Abstract
While some recent neuroimaging studies have implicated medial rostral prefrontal cortex (MPFC) in ‘mentalizing’ and self-reflection, others have implicated this region in attention towards perceptual vs self-generated information. In order to reconcile these seemingly contradictory findings, we used fMRI to investigate MPFC activity related to these two functions in a factorial design. Participants performed two separate tasks, each of which alternated between ‘stimulus-oriented phases’ (SO), where participants attended to task-relevant perceptual information, and ‘stimulus-independent phases’ (SI), where participants performed the same tasks in the absence of such information. In half of the blocks (‘mentalizing condition’), participants were instructed that they were performing these tasks in collaboration with an experimenter; in other blocks (‘non-mentalizing condition’), participants were instructed that the experimenter was not involved. In fact, the tasks were identical in these conditions. Neuroimaging data revealed adjacent but clearly distinct regions of activation within MPFC related to (i) mentalizing vs non-mentalizing conditions (relatively caudal/superior) and (ii) SO vs SI attention (relatively rostral/inferior). These results generalized from one task to the other, suggesting a new axis of functional organization within MPFC.
Keywords: area 10, attention, medial prefrontal cortex, mentalizing, theory of mind
Recent studies have pointed to the medial rostral prefrontal cortex (MPFC) as a region of the human brain that plays a crucial role in social cognition (Amodio and Frith, 2006). Functional neuroimaging studies have consistently reported MPFC activation related to mentalizing (Frith and Frith, 2003), i.e. attributing mental states to other agents. Additional studies have reported MPFC activation associated with reflection upon one's own emotions (Lane et al., 1997; Gusnard et al., 2001) and character traits (Johnson et al., 2002; Macrae et al., 2004). It has therefore been proposed that this region ‘is engaged when we attend to our own mental states as well as the mental states of others’ (Frith and Frith, 2003, 467).
Other studies have suggested a role of MPFC [approximating Brodmann Area (BA) 10] in attentional selection between stimulus-oriented (SO) and stimulus-independent (SI) thought (Burgess et al., 2005). For example, in one study (Gilbert et al., 2005) MPFC activity was consistently observed in three separate tasks whilst participants attended to visually presented information (‘SO phases’), compared with when they performed the same tasks ‘in their heads’ (‘SI phases’). This activity was unrelated to task difficulty and a subsequent study ruled out an explanation in terms of task-unrelated thought during SO phases because MPFC activity was positively related to performance (Gilbert et al., 2006a, 2006b). In addition, potential differences in ‘working memory’ demands between conditions were unable to explain these results, because greater MPFC activity was observed in the conditions that, if anything, had reduced working memory demands. Consistent with these findings, other studies have reported medial rostral PFC activation in a variety of tasks requiring strong attentional engagement with the external environment. For example, Small et al. (2003) found that activity in this region was associated with deployment of visual attention toward specific regions of space, in a spatial cueing paradigm (Posner, 1980) and Janata et al. (2002) found that activity in medial rostral PFC varied systematically according to the musical key of a melody in an auditory vigilance task.
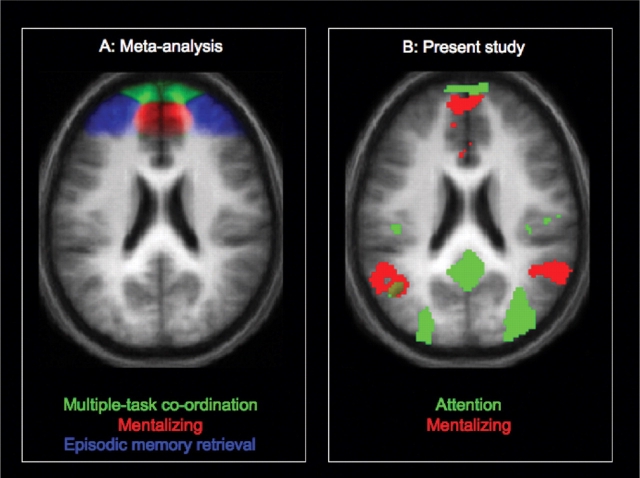
These results present a paradox. While studies investigating social cognition have suggested that MPFC activity reflects self-referential mental processes, studies investigating attentional selection have suggested a role for MPFC in attention towards perceptual information. A possible resolution of this paradox was suggested by a recent meta-analysis of functional imaging studies that reported activation peaks within BA 10 (Gilbert et al., 2006c). Activation peaks from studies involving mentalizing and self-reflection tasks were significantly caudal to those from studies involving other tasks. Conversely, activation peaks from studies involving multiple-task co-ordination (previously argued to depend upon selection between SO and SI thought; Burgess et al., 2003) were significantly rostral to those from other studies. This suggests that caudal and rostral MPFC may be preferentially involved in social cognition and attentional selection respectively. However, convincing segregation of function is only given by imaging data for which the two kinds of task have been performed by the same subject in the same experiment. The present study therefore employed a 2 × 2 factorial design crossing the factors of attentional focus (SO vs SI) and mentalizing (mentalizing vs non-mentalizing).
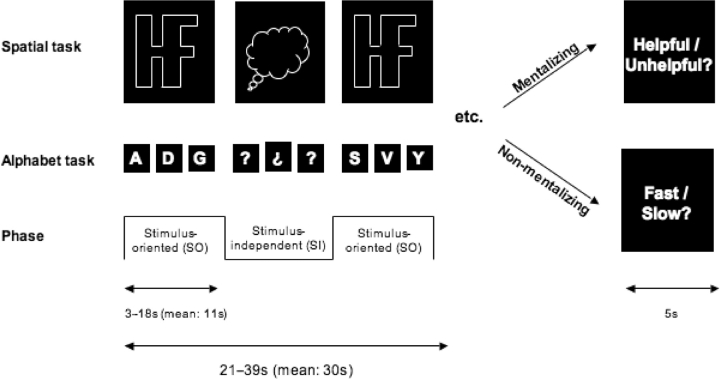
We investigated two of the three tasks originally studied by Gilbert et al. (2005). In both tasks, participants alternated between SO phases, where visual information was task-relevant, and SI phases, where visual information was no longer informative (Figure 1). The transitions between these phases were cued by changes in the appearance of the visual stimuli, and occurred at unpredictable times. Unlike our earlier study, the tasks in the present study were presented in two conditions: mentalizing and non-mentalizing. In mentalizing blocks, participants were told that they were performing the tasks in collaboration with an experimenter (Gallagher et al., 2002), who was able to control the timing of transitions between the SO and SI phases with a button-press. At the end of these blocks (mean duration: 30 s) participants made a judgment as to whether the experimenter was trying to be helpful or unhelpful in his timing of the transitions in that block. In non-mentalizing blocks, participants were told that the timing of these transitions was randomly chosen by the computer. At the end of these blocks, participants judged whether the transitions between phases occurred faster or slower than usual. Thus, both types of blocks were matched in that participants saw identical stimuli and made judgments on precisely the same source of information (the timing of switches between SO and SI phases). However, only in the mentalizing blocks were participants required to interpret this information in terms of the mental state of another agent, i.e. to ‘mentalize’. In actuality, the timing of SO/SI transitions was randomly selected in all blocks.
Fig. 1.
Schematic illustration of the two behavioral tasks. In the ‘spatial’ task (SO phase), participants repeatedly pressed one of two response buttons, as if navigating around the edge of a complex shape in a clockwise direction, to indicate whether the next corner would require a left or a right turn. During the SI phase this shape was replaced by a ‘thought-bubble’ shape and participants were required to imagine the shape that was presented in the SO phase and continue navigating as before. In the ‘alphabet’ task (SO phase), participants classified upper-case letters of the alphabet according to whether they were composed of straight lines or curves. The stimuli cycled through the alphabet, skipping two letters between each stimulus and the next. In the SI phase the letters were replaced with question marks. Participants mentally continued the sequence and continued classifying letters as before.
METHODS
Participants
There were 16 healthy right-handed participants (mean age: 21, range 18–27; nine female). All were healthy UCL students whose first language was English, with no significant medical history of substance abuse, mental illness, head injury or other neurological condition necessitating hospital admission. All provided written informed consent before participating.
Tasks and procedure
In SO phases of the ‘spatial task’ (task 2 of the study of Gilbert et al., 2005), participants repeatedly pressed one of two buttons, as if navigating around the edge of a complex shape in a clockwise direction, to indicate whether the next corner would require a left or a right turn. The stimulus presented during this phase was white, approximately 7°, tall and wide, and shaped similarly to the outlines of the letters H and F placed adjacent to one another, with the vertical line between them removed (Figure 1). A green arrow at the top-right corner of the shape indicated the position from which to start, at the beginning of each block. Following the first button-press response this arrow was removed. During SI phases, the shape was replaced by a similarly sized white ‘thought-bubble’ shape; subjects were required to imagine the shape that was presented in the SO phase and continue navigating from their current position.
In SO phases of the ‘alphabet task’ (task 3 of the study of Gilbert et al., 2005), participants classified capital letters by pressing one of two buttons, according to whether the letter was composed entirely of straight lines, or whether it had any curves. Subsequent letters were presented immediately following each button press, forming a regular sequence that cycled through the alphabet, skipping two letters between each stimulus and the next. Stimuli were presented in white Arial typeface, approximately 1° tall and wide. During the SI phase these letters were replaced with alternating question marks and upside-down question marks. Participants were required to mentally continue the sequence from their current position in the alphabet, performing the same classification task for each self-generated letter. The first letter to be presented in each SO phase was the appropriate continuation of the sequence, assuming that the sequence had been correctly maintained during the preceding SI phase.
Each task was performed in two out of four runs in an AABB order counterbalanced across participants. Within each run, participants performed a total of eight blocks, which alternated between mentalizing and non-mentalizing conditions. A different screen background (dark blue or dark red) was used for each condition, counterbalanced across participants. The length of each block varied randomly between 21 s and 39 s (mean: 30 s). In a randomly selected half of blocks (‘fast blocks’) transitions between the SO and SI phases occurred with a mean rate of every 7.6 s (range 3–18 s). In other blocks (‘slow blocks’) transitions occurred at a mean rate of every 13.5 s (range: 3–18 s). At the end of each block, there was a 1 s pause, followed by a 5 s period during which participants indicated with a button press whether they believed the experimenter was trying to be helpful or unhelpful (in mentalizing blocks) or whether they believed the SO/SI transitions were faster or slower than average (in non-mentalizing blocks). Following this judgment, there was a 5 s reminder whether transitions were to be controlled by the computer or the experimenter in the following block. There was then a variable pause between 5 and 11 s (mean: 8 s) before the next block began (this is referred to below as the ‘stimulus-expectation condition’).
Pre-scan training
Participants took part in a pre-scan training session lasting ∼40 min. They were first read a cover story explaining that the experiment would sometimes involve collaboration with the experimenter (see Supplementary Material). They were then trained on each of the two tasks. Following this, they performed one run of six blocks of each task. These runs were identical to the tasks performed in the experimental session, except that transitions between SO and SI phases in mentalizing blocks were controlled by button presses of the experimenter, who sat next to the participant (in accordance with the cover story).
Scanning procedure
A 3T Siemens Allegra head-only system was used to acquire both T1-weighted structural images and T2*-weighted echoplanar (EPI) images [64 × 64; 3 × 3 mm pixels; echo time (TE), 30 ms] with BOLD contrast. Each volume comprised 36 axial slices (2 mm thick, separated by 1.7 mm, oriented at approximately 10° to the AC-PC plane), covering the whole brain. Functional scans were acquired during four sessions, each comprising 174 volumes (lasting ∼7 min). Volumes were acquired continuously with an effective repetition time (TR) of 2.34 s per volume. The first six volumes in each session were discarded to allow for T1 equilibration effects. Following the functional scans, a 12-min structural scan was performed.
Data analysis
Behavioral data were analyzed as in the previous study of Gilbert et al. (2005). fMRI data were analyzed using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). The volumes were realigned, corrected for different slice acquisition times, normalized into 2 mm cubic voxels using the Montreal Neurological Institute (MNI) reference brain using 4th-degree B-spine interpolation, and smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel.
The volumes acquired during the four sessions were treated as separate time series. For each series, the variance in the BOLD signal was decomposed with a set of regressors in a general linear model (Friston et al., 1995). Separate regressors coded for sustained activity in each of the four main conditions of interest (SO mentalizing, SI mentalizing, SO non-mentalizing, SI non-mentalizing), and the pre-task instruction periods, convolved with a canonical hemodynamic response function. A pair of additional regressors (one for the mentalizing and one for the non-mentalizing condition) indexed the period during which participants made their end-of-block judgments, and a further pair indexed the pause before each run of trials. These regressors, together with the regressors representing residual movement-related artifacts and the mean over scans, comprised the full model for each session. The data and model were high-pass filtered to a cut-off of 1/128 Hz.
Parameter estimates for each regressor were calculated from the least mean squares fit of the model to the data. Effects of interest were assessed in a random effects analysis as follows. Eight contrasts were performed, each contrast individually assessing the variance explained by the regressors representing each of the four main conditions of interest in the two tasks (i.e. Alphabet SO Non-mentalizing; Alphabet SO Mentalizing; Alphabet SI Non-Mentalizing, etc.). These contrasts were entered into a repeated-measures analysis of variance (ANOVA) using non-sphericity correction (Friston et al., 2002). Appropriate contrasts for effects of interest were conducted at the second level, averaging over the two tasks. Contrasts were thresholded at P < 0.05, corrected for multiple comparisons across the whole brain volume (except where stated).
RESULTS
Post-experiment debriefing indicated that no participant was aware that the timing of SO/SI transitions was always random, rather than being under experimenter control during mentalizing blocks, and a pilot study found that participants unanimously described the timing of these switches in terms of the mental state of the experimenter (see Supplementary Material).
Behavioral data: post-block responses
Table 1 shows the mean percentage of ‘slow’ (vs ‘fast’) responses in non-mentalizing blocks, and the mean percentage of ‘unhelpful’ (vs ‘helpful’) responses in mentalizing blocks, separately for ‘fast blocks’ (where transitions between SO and SI phases were relatively rapid) and ‘slow blocks’ (where such transitions were less frequent). Participants distinguished between fast and slow blocks in both mentalizing [F(1,15) = 6.0, P = 0.027] and non-mentalizing [F(1,15) = 9.3, P = 0.008] conditions. In mentalizing conditions, participants were more likely to respond ‘helpful’ in fast vs slow blocks (see Supplementary Material). In neither type of block was there a main effect or interaction involving Task [Spatial or Alphabet; F(1,15) < 2.2, P > 0.16].
Table 1.
Post-block responses
| Non-mentalizing blocks (% ‘slow’ responses) |
Mentalizing blocks (% ‘unhelpful’ responses) |
|||
|---|---|---|---|---|
| Fast blocks | Slow blocks | Fast blocks | Slow blocks | |
| Spatial task | 48.4 (8.1) | 79.7 (6.6) | 43.8 (7.0) | 64.1 (7.6) |
| Alphabet task | 50.0 (7.6) | 79.7 (6.9) | 42.2 (6.3) | 56.3 (7.4) |
Standard errors are shown in parentheses.
Behavioral data: task performance
Behavioral data are presented in Table 2. The two tasks were analyzed separately in 2 (Phase: SO/SI) × 2 (Trial-type: switch, i.e. the trial immediately following a switch between the SO and SI phases vs non-switch) × 2 (Mentalizing: mentalizing/non-mentalizing) repeated measures ANOVAs. The Trial-type factor was included because the present experimental design can be seen as a variant on the task-switching paradigm (see Gilbert et al., 2005 for discussion). In the reaction time (RT) data, there was a main effect of Phase in the Alphabet task [F(1,15) = 139, P < 10−9], with SI trials slower than SO trials, but no significant difference in the Spatial task [F(1,15) = 1.9, P = 0.19]. In both tasks there was a main effect of Trial-type [F(1,15) > 16.6, P < 0.001], switch trials being slower than non-switch trials. In addition, there was a significant Phase × Trial-type interaction in both tasks [F(1,15) > 15.8, P < 0.002]. However, while in the Spatial task this resulted from a greater difference between switch and non-switch trials in SO than SI phases, the interaction resulted from the reverse pattern of results in the Alphabet task. In neither task was there a main effect of Mentalizing, nor any significant interaction involving the Mentalizing factor [F(1,15) < 1.3, P > 0.28]. Thus, participants performed the two tasks equivalently in the mentalizing and non-mentalizing conditions.
Table 2.
Mean reaction time (RT) and error rate in each condition of the two tasks
| Task | Phase | Trial type | Non-mentalizing |
Mentalizing |
||
|---|---|---|---|---|---|---|
| RT | Error (%) | RT | Error (%) | |||
| Spatial | Stimulus-oriented | Non-switch | 965 (99) | 9.3 (2.0) | 993 (95) | 9.4 (1.9) |
| Switch | 1173 (87) | 8.6 (2.7) | 1252 (80) | 12.9 (2.7) | ||
| Stimulus-independent | Non-switch | 1116 (114) | 11.3 (3.3) | 1120 (108) | 9.3 (1.9) | |
| Switch | 1161 (111) | 11.4 (3.6) | 1163 (105) | 9.4 (2.7) | ||
| Alphabet | Stimulus-oriented | Non-switch | 836 (68) | 2.6 (0.5) | 840 (61) | 2.0 (0.5) |
| Switch | 1075 (66) | 3.7 (2.0) | 1054 (72) | 4.0 (2.1) | ||
| Stimulus-independent | Non-switch | 1575 (107) | 13.6 (3.3) | 1570 (107) | 12.0 (3.8) | |
| Switch | 2312 (116) | 15.4 (3.1) | 2321 (151) | 13.3 (4.2) | ||
In the error data, the only significant effect was a main effect of Phase in the Alphabet task [F(1,15) = 14.8, P = 0.002], with more errors being committed in SI than SO phases.
Functional imaging results
Table 3 lists all regions of activation in (i) the contrast of SI vs SO conditions, (ii) the contrast of SO vs SI conditions conditions, and (iii) the contrast of mentalizing vs non-mentalizing conditions.
Table 3.
Regions showing significant differences in BOLD signal between conditions (P < 0.05 corrected for whole-brain volume). Brodmann Areas (BAs) are approximate
| Region | BA | Hemisphere | x | y | z | Zmax | Voxels |
|---|---|---|---|---|---|---|---|
| Stimulus-Independent > Stimulus-Oriented | |||||||
| Insula | 13 | R | 34 | 26 | −4 | 4.8 | 1 |
| 13 | L | −30 | 22 | 2 | 4.8 | 2 | |
| SMA/cingulate gyrus | 6/32 | L | −6 | 18 | 48 | 6.2 | 297 |
| Premotor cortex | 6 | L | −26 | 8 | 54 | 5.4 | 36 |
| Inferior parietal lobule | 40 | L | −44 | −44 | 40 | 5.5 | 40 |
| Medial occipital cortex | 18/19 | L | −8 | −96 | 22 | 4.9 | 6 |
| Stimulus-Oriented > Stimulus-Independent | |||||||
| Medial frontal cortex | 10 | R | −10 | 68 | 20 | 5.0 | 12 |
| 10/9 | R | 2 | 66 | 26 | 5.0 | 9 | |
| 10/11 | – | 0 | 58 | −14 | 5.1 | 50 | |
| 6 | L | −22 | −6 | 74 | 4.8 | 1 | |
| 6 | L | −4 | −18 | 52 | 5.7 | 76 | |
| Postcentral gyrus | 3 | L | −40 | −20 | 56 | >8 | 1490 |
| Inferior parietal cortex | 40 | L | −50 | −22 | 18 | 5.7 | 38 |
| Posterior cingulate/precuneus | 31 | R | 4 | −52 | 32 | 5.6 | 176 |
| Superior parietal cortex | 7 | R | 30 | −56 | 60 | 6.7 | 182 |
| 7 | L | −20 | −62 | 60 | 5.0 | 9 | |
| 7 | R | 26 | −66 | 52 | 4.8 | 6 | |
| Lateral occipital cortex | 18 | R | 34 | −94 | 8 | >8 | 4483 |
| 18 | L | −32 | −94 | 6 | >8 | 2800 | |
| Mentalizing > Non-mentalizing | |||||||
| Medial frontal cortex | 10/9 | L | −8 | 54 | 30 | 4.8 | 2 |
| Temporal pole | 21/38 | R | 48 | 8 | −34 | 5.2 | 33 |
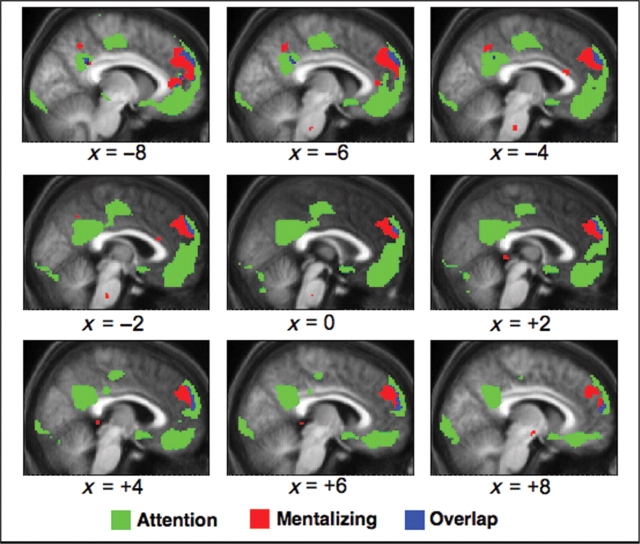
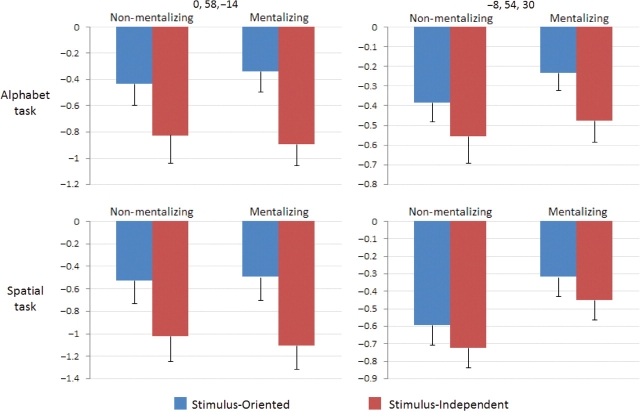
In the SI > SO contrast, there were significant activations in bilateral insula, left supplementary motor area/cingulate gyrus and premotor cortex, left inferior parietal lobule and left medial occipital cortex. In the contrast of non-mentalizing vs mentalizing conditions, there were no significant voxels. Importantly, both the SO > SI contrast and the mentalizing > non-mentalizing contrast revealed activation in MPFC. However, consistent with the meta-analysis (Gilbert et al., 2006c), the activation peak associated with attentional selection was rostral to the activation peak associated with mentalizing (Figure 2). There was virtually no overlap between the regions of activation for these two contrasts, even at a more liberal threshold of P < 0.001 uncorrected (Figure 3). Moreover, analysis of the interaction between the two factors (attentional focus and mentalizing) revealed no active voxels. This interaction was not significant in any of the peak MPFC regions identified in the above contrasts [F(1,15) < 4.3]. Thus, there was no evidence for shared processes underlying attentional selection and mentalizing. The results of this last analysis are presented in Figure 4, with results plotted separately for the peak MPFC regions in the two contrasts and the two tasks. In this graph, the ‘stimulus-expectation condition’ is used as a common reference condition, because it was present in all scans. As shown in the figure, the stimulus-expectation condition was associated with greater signal than any other condition.
Fig. 2.
Panel A: functional subdivision of rostral PFC according to an earlier meta-analysis of functional neuroimaging studies (Gilbert et al., 2006c, adapted from Figure 6). Panel B: regions of activation in the present study for contrasts related to attention (SO > SI, shown in green) and mentalizing (mentalizing > non-mentalizing, shown in red), thresholded at P < 0.001 uncorrected. Results are displayed on axial slices (z = 24) of the participants’ mean normalized structural image.
Fig. 3.
Regions of activation related to attention (SO > SI, shown in green), mentalizing (mentalizing > non-mentalizing, shown in red), and their overlap (blue). Contrasts were thresholded at P < 0.001 uncorrected and plotted on the participants’ mean normalized structural image.
Fig. 4.
BOLD signal associated with the four experimental conditions, in each task. Left panel: results from the peak MPFC region identified in the contrast of SO > SI. Right panel: results from the peak MPFC region identified in the contrast of mentalizing > non-mentalizing. Results indicate the mean parameter estimate in each condition from a 6 mm-radius sphere centered on the relevant activation peak. All results are plotted relative to the stimulus-expectation condition, i.e. the period at the beginning of each block before any stimulus is presented. Error bars represent standard errors.
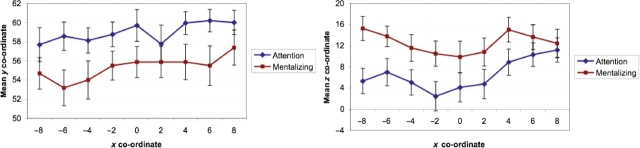
In order to test formally for whether the regions activated by these two contrasts were spatially distinct, peak y and z co-ordinates within BA 10 were extracted on a subject-by-subject basis for each contrast, at each sagittal slice between x = −8 and x = +8 (in this analysis, BA 10 was defined as y ≥ 40; −12 ≤ z ≤ 30). Analysis of these co-ordinates confirmed that activations associated with attentional selection were significantly rostral to those associated with mentalizing [y = 59.9 vs 55.3; F(1,15) = 5.6, P = 0.03], as well as being significantly inferior [z = 6.6 vs 12.5; F(1,15) = 5.2, P = 0.04; Figure 5].
Fig. 5.
Mean peak y (left panel) and z (right panel) co-ordinates within BA 10 for the contrasts related to attention (SO > SI) and mentalizing (mentalizing > non-mentalizing), at each sagittal slice between x = −8 and x = +8. Error bars represent standard errors.
As well as contrasting mentalizing and non-mentalizing conditions during performance of the Alphabet and Spatial tasks, we also compared these conditions during the 5-s period at the end of each block where participants indicated whether the experimenter was being helpful or unhelpful (mentalizing blocks) or whether switches between phases were relatively fast or slow (non-mentalizing blocks). There were no areas of activation at a whole-brain corrected threshold. However, applying a small volume correction to an 8 mm radius sphere centered on the peak MPFC co-ordinate in the contrast between mentalizing and non-mentalizing conditions during task performance (−8, 54, 30), this contrast revealed significant activation in a nearby region of MPFC (−8, 50, 30, 5 voxels, P = 0.035 corrected for multiple comparisons across search volume). In addition, there was significant activation in the original activation peak (−8, 54, 30) at an uncorrected threshold [t(15) = 2.3, P = 0.03]. Thus, a similar region of MPFC was activated both during task performance, when participants were told that they were performing the task with an experimenter, and also at the end of each block, when participants decided whether the experimenter was being helpful or unhelpful.
In a further set of analyses, we investigated potential differences between the two tasks, by examining interactions between the main experimental factors (Phase: SO/SI; Mentalizing: mentalizing/non-mentalizing) and Task (Alphabet/Spatial). There were no regions showing significant Task × Mentalizing activations, suggesting that the mentalizing manipulation had similar effects in the two tasks. In the Task x Phase analyses (Table 4), several posterior brain regions showed significant activations. There was bilateral activation in lateral occipito-temporal cortex, which showed a greater difference between the SO and SI conditions in the Alphabet task than the Spatial task. The reverse contrast revealed activation in left lateral premotor cortex, right superior parietal cortex and widespread activation in medial and lateral occipital cortex, all of which showed a greater difference between the SO and SI conditions in the Spatial task than the Alphabet task.
Table 4.
Regions showing significant Task x Phase interactions (P < 0.05 corrected for whole-brain volume). Brodmann Areas (BAs) are approximate
| Region | BA | Hemisphere | x | y | z | Zmax | Voxels |
|---|---|---|---|---|---|---|---|
| Alphabet (SO > SI) > Spatial (SO > SI) | |||||||
| Lateral occipito-temporal cortex | 37 | R | 54 | −68 | −2 | 7.0 | 222 |
| 37 | L | −50 | −70 | 2 | 5.0 | 21 | |
| Spatial (SO > SI) > Alphabet (SO > SI) | |||||||
| Lateral premotor cortex | 6 | L | −56 | −2 | 46 | 5.0 | 15 |
| Superior parietal cortex | 7 | R | 22 | −64 | 60 | 5.4 | 28 |
| Lateral occipital cortex | 19 | R | 30 | −80 | 18 | 6.4 | 148 |
| Medial occipital cortex | 18 | R | 10 | −96 | 6 | 7.1 | 611 |
It important to note that the Task × Phase interactions failed to reveal any significant voxels in medial prefrontal cortex. In the behavioral data, there was a significant difference in reaction time between SO and SI conditions in the Alphabet task, but not the Spatial task. This resulted in a highly significant Task × Phase interaction [F(1,15) = 130; P < 10−9). If differences in BOLD signal between the SO and SI conditions reflected these behavioral differences (e.g. due to the influence of ‘task difficulty’), a similar Task × Phase interaction would be expected in the BOLD data. However, even at a threshold of P < 0.05 uncorrected, none of the three MPFC regions identified by the SO > SI contrast showed such an interaction. Moreover, even in the Spatial task, where there was no significant difference in reaction time between the SO and SI phases, there was a significant difference in BOLD signal in all three of these regions [F(1,15) > 13, P < 0.003). In neither task was there a significant correlation between behavioral differences between SO and SI conditions and the corresponding BOLD differences in any of these three regions (|r| < 0.3, P > 0.26). Thus, the present results cannot be explained simply by differences in task difficulty between conditions.
Finally, we analyzed the degree to which signal in medial rostral PFC (defined using the same co-ordinates as above) generalized from one task to the other. For each participant we extracted signal at every voxel within this region for each of the four orthogonal contrasts resulting from the factorial crossing of Task and Contrast (i.e. Alphabet Attention, Alphabet Mentalizing, Spatial Attention, Spatial Mentalizing). Because we were interested in the spatial distribution of responses to each of these contrasts, rather than the overall level of activity, the results for each contrast were normalized so that throughout medial rostral PFC there was a mean response of zero, with standard deviation of one. We then calculated the correlation matrix between responses at each voxel to each of these contrasts, separately for each participant. The resulting correlations were entered into one-sample t-tests to test for consistent results across participants (Table 5). Of the six possible pairwise correlations, only the correlations between the two Attention contrasts and the two Mentalizing contrasts were significantly different from zero. Thus, results from the Attention contrast generalized significantly from one task to the other, as did results from the Mentalizing contrast. However, medial rostral PFC responses did not generalize consistently from the Attention to the Mentalizing contrast, or vice versa, even within the same task.
Table 5.
Mean correlation coefficients between medial rostral PFC contrast estimates
| Alphabet task |
Spatial task |
||||
|---|---|---|---|---|---|
| Attention | Mentalizing | Attention | Mentalizing | ||
| Alphabet task | Attention | – | 0.11 | 0.34** | 0.03 |
| Mentalizing | – | 0.04 | 0.17* | ||
| Spatial task | Attention | – | −0.11 | ||
| Mentalizing | – | ||||
*P < 0.05. **P < 0.0005.
DISCUSSION
These results confirm a new axis of functional organization within MPFC, with the most rostral part preferentially involved in tasks requiring SO vs SI thought, and an adjacent caudal (and superior) region preferentially involved in mentalizing (i.e. reflecting on the mental states of another agent). One consequence of this finding is that there need be no contradiction between functional imaging studies reporting MPFC activation associated with mentalizing/self-reflection (involving attention to self-generated information) and those reporting MPFC activation associated with attention to current perceptual input, because different regions of MPFC were activated by these two types of contrast. In addition, there were no voxels yielding a significant interaction between the attention and mentalizing factors, and the interaction effect was not significant in the peak MPFC regions identified by the main effects. Thus, insofar as any MPFC regions showed an effect of both factors, these effects were additive rather than interactive, suggesting an absence of shared underlying processes (cf. additive factors logic; Sternberg, 1969, 1998. For an application of the additive factors logic to fMRI see Reynolds et al., 2006). Of course, a wide variety of processes are likely to contribute to mentalizing tasks. Indeed, recent studies have begun to subdivide such processes and provide evidence for distinct neuroanatomical substrates (e.g. Saxe and Powell, 2006). In this context, it is perhaps even more remarkable that there was so little overlap between MPFC regions involved in mentalizing and attentional selection, given that the mentalizing manipulation is likely to have affected a large range of underlying cognitive processes. Before discussing the implications of these findings, we first consider their relationship with (i) potential differences in ‘task difficulty’ between conditions; (ii) potential differences in ‘working memory’ demands between conditions; and (iii) the issue of activation ‘increases’ or ‘decreases’, compared with a baseline condition.
The finding of increased BOLD signal in medial rostral PFC during SO vs SI attention replicates the earlier findings of Gilbert et al. (2005, 2006a). This increased activation during SO attention is unlikely merely to reflect differences in task difficulty between SO and SI phases. In common with earlier studies (Gilbert et al., 2005, 2006a), signal change in medial rostral PFC was unrelated to task difficulty, as indexed by RT. In addition, there was a significant behavioral difference between the two phases in only one of the tasks (Alphabet task), yet activity in medial rostral PFC was significantly different between the SO and SI phases both Alphabet and Spatial tasks, and signal change associated with the SO > SI contrast did not differ significantly between the two tasks. Another possibility is that activity in medial rostral PFC reflects demands for rehearsal or maintenance of information (i.e. ‘working memory’). However, in the present study increased BOLD signal was observed in SO phases. If anything, these phases had reduced working memory demands, because task-relevant information was perceptually available. Thus, this hypothesis cannot provide a full account of the present results.
In the analysis of BOLD signal associated with the various conditions relative to the ‘stimulus expectation condition’ (i.e. lying in the scanner with no task apart from waiting for upcoming task-relevant stimuli), all conditions were associated with decreased signal. Although this condition was not matched with the other conditions in terms of stimuli, task or any other factor, these results are consistent with previous demonstrations that low-demand conditions are associated with relatively high signal in MPFC (e.g. McKiernan et al., 2003; Gilbert et al., 2006a). However, the suitability of such low-demand conditions (e.g. ‘rest’) as a baseline in neuroimaging studies is at present a matter of considerable debate (Morcom and Fletcher, in press). Because psychological processes are so unconstrained in such conditions, it is unclear whether they are best characterized as involving an unusual degree of SI cognition (i.e. ‘mind-wandering’, e.g. McKiernan et al., 2003), an unusual degree of SO cognition (i.e. watchfulness towards the external environment, e.g. Gilbert et al., 2006a) or a combination of the two (i.e. ‘surveillance of the internal and external environments’, Gusnard et al., 2001, 4259). Thus, rather than relying on comparisons with an arbitrary baseline condition, conclusions in the present study were drawn from comparisons between more constrained experimental conditions.
The finding that activation peaks related to mentalizing were caudal to those related to attentional selection fits well with our earlier meta-analysis (Gilbert et al., 2006c), which showed that studies involving mentalizing tended to produce relatively caudal activations within rostral PFC, compared with studies involving other types of task. Thus, the present results confirm that as well as there being cytoarchitectonic differences between relatively rostral and caudal regions within rostral PFC (Carmichael and Price, 1994), there are corresponding functional differences. In addition to variation along a rostral-caudal axis, we also found that activation peaks related to mentalizing were significantly superior to those involving attentional selection, consistent with previous social cognitive neuroscience studies indicating functional variation along this axis (e.g. Mitchell et al., 2006). Patterns of activity generalized significantly from one task to the other, despite differences in stimulus-materials between the two tasks. However, there were no significant associations between patterns of results within either task when different processes (attention-related vs mentalizing-related) were compared. Thus, the present results are more consistent with a process-specific model of PFC subdivisions (i.e. the idea that different subregions of PFC support different cognitive functions, regardless of the nature of the stimulus materials) rather than a material-specific model (i.e. the idea that different subregions of PFC support the same fundamental cognitive process operating on different categories of stimulus; see Gilbert et al., 2006c for further discussion). Recent studies have suggested a gradient of functional specialization within prefrontal cortex, with representations becoming increasingly abstract in more rostral regions (e.g. Koechlin et al., 2003; Amodio and Frith, 2006). The present results would be consistent with such an account, in the sense that the most rostral part of MPFC showed activity related to the SO vs SI constrast that was not dependent on the particular type of stimulus that was presented, or the specific task being carried out.
One potential interpretation of these results is that the two regions of rostral MPFC identified in the present study both play a role in directing attention towards task-relevant information. However, whereas the most rostral part of MPFC may be preferentially involved in non-social tasks that require biasing of attention towards current perceptual information, the adjacent caudal region may be preferentially involved in social tasks that may require biasing of attention towards other types of information (e.g. emotional information; cf. Lane et al., 1997; Gusnard et al., 2001; Lieberman et al., in press). This view is able to accommodate the wide variety of social and non-social tasks that activate MPFC (Gilbert et al., 2006c), unlike accounts of MPFC function that focus on social or non-social functions alone. Furthermore, the suggestion that rostral PFC is involved in attentional selection between perceptual and self-generated information may potentially explain the role of this brain region in a wide variety of cognitive domains (Burgess et al., 2005). In particular, situations involving co-ordination of multiple tasks and prospective memory demands may depend critically on the ability to select between attending to incoming perceptual information related to the current task and attending to internally represented intentions (Burgess et al., in press). Such situations present particular difficulties to patients with circumscribed rostral PFC damage (Shallice and Burgess, 1991; Burgess, 2000; Bird et al., 2004).
Aside from rostral PFC, the present study revealed signal change in several other brain regions. In the contrast of SI vs SO conditions, activation peaks in the insula, supplementary motor area/cingulate gyrus, premotor cortex and inferior parietal lobule matched closely the activation peaks identified in the study of Gilbert et al. (2006a), which also involved a comparison between SO and SI conditions. The analysis of task-specific effects revealed that the SO vs SI comparison preferentially activated lateral occipito-temporal cortex in the Alphabet task, consistent with a role of this region in processing letter stimuli (Flowers et al., 2004). In the Spatial task, the SO vs SI comparison was associated with other regions of visual cortex (BA 18/19), which may have been involved in perceptual analysis of the shape that participants navigated, along with superior parietal cortex (BA 7), which has previously been implicated in visually guided navigation (e.g. Shelton and Gabrieli, 2002).
In the comparison of SO vs SI conditions, significant activation in brain regions outside MPFC corresponded well with the regions implicated in the earlier studies of Gilbert et al. (2005, 2006a), including posterior cigulate/precuneus and both superior and inferior regions of lateral parietal cortex. In addition, the present study revealed large occipital activations associated with SO vs SI conditions. This may reflect attentional modulation of visual cortical areas, depending on SO vs SI conditions. However, since in this study the visual stimuli were not perfectly matched between conditions, these occipital activations may simply reflect differences between the stimuli used in the two conditions (for evidence of attentional modulation of visual cortical areas depending on SO vs SI conditions, see Gilbert et al., 2006a). Turning now to the mentalizing vs non-mentalizing contrast, the only region showing significant activity besides MPFC was right temporal pole. This region is frequently activated in studies of mentalizing (Frith and Frith, 2003), consistent with its strong anatomical projections with MPFC (Barbas et al., 1999). At an uncorrected threshold, additional activity for the mentalizing vs non-mentalizing contrast was observed in bilateral tempero-parietal junction (Figure 2). This fits well with previous studies suggesting an important role of this region in mentalizing (e.g. Saxe and Wexler, 2005). Thus, the present study adds to the growing literature indicating that both mentalizing and selection between SO and SI thoughts are associated with robust, reproducible patterns of activation (Frith and Frith, 2003; Burgess et al., 2005). Indeed, even within the present study, activity associated with mentalizing and attention generalized significantly from one task to another (although there was no significant generalization between these two contrasts themselves). In addition, despite the anatomical proximity of the MPFC regions associated with attention and mentalizing, the present results indicate that these regions can be dissociated within a single experiment (see also Simons et al., in press), as well as on the basis of a statistical trend across a large number of studies (Gilbert et al., 2006c).
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by the Wellcome Trust (061171).
Footnotes
Conflict of Interest
None declared.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. Journal of Comparative Neurology. 1999;410:343–67. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘theory of mind’ and cognition. Brain. 2004;127:914–28. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Strategy application disorder: the role of the frontal lobes in human multitasking. Psychological Research. 2000;63:279–88. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ, Okuda J, Schölvinck ML, Simons JS. On the role of rostral prefrontal cortex (area 10) in prospective memory. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective Memory: Cognitive, Neuroscience, Developmental, and Applied Perspectives. Mahwah: Erlbaum; in press. [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of rostral frontal cortex (area 10) in prospective memory: a lateral vs medial dissociation. Neuropsychologia. 2003;41:906–18. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan J, Phillips L, McLeod P, editors. Measuring the Mind: Speed, Control and Age. Oxford, England: Oxford University Press; 2005. pp. 217–48. [Google Scholar]
- Carmichael ST, Price JL. Architectoic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. Journal of Comparative Neurology. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Flowers DL, Jones K, Noble K, et al. Attention to single letters activates left extrastriate cortex. Neuroimage. 2004;21:829–39. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RNA, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions Royal Society London B. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. Neuroimage. 2002;16:814–21. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. European Journal of Neuroscience. 2005;21:1423–31. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. Journal of Experimental Psychology: Human. 2006a;32:45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognitive Neuroscience. 2006c;18:932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Frith CD, Burgess PW. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain-behavior associations. Cerebral Cortex. 2006b;16:1783–1789. doi: 10.1093/cercor/bhj113. (in press) [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA; 2001. pp. 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P, Birk JL, Van Horn JD, Leman M, Tillmann B, Bharucha JJ. The cortical topography of tonal structures underlying Western music. Science. 2002;298:2167–70. doi: 10.1126/science.1076262. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM-L, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8:3969–72. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisengerger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Psychological Science. Putting feelings into words: affect labeling disrupts amygdala activity to affective stimuli. (in press) [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Neuroimage. Does the brain have a baseline? Why we should be resisting a rest. (in press) [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, McDermott KB, Braver TS. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cerebral Cortex. 2006;16:519–28. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell L. It's the thought that counts: specific brain regions for one component of theory of mind. Psychological Science. 2006;17:692–9. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal-lobe damage in man. Brain. 1991;114:727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shelton AC, Gabrieli JDE. Neural correlates of encoding space from route and survey perspectives. Journal of Neuroscience. 2002;22:2711–7. doi: 10.1523/JNEUROSCI.22-07-02711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Henson RNA, Gilbert SJ, Fletcher PC. Journal of Cognitive Neuroscience. Separable forms of reality monitoring supported by anterior prefrontal cortex. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18:633–41. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: extensions of Donders’ method. Acta Psychologica. 1969;30:276–315. [Google Scholar]
- Sternberg S. Discovering mental processing stages: the method of additive factors. In: Scarborough D, Sternberg S, editors. An Invitation to Cognitive Science, Volume 4: Methods, Models, and Conceptual Issues. Cambridge: MIT Press; 1998. pp. 704–863. [Google Scholar]