virol. 1981 Aug;39(2):463–470. doi: 10.1128/jvi.39.2.463-470.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. C., Famulari N. G., O'Donnell P. V., Compans R. W. Glycopeptides of murine leukemia viruses. II. Comparison of xenotropic and dual-tropic viruses. J Virol. 1980 Apr;34(1):154–161. doi: 10.1128/jvi.34.1.154-161.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. C., Wise K. S., Edlund L. E., Acton R. T., Compans R. W. Origin of the minor glycoproteins of murine leukemia viruses. J Virol. 1978 Oct;28(1):84–94. doi: 10.1128/jvi.28.1.84-94.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger P. W., Uchida N., Tanzer M. L. Immunocytochemical localization of procollagen and fibronectin in human fibroblasts: effects of the monovalent ionophore, monensin. J Cell Biol. 1980 Dec;87(3 Pt 1):663–671. doi: 10.1083/jcb.87.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren H. W., Prins F. A., Herbrink P., Warnaar S. O. Electron microscopic studies on the role of the envelope antigens of R-MuLV-ts29 in budding. Virology. 1981 Aug;113(1):254–262. doi: 10.1016/0042-6822(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Kabat D. Genetic and sialylation sources of heterogeneity of the murine leukemia virus membrane envelope glycoproteins gp69/71. J Biol Chem. 1979 Feb 25;254(4):1340–1348. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pinter A., deHarven E. Protein composition of a defective murine sarcoma virus particle possessing the enveloped type-A morphology. Virology. 1979 Nov;99(1):103–110. doi: 10.1016/0042-6822(79)90041-2. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Koch G. Viral protein synthesis in Friend erythroleukemia cell lines. J Virol. 1977 Jan;21(1):328–337. doi: 10.1128/jvi.21.1.328-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Murray M. J., Webb M. C., Kabat D. A murine leukemia virus mutant with a temperature-sensitive defect in membrane glycoprotein synthesis. Cell. 1979 Jan;16(1):77–88. doi: 10.1016/0092-8674(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. Tunicamycin inhibits glycosylation of precursor polyprotein encoded by env gene of Rauscher murine leukemia virus. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1206–1213. doi: 10.1016/0006-291x(79)90245-6. [DOI] [PubMed] [Google Scholar]
- Smilowitz H. Routes of intracellular transport of acetylcholine receptor and esterase are distinct. Cell. 1980 Jan;19(1):237–244. doi: 10.1016/0092-8674(80)90405-5. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Bennett M., Mirand E. A., Trudel P. J. Isolation and characterization of a lymphatic leukemia virus in the Friend virus complex. J Natl Cancer Inst. 1971 Jun;46(6):1209–1217. [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Oncornavirus envelope glycoprotein in serum of mice. Virology. 1976 Nov;75(1):130–144. doi: 10.1016/0042-6822(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Hoessli D., Vassalli P. Intracellular transport of lymphoid surface glycoproteins. Role of the Golgi complex. J Mol Biol. 1981 Aug 25;150(4):525–535. doi: 10.1016/0022-2836(81)90378-8. [DOI] [PubMed] [Google Scholar]
- Uchida N., Smilowitz H., Tanzer M. L. Monovalent ionophores inhibit secretion of procollagen and fibronectin from cultured human fibroblasts. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1868–1872. doi: 10.1073/pnas.76.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Tsukamoto-Adey A., Weissman I. L. Cellular maturation of oncornavirus glycoproteins: topological arrangement of precursor and product forms in cellular membranes. Virology. 1977 Feb;76(2):539–553. doi: 10.1016/0042-6822(77)90236-7. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Oncornavirus budding: kinetics of formation and utilization of viral membrane glycoprotein. Virology. 1976 Feb;69(2):464–473. doi: 10.1016/0042-6822(76)90477-3. [DOI] [PubMed] [Google Scholar]