Abstract
Single pulse transcranial magnetic stimulation (TMS) was used to disrupt the right inferior parietal lobe (rIPL) whilst neurologically intact participants made self/other judgments about whole arm reaching movements. Visual feedback of a physically coincident virtual hand was perturbed or left unperturbed (randomly) while TMS was delivered to either the rIPL or the vertex (blocked). Visual feedback of the virtual hand was veridical until the hand became occluded by a virtual bar approximately half way through the movement. TMS was delivered on 50% of trials at random during occlusion of the hand. The position of the virtual hand relative to the real hand was also perturbed during occlusion of the virtual hand on 50% of trials at random. At the end of the reach participants were required to make a verbal judgment as to whether the movement they had seen was self (unperturbed) or other (perturbed). The results revealed that when TMS was applied over rIPL, participants were more likely to misattribute agency to the computer, making more other responses for both perturbed and unperturbed trials. These findings highlight the role of a parietal neural comparator as a low-level mechanism in the experience of agency.
Keywords: agency attribution, inferior parietal lobe, transcranial magnetic stimulation, self-awareness, social cognition
INTRODUCTION
There is a great similarity between our own actions and those of other people, not only in terms of the movement's characteristics, but also in the way that the movements are processed in the brain (Rizzolatti and Craighero, 2004). It is this similarity that allows us to interpret the intentions and desires of other people. Before we can begin to do this, however, we need to be aware of whether the source of a perceived action is that of someone else or ourselves. The ability to correctly identify our own actions from those produced by other people (agency attribution) is a fundamental component of human social interaction and while ambiguity of this experience is rare, the process can become compromised in mental illness or following brain injury.
Neuropsychological evidence has associated self/other processing with the right parietal lobe. Symptoms following damage to this area frequently involve disorders of self-awareness, examples of which include: hemi-spatial neglect, a disorder in which a patient is unaware of the side of space contralateral to the lesion, often including their own body parts (Vallar and Perani, 1986; Driver and Mattingley, 1998); anosognosia, in which the patient is unaware of their contralesional disabilities (for example, their hemiplegia) (Paysant et al., 2004; Jehkonen et al., 2006) asomatognosia, in which patients can deny ownership of their own limb (Pia et al., 2004) and some cases of alien limb syndrome (Groom et al., 1999) in which patients report the absence of volitional control over the affected limb—often referring to it in the third person. Another patient group with a disrupted sense of agency are those presenting with passivity (delusions of control). This is one of the first-order positive symptoms in schizophrenia and involves the patient believing that his or her actions are being controlled or influenced by an external agent. This specific symptom has been associated with abnormal activity in the right inferior parietal lobe (rIPL). For example Spence et al. (1997) observed hyperactivity in the rIPL using positron emission tomography (PET) in schizophrenic patients experiencing passivity compared with patients without this symptom and a non-schizophrenic control group. This specific patient group has also been found to have reduced cortical volume in the rIPL (Maruff et al., 2005).
While attribution errors in brain-damaged and schizophrenic individuals have been studied extensively, reports of misattribution in healthy people are rare. However, evidence associating the rIPL with successful self/other action discrimination has been demonstrated in neurologically intact individuals using functional imaging. Farrer et al. (2003a) tested participants making joystick movements in a self/other judgment task while measuring brain activity using PET. Participants were given visual feedback of their movements by the presentation of a virtual hand and joystick on a mirror positioned in front of their moving hand. In some trials, the visual feedback deviated from their actual movements by 25° or 50° (defined as other) while the remaining trials were left unperturbed (defined as self). The results of this experiment revealed an increase of blood flow to the rILP when participants made other judgments and an increase in blood flow to the insula (primarily right hemisphere) when participants made self judgments. The PET 2003 study was a follow-up to a previous functional magnetic resonance imaging (fMRI) study that also highlighted the involvement of the anterior insula and rIPL for self- vs other-generated movements, respectively (Farrer and Frith, 2002). However, it is not clear from the study of Farrer et al. (2003a), whether the activation detected in rIPL reflected the locus of primary mechanisms involved in the sense of agency or simply the detection of spatial discordance between the seen and felt positions of the joystick which might then be used to inform self/other judgments.
The current study further investigated the role of the rIPL in self/other judgments by disrupting this area in neurologically normal participants using single pulse transcranial magnetic stimulation (TMS). TMS is a technique that allows the study of causal relationships between brain and behavior by producing a transient and localized disruption to normal brain activity. The focal magnetic pulses produced by TMS create time-locked ‘virtual lesions’ over a specific region of interest on the cortex, which, if essential to the task, can alter the participant's performance (Pascual-Leone et al., 2000). In the current experiment, it was important to deliver the TMS pulse at a time when the participant had no vision of the virtual hand. In a paradigm [similar to that described earlier by Farrer et al. (2003a)], participants made judgments about whole limb movements while receiving TMS (or no TMS) to either rIPL or the vertex. Crucially, the TMS was delivered when movement of the hand was occluded from view (i.e. at a time when there was no discordance between the seen and felt positions of the hand). Although the finger was obscured from view for an average of 155 ms, total hand occlusion was only achieved for ∼25 ms, restricting TMS delivery to a single pulse. The vertex was chosen as the control site for this experiment as it is a frequently used control site to test for non-specific effects of TMS (Bestman et al., 2002; Muggleton et al., 2006; Nyffeler et al., 2006). The analogous left hemisphere location, the left inferior parietal lobe, was not used as a control site due to the associations between this location and other (temporal) aspects of agency attribution (MacDonald and Paus, 2004). Planned comparisons were conducted to test directly whether TMS stimulation disrupted self/other judgments when applied to the rIPL (and not when applied to the vertex) at a time when spatial discordance could not be detected.
MATERIALS AND METHODS
Right-handed 10 healthy volunteers (eight females and two males: mean age 22 years) gave fully informed written consent to participate in the study. All were screened for contraindications to TMS using a self-report questionnaire based on the TMS Adult safety Screen (Keel et al., 2001). The study was approved by the University of Nottingham, School of Psychology. Ethics committee and conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Participants were seated in front of a horizontal mirror (450 × 300 mm) raised on a wooden board 300 mm above a 900 × 900 mm table. A projection screen (800 × 540mm) was suspended horizontally 300 mm above the mirror. A Toshiba TLP560 projector was also suspended a further 910 mm above the projection screen. Thus, the image projected onto the screen appeared to the participant to be in the same plane as the table surface (Figure 1A). Hand movements were recorded at 240 Hz by an electromagnetic sensor (Polhemus Liberty) attached to the index finger of the reaching (right) hand. A circular plastic disc (10 mm diameter) attached to the leading edge of the table was used to mark the start point and a life-sized projected colour image of the experimenter's (female) hand positioned in a pointing posture (extended index finger) was used as a representation of the participant's own hand. A Magstim Rapid TMS machine (the Magstim Company Ltd) with double 70 mm coil was used to deliver the magnetic pulse to the appropriate areas on the scalp marked out using disposable surgical caps. The coil was placed tangentially to the skull and was set to stimulate at 110% of motor threshold (defined as the minimal TMS intensity required to cause involuntary twitching of the contralateral hand in at least 5 out of 10 trials). For the rIPL condition the TMS wand was positioned 50 mm posterior to the motor hand area (Nager et al., 2004) and the vertex was found at the intersection between the naison-inion line and the line between the pre-auricular points.
Fig. 1.
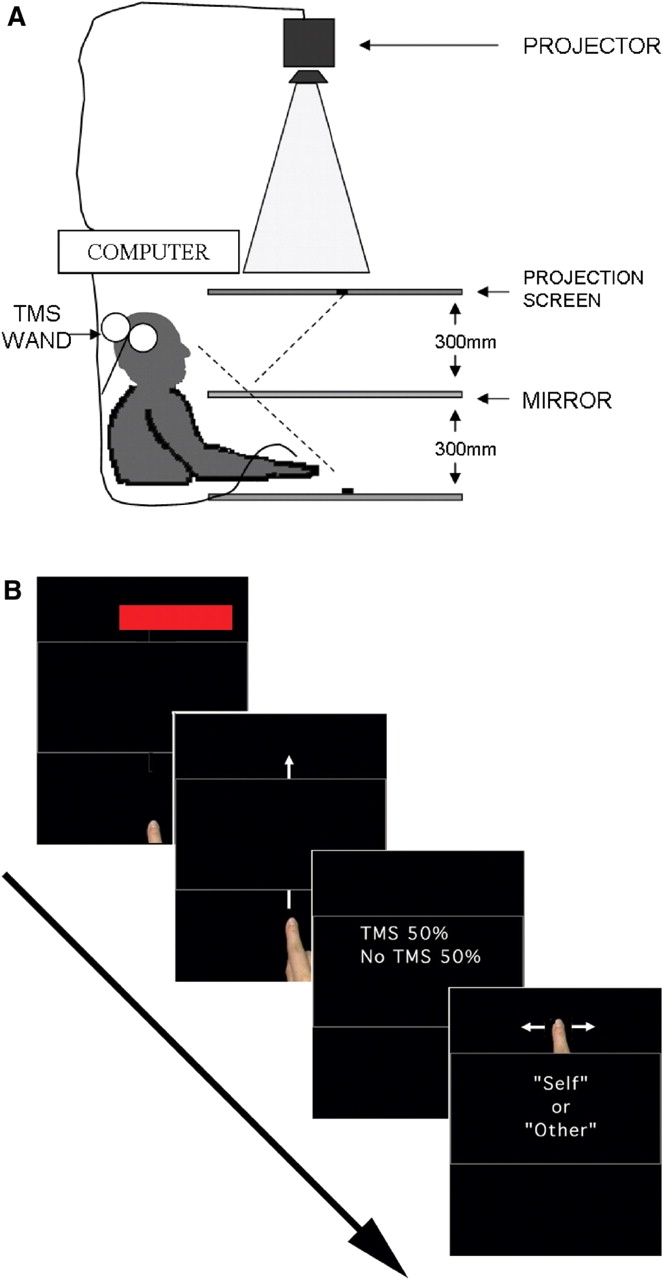
(A) Schematic representation of experimental set up. When looking into the mirror, images projected into the upper screen appear to be in the same plane as the table surface. Thus, an unperturbed image of a virtual hand appears to be in the same location as the participant's actual hand. (B) At the beginning of each trial a red target bar appeared for 500 ms and then disappeared again before movement onset. During the reach, participants saw the virtual hand pass beneath a virtual occluding bar before re-emerging on the other side. TMS was applied on 50% of trials at random while the hand was occluded. On 50% of trials the hand was also perturbed from its veridical position while occluded. At the end of the trial participants made a verbal self vs other judgment.
Procedure
Participants sat at the table and looked down into the mirror in which they could see a black screen (a reflection of the image from the projector on the projection screen). Participants placed their index finger on the start position with their hand in the same posture as the image that represented their movements (a pointing posture) and were required to return to the same start position at the end of each trial. At the beginning of each trial a red target bar appeared for 500 ms (bar: 160 × 20 mm, with the inner edge 40 mm from the midline and 300 mm from the start point). Immediately following removal of the target bar image a tone indicated to the participant that they should make a unimanual reaching movement with their right hand toward where the target bar had been. During the reach, participants saw the virtual hand image pass beneath a virtual occluding bar (440 × 160mm) before re-emerging on the other side (Figure 1B).
For the first 100 mm of the movement the trajectory and velocity of the virtual hand was calculated online using position data from the motion tracker on the index finger and was thus the same as the actual movement (delay <10 ms) before becoming occluded by the virtual bar. In half the trials, following occlusion, the virtual hand continued to accurately represent the actual reach trajectory (‘self’). In the other half, whilst occluded, the image undertook a lateral shift (‘other’) equivalent to a cursor rotation of four degrees beginning at the initial start location so that when the hand re-appeared its spatial position was to one side of the actual hand position. In half the ‘other’ trials (25% of total trials), this shift was to the left and in the remaining trials it was to the right. Each participant took part in two sequential experimental blocks, one for each stimulation site, the order of which was counterbalanced between participants. Within each block there were 96 trials (192 in total), which were conducted in a pseudo-randomized order (perturbed and unperturbed trials with and without TMS) in half the trials TMS was applied as a single pulse delivered at the moment the virtual hand became fully obscured behind the occluding bar. In the remaining trials no TMS pulse was delivered.
Participants were informed that visual feedback up to the occluding bar would accurately represent their own movements and that this would also be true of half the trials following occlusion. It was explained that in the remaining trials, when the virtual hand emerged from behind the occluding bar, visual feedback would be controlled by the computer and would deviate the path of the virtual hand laterally either left or right of their actual hand path. Participants made their responses verbally: being instructed to respond ‘self’ if they felt that the virtual hand accurately represented their movement throughout the entire reach (i.e. that they were in control of the movement) and ‘other’ if they felt that the hand path, after re-emerging, had been controlled by the computer. Participants were instructed to move at a comfortable and natural speed. It was also explained that the target bar that extended across most of the display, was intended only as an indicator of the distance that they should travel and that there were no directional accuracy requirements. The width of the target bar was such that it prevented participants from using its remembered location as a target to use as an indicator of the relative difference between their intended and actual reach direction. Note, also, that perturbations were in the lateral direction only so that it was not possible to use memory of the distance of the bar to detect perturbations. Participants were aware that the representational image of their hand position was not their own real hand (although one participant did make this mistake).
RESULTS
Responses were converted into percent correct scores for each participant (correct responses were self for ZERO-degree perturbations and other for FOUR-degree perturbations). These data were entered in a 2 × 2 × 2 repeated measures ANOVA with the factors STIMULATION (TMS and NO-TMS), BRAIN AREA, (RIPL and VERTEX) and PERTURBATION, (ZERO and FOUR degrees). There were no significant main effects of STIMULATION [F(1, 9) = 0.031, P = NS] or BRAIN AREA [F(1, 9) = 0.909, P = NS]. There was, however, a significant main effect of PERTURBATION [F(1, 9) = 17.183, P < 0.01] with mean percent correct responses being greater for ZERO (mean = 76.3, s.d. = 16.24) than FOUR degrees (mean = 60.4% s.d. = 12.62). There was also a significant two-way STIMULATION * PERTURBATION interaction [F(1, 9) = 12.27, P < 0.01], but this interaction was not informative as it used data collapsed across brain areas and can be completely explained by the results of the planned comparisons. There were no significant interactions for STIMULATION * BRAIN AREA [F(1, 9) = 1.56, P = NS], BRAIN AREA * PERTURBATION [F(1, 9) = 0.43, P = NS] or STIMULATION * BRAIN AREA * PERTURBATION [F(1, 9) = 2.794, P = NS.]
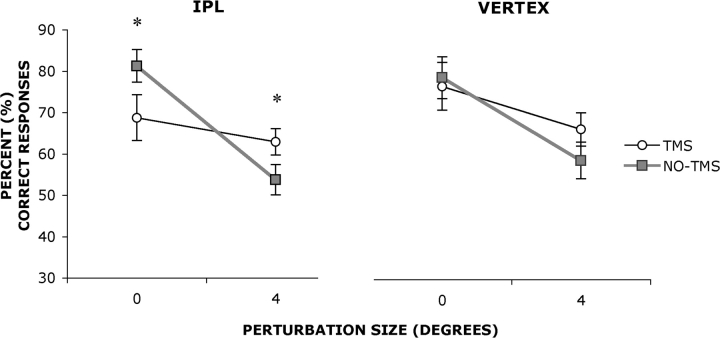
Planned comparisons were conducted between the predicted variables of interest (Figure 2) revealing significant differences in percent correct responses between TMS and NO-TMS over the RIPL for the ZERO-degree perturbation [F(1, 9) = 11.96, P < 0.01] with percent correct responses being greater for NO-TMS (mean = 81.25%) than TMS (mean = 68.75%) trials. A significant difference was also found at the RIPL at the FOUR-degree perturbation [F(1, 9) = 6.432, P < 0.05] with percent correct responses being greater for TMS (mean = 62.92%) than NO-TMS (mean = 53.75%). No difference was found at the VERTEX between TMS and NO-TMS trials for either ZERO- [F(1, 9) = 0.332, P = NS] or FOUR- [F(1, 9) = 4.3, P = 0.07] degree perturbations.
Fig. 2.
Percent correct responses for zero-degree (self) and four-degree (other) perturbations when TMS was delivered (open circles) or not delivered (filled squares) over rIPL (left figure) or vertex (right figure). Asterisks denote significant differences revealed by planned comparisons.
DISCUSSION
When TMS is applied over the rIPL, participants are more likely to give a judgment of other (for both present and absent perturbations) compared with when no TMS is applied (that is: percent correct responses were reduced for the zero-degree perturbation, but increased for the four-degree perturbation). In contrast, this TMS effect was not observed when stimulation was over the vertex. At first glance, these results may seem counter-intuitive given the results of previous imaging experiments using similar paradigms. For example, Farrer and colleagues (Farrer and Frith, 2002; Farrer et al., 2003a) reported increased activation in the rIPL when participants made other judgments compared with self judgments in tasks involving perturbed vs real feedback of cursor or joystick movements. In accordance with these findings, one might expect that disrupting the area thought to be heavily involved in other attribution (rIPL) would lead to a disruption in the ability to make other judgments—and hence an increase in self judgments. However, the results are compatible with comparator model accounts of the processes underlying rIPL activity. Comparator models have been proposed as one of the mechanisms responsible for successful agency attribution and it has been suggested that lesions to the system can result in the abnormal agency attribution seen in, for example, anosognosia and schizophrenia (Frith et al., 2000).
Schizophrenic patients experiencing passivity can accurately carry out movements as intended, but feel as if their movements are under the control of another (external) agent. The comparator model proposed by Frith et al. (2000), supported by the current results, helps to create an understanding of the processes that might underlie this experience (Figure 3). Whenever the central nervous system (CNS) plans a movement, a copy of the motor command is generated (efference copy) and this can be used by the CNS to predict the consequences of that movement. Such a prediction mechanism can be used in many ways, but importantly it allows the CNS to anticipate and correct for movement errors, filter expected sensory input and help maintain the estimate of the current state of the motor system. An accurate representation of one's own current limb position depends on accurate sensory feedback as well as accurate current state predictions.
Fig. 3.
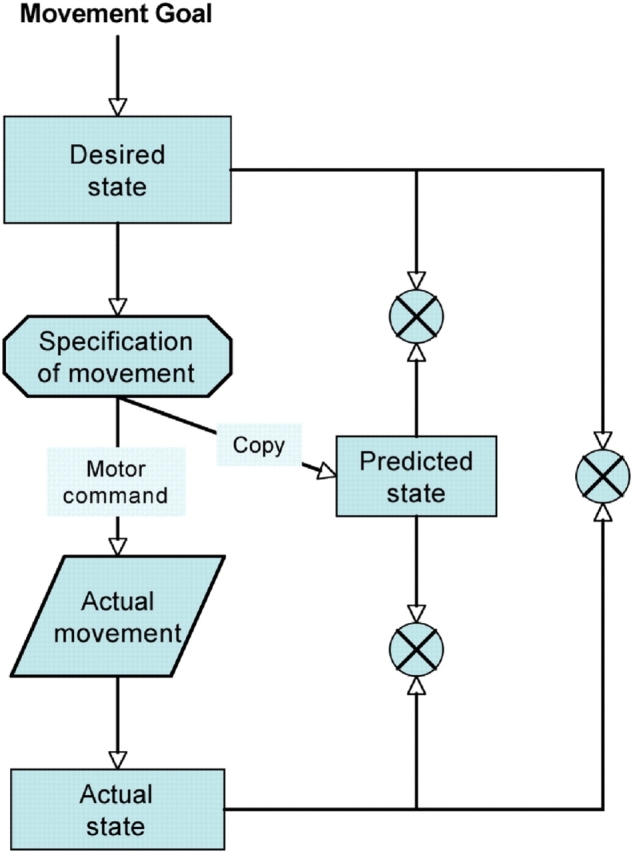
Starting from the top left of the diagram and working down the left hand side: the intended goal of an action is necessary to specify the desired (next) state of the limb and also the movement required to achieve that state. At this stage a motor command is generated to execute the necessary movements and a parallel efference copy is sent to the predictor in order to calculate the consequences of that particular motor command. In addition to this and following on from the motor command, there is the (estimated) actual state of the motor system based on the outgoing motor commands and sensory feedback. This is an iterative loop and is constantly active both before and throughout the movement and as the movement unfolds there are a number of comparisons that can be made in order to monitor and update it. Comparisons (represented by crossed circles) can be made between the desired state and the predicted state, between the desired state and the actual state and between the actual state and the predicted state. Any discrepancies can be used to modify and correct the motor command on-line during the movement and so make it as accurate as possible. Thus, feelings of agency are apparent when the comparisons match, but if the discrepancies between any of the comparisons become too large then the CNS may treat any observed or internally monitored self movement as belonging to, or being under the influence of, an external agent. When the system dysfunctions, therefore, perhaps through brain abnormality or lesion, misperceptions of agency can occur. Adapted from Frtih et al. (2000).
Frith and colleagues suggest that schizophrenic patients with passivity symptoms have impaired predicted state representations and as a consequence they perceive a false discordance between the predicted and actual states of their movement. As a result they feel as though an external agent is controlling their actions even though the intended goal is still achieved (the rest of the system remains intact so the patient can still successfully construct and execute the desired movement and their intended goals match their perceived outcome). The misattribution of agency observed in the current experiment, mirrors that seen in schizophrenic delusions of control and is also explained by a disruption of predicted state mechanisms. As outlined above, the comparator model predicts that disrupting predicted state representations would lead to an increase in other judgments that is precisely what happens in the current experiment when the rIPL is disrupted by TMS. A unique and important factor in the current study relates to the timing of the TMS pulse: crucially it occurred when vision of the virtual hand was not available to the participant. Correct self/other judgments in a task such as this, requires that the participant accurately predict where their hand will re-emerge from behind the occluding bar. Occluding the hand for a portion of the reach places an extra burden on predictive mechanisms at precisely the time at which the TMS pulse is delivered. In relation to previous studies, it has been argued that the rIPL activation observed in the Farrer et al. (2003a) study might simply reflect the detection of spatial discordance rather than the sense of agency itself. In the current experiment however, TMS is delivered at a time at which there is no sensory discordance. The felt position of the limb remains unperturbed and the seen position of the limb is occluded. While the rIPL may indeed be heavily implicated in the detection of sensory discordance, that is not the process that is being disrupted by TMS in the current experiment.
It is interesting to note that the planned comparison between TMS and NO-TMS at the vertex stimulation site also approached significance (P = 0.07) with perturbations of four degrees. The direction of the difference here was in the same direction as the equivalent TMS vs NO-TMS comparison at the rIPL stimulation site. This probably reflects a general effect of TMS on the frequency of other responses (that is, participants might generally report ‘other’ more frequently when receiving TMS regardless of stimulation location). However, unlike rIPL TMS, the difference for zero-degree perturbations at the vertex did not approach significance. Thus, the effect of TMS over the rIPL (significantly affecting responses at both the zero- and four-degree perturbations) suggests that parietal TMS has an effect over and above any general effects of TMS. In addition, there appears to be a substantial self response bias which is most likely a consequence of the inherent difficulty of the task: previous research (Farrer et al., 2003b) has demonstrated that whereas participants can easily detect perturbations of around 10–15 degrees, they find perturbations as small as 5 degrees particularly difficult and tend to give many more self than other responses. If the default response, when uncertain, is self, then more self responses would be expected if TMS increased uncertainty. This, however, was not the outcome of the current experiment: more other, rather than self, responses were observed. In an attempt to disentangle the source of this change in bias, data were subsequently re-analyzed in the following manner: The original results were converted into percent self responses and re-entered in a 2 × 2 × 2 ANOVA as before. Planned comparisons between TMS and No-TMS for each condition revealed a significant reduction in self responses at the rIPL site for both zero- [F(1, 9) = 12.7, P < 0.01] and four- [F(1, 9) = 6.8, P < 0.05] degree perturbations, a marginal effect at the vertex site for the four-degree perturbation [F(1, 9) = 4.5, P < 0.06] and no effect at the vertex site for the zero-degree perturbation [F(1, 9) = 0.4, P = 0.57]. Taken together with the initial results, it seems as though TMS over rIPL might preferentially affect the ability to detect self on self trials (i.e. when there is no perturbation) If, as argued here, the comparator's representation of the predicted state of the motor system is disrupted by rIPL TMS, then the CNS would no longer have access to what self is, which would, indeed, lead to an increase in the number of inaccurate other responses on self trials.
Interestingly, other non-action-related forms of self/other discrimination have been associated with this same region. In an fMRI study, Uddin et al. (2005) found an increase in activity in the rIPL during a self-face recognition task in which participants had to make self/other judgments of faces morphed to different degrees between the participants own face and that of a gender-matched familiar other. The activity observed in this instance, however, was an increase when making self judgments (opposite to that observed with self/other action discrimination) and a follow-up TMS study (Uddin et al., 2006) demonstrated that disruption to the rIPL lead to a reduction in other judgments. These findings highlight one of the inherent problems with self-other judgment tasks, as they involve two different processes: a lower-level feeling of agency and a higher-level judgment of agency. This is a key criticism of both self/other judgment tasks and comparator model explanations in which there is no distinction between the lower-order sensations of otherness from higher-order overt categorical judgments (Gallagher, 2007; Synofzik et al., 2007).
There are, however, two crucial differences between the Uddin et al. (2006) study and the current experiment: first, in Uddin et al.' s work TMS was applied prior to the experimental procedure for 20 min at 1 Hz which has the effect of depressing the stimulated area for a prolonged period rather than at a specific time period during an individual process; second, Uddin et al.' s task would not have engaged the neural motor comparator mechanism as there was no motor component as in the current task. It is difficult to determine whether depressing rIPL in Uddin et al.' s experiment depressed the activity of lower-order face processing mechanisms or depressed part of the higher-order network involved in judgments of agency. Due to the transitory nature of the stimulation in the current experiment, however, it was more likely to have influenced lower-order mechanisms than higher-order networks.
In conclusion, the result of the current study supports the involvement of a neural comparator in agency attribution and adds further support to the idea that the inability to accurately predict the consequences of self-generated actions underlies delusions of control in schizophrenia (Farrer et al., 2004). The data presented here expands upon previous findings in that it offers a more detailed account of the processes underlying rIPL activation (Farrer et al., 2003a). We propose that such activity reflects low-level sensational aspects of agency (detection of mismatches between predicted and actual state representations by the comparator) rather than higher-level self/other judgments. It is important that future research should attempt to further tease apart these two processes as both are crucial to agency attribution and only through an understanding of the mechanisms underlying both processes can we begin to form conclusions as to the nature of normal and abnormal experiences of agency.
REFERENCES
- Bestmann S, Thilo KV, Sauner D, Siebner HR, Rothwell JC. Parietal magnetic stimulation delays visuomotor mental rotation at increased processing demands. Neuroimage. 2002;17:1512–20. doi: 10.1006/nimg.2002.1266. [DOI] [PubMed] [Google Scholar]
- Driver J, Mattingley JB. Parietal neglect and visual awareness. Nature Neuroscience. 1998;1:17–22. doi: 10.1038/217. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Frith CD, et al. Neural correlates of action attribution in schizophrenia. Psychiatry Research-Neuroimaging. 2004;131:31–44. doi: 10.1016/j.pscychresns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003a;18:324–33. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Paillard J, Jeannerod M. The role of proprioception in action recognition. Consciousness and Cognition. 2003b;12:609–19. doi: 10.1016/s1053-8100(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Frith C, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philosophical Transactions Of The Royal Society Of London Series B-Biological Sciences. 2000;355:1771–88. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. The natural philosophy of agency. Philosophy Compass. 2007;2:347–57. [Google Scholar]
- Groom KN, Ng WK, Kevorkian CG, Levy JK. Ego-syntonic alien hand syndrome after right posterior cerebral artery stroke. 1999;80:162–5. doi: 10.1016/s0003-9993(99)90114-4. [DOI] [PubMed] [Google Scholar]
- Jehkonen M, Laihosalo M, Kettunen JE. Anosognosia after stroke: assessment, occurrence, subtypes and impact on functional outcome reviewed. Restorative neurology and neuroscience. 2006;24:209–15. [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clinical Neurophysiology. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- MacDonald PA, Paus T. The role of parietal cortex in awareness of self-generated movements: a trascranial magnetic stimulation study. Cerebral Cortex. 2004;13:962–7. doi: 10.1093/cercor/13.9.962. [DOI] [PubMed] [Google Scholar]
- Maruff P, Woods SJ, Velakoulis D, et al. Reduced volume of parietal and frontal association areas in patients with schizophrenia characterized by passivity delusions. Psychological Medicine. 2005;35:99–113. doi: 10.1017/s0033291704003113. [DOI] [PubMed] [Google Scholar]
- Muggleton N, Postma GP, Moutsopoulou K, Nimmo-Smith I, Marcel A, Walsh V. TMS over right posterior parietal cortex induces neglect in a scene-based frame of reference. Neuropsychologia. 2006;44:1222–9. doi: 10.1016/j.neuropsychologia.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Nager W, Wolters C, Munte TF, Johannes S. Transcranial magnetic stimulation to the parietal lobes reduces detection of contralateral somatosensory stimuli. Acta Neurologica Scandinavica. 2004;109:146–50. doi: 10.1034/j.1600-0404.2003.00194.x. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Wurtz P, Luscher HR, et al. Repetitive TMS over the human oculomotor cortex: comparison of 1-Hz and theta burst stimulation. Neuroscience Letters. 2006;409:57–60. doi: 10.1016/j.neulet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience—virtual lesion, chronometry, and functional connectivity. Current Opinion In Neurobiology. 2000;10:232–7. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Paysant J, Beis JM, Le Chapelain L, Andre JM. Mirror asomatognosia in right lesions stroke victims. Neuropsychologia. 2004;42:920–5. doi: 10.1016/j.neuropsychologia.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Pia L, Neppi–Modona M, Ricci R, Berti A. The anatomy of anosognosia for hemiplegia: a meta-analysis. Cortex. 2004;40:367–77. doi: 10.1016/s0010-9452(08)70131-x. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review Of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997;120:1997–2011. doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Vosgerau G. Consciousness and Cognition. 2007. Beyond the comparator model: a mutifactorial two-step account of agency. (Available online May 2007) [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar–Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. Neuroimage. 2005;25:926–35. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Molnar–Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Social Cognitive and Affective Neuroscience. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy Of unilateral neglect after right-hemisphere stroke lesions—a clinical Ct-Scan correlation study in man. Neuropsychologia. 1986;24:609–22. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]