Abstract
The ability to detect agency is fundamental for understanding the social world. Underlying this capacity are neural circuits that respond to patterns of intentional biological motion in the superior temporal sulcus and temporoparietal junction. Here we show that the brain's blood oxygenation level dependent (BOLD) response to such motion is modulated by the representation of the actor. Dynamic social interactions were portrayed by either live-action agents or computer-animated agents, enacting the exact same patterns of biological motion. Using an event-related design, we found that the BOLD response associated with the perception and interpretation of agency was greater when identical physical movements were performed by real rather than animated agents. This finding has important implications for previous work on biological motion that has relied upon computer-animated stimuli and demonstrates that the neural substrates of social perception are finely tuned toward real-world agents. In addition, the response in lateral temporal areas was observed in the absence of instructions to make mental inferences, thus demonstrating the spontaneous implementation of the intentional stance.
Keywords: biological motion, intentional action, social perception, superior temporal sulcus, temporoparietal junction
PERCEIVING AND UNDERSTANDING THE ACTIONS OF INTENTIONAL AGENTS, BOTH REAL AND ABSTRACT
Central to successful daily functioning is the ability to identify, understand and respond to other autonomous agents (Frith and Frith, 1999, 2001; Gallagher and Frith, 2003). From relatively simple perceptual cues, people can readily compute the complex motives and intentions that guide the behavior of others (Fletcher et al., 1995; Castelli et al., 2000; Gallagher et al., 2000; Pelphrey et al., 2003a; Shultz et al., 2004). This effortless adoption of what has been termed the ‘intentional stance’ is a pivotal component of social cognition (Dennet, 1987; Frith and Frith, 2003). Prior to attributing intentions, however, people must first discern the presence of agents. That is, they must parse perceptual information into animate and inanimate categories and classify interactions between objects as mechanical or intentional (Frith and Frith, 1999). For different patterns of motion, critical distinctions are made between animate movement (e.g. self-propelled, possibly non-human, motion) and biological motion (e.g. movement of limbs, faces). Whereas animate movement is characterized by mechanical causation, biological motion reflects non-mechanical contingency or causation at a distance (i.e. psychological causation or intentional movement; Castelli et al., 2000). Importantly, detecting agency from motion cues is believed to be a critical precursor of mentalizing, the ability to construe human behavior as intentional in nature (Scholl and Tremoulet, 2000; Baldwin and Baird, 2001; Gallagher and Frith, 2003). The perception of agency from such cues is often immediate, effortless and automatic, thus distinguishable from more complicated forms of social cognition such as imputing motivations or making judgments regarding beliefs (Scholl and Tremoulet, 2000).
Recent investigations of perceiving biological motion and mentalizing have identified brain areas that are involved in the explicit evaluation of moving objects, usually animated cartoon characters or geometric shapes (Gallagher and Frith, 2003); these include the superior temporal sulcus (STS), medial prefrontal cortex (mPFC) and temporal-parietal junction (TPJ). The presence of this mentalizing network has also been demonstrated in a number of studies that employed non-dynamic representations, such as stories about others (Saxe and Powell, 2006; Saxe and Kanwisher, 2003), photographs of faces (Mitchell et al., 2005b), and semantically presented trait and mental state information (Mitchell et al., 2004; Mitchell et al., 2005a). A parallel though related stream of research related to the understanding of biological motion and intentional action has focused on the pivotal role of the superior temporal sulcus, confirmed by both single-cell recordings in macaques (Jellema et al., 2000) and neuroimaging techniques in humans (Allison et al., 2000; Pelphrey et al., 2004a; Saxe et al., 2004; Thompson et al., 2005).
Studies that examine the mentalizing network typically involve presenting abstract representations of social agents (e.g. story characters, animated cartoon characters), and asking participants to make a social judgment or mental inference. While informative, this methodological strategy nevertheless raises two interesting theoretical issues. First, how are the neural substrates of elicited and explicit social judgments related to the response that accompanies the spontaneous (i.e. implicit) implementation of the intentional stance? Second, the employment of abstract representations (such as geometric shapes) begs the question of whether the neural system that detects agency is sensitive to the form of the cue provider (i.e. the moving agent)? Does it make a difference if identical patterns of biological motion are generated by real people or animated characters? Stated differently, to what degree can research using abstract shapes be generalized to real-world implementations of the intentional stance? While we are certainly able to view a wide variety of stimuli as intentional, the degree to which this process reflects our everyday perception of conspecifics is unclear. One previous investigation into this topic exists, but the conclusions that can be drawn from it are limited as the biological motion presented in the cartoon and live-action conditions were not identical (Han et al., 2005). Our own unique stimuli overcame this important problem.
METHODS
Matching cartoon and live-action actors on biological motion
To address these questions, we used a unique set of stimuli derived from the film Waking Life (Palotta and Linklater, 2001) and a paradigm in which no explicit judgments were solicited. Originally shot with human actors, the footage of Waking Life was transformed by computer animators into a cartoon in which the motion of its characters is identically matched to the original movie. We edited segments of the original footage together with the relevant cartoon sequences to create a video in which animated and live-action shots were interleaved (Figures 1 and 2A). Importantly, each shot was shown both in its animated and its live-action format, thereby ensuring that subjects saw identical biological movement in both conditions. The videos contained 29 different scenes (situations involving the same characters and setting) that were each composed of between 1 and 15 shots (continuous footage between edits; 120 different shots total) which alternated between live-action and animated versions. Alternation of the live-action and animated shots within scenes was employed to prevent adaptation to either of the presentation-types. Each shot included at least one person within the frame, and scenes typically depicted characters moving or engaging in social interaction. Shots were on average 4.7 s (s.d. = 3.7, Range = 0.23—18.9), and scenes averaged 19.9 s (s.d. = 12.5, Range = 3.2–55.7). Each scene was presented twice over the course of a single block of presentations, and the alternation was mirrored, so that if the first viewing began with a live-action shot followed by an animated shot, the second viewing started with an animated shot followed by a live-action shot (Figure 1). A fixation cross was presented between scenes, for either 4 (32 instances), 8 (17 instances) or 16 s (10 instances), with duration pseudo-randomly determined. Scenes were pseudo-randomly ordered so that no scene was presented twice back-to-back and the alternation was balanced so that scenes beginning with a live-action shot were seen first half of the time; this controlled for adaptation effects that may have occurred upon seeing the same scene twice (cartoon or live-action presentation was not confounded with second-viewings). Although the same order of scenes was presented to all participants, cartoon and live-action footage was evenly distributed across the presentation block so order effects cannot account for differences between the two stimulus types.
Fig. 1.
Example scene from stimuli movie in which real and cartoon shots were interleaved, creating two separate versions of the same scene that together presented identical footage in both cartoon and live-action forms.
Fig. 2.
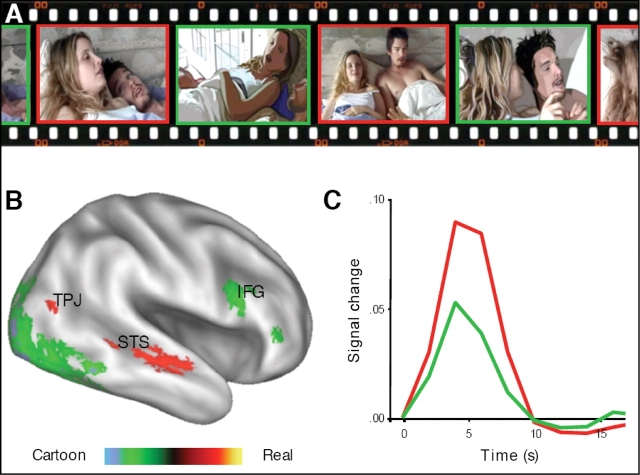
(A) Sample of video with real (red) and computer-generated (green) actors. (B) An inflated cortical representation of the right-hemisphere shows greater activation for live-action than for animated sequences in the TPJ and STS. Greater activation for animated than for live-action sequences was observed in the lateral occipital cortex (LOCC; see Table 2), and the inferior frontal gyrus (IFG). (C) Hemodynamic response function observed in the STS for live-action (red) and animated (green) sequences. The TPJ showed a similar pattern of activation.
Participants were told to simply watch the videos closely; unlike in most previous studies, no explicit instructions to draw mental inferences were given.
Brain scanning methods, procedure, and analysis
A total of 19 participants (10 female) aged 17–43 years (M = 22.1, s.d. = 6.2) passively viewed the video without sound while undergoing fMRI scanning with a GE 1.5T MRI scanner using a standard birdcage headcoil. A screening process ensured that no participant had previously seen Waking Life. T2*-weighted functional images were collected using a gradient echo EPI sequence (20 axial slices, 5.5 mm thick, 1 mm apart; TR = 2000 ms; TE = 35 ms; flip angle = 90°; FOV = 24 cm) during viewing. The stimuli were presented in two runs, each run 410 volumes in length (the first 5 volumes were discarded to ensure signal equilibration). T1-weighted high-resolution anatomical scans were also collected for each participant using a 3D sequence (SPGR; 128 saggital slices, 1.2 mm thick; TR = 25 ms; TE = 6 ms; flip angle = 15°; FOV = 24 cm).
All preprocessing and statistical analysis was performed using SPM99 (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm). Functional images were corrected for motion, coregistered to anatomical images, then both were normalized to standardized Montréal Neurological Institute (MNI) space using the filT1 template, and smoothed using a 6 mm full-width-at-half-maximum Gaussian kernel. Contrasts (t-tests) comparing blood oxygenation level dependent (BOLD) responses for events time-locked to the onset of realistic footage vs the onset of cartoon stimuli (and vice versa) were calculated (P < 0.001, minimum cluster-size of 5 voxels) for each individual, and a random-effects model was applied to all contrasts in order to examine group-level effects.
RESULTS
Social brain areas prefer live-action actors to cartoon characters
Medial areas of occipital cortex (e.g. cuneus, lingual gyrus) and subcortical sensory pathways (e.g. thalamus, caudate) showed a greater BOLD response during viewing of real scenes than during viewing of animated scenes (Real > Animated contrast; Table 1). More interestingly, however, the right STS and right TPJ also showed a greater response during the live-action portrayals compared to the animated reconstructions (Figure 2B, Table 1). While these areas were engaged by the presentation of cartoon characters, in line with previous research, the STS and TPJ are clearly more engaged when participants view live-action agents (Figure 2C). Another brain region associated with social-cognitive processing, the right middle frontal gyrus (MFG), was also more active during live-action portrayals of social interaction (See Table 1).
Table 1.
Brain regions more associated with live-action depictions relative to animated depictions of action
| Region | BA | x | y | z | # Voxels | Peak Z-Score |
|---|---|---|---|---|---|---|
| R STS | 21/22 | 56 | −35 | 5 | 87 | 4.23 |
| 53 | −21 | −4 | 3.73 | |||
| 53 | −12 | −12 | 3.72 | |||
| R TPJ | 39 | 50 | −63 | 20 | 10 | 3.75 |
| R MFG | 8/9 | 24 | 40 | 37 | 11 | 3.75 |
| B mOCC (Lingual gyrus) | 17/18 | 9 | −76 | 4 | 1303 | 5.53 |
| (Lingual gyrus) | 17/18 | −9 | 73 | 4 | 5.22 | |
| (Cuneus) | 18/19 | 12 | −74 | 26 | 4.92 | |
| R cuneus | 17/18 | 24 | −55 | 14 | 40 | 3.89 |
| B mOCP | 18 | 0 | −88 | −13 | 6 | 3.71 |
| PCC | 23/31 | 0 | −45 | 30 | 105 | 4.16 |
| (R precuneus) | 7 | 3 | −44 | 49 | 3.96 | |
| (L precuneus) | 7/31 | −6 | −47 | 47 | 3.46 | |
| L PCC | 23 | −12 | −48 | 33 | 5 | 3.49 |
| R precuneus | 7 | 3 | −58 | 61 | 55 | 3.85 |
| L precuneus | 7 | −9 | −67 | 61 | 5 | 3.45 |
| B THL | 3 | −26 | 10 | 19 | 4.03 | |
| L CAUD | −15 | −2 | 17 | 20 | 4.02 | |
| L SCB | −18 | −44 | −10 | 12 | 4.02 |
Threshold P < 0.0005 (uncorrected), extent threshold = 5 voxels; coordinates follow atlas by Talairach and Tournoux (1988); BA, Brodmann area; STS, superior temporal sulcus; TPJ, temporoparietal junction; MFG, middle frontal gyrus; mOCC, medial occipital cortex; mOCP, medial occipital pole; PCC, posterior cingulate cortex; THL, Thalamus; CAUD, caudate; SCB, superior cerebellum. A slightly higher than traditional threshold was employed since at P < 0.001 medial occipital activations obscured unique medial parietal peaks. At P < 0.001, activations are present in the L STS (−50, 72, 2; k = 18; Z = 3.70), L TPJ (−48, −60, −12; k = 6; Z = 3.42), left posterior dorsomedial frontal cortex (−3, −3, 50; k = 8; Z = 3.37), and bilateral posterior cerebellum (3, −83, −26; k = 6; Z = 3.30).
Split-half reliability analysis
A formal replication analysis was conducted in order to test the reliability of these results for the STS and TPJ. Data for each participant was split into two separate data sets: a hypothesis-generating data set and a hypothesis-testing data set. Each set represented the viewing of equivalent video content but was mirrored in presentation format (i.e. video segments that were real in the first data set were animated in the second data set and vice versa). Regions of interest were defined based on peak activations in the STS and TPJ from the hypothesis-generating data set for the Real > Animated contrast [all significant voxels (P < 0.01) within 8 mm of the peak]. This analysis identified activations in the right STS (60, −42, 6; 51, −24, −6) and TPJ (54, −63, 12). Activations in these locations were then formally tested for replication in the hypothesis-testing data set. For each participant, parameter estimates of signal change for each region were computed, averaged and submitted to a one-sample t-test (H0 = 0). Both activations replicated in the hypothesis-testing data set, for the STS (P < 0.001), and TPJ (P < 0.05), heightening our confidence in the reliability of this result.
Brain regions more sensitive to cartoon than live-action agents
The reverse contrast, examining greater activations for perceiving cartoon agents relative to live-action actors (Animated > Real), failed to reveal a response in any area characteristically associated with mentalizing. Instead, activations were observed in the ventrolateral occipitotemporal cortex (including the fusiform gyrus) extending dorsally up to the lateral occipital cortex, intraparietal sulci, and premotor and somatosensory cortices (Table 2).
Table 2.
Brain regions more associated with animated depictions relative to live-action depictions of action
| Region | BA | x | y | z | # Voxels | Peak Z-Score |
|---|---|---|---|---|---|---|
| L VLOCC | 18/19, 37 | −33 | −90 | 7 | 1385 | 5.67 |
| −30 | −92 | 24 | 5.66 | |||
| −33 | −82 | −9 | 5.50 | |||
| R VLOCC* | 18/19, 37 | 39 | −88 | −3 | 1686 | 5.47 |
| 33 | −87 | 10 | 5.29 | |||
| 30 | −83 | 24 | 5.21 | |||
| R anterior IFG | 10 | 53 | 38 | 1 | 34 | 4.38 |
| 56 | 43 | −7 | 3.87 | |||
| L IPS | 7 | −27 | −61 | 53 | 71 | 4.35 |
| −33 | −50 | 49 | 3.43 | |||
| R PMC | 44 | 48 | 13 | 19 | 121 | 4.31 |
| 9 | 53 | 13 | 27 | 4.12 | ||
| 6/8 | 59 | 14 | 38 | 3.55 | ||
| R OFC | 11 | 24 | 40 | −20 | 14 | 4.28 |
| L PMC | 6/8 | −48 | 1 | 28 | 6 | 3.91 |
| L OFC | 11 | −36 | 48 | −23 | 9 | 3.76 |
| R SFG | 6 | 33 | 0 | 55 | 7 | 3.64 |
| R inferior PoCG | 2 | 48 | −30 | 35 | 8 | 3.58 |
| R superior PoCG | 3 | 53 | −18 | 56 | 7 | 3.44 |
Threshold P < 0.0005 (uncorrected), extent threshold = 5 voxels; coordinates follow atlas by Talairach and Tournoux (1988); BA, Brodmann area; VLOCC, ventrolateral occipital cortex; IFG, inferior frontal gyrus; IPS, intraparietal sulcus; PMC, premotor cortex; OFC, orbitofrontal cortex; SFG, superior frontal gyrus; PoCG, post-central gyrus; *extends to R IPS, (36, −59, 53, Z = 4.77). At P < 0.001, no additional activations are revealed.
DISCUSSION
A number of areas that were preferentially activated by live-action portrayals of social agents have been implicated in research on biological motion and mental inference. Some of the conclusions drawn by these previous studies, however, bear closer examination in light of the findings reported herein.
Lateral temporal areas and intentional motion
The STS and TPJ have previously been implicated in both the detection of biological motion (Beauchamp et al., 2002) and the process of mental state attribution (Frith and Frith, 1999; Gallagher and Frith, 2003). Pelphrey and colleagues (2003a), for example, have demonstrated that the right STS is preferentially involved when biological motion is presented, but not for non-biological but coherent and meaningful movements. In this case, biological motion was operationalized in two ways, as a computer-generated person walking in profile, or a ‘robot’ that moved identically, composed of cylinders and spheres. These researchers found that the STS did not discriminate between these two presentations, leading them to conclude that this area is sensitive to biological motion but not the surface features of a moving object. While our own results appear to contradict their conclusion, this divergence may be explained by differences in stimuli. The computer-generated animations employed by Pelphrey and colleagues (2003a) were clearly not accurate representations of a real intentional human, in contrast to the actors in our live-action segments. The STS may thus be preferentially activated by representations of social agents that closely approximate the real-world, while responding less to all cartoon and unrealistic representations, perhaps equally (cf. Perani et al., 2001).
By examining other aspects of biological motion, such as shifts in gaze (Pelphrey et al., 2003b; cf. Pelphrey et al., 2004b) and reaching movements (Pelphrey et al., 2004a), Pelphrey and his colleagues have concluded that the STS not only codes for biological motion, but it appears to be most involved when decoding the intentions behind actions. Saxe and colleagues (2004) have reported a similar conclusion, showing that with biological motion held constant, the STS is more engaged when this motion implies the operation of intention or motivation. It seems plausible, then, that the current findings demonstrate that our brains perceive biological motion enacted by real people as more intentional in flavor.
In sum, previous research has shown that the STS and TPJ are active during the presentation of articulated motion (Beauchamp et al., 2002; Beauchamp et al., 2003), and for very abstract representations of intentional action such as moving shapes (Allison et al., 2000; Beauchamp et al., 2003; Shultz et al., 2004), leading some to conclude that form is irrelevant for processing biological motion (Pelphrey et al., 2003a) and in mentalizing (Shultz et al., 2004). Here we show, however, that these temporal social perception areas are most responsive when subjects view real people compared to viewing animated cartoon characters.
Right middle frontal gyrus and mentalizing
The right MFG, also more sensitive to live-action relative to cartoon agents, has been implicated in a number of studies that pertain to mentalizing and perceptions of agency. Blakemore and colleagues (2003) observed that this area was only engaged when attention was explicitly directed (via task-instructions) toward the contingent relationships between moving shapes, and thus concluded that it plays a top-down role in social perception. Congruent with this idea, the right MFG is more active when people are explicitly asked to make judgments about persons as opposed to dogs (Mason et al., 2004), or when instructed to make mental inferences based upon a person's eye-region (Platek et al., 2004). This area has also been activated in a study on decoding intentionality that lacked task instructions (Pelphrey et al., 2004a), as did the current investigation. Preferential activity in this area may thus provide evidence that greater explicit mentalizing, or perceptions of intentionality, spontaneously occurred when participants were viewing the live-action actors.
Medial parietal regions and mentalizing
Medial parietal areas, such as the precuneus and posterior cingulate also exhibited a greater BOLD response to the live-action actors compared to the cartoon agents. These regions have often been implicated in studies of social cognition and mentalizing (Saxe and Wexler, 2005). A number of studies have found preferential involvement of this region when taking another's perspective (Ruby and Decety, 2001; Jackson et al., 2006), perhaps especially with respect to their thoughts rather than bodily sensations like hunger (Saxe and Powell, 2006). It is also involved when making judgments regarding the self (Johnson et al., 2002) and appears to be involved in the processing of emotions (Ochsner et al., 2004), possibly social emotions in particular (Britton et al., 2006). Thus, its preferential engagement during the presentation of live-action social agents relative to cartoon agents supports our claim that social processing brain areas are especially tuned to realistic representations of conspecifics. However, the precuneus is also known to be involved in the encoding of spatial relations (Frings et al., 2006) and its greater response for live-action segments might also reflect either more, or more realistic, spatial information for these stimuli (e.g. depth perspective).
Spontaneous social perception and solicited social judgments
An overlap exists between the network of brain areas we observed during the spontaneous adoption of the intentional stance and those normally associated with explicit social judgments (Frith and Frith, 2003). Saxe (2006) has argued that the TPJ supports reasoning about mental states (Saxe and Kanwisher, 2003). If this is true, here we show that mentalizing processes implicitly and spontaneously engaged are neurally instantiated in similar ways to explicitly solicited mental inferences. German and colleagues (2004) have published corroborating data, demonstrating that displays of pretense evoke activation in social brain areas without instructions to engage in mentalizing. This finding demonstrates that the activations found by previous researchers are not simply the result of instructions directing participants to engage in mentalizing tasks, but that these regions of the brain are also employed when individuals infer mental states in a natural, non-directed and more ecologically valid manner.
Other brain regions more associated with live-action actors
The live-action footage resulted in more BOLD activation throughout visual-processing areas (e.g. medial occipital cortex, lingual gyrus, cuneus) and subcortical sensory pathways (e.g. caudate, thalamus). These early visual areas are sensitive to cues of perceptual depth (Backus et al., 2001), and the live-action footage certainly presented more such information compared to the cartoon segments, which appeared somewhat flat in comparison. Subcortical sensory pathways may have been more activated by the greater information contained within the live-action video segments.
Brain regions associated with cartoon actors
A number of the activations preferentially engaged by cartoon agents have been found to prefer non-human (e.g. tool) motion over biological motion, or fail to discriminate between such stimuli, such as the premotor cortices and intraparietal sulci (Beauchamp et al., 2002, 2003; Pelphrey et al., 2003a). The superior frontal gyrus activation witnessed during this contrast was rather caudally located, and is best described as belonging to part of the former structure. Greater activation in ventrolateral occipitotemporal visual areas (e.g. fusiform gyrus) were likely due to the fact that the cartoon segments contained far brighter colors (Beauchamp et al., 1999). These activations extended along the lateral surface of the occipital cortex, into areas of the brain that have been associated with subjective percepts, specifically of objects and shapes (Grill-Spector and Malach, 2004), possibly indicating that more effort was required to parse the cartoon presentations into recognizable objects. Perhaps the most interesting activations for this contrast were in the bilateral orbitofrontal cortex. This region is most often associated with reward, with the anterior portion responding to even quite abstract reinforcers such as music (Kringelbach, 2005). Our own activations in this region correspond to the anterior orbitofrontal cortex, and may indicate that the cartoon stimuli were viewed as more novel and thus more rewarding.
Possible confounds
A possible weakness in the design of this study is that the BOLD differences observed could be the result of other, more superficial, differences between the cartoon and live-action video. Although biological motion was controlled across conditions, the two types of stimuli did differ along some other visual dimensions and perhaps these differences can account for the preferential activations observed, rather than the perception of these stimuli as either cartoon or realistic. Mentalizing brain areas have not previously been shown to respond to simple visual manipulations such as depth, brightness and contrast, however (Grill-Spector and Malach, 2004). Future investigations employing a parametric or factorial design would clarify this issue.
CONCLUSION
Utilizing a set of unique stimuli, we have shown that areas of the brain associated with social perception and mentalizing (rSTS, rTPJ, rMFG) are preferentially responsive to live-action portrayals of human action, even in the absence of explicit mentalizing instructions. Thus, while a range of moving stimuli can trigger the implementation of the intentional stance, this mindset is most successfully activated when biological motion is generated by another person. The neural system that supports mentalizing thus appears to be finely tuned to an agentic understanding of real human movements. Moreover, our data show that the intentional stance, spontaneously engaged without explicit instructions, involves a network of brain areas similar to that which supports overt social judgments.
Acknowledgments
The authors would like to extend thanks toward K. Demos, C. Hynes, L. Somerville, and especially J. Moran for help with the statistical analyses. They would also like to thank the Dartmouth Brain Imaging Center for funding this study.
Footnotes
Conflict of Interest
None declared.
REFERENCES
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Backus BT, Fleet DJ, Parker AJ, Heeger DJ. Human cortical activity correlates with stereoscopic depth perception. Journal of Neurophysiology. 2001;86:2054–68. doi: 10.1152/jn.2001.86.4.2054. [DOI] [PubMed] [Google Scholar]
- Baldwin DA, Baird JA. Discerning intentions in dynamic human interaction. Trends in Cognitive Sciences. 2001;5:171–8. doi: 10.1016/s1364-6613(00)01615-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Haxby JV, Jennings JE, DeYoe EA. An fMRI version of the Farnsworth–Munsell 100-Hue test reveals multiple color-selective areas in human ventral occipitotemporal cortex. Cerebral Cortex. 1999;9:257–63. doi: 10.1093/cercor/9.3.257. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:149–59. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. fMRI responses to video and point-light displays of humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Boyer P, Pachot-Clouard M, Segebarth C, Decety J. The detection of contingency and animacy from simple animations in the human brain. Cerebral Cortex. 2003;13:837–44. doi: 10.1093/cercor/13.8.837. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, LIberzon I. Neural correlates of social and nonsocial emotions: an fMRI study. NeuroImage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Dennet D. The Intentional Stance. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- Fletcher PC, Happé F, Frith U, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Frings L, Wagner K, Quiske A, et al. Precuneus is involved in allocentric spatial location encoding and recognition. Experimental Brain Research. 2006;173:661–72. doi: 10.1007/s00221-006-0408-8. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds–A biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10:151–5. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London, Series B. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- German TP, Niehaus JL, Roarty MP, Giesbrecht B, Miller MB. Neural correlates of detecting pretense: automatic engagement of the intentional stance under covert conditions. Journal of Cognitive Neuroscience. 2004;16:1805–17. doi: 10.1162/0898929042947892. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. The human visual cortex. Annual Review of Neuroscience. 2004;27:647–77. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- Han S, Jiang Y, Humphreys GW, Zhou T, Cai P. Distinct neural substrates for the perception of real and virtual worlds. NeuroImage. 2005;24:928–35. doi: 10.1016/j.neuroimage.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jellema T, Baker CI, Wicker B, Perrett DI. Neural representation for the perception of the intentionality of actions. Brain and Cognition. 2000;44:280–302. doi: 10.1006/brcg.2000.1231. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Mason MF, Banfield JF, Macrae CN. Thinking about actions: the neural substrates of person knowledge. Cerebral Cortex. 2004;14:209–14. doi: 10.1093/cercor/bhg120. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji M, Macrae CN. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. Journal of Neuroscience. 2004;24:4912–7. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji M, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. NeuroImage. 2005a;28:757–62. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji M, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Science. 2005b;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Palotta T., (Producer), Linklater R., (Director) Waking Life, [Motion Picture] Los Angeles, USA: Fox Searchlight Pictures; 2001. [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeowen MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. The Journal of Neuroscience. 2003a;23:6819–25. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003b;41:159–70. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004a;16:1706–16. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004b;15:598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Perani D, Fazio F, Borghese NA, et al. Different brain correlates for watching real and virtual hand actions. NeuroImage. 2001;14:749–58. doi: 10.1006/nimg.2001.0872. [DOI] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Jr, Mohamed FB. Where am I? The neurological correlates of self and other. Cognitive Brain Research. 2004;19:114–22. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4:546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16:1–5. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about people: the role of temporoparietal junction in “theory of mind”. NeuroImage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's the thought that counts: specific brain regions for one component of theory of mind. Psychological Science. 2006;17:692–9. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao D-K, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–49. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Scholl BJ, Tremoulet PD. Perceptual causality and animacy. Trends in Cognitive Sciences. 2000;4:299–309. doi: 10.1016/s1364-6613(00)01506-0. [DOI] [PubMed] [Google Scholar]
- Shultz J, Imamizu H, Kawato M, Frith CD. Activation of the human superior temporal gyrus during observation of goal attribution by intentional objects. Journal of Cognitive Neuroscience. 2004;16:1695–705. doi: 10.1162/0898929042947874. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Thompson JC, Clarke M, Stewart T, Puce A. Configural processing of biological motion in human superior temporal sulcus. Journal of Neuroscience. 2005;25:9059–66. doi: 10.1523/JNEUROSCI.2129-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]