Abstract
Extraversion has been shown to positively correlate with activation within the ventral striatum, amygdala and other dopaminergically innervated, reward-sensitive regions. These regions are implicated in emotional responding, in a manner sensitive to attentional focus. However, no study has investigated the interaction among extraversion, emotion and attention. We used fMRI and dynamic, evocative film clips to elicit amusement and sadness in a sample of 28 women. Participants were instructed either to respond naturally (n = 14) or to attend to and continuously rate their emotions (n = 14) while watching the films. Contrary to expectations, striatal response was negatively associated with extraversion during amusement, regardless of attention. A negative association was also observed during sad films, but only when attending to emotion. These findings suggest that attentional focus does not influence the relationship between extraversion and neural response to positive (amusing) stimuli but does impact the response to negative (sad) stimuli.
INTRODUCTION
Philosophers dating back over two millennia to Plato (and beyond) have speculated about the nature of individual differences in motivation and emotion. One fundamental personality dimension that has received considerable attention in this regard is extraversion (Costa and McCrae, 1980; Goldberg, 1990; John and Srivastava, 1999). A consensus has emerged that differences in extraversion involve differences in emotional reactivity (Gross et al., 1998; Depue and Collins, 1999); however, the literature has also been characterized by conflicting findings that make definitive categorization of this affective response style elusive. With the advent of modern neuroimaging techniques, new insights into the biological bases of personality are emerging, and scientists have begun to link variation in brain function to extraversion, promising a better understanding of this trait.
Behaviorally, extraversion is associated with a tendency to be assertive, to experience excitement and positive affect and to enjoy social situations (Costa and McCrae, 1980; Depue and Collins, 1999; Lucas et al., 2000). Researchers have attempted to link this profile to differences in affective reactivity to specific positive and negative stimuli, but it remains unclear how best to characterize these differences. Extraversion is consistently linked to greater sensitivity to positive or rewarding stimuli (Depue and Collins, 1999; Lucas et al., 2000). Extraverts report more positive affect while watching funny films (Gross et al., 1998; Morrone et al., 2000), imagining themselves in happy events (Larsen and Ketelaar, 1991), and in a variety of other situations (Lucas and Fujita, 2000). However, there is disagreement over whether extraverts respond more strongly than introverts to all classes of rewarding stimuli generally, or more specifically to social rewards (Diener et al., 1984; Lucas et al., 2000; Lucas and Diener, 2001; Ashton et al., 2002).
Extraversion has also been linked with negative emotional responding, although findings have been considerably more mixed. Most researchers note little relationship with negative affect, which is more strongly associated with neuroticism (Diener et al., 1984; Gross et al., 1998). Others have found extraversion to be associated with decreased experience of negative affect. Extraverts experience less negative affect during sad films (Lischetzke and Eid, 2006) and when imagining themselves in unpleasant situations (Larsen and Ketelaar, 1991), and report lower levels of negative mood across the day than introverts (Stewart et al., 2005). On the other hand, some researchers have reported a positive relationship between extraversion, crying and sadness (Choti et al., 1987; Peter et al., 2001). It is not clear how to reconcile these conflicting findings, and it may be that differences in the context, or the method of induction and measurement of emotion and personality exert an important, although as yet unspecified, moderating effect on these relationships.
Recent advances in the ability to dynamically, non-invasively image the human brain promise new insight regarding these issues. As our understanding about the functions of different neural circuits grows, and as we link differences within those circuits to individual differences in personality, we may develop a more nuanced understanding of the relationship between extraversion and emotion. Yet, only a relatively small number of studies have examined the neural bases of extraversion, in a limited number of contexts.
In these studies, researchers frequently observe a correlation between extraversion and a set of dopaminergically innervated regions, including the ventral striatum [comprised of the nucleus accumbens (NAcc), as well as ventral caudate and putamen], amygdala and medial prefrontal cortices (Depue and Collins, 1999). These regions appear to be critical to incentive processing (Olds and Milner, 1954; Breiter et al., 2001; Knutson et al., 2001a, b; O’Doherty et al., 2002; Kringelbach et al., 2003). However, which specific components of this response relate to extraversion and in what direction, remains uncertain. Extraversion has been found to correlate positively with the response in the nucleus accumbens, medial prefrontal (MPFC) and orbitofrontal cortex (OFC) to receipt (but not anticipation) (Cohen et al., 2005) and to anticipation (but not receipt) (Knutson and Bhanji, 2006) of monetary rewards. Researchers have also found that extraverts respond more strongly in the amygdala to smiling faces and positive pictures (Canli et al., 2001, 2002) and to pleasant smells (Vaidya et al., 2007); however, Mobbs et al. (2005) find that extraverts respond less strongly in the amygdala to amusing cartoons. These findings support the link between extraversion and positive emotion; however, their discrepancies also highlight the poorly understood difference between various types or components of the reward response. Interpretation of these results is further complicated by data demonstrating the involvement of both the amygdala and ventral striatum in the processing of negative stimuli (Adolphs et al., 1999; Becerra et al., 2001; Phan et al., 2002; Reynolds and Berridge, 2001, 2002; Yacubian et al., 2006; Seymour et al., 2007), although the conditions under which either structure participates in negative or positive processing has yet to be fully specified (Cooper and Knutson, 2007).
One important additional consideration is that recent work suggests that processing within these regions may be modulated by attentional and contextual factors. Attending to emotion activates the medial prefrontal and anterior cingulate cortices (Lane et al., 1997, 1998; Hariri et al., 2000; Ochsner et al., 2004; Taylor et al., 2003; Hutcherson et al., 2005), and also modulates activation in the amygdala (Hariri et al., 2000; Taylor et al., 2003) and ventral striatum (Gorno-Tempini et al., 2001). Furthermore, amygdala activation to ambiguous stimuli, such as surprised faces, varies depending on contextual cues (Kim et al., 2004) and striatal response can depend on the contextual salience of a stimulus (Zink et al., 2004). Such effects may occur because attention alters the salience of aspects of the emotional events with which the amygdala and ventral striatum are concerned.
Attentional factors may play a role in the pattern of affective responding observed in extraverts, who consistently react more strongly to positive stimuli, across a variety of contexts, but show less consistent differences for negative stimuli. If extraversion is associated with responding in the striatum, amygdala and MPFC, and these regions are sensitive to manipulations of attention, then attention may modulate the relationship between extraversion and response to affective stimuli. This hypothesis may explain some of the inconsistencies in the neural or behavioral literatures regarding the relationship between extraversion and positive or negative emotion. In studies where amygdala responds more strongly to positive stimuli in extraverts, participants have either actively attended away from the emotion (e.g. judging gender in facial expression tasks: Canli et al., 2001) or passively experienced the emotion (Canli et al., 2001; Vaidya et al., 2007). In contrast, the sole study to find a negative relationship between amygdala response and funny jokes required participants to actively attend to and rate their emotional responses. In the behavioral literature, too, the extent to which studies focus participants on emotion may help to explain divergent results. Studies that obscure their interest in emotion by focusing participants on cognitive or memory aspects of a task (Larsen and Ketelaar, 1991) tend to find that extraversion predicts less negative emotion, whereas studies finding weak (Gross et al., 1998) or strong (Choti et al., 1987) positive correlations between extraversion and negative emotion tend to focus participants on the emotional experience. Such findings suggest the need to more carefully assess the attentional constraints of a task when exploring personality, although as yet no formal comparison has been made of the relationship between personality and emotion under varying attentional contexts.
Is extraversion differentially related to neural response to negative or positive emotion in more evocative, temporally varying, socially meaningful contexts or under particular attentional conditions? The present study examined the neural correlates of extraversion in dynamic, social, emotionally engaging amusing, sad and neutral films, a context not previously used in neuroimaging studies of this personality trait. Amusement activates reward circuitry (Mobbs et al., 2003). Although sadness has not been linked to the ventral striatum, it has been shown to activate the amygdala, as well as regions involved in social cognition (Goldin et al., 2005). We also identified neutral films that included social interaction, to allow us to fully leverage contrasts between emotions. In order to understand the degree to which attentional factors contribute to heterogeneous responding in extraversion, we included an attentional manipulation: participants either simply watched films, or continuously rated their emotional experience while watching, allowing us to compare effects in the two groups.
Using this approach, we tested (i) whether extraversion is related to positive responsivity only, or also to negative responsivity and (ii) whether the relationship between extraversion and response in regions such as the ventral striatum and amygdala are modulated by attentional focus for positive or negative emotion.
METHOD
Participants
Twenty-eight women (18–21 years) were recruited from the Stanford University community. All participants were right-handed, had normal visual acuity and were screened for history of psychiatric or medical disorders. Only women were enrolled to limit potential gender differences in emotional responding (Wager et al., 2003). Participants provided informed consent in accordance with guidelines set forth by the Medical Human Subjects Committee of Stanford University.
Materials and measures
Personality
Extraversion was assessed using the 48-item extraversion scale of the Revised NEO Personality Inventory (NEO-PI-R: Costa and McCrae, 1992).1 Mean levels of extraversion did not differ between the two groups (MView = 170.71, s.d. = 20.90; MRate = 162.14, s.d. = 12.61, t(26) = 1.31, P < 0.2).
Film stimuli
Participants viewed a series of nine 2 min color film clips. Two amusing and two sad film clips were drawn from a set of previously validated film stimuli (Gross and Levenson, 1995). These included amusing clips involving a single actor in a comedic routine and sad clips involving an adult and a child in a sad interaction. We selected five neutral clips that were matched to the emotional film clips in duration, number of actors and social interaction. The neutral clips consisted of a single actor demonstrating cooking skills or two actors demonstrating home repair, sewing or commercial sales, and were interleaved between each of the emotional films. The clips were presented in one of two counterbalanced orders, beginning with a neutral clip followed either by an amusing or sad clip and alternating emotion type with interleaved neutral films.
Procedure
In a separate session, 1 week prior to scanning, participants completed a packet of questionnaires including the measure of extraversion. During the scanning session, participants viewed the series of amusing, neutral and sad clips within the MR scanner in a single functional run, during which they were instructed to allow themselves to respond as naturally as possible. Participants were randomly assigned to one of two instructional groups: ATTEND (n = 14) or WATCH (n = 14). Participants in the ATTEND group were instructed to attend and make continuous ratings of their emotion experience while watching the films (for a more complete description of the rating manipulation and main effects on neural activity, see Hutcherson et al., 2005). Participants in the WATCH group viewed the films without making ratings. After exiting the scanner, participants completed a questionnaire assessing their emotional responses to the films.
Image acquisition
Imaging was performed on a General Electric 3 Tesla Signa magnet using a T2*-weighted gradient echo spiral in/out pulse sequence using blood oxygenation level-dependent (BOLD) contrast (Glover and Lai, 1998) collected via a custom-built quadrature ‘dome’ elliptical bird cage head coil. Head movement was minimized using a bite bar formed with the subject's dental impression. Functional images (560 volumes per functional run) were obtained from 25 sequential axial slices using the following parameters: TR (relaxation time) = 2000 ms, TE (echo time) = 30 ms, flip angle = 60 degrees, FOV = 24 cm, matrix = 64 × 64, single shot, in-plane resolution = 3.75 × 3.75 mm, slice thickness = 5 mm, no gap. A T1-weighted fast spin echo anatomical scan was acquired in the same plane as the functional slices prior to acquisition of functional scans (TR = 500 ms, TE = 14 ms, in-plane resolution = 0.9375 mm and slice thickness = 5 mm).
Preprocessing of fMRI data
Preprocessing and statistical analysis of the data was performed using Analysis of Function NeuroImages software (AFNI: Cox, 1996). To correct for head movement, each functional time series was aligned to a base image in the middle of the first 2 min film clip. Each voxel was also subjected to an outlier detection and interpolation algorithm to correct potentially spurious time points. Individual subject statistical maps were spatially smoothed using an 8 mm3 FWHM Gaussian kernel, resampled into 3.75 mm3 isotropic voxels and spatially normalized into Talairach and Tournoux atlas coordinate space.
Extraversion, emotion and BOLD response to films
To understand the relationship between extraversion and BOLD response to the films, we calculated separately for each subject the BOLD signal to amusing and sad films, contrasted to the neutral baseline films.2 All five neutral films were used to ensure a stable estimate of baseline BOLD response. These contrast maps were used as input into a regression with extraversion. In order to examine the effects of attention, regression analyses were calculated separately for each group (ATTEND and WATCH), and compared to each other using Fisher's test for equality of two independent sample correlation coefficients (Fisher, 1921), using a joint probability threshold that consisted of a voxel-wise significance of P < 0.005 and a minimum cluster extent of 27 voxels (1423 mm3).3 This threshold protects against detection of false positives at the cluster level with a probability P < 0.01, as determined using by a MonteCarlo simulation program in the AFNI library (AlphaSim). Where these analyses revealed no effects of the ATTEND vs WATCH manipulation, we collapsed across groups and report overall correlations. We report regions in which overall correlations were significant using a voxel-level significance of P < 0.001 and a minimum cluster extent of 10 voxels (527 mm3), yielding a cluster-level P < 0.05. Within the amygdala, a subcortical a priori region of interest, we report results within a search region anatomical mask at a voxel-level of P < 0.05.
RESULTS
Because the effects of personality and attention were significantly different depending on the emotion examined, we report the results below by emotion. For each, we describe the behavioral and neural correlates of extraversion, first comparing groups and then where appropriate collapsing across groups.
Amusement
Extraversion and emotion experience
To assess whether extraversion was related to amusement, we correlated extraversion and post-session questionnaire ratings of amusement. Extraversion was marginally associated with the intensity of amusement experience during the two amusing films in both the WATCH (rWatch = 0.45, P < 0.10) and ATTEND (rAttend = 0.56, P < 0.06) groups. The strength of association did not differ between attentional groups (P < 0.74). Collapsing across groups yielded a significant correlation between extraversion and amusement (rAll = .42, P < 0.05).
Extraversion and BOLD response to amusing films
A comparison of the effects of attention (i.e. ATTEND vs WATCH) on the correlation between extraversion and BOLD response to amusing films revealed no regions that showed a significantly different relationship to extraversion in the two groups. We thus collapsed across these groups and report results for amusement from the overall analysis (N = 28). A significant negative correlation emerged between extraversion and BOLD response to amusing compared to neutral films in the right ventral striatum (including nucleus accumbens) as well as other regions, including right lateral prefrontal cortex (LPFC), left putamen and somatosensory cortex (Table 1; Figure 1A). A small cluster of voxels within a search ROI centered on the amygdala also revealed a positive correlation between extraversion and response to amusing films (r = 0.45, P < 0.01). However, inspection of this correlation suggested that it was largely driven by a single outlier in the data (>3 s.d. from the mean). Removing the outlier resulted in a non-significant correlation within this region (r = 0.16, P > 0.44).
Table 1.
Extraversion and BOLD response to amusing and sad films
| Region | BA | Volume | x | y | z | rAttend | p | rWatch | p | rAll | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraversion and Response to Amusing vs. Neutral Films (All Participants) | ||||||||||||
| R | Sup. frontal gyrus | 10 | 949 | 19 | 64 | 12 | −0.33 | 0.25 | −0.87 | <0.001 | −0.73 | <0.001 |
| R | Mid. frontal gyrus | 10 | 3480 | 38 | 38 | 16 | −0.11 | 0.71 | −0.82 | <0.001 | −0.70 | <0.001 |
| R | Postcentral gyrus | 4 | 580 | 15 | −41 | 68 | −0.38 | 0.18 | −0.81 | <0.001 | −0.69 | <0.001 |
| R | Nucleus Accumbens | 1582 | 11 | 19 | 1 | −0.43 | 0.12 | −0.78 | 0.001 | −0.71 | <0.001 | |
| L | Putamen/ | 580 | −19 | 11 | −3 | −0.67 | 0.01 | −0.64 | 0.01 | −0.67 | <0.001 | |
| Nucleus Accumbens | ||||||||||||
| Extraversion and Response to Sad vs. Neutral Films (ATTEND vs. WATCH participants) | ||||||||||||
| B | Ventral striatum/ | 3586 | −19 | 8 | −11 | −0.59 | 0.03 | 0.74 | 0.003 | 0.34 | 0.23 | |
| Nucleus Accumbens | ||||||||||||
Note: BA = Brodmann's Area. Coordinates specify peak voxel in cluster according to Talairach and Tournoux brain atlas.
Fig. 1.
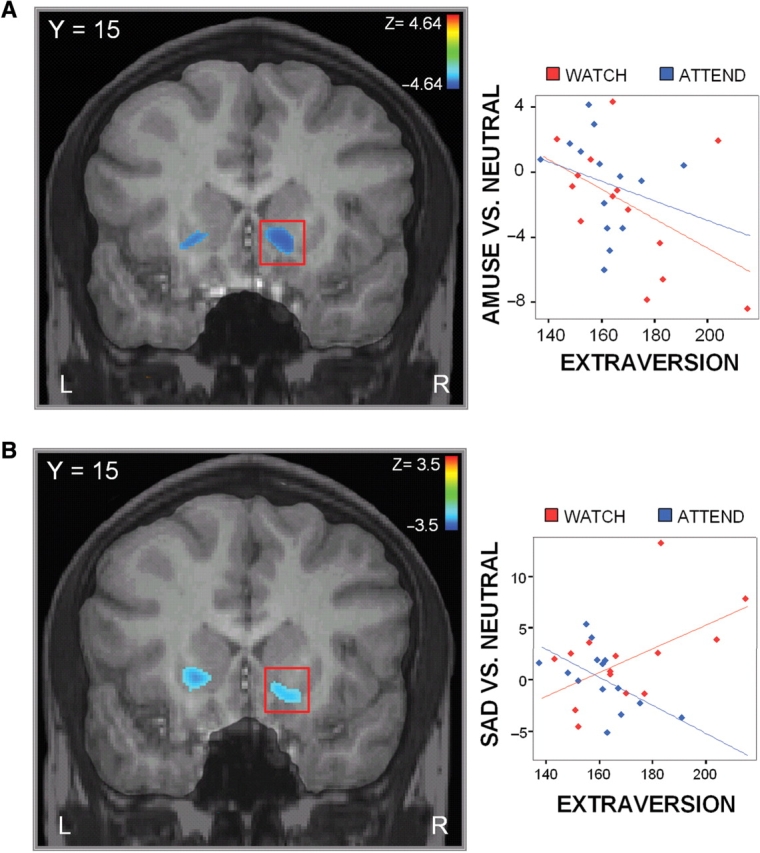
Extraversion and neural response to emotional films. (A) Correlation between extraversion and response in the ventral striatum to amusing films, collapsing across groups. (B) Contrast of ATTEND group vs WATCH group in the correlation between extraversion and response in the ventral striatum to sad films.
Sadness
Extraversion and emotion experience
Extraversion was not correlated with sadness on the post-session questionnaire ratings in the WATCH group (r = −0.28, P < 0.34) but was significantly positively correlated to sadness in the ATTEND group (r = 0.66, P < 0.02). The correlations within the WATCH and ATTEND groups differed significantly (P < 0.02).
Extraversion and neural response to sad films
An initial comparison of the two groups to investigate the impact of attention revealed a strong effect within a large region encompassing both right and left ventral striatum (including nucleus accumbens) (Table 1; Figure 1B). Within the WATCH group, there was a strong positive correlation between extraversion and response to sad films in the ventral striatum (rWatch = 0.74). Within the ATTEND group, there was a strong negative correlation in this region (rAttend = −0.56).
Secondary analyses
Because our results revealed an unexpected negative correlation between extraversion and activation in bilateral ventral striatum/NAcc, we wished to test the robustness of these findings by examining the response to the films in more detail.
Effect of film
In order to ascertain whether our results were driven by unique and idiosyncratic effects of one of the films, we performed analyses using only one of the amusing or sad films, and compared it to results obtained when using only the other. Within the ventral striatum, this analysis yielded essentially identical results to those obtained using both films, albeit at lower thresholds (P < 0.01, uncorrected). Moreover, the results from each film looked nearly identical to the other. Extraversion predicted a negative response in the ventral striatum to either amusing film that was not sensitive to attention; it also predicted a negative response to either sad film, but only in the group that attended to their emotions.
1st half vs 2nd half
Another potential explanation for the decreased BOLD signal we observed in the ventral striatum concerns habituation. Perhaps extraverts actually respond more in the NAcc, but then also habituate or adapt more quickly, leading to an overall negative response. In this case, block analysis of the whole film would obscure early activations compared to late deactivations. We therefore examined whether using only the first half or only the second half of each emotional film would yield different results. These results again mirrored results from the full analyses, although at a reduced significance level (P < 0.01, uncorrected). Analyzing just the first half of amusing or sad films yielded a negative correlation with ventral striatum that did not differ depending on attention; analyzing just the second half yielded a nearly identical relationship.
DISCUSSION
This study examined relations among extraversion, emotion experience and fMRI BOLD responses during potent film clips that evoked amusement and sadness. Our goal was to understand how extraversion relates to positive or negative emotional reactivity and patterns of BOLD responding. Based on a literature in neuroimaging concerning the modulation of emotional processing by attentional factors (Hariri et al., 2001; Taylor et al., 2003), we tested whether a rating manipulation that focuses attention on emotion experience, as opposed to a more passive stance, would moderate the relationship between personality and emotional responding. In the context of amusing and sad films, we found an interaction of extraversion, emotion type and attentional condition, evident in both behavior and neural reactivity.
Extraversion and amusement
Consistent with prior behavioral results, we found that extraversion was related to subjective experience of amusement in both groups, although this effect only achieved statistical significance in the larger, combined sample. Neurally, we found that extraversion correlates with response in the ventral striatum (including NAcc), though in an opposite direction from that typically reported in the literature. This relationship was evident in two groups, regardless of attentional focus. It was also reliably observed whether examining each film independently and also when examining just the first or second half.
These results provide converging evidence to support the theory that extraversion results from differences in the functioning of a dopaminergic circuit involving the ventral striatum (Depue and Collins, 1999). This system may contribute to the consistent effects of extraversion on response to positive stimuli. However, interpretation of these results is complicated by the fact that, despite greater self-reported amusement, extraverts demonstrated a greater negative response within the striatum to positive stimuli, a relationship that held regardless of attentional condition. We return to this issue subsequently.
Extraversion and sadness
Whereas response to amusing stimuli in extraverts was not strongly affected by attention, the manipulation had a substantial impact on responses to sad films. Extraversion predicted greater subjective sadness, but only in the group rating their emotions. Similarly, although there was no overall relationship between extraversion and sadness in the NAcc, accounting for attentional condition unmasked a strong relationship to both experiential and neural responses when participants attended to their emotions, but not when they simply watched sad films. There are several interpretations of this observation. On one hand, the correlation between extraversion and NAcc response to sad films within the ATTEND group looked surprisingly similar to that seen in both groups to amusing films, and may indicate that extraverts find sad films rewarding, but only when focusing on the emotionally salient aspects of the films. On the other hand, based on the larger extant literature suggesting that positive NAcc response is associated with positive affect (Breiter et al., 2001; Knutson et al., 2001a, b), this could indicate that when extraverts attend to sad aspects of a social situation, they feel more negative. This conclusion is supported by greater reports of sadness when rating, but not when simply watching sad films.
Even setting aside the issue of directionality, this finding may clarify the inconsistent relationship between extraversion and negative affect, with studies reporting no relationship (Lucas and Diener, 2001), an inverse correlation (Larsen and Ketelaar, 1991), or even a positive correlation (Choti et al., 1987). As alluded to in the introduction, there are hints in the literature that the degree to which emotion is made an explicit, central focus of a study may influence the relationship between extraversion and negative emotion. To our knowledge, however, this study is the first to directly examine the impact of attention to emotion on personality. A complete understanding awaits further studies and will require more careful consideration of the contextual and attentional factors under which negative events are processed.
If attention proves to modulate the relation between personality and emotional response, what are the processes driving such an effect? Do extraverts respond to negative as well as positive stimuli, but in a more context-dependent manner? One possibility is that the additional task of rating impacts extraverts differentially. A growing literature suggests that extraverts, possibly due to differences in striatal dopamine, have greater working memory capacity (Lieberman, 2000), and therefore may have greater freedom than introverts to attend to particular aspects of an emotional stimulus under cognitive load (Lieberman and Rosenthal, 2001).
Our findings may also be due to the type of negative emotion examined. Studies reporting a positive relationship between extraversion and negative emotion have focused on sadness, and other research suggests that extraverts may be more empathetic (Hekmat et al., 1974; Richendoller and Weaver, 1994), a feature that could lead to greater empathy-induced sadness. Our research, however, suggests that an empathy–sadness–extraversion link may be limited to those occasions in which the emotional or social aspects of a situation are made more attentionally salient. However, future work will be needed to determine exactly what aspects of the rating manipulation affected responding. Perhaps, consistent with the idea that extraversion is associated particularly with social stimuli (Ashton et al., 2002), highlighting the affiliative bonds (or lack thereof) between people are sufficient to induce greater negative emotion, particularly sadness, in extraverts.
Limitations and future directions
As noted earlier, our interpretation of these results is complicated by the puzzling negative correlation between NAcc activation and extraversion. Extraversion has typically been associated with increased response in the amygdala, NAcc and associated prefrontal areas to rewarding stimuli. We observed a strong, consistent decrease in response within the NAcc correlated with extraversion. Only one other study, using PET rather than fMRI, has reported an inverse association between extraversion and resting baseline glucose metabolism in the ventral striatum (Fischer et al., 1997), although some have reported a decreased response in the amygdala correlated with extraversion (Mobbs et al., 2005). Several notable differences between our paradigm and those of others may account for our observations. One important difference concerns the nature of the stimuli. We operationalized positive and negative emotion using humor and sadness in complex social films, whereas many other studies have used simpler or non-social stimuli. Given the importance that some authors have placed on the specifically social nature of extraversion, and the observation that the NAcc is more responsive to films inducing positive emotions in social than non-social contexts (Britton et al., 2006), it will be important in future studies to distinguish between levels of complexity and social salience in experimental stimuli and to observe if and how extraversion relates to parametric modulation of these dimensions.
Another notable feature of our study concerns the temporal profile of emotional stimuli. Recent work suggests that the NAcc may be particularly concerned with rewards during anticipation, rather than outcome (Knutson et al., 2001a, b). Other studies also show that dopaminergic neurons fire not at the onset of reward per se, but at the earliest cue indicating the imminent delivery of reward (Schultz, 2000). Although these studies are suggestive, relatively little is currently known about the precise temporal pattern of responding within the NAcc during anticipation and receipt of reward, or whether and how it is modulated by the type or intensity of reward.
One possibility is that the temporal characteristics of the films we used may account for the differences we see. Some researchers have noted that in some regions, BOLD responses showing larger initial increases are followed by larger decreases. In these cases, analyses at the trial level can yield positive activations, while analyses at the block level can yield quite different results (Meltzer et al., 2007). Notably, some studies have observed precisely such a response in the NAcc, reporting initial increases in activation in response to a stimulus followed by larger decreases (Yacubian et al., 2006). If such a response occurs to amusing stimuli, the deactivation we observe at the block level may result from greater reward response on a more immediate time scale. Unfortunately, a tradeoff exists between increased complexity and temporal precision. Although we performed an analysis that demonstrated similar results using either the first or second half of our 2-min long blocks, the nature of our stimuli prevent us from teasing apart the contributions of different temporal components of the reward response at a smaller scale (on the order of seconds). Future studies will need to assess the relative contribution of anticipatory and consummatory phases of emotional processing to extraversion.
A further limitation of this study—and of many neuroimaging studies—is its relatively small and limited sample. Behavioral studies of personality typically analyze responses within a sample that is larger than ours by a factor of five to ten and includes both men and women from a wide variety of backgrounds. One concern in studies like ours that have fewer participants or examine only one gender is whether findings will prove replicable. Several features lead us to believe that our findings will prove to be robust. First, our results converge on many of the same regions identified in other studies, albeit in the opposite direction within the NAcc. Second, when considering amusement processing, we found that results looked similar in two independent groups, regardless of an attentional manipulation. Third, these neural findings were observed in the context of theoretically consistent differences in subjective reports of amusement. NAcc deactivation to amusing films in extraverts accompanied greater experience of positive emotion that was predicted by extraversion. These factors combine to give us greater confidence in the stability and replicability of our findings. However, whether such results would generalize to men or to participants of different age, socioeconomic status or cultural background remains to be seen.
Future work will also need to consider several aspects of emotion, including how extraversion relates to different temporal components of emotional responding, a direct comparison of social and non-social reward processing in extraverts, how empathy, reward seeking and pleasure at reward outcome are differentially related to extraversion, and how different specific positive emotions (such as pride and compassion) or negative emotions (such as shame or anger) are experienced by extraverts. It will also be important to understand how emotional responsivity within different biological systems underlie other important personality traits, such as neuroticism, or impulsivity, and if and how attention influences such relationships. We believe that these questions have the potential to generate fruitful and exciting research in the years to come.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was funded by a grant to Dr J.J.G. from the National Institute of Health (R01 MH58147).
Footnotes
In addition, the 48-item version of the Neuroticism scale, and short, 12-item measures for openness, conscientiousness and agreeableness were included. Inspection of these measures indicated that none were significantly correlated with extraversion except conscientiousness, and all results reported in the paper hold when controlling statistically for the influence of these other personality variables, including neuroticism.
We also conducted analyses directly contrasting amusing to sad clips. These results largely confirm the findings compared to neutral, giving us confidence that our results are not solely driven by arousal or other non-affective differences between emotional and neutral films. The results of these comparisons can be found in Supplementary Table 1 online.
Although this extent threshold is relatively large when considering subcortical regions such as the nucleus accumbens, results in the ventral striatum do not change if the threshold is reduced, or when using the uncorrected P-value with no extent threshold.
REFERENCES
- Adolphs R, Tranel D, Hamann S, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–7. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Ashton MC, Lee K, Paunonen SV. What is the central feature of extraversion? Social attention versus reward sensitivity. Journal of Personality and Social Psychology. 2002;83:245–52. [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–46. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: an fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JDE. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Choti SE, Marston AR, Holston SG, Hart JT. Gender and personality variables in film-induced sadness and crying. Journal of Social and Clinical Psychology. 1987;5:535–44. [Google Scholar]
- Cohen MX, Young J, Baek J, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Research Cognitive Brain Research. 2005;25:851–61. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. NeuroImage. 2007 doi: 10.1016/j.neuroimage.2007.08.009. doi:10.1016/j.neuroimage.2007.08.009 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Influence of extraversion and neuroticism on subjective well-being: happy and unhappy people. Journal of Personality and Social Psychology. 1980;38:668–78. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory and NEO Five Factor Inventory Profession Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cox RW. Software for analysis and visualization of function magnetic neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Diener E, Larsen RJ, Emmons RA. Person x situation interactions: choice of situations and congruence response models. Journal of Personality and Social Psychology. 1984;47:580–92. doi: 10.1037//0022-3514.47.3.580. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. An alternative “description of personality”: the Big-Five factor structure. Journal of Personality and Social Psychology. 1990;59:1216–29. doi: 10.1037//0022-3514.59.6.1216. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wik G, Fredrikson M. Extraversion, neuroticism and brain function: a PET study of personality. Personality and Individual Differences. 1997;23:345–52. [Google Scholar]
- Fisher RA. On the probably error of a coefficient of correlation deduced from a small sample. Metron. 1921;1:1–32. [Google Scholar]
- Gorno-Tempini ML, Pradelli S, et al. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–73. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. Neuroimage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Journal of Magnetic Resonance Medicine. 1998;39:361–8. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Gross JJ, Sutton SK, Ketelaar TK. Relations between affect and personality: support for the affect-level and affective-reactivity views. Personality and Social Psychology Bulletin. 1998;24:279–88. [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hekmat H, Khajavi F, Mehryar A. Psychoticism, neuroticism and extraversion: the personality determinants of empathy. Journal of Clinical Psychology. 1974;30:559–61. doi: 10.1002/1097-4679(197410)30:4<559::aid-jclp2270300427>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? Neuroimage. 2005;27:656–68. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- John OP, Srivastava S. The big five trait taxonomy: history, measurement, and theoretical perspectives. In: Pervin LA, John OP, editors. Handbook of Personality: Theory and Research. 2nd. New York: Guilford Press; 1999. pp. 102–38. [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12(17):3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji J. Neural substrates for emotional traits. In: Canli T, editor. Biology of Personality and Individual Differences. New York: Guilford Press; 2006. pp. 116–32. [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–71. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8:3969–72. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. Journal of Personality and Social Psychology. 1991;61:132–40. doi: 10.1037//0022-3514.61.1.132. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Introversion and working memory: central executive differences. Personality and Individual Differences. 2000;28:479–86. [Google Scholar]
- Lieberman MD, Rosenthal R. Why introverts can't always tell who likes them: multitasking and nonverbal encoding. Journal of Personality and Social Psychology. 2001;80:294–310. doi: 10.1037/0022-3514.80.2.294. [DOI] [PubMed] [Google Scholar]
- Lischetzke T, Eid M. Why extraverts are happier than introverts: the role of mood regulation. Journal of Personality. 2006;74:1127–62. doi: 10.1111/j.1467-6494.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Diener E, Grob A, Suh EM, Shao L. Cross-cultural evidence for the fundamental features of extraversion. Journal of Personality and Social Psychology. 2000;79:452–68. doi: 10.1037//0022-3514.79.3.452. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Fujita F. Factors influencing the relation between extraversion and pleasant affect. Journal of Personality and Social Psychology. 2000;79:1039–56. doi: 10.1037//0022-3514.79.6.1039. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Diener E. Understanding extraverts’ enjoyment of social situations: the importance of pleasantness. Journal of Personality and Social Psychology. 2001;81:343–56. doi: 10.1037//0022-3514.81.2.343. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Constable RT. Biphasic hemodynamic responses influence deactivation and may mask activation in block-design fMRI paradigms. Human Brain Mapping. 2007 doi: 10.1002/hbm.20391. doi: 10.1002/hbm.20391 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Greicius MD, Abdel-Azim E, Menon V, Reiss AL. Humor modulates the mesolimbic reward centers. Neuron. 2003;40:1041–8. doi: 10.1016/s0896-6273(03)00751-7. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Hagan CC, Azim E, Menon V, Reiss AL. Personality predicts activity in reward and emotional regions associated with humor. Proceedings of the National Academic Science USA. 2005;102:16502–6. doi: 10.1073/pnas.0408457102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone JV, Depue RA, Scherer AJ, White TL. Film-induced incentive motivation and positive activation in relation to agentic and affiliative components of extraversion. Personality and Individual Differences. 2000;29:199–216. [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Deichman R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Peter M, Vingerhoets AJJM, Van Heck GLV. Personality, gender, and crying. European Journal of Social Psychology. 2001;15:19–28. [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KB. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. Journal of Neuroscience. 2001;21:3261–70. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KB. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22:7308–20. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendoller NR, Weaver JB. Exploring the links between personality and empathic response style. Personality and Individual Differences. 1994;17:303–11. [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. Journal of Neuroscience. 2007;27:4826–31. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ME, Ebmeier KP, Deary IJ. Personality correlates of happiness and sadness: EPQ-R and TPQ compared. Personality and Individual Differences. 2005;38:1085–96. [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Paradiso S, Andreason NC, Johnson DL, Boles Ponto LL, Hichwa RD. Correlation between extraversion and regional cerebral blood flow in response to olfactory stimuli. American Journal of Psychiatry. 2007;164:339–41. doi: 10.1176/ajp.2007.164.2.339. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. Journal of Neuroscience. 2006;26:9530–7. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–17. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]


