Abstract
To investigate how individual differences in moral judgment competence are reflected in the human brain, we used event-related functional magnetic resonance imaging, while 23 participants made either socio-normative or grammatical judgments. Participants with lower moral judgment competence recruited the left ventromedial prefrontal cortex and the left posterior superior temporal sulcus more than participants with greater competence in this domain when identifying social norm violations. Moreover, moral judgment competence scores were inversely correlated with activity in the right dorsolateral prefrontal cortex (DLPFC) during socio-normative relative to grammatical judgments. Greater activity in right DLPFC in participants with lower moral judgment competence indicates increased recruitment of rule-based knowledge and its controlled application during socio-normative judgments. These data support current models of the neurocognition of morality according to which both emotional and cognitive components play an important role.
Keywords: moral judgment, individual differences, moral judgment competence, right dorsolateral prefrontal cortex, fMRI
INTRODUCTION
Moral judgment can be defined as the evaluation of actions with respect to norms and values established in a society (such as not stealing or being an honest citizen). When judging a behavior as morally good or bad, people refer to their internal representations of these norms and values (i.e. emotionally laden internal moral orientations or principles).
Psychological research on moral judgment has long been dominated by a developmental approach investigating the maturation of moral orientations and principles and emphasized the role of conscious and rational reasoning processes (Kohlberg, 1969). Conversely, more recent models emphasize the role of unconscious and intuitive processes in moral judgment (Blair, 1995; Haidt, 2001, 2007; Hauser, 2006; Hauser et al., 2007; Mikhail, 2007). The social intuitionist model by Haidt (2001), for example, posits that fast and automatic intuitions are the primary source of moral judgments, whereas conscious deliberations are only used to construct post hoc justifications for judgments that have already occurred. Although there is some evidence supporting this view, others argue that immediate intuitions can also be informed by conscious deliberation (Pizarro and Bloom, 2003) and that some moral principles are available to conscious reason while others are not (Cushman et al., 2006).
In addition to this current debate, neuropsychological models claim that emotions are important to adapt behavior to environmental demands (Damasio, 1996). In line with this view, studies on patients with brain lesions showed that damage to the prefrontal cortex (especially its ventromedial and orbitofrontal portions) leads to deficits in social behavior and moral decision making (Damasio et al., 1994; Dimitrov et al., 1999; Koenigs and Tranel, 2007; Koenigs et al., 2007).
Investigating the question of how moral judgments are made in healthy subjects, a number of recent neuroimaging studies identified a network of brain regions contributing to moral cognition. Although these studies used different tasks ranging from simple moral decisions (Moll et al., 2001, 2002a, b; Heekeren et al., 2003, 2005; Luo et al., 2006) to complex dilemmatic moral judgments (Greene et al., 2001, 2004; Borg et al., 2006), the results are remarkably consistent and revealed a functional network of brain regions including the ventromedial prefrontal cortex (VMPFC), orbitofrontal cortex (OFC), the temporal poles, the amygdala, the posterior cingulate cortex (PCC), and the posterior superior temporal sulcus (PSTS), that is, brain regions which are involved in emotional as well as in cognitive information processing (see Greene and Haidt, 2002; Casebeer, 2003; Casebeer and Churchland, 2003; Moll et al., 2003, 2005; Goodenough and Prehn, 2004; Lieberman, 2007 for reviews). Those studies have variously focused on the evaluation of one's own actions and whether actions are intentionally or accidentally (Berthoz et al., 2002; Berthoz et al., 2006; Borg et al., 2006), on the influence of bodily harm on neural correlates of moral decision making (Heekeren et al., 2005), on the regulation of emotional responses (Harenski and Hamann, 2006), on the role of cognitive control and conflict processing (Greene et al., 2004), and on the impact of audience on moral judgments (Finger et al., 2006). In summary, the results of the previous functional magnetic resonance imaging (fMRI) studies support a theory of moral judgment according to which both emotional and cognitive components play an important role (Greene et al., 2004).
So far, neural correlates of moral decision making have been looked at in group analyses and individual differences in information processing have been treated as ‘noise’. The results of these studies may therefore crucially depend on the specific sample and their characteristics in information processing (Thompson-Schill et al., 2005; Mériau et al., 2006).
A current approach (Lind, 2007) points out the role of individual differences within the moral domain. Here, morality is defined as consisting of two inseparable, yet distinguishable aspects: (i) a person's moral orientations and principles and (ii) a person's competence to act accordingly. According to this theory, moral judgment competence is the ability to apply moral orientations and principles in a consistent and differentiated manner in varying social situations. Thus, social norms and values represented as affectively laden moral orientations are linked by means of moral judgment competence with everyday behavior and decision making. While most people commonly agree upon moral orientations and principles that are considered to be virtuous in their society, it is evident that people differ considerably with respect to their moral judgment competence (Lind, 2007).
Thus, relating individual differences in moral judgment competence to brain-imaging data derived from group analyses may lead to a more comprehensive understanding of the neural mechanisms involved in moral judgment. In the present study, we therefore investigated how individual differences in moral judgment competence are reflected in changes in brain activity during a simple socio-normative judgment task. We used event-related fMRI to measure neural activity, while 23 participants made either socio-normative or grammatical judgments and correlated neural activity with individual scores in moral judgment competence.
Based on the previous findings on the neural correlates of moral decision making, we hypothesized that individual differences in moral judgment competence correlate with blood oxygen level-dependent (BOLD) activity in the functional network of brain regions that contribute to moral decision making.
METHODS
Subjects
Twenty-three healthy female subjects [age: mean (M) = 25.17, standard deviation (s.d.) = 6.56] participated in this study. All participants were native German speakers, right-handed as assessed using the Edinburgh Handedness Inventory (Oldfield, 1971), with a similar educational level (general qualification for university entrance), and without any history of neurological or psychiatric diseases. We included only female participants because gender differences in the neural substrates of various aspects of cognition and emotion processing have been reported (Piefke et al., 2005; Cahill, 2006). The study was approved by the local ethics committee of the Charité University Medicine Berlin. Subjects were paid for their participation and gave written informed consent prior to investigation according to the Declaration of Helsinki (1991).
Task and material
To investigate neural correlates of moral judgment, we compared neural activity during a socio-normative judgment task with neural activity during a grammatical judgment task. We contrasted socio-normative judgments with grammatical judgments, because both kinds of judgments are rule-based.
During the socio-normative and the grammatical judgment tasks pairs of sentences were presented. An introductory sentence was presented first, introducing the participant to a specific situation. This introductory sentence was followed by a second sentence, which could contain either a violation of a social norm or a grammatical rule or no violation of such, respectively (Table 1).
Table 1.
Examples of sentence material
| Violation | Non-violation | ||
|---|---|---|---|
| Socio-normative judgment | First sentence | A uses public transportation [A fährt mit der S-Bahn] | A uses public transportation [A fährt mit der S-Bahn] |
| Second sentence | He smashes the window [Er wirft das Fenster ein] | He looks out of the window [Er sieht aus dem Fenster] | |
| Grammatical judgment | First sentence | B goes to a restaurant [B geht in ein Restaurant] | B goes to a restaurant [B geht in ein Restaurant] |
| Second sentence | He order a starter [Er bestellen eine Vorspeise] | He orders a starter [Er bestellt eine Vorspeise] |
During both tasks, the first sentence of a trial introduced the participants to a specific situation. Half of the second sentences contained a violation of a social norm or grammatical rule. After the appearance of the second sentence, participants were instructed to decide whether the action described in the second sentence was a social norm violation or not, or whether the sentence was grammatically correct or incorrect.
The participants were instructed to either decide whether the action described in the second sentence was a social norm violation or not (socio-normative judgment) or to decide whether the second sentence was grammatically correct or incorrect (grammatical judgment). To avoid neural activity due to an automated detection of social norm violations or grammatical errors as ‘confounding’ activity, the sentences used in the socio-normative judgment task did not contain grammatical errors and sentences used in the grammatical judgment task did not contain social norm violations. Thus, sentence material for the different tasks was similar but not identical.
Responses were given by pressing one of two buttons of an MRI compatible response device (labeled ‘yes’ or ‘no’), with middle and index finger of the right hand as quickly and correctly as possible. The assignment of ‘yes’ and ‘no’ to the response finger was counterbalanced across participants.
A reading condition was used as a low-level baseline task during which the participants had to read pairs of socio-normatively and grammatically correct sentences without making a decision. Here, participants also had to respond with a button press after they finished reading the second sentence.
Each task comprised 48 trials (24 violations and 24 non-violations of social norms, 24 violations and 24 non-violations of grammatical rules and 48 pairs of sentences for the reading condition). We thus used three tasks containing a total of five different conditions: socio-normative judgment containing either a violation of a social norm (NormJ/v) or not (NormJ/nv), grammatical judgment containing either a violation of a grammatical rule (GramJ/v) or not (GramJ/nv) and a reading task (Reading). Thus, the factors ‘task’ (socio-normative vs grammatical judgment) and ‘correctness’ (rule violation vs non-violation) were independently varied in a 2 × 2 factorial design.
Sentence material was matched for number of syllables and word frequencies (Baayen et al., 1993). Violations of grammatical rules and social norms were simple and unambiguous. To control for strong differences in emotional arousal between the tasks, sentences were devoid of bodily harm.
The sentence material has been validated in a previous questionnaire-based investigation (n = 80), confirming that all violations and non-violations of social norms could easily be judged as correct or incorrect, respectively.
Experimental procedure
Prior to the experiment, participants completed a practice session with similar stimulus material from a different material set. Sentences were presented visually in a mixed blocked/event-related design using a customized experimental control software (Presentation, Neurobehavioral Systems Inc., Albany, CA, USA) running on a Microsoft Windows 98 operating system.
The order of the experimental blocks (four blocks per task) was counterbalanced across participants. Each block was preceded by an instruction cue for 5 s (which stated ‘Socio-normative judgment’, ‘Grammatical judgment’ or ‘Reading’, respectively) and followed by 12 pairs of sentences. Each part (introductory sentence and second sentence) was presented for 2 s. Between introductory and second sentence, a black screen was presented for 1.5 s. Trials were presented in a pseudo randomized order with jittered interstimulus intervals (ISI) (minimum = 2 s, maximum = 12 s, mean = 4.5 s) optimized using OptSeq2 (www.surfer.nmr.mgh.harvard.edu). Between two trials participants were instructed to fixate a cross presented foveally.
While brain activity was monitored using fMRI, response times (RTs) and error rates were recorded. To assess potential differences in emotional arousal between the tasks, we simultaneously recorded skin conductance level (see below).
Immediately after the experiment participants rated all sentences from the experiment regarding immorality, emotionality (emotional arousal and valence), imagery, and familiarity on seven-point rating scales from 0 (no immorality, no emotional arousal, unpleasant, not imaginable, not personally familiar, respectively) to 6 (high immorality, high emotional arousal, pleasant, highly imaginable, highly personally familiar, respectively) and completed a paper- and pencil-version of the Moral Judgment Test (MJT) (Lind, 1998, 2007; www.uni-konstanz.de/ag-moral/mut/mjt-intro.htm; see subsequently) and the Social Desirability Scale (SDS-17) (Stöber, 2001).
Skin conductance data acquisition and analyses
As an indicator for emotional arousal, we continuously recorded skin conductance level (in µS) during the experiment at a sampling rate of 100 Hz. We used a pair of Ag/AgCl electrodes placed on the palm of the left hand and a commercial skin conductance sampling device (Psylab®, Contact Precisions Instruments, Boston, USA). A double-shielded cable protected the skin conductance signal from scanner-related artifacts. Skin conductance data were analyzed using Matlab® 7.0.4. (The MathWorks, Inc., MA, USA). Because we used short sentence material devoid of bodily harm, we expected changes in skin conductance to be very small. As recommended in the literature (Boucsein, 1992), we therefore only analyzed skin conductance level during experimental blocks (normative judgment and grammatical judgment) instead of event-related skin conductance responses for each of the four conditions (NormJ/v, NormJ/nv, GramJ/v, GramJ/nv, respectively). For each experimental block data were detrended, baseline corrected, and averaged across tasks (socio-normative judgment, grammatical judgment, and reading) and participants (Boucsein, 1992). For technical reasons data were not available for one subject.
Assessment of individual moral judgment competence
We assessed moral judgment competence with the MJT (Lind, 1998, 2007; www.uni-konstanz.de/ag-moral/mut/mjt-intro.htm; Lind and Wakenhut, 1980, 1985; Lind, 1982). The MJT confronts a participant with two complex moral dilemmas. In one dilemma (the doctor dilemma), for example, a woman had cancer with no hope for being cured. She suffered terrible pain and begged the doctor to aid her in committing medically assisted suicide. She said she could no longer endure the pain and would be dead in a few weeks anyway. The doctor complied with her wish. After presentation of this short story, the participant indicates to which degree he or she agrees or disagrees with the solution chosen by the protagonist. After that, the participant is presented with six arguments supporting (pro-arguments) and six arguments rejecting (counter-arguments) the protagonist's solution, which the participant has to rate with regard to its acceptability on a nine-point rating scale ranging from −4 (highly unacceptable) to +4 (highly acceptable). Each argument represents a certain moral orientation (according to the six Kohlbergian stages; Kohlberg, 1969). An example for a low-level argument against the doctor's solution would be: ‘The doctor acted wrongly because he could get himself into much trouble. They have already punished others for doing the same thing’, whereas the argument: ‘The doctor acted wrongly because the protection of life is everyone's highest moral obligation. We have no clear moral criteria for distinguishing between mercy-killing and murder’ represents a more elaborated argument against the given solution.
A person's moral orientation can be assessed by calculating the median acceptability for all four arguments, which refer to a certain moral orientation. In general, adult participants prefer more elaborated arguments, but differ in their ability of applying this moral orientation consistently especially when confronted with counter-arguments. The moral judgment competence score (C-score, the MJT's main score) is calculated as an individual's total response variation concerning the underlying moral orientations of the given arguments. It reflects the degree to which a participant's judgments about the pro- and counter-arguments are consistent and thus assesses how consistently or, in Lind's terms, how competently a person applies a certain moral orientation in the decision-making process independently of whether arguments are in line with the personal opinion on a particular issue or not. A highly competent person (indicated by a high C-score close to 100) will consistently appreciate all arguments referring to a certain socio-moral perspective, irrespective of whether this argument is a pro- or counter-argument. In contrast, a person with low moral judgment competence will appreciate only arguments, which support their own solution of the dilemma (only pro- or counter-arguments, respectively).
The concept of moral judgment competence is based on Kohlberg who introduced the term moral judgment competence as ‘the capacity to make decisions and judgments, which are moral (i.e. based on internal principles) and to act in accordance with such judgments’ (Kohlberg, 1964, p. 425). However, by defining moral judgment competence more precisely, Lind's approach clearly goes beyond what we may ordinarily call ‘moral competence’ as well as the Kohlbergian approach, which focused merely on moral orientations and the level of reasoning (see Haidt, 2001 for a critique on the Kohlbergian methodology).
To our knowledge the MJT is the only available test that provides a measure of moral judgment competence and differs from other instruments such as Kohlberg's Moral Judgment Interview (MJI) (Colby et al., 1987), the Defining Issue Test (DIT) (Rest, 1974) or the Sociomoral Reflection Measure (SRM) (Gibbs et al., 1992) that rather assess individual moral attitudes. The MJT has proved to be a valid and reliable psychometric test. For instance, moral judgment competence has been associated with responsible and democratic behavior (Heidbrink, 1985; Sprinthall et al., 1994; Gross, 1997). Translated in many languages, it also has been successfully used in scientific research (i.e. testing theoretical assumptions on moral development) and in evaluation of educational programs (Lind, 2006, 2007; Lerkiatbundit et al., 2006).
fMRI data acquisition and analyses
We used a 1.5-T MRI scanner (Siemens Magnetom Vision, Erlangen, Germany) with a standard head coil to acquire whole brain MRI data. Head movement was minimized using a vacuum pad. Axially oriented functional images (T2*-weighted volumes) were acquired using standard parameters (TE: 40 ms; TR: 2500 ms; flip angle: 90°; FOV: 256 mm; matrix: 64 × 64; voxel size: 4 × 4 × 4.6 mm; 26 slices). After acquisition of functional images, a sagittally oriented T1-weighted volume (TE: 5 ms; TR: 20 ms; flip angle: 30°; matrix: 256 × 256; voxel size: 1 × 1 × 1 mm) and a proton-density-weighted volume (TE: 15 ms; TR: 4350 ms; flip angle: 180°; matrix: 252 × 256; voxel size: 1 × 1 × 4.6 mm) were acquired for registration of the functional images.
MRI data were analyzed using a mixed effects approach within the framework of the general linear model as implemented in FMRI Expert Analysis Tool (FEAT), part of FSL (FMRIB's Software Library; www.fmrib.ox.ac.uk/fsl; Smith et al., 2004) and AFNI (Analysis of Functional NeuroImages; www.afni.nimh.nih.gov; Cox, 1996).
Prior to statistical analyses the following preprocessing was applied: slice-time correction and motion correction using MCFLIRT (Motion Correction using FMRIB's; Linear Image Registration Tool; Jenkinson et al., 2002), non-brain removal using BET (Brain Extraction Tool; Smith, 2002), spatial smoothing using a Gaussian kernel of 12 mm FWHM (Full-Width Half-Maximum), and high pass temporal filtering (Gaussian-weighted least squares straight line fitting with sigma = 50.0 s). Registration to high resolution and standard images was done using FLIRT (FMRIB's; Linear Image Registration Tool; Jenkinson and Smith, 2001).
Time series were modeled using event-related regressors for all five conditions (NormJ/v, NormJ/nv, GramJ/v, GramJ/nv, Reading) as well as the instruction periods and convolved with a hemodynamic response function. The instruction periods and the first introductory sentence of each trial were modeled as regressors of no interest, whereas the second sentences, which contained the experimental manipulation were modeled as regressors of interest. To control for differences in RTs between the conditions, we used an additional regressor that was modeled using RTs during each trial as a parametric modulation. Error trials (5.7% in total) were also modeled as an additional regressor of no interest. Contrast images (e.g. socio-normative judgment vs grammatical judgment) were computed for each subject and, after spatial normalization, transformed into standard space (Jenkinson et al., 2002).
All group analyses were performed using the transformed contrast images in a mixed effects model treating subjects as random. In the higher level analyses, we report clusters of maximally activated voxels that (i) survived statistical thresholding at Z = 3.09 and (ii) had a cluster size of at least 221 mm3, resulting in a corrected mapwise P < 0.05 as determined using Monte Carlo simulations as implemented in AFNI's AlphaSim.
To determine in which brain regions task-related changes in BOLD signal covaried with moral judgment competence, we used the individual C-score as a covariate in the higher level analysis. C-scores covaried with BOLD responses in the right dorsolateral prefrontal cortex (DLPFC) during socio-normative judgments (see ‘Results’ section). To further explore this effect, we split the sample into a ‘low’- and a ‘high’-moral judgment competence group using the median value (MD = 37.36). Note that moral judgment competence in our sample was higher than average compared to other studies (see ‘Results’ section; Lind, 2007). Therefore, we use the label ‘low’ purely in a statistical sense, which means relatively low compared to the ‘high’ moral judgment group. The resulting subgroups differed with respect to the C-score [t(21) = −7.31; P < 0.001], but not with respect to RTs [socio-normative judgments: t(21) = 0.594; P = 0.56; grammatical judgments: t(21) = 1.09; P = 0.29] or error rates [socio-normative judgments: t(21) = −0.92; P = 0.37; grammatical judgments: t(21) = −0.05; P = 0.96].
We further investigated whether individual differences in moral judgment competence also modulated BOLD activity in brain regions contributing to moral decision making. First, we identified regions of interest (ROIs) based on the contrast socio-normative judgments vs grammatical judgments (main effect of task) thresholded at Z = 3.09 (left VMPFC, right temporal pole, left PSTS). The ROIs in the left OFC and the left temporal pole were based on a map thresholded at Z = 3.89. All ROIs were clusters with a size of at least 28 voxels (cluster size corresponding to a corrected cluster threshold of P < 0.05). Second, we correlated BOLD responses in these regions with C-scores and performed a multivariate analysis of variance (ANOVA) with the factor group (‘low’ vs ‘high’ moral judgment competence).
RESULTS
Behavioral data, post hoc ratings and skin conductance level
Mean RTs (only for correctly answered trials) and error rates were computed for each of the five experimental conditions and averaged across participants (Table 2).
Table 2.
Response times and error rates for the five conditions
| NormJ/v | NormJ/nv | Gram/v | Gram/nv | Reading | |
|---|---|---|---|---|---|
| Mean response times in ms (s.d.) | 1193.10 (231.35) | 1218.88 (216.30) | 1273.47 (165.09) | 1408.33 (239.30) | 960.20 (201.93) |
| Mean error rates (s.d.) | 0.03 (0.04) | 0.04 (0.04) | 0.10 (0.08) | 0.04 (0.04) | – |
n = 23; NormJ/v, violations of social norms; NormJ/nv, non-violations of social norms; GramJ/v, violations of grammatical rules; GramJ/nv, non-violations of grammatical rules; Reading, reading control task.
For RTs a 2 × 2 repeated measure ANOVA (n = 23) and paired t-tests were performed. There was a significant main effect for both factors: task [socio-normative judgment vs grammatical judgment; F(1,22) = 40.88; P < 0.001] and correctness [social norm or grammatical rule violations vs non-violations; F(1,22) = 16.01; P = 0.001], as well as their interaction [F(1,22) = 6.81; P = 0.016]. That is, participants responded faster during socio-normative judgments than during grammatical judgments, and RTs were shorter when participants identified a violation of a social norm or a grammatical rule than a non-violation.
Paired t-tests for RTs revealed that there was neither a significant difference in RTs between violations and non-violations of social norms [t(22) = −1.26; P = 0.22] nor between violations of social norms and violations of grammatical rules [t(22) = −2.55; P = 0.02, only trend because of adjusted α = 0.008]. However, there was a significant difference between non-violations of social norms and grammatical rules [t(22) = −6.81; P < 0.001] as well as between violations and non-violations of grammatical rules [t(22) = −3.8; P = 0.001]. Thus, the task by correctness interaction appears to be driven by the particularly slow RTs when participants decided that grammatical rules were not violated (Table 2).
Error rates were low (5.7% in total). Most errors occurred during identification of grammatical rule violations (GramJ/v; Table 2).
Post hoc ratings (n = 23) of the sentence material regarding immorality, emotionality (emotional arousal and valence), familiarity, and imagery acquired outside the scanner after the scanning session were compared using non-parametric Wilcoxon tests. As expected, violations of social norms were rated more immoral (Wilcoxon Z = −4.14; P < 0.001), more emotionally arousing (Wilcoxon Z = −4.37; P < 0.001), more unpleasant (Wilcoxon Z = −3.90; P < 0.001), less personally familiar (Wilcoxon Z = −3.97; P < 0.001), and less imaginable (Wilcoxon Z = −3.47; P < 0.001) than non-violations of social norms (this was also confirmed by the questionnaire-based pilot study of n = 80 subjects, see above).
The continuously measured skin conductance level showed no significant difference between socio-normative (M = −0.07, s.d. = 0.38) and grammatical judgments [M = 0.03, s.d. = 0.35, n = 22, t(21) = −1.11; P = 0.28].
Individual moral judgment competence
As described earlier, the MJT provides measures for both a person's moral orientation and the moral judgment competence. In line with the literature, the participants in our sample did not differ from each other regarding their moral orientation and showed most preferences for arguments referring to advanced levels of moral reasoning (Kohlberg, 1969). However, their individual moral judgment competence (C-score) was normally distributed within our sample (n = 23, M = 36.93, s.d. = 16.67; Kolmogorov–Smirnov Z = 0.5; P = 0.96). Compared to the mean reported in other studies the mean C-score of 36.93 is relatively high (Lind, 2007). That is, moral judgment competence on average was well pronounced in our sample. However, the standard deviation (s.d. = 16.67, maximum score = 62.74, minimum score = 5.55) indicates that the range of C-scores was also reasonably wide. Notably, C-scores were not correlated with individual tendency to respond in a norm-congruent way as measured with the SDS-17 (r = −0.28; P = 0.20).
In both experimental conditions, there was no correlation between C-scores and RT (socio-normative judgments: r = −0.06; P = 0.78; grammatical judgments: r = −0.16; P = 0.46), error rates (socio-normative judgments: r = 0.34; P = 0.12; grammatical judgments: r = 0.03; P = 0.88) and skin conductance level (socio-normative judgments: r = −0.06; P = 0.80; grammatical judgments: r = 0.004; P = 0.99). There was also no correlation between C-scores and post hoc ratings of social norm violations (immorality: r = −0.12; P = 0.60; emotional arousal: r = 0.19; P = 0.40; emotional valence: r = −0.09; P = 0.69; familiarity: r = 0.10; P = 0.66; imagery: r = −0.15; P = 0.50).
fMRI data
Main effects (factors task and correctness) and interaction
In the first step, we analyzed the fMRI data in a mixed effects group analysis (n = 23) without taking individual differences in moral judgment competence into account.
The comparison of socio-normative judgments vs grammatical judgments (main effect of task) revealed greater BOLD responses in the left VMPFC (BA 10/11), the left OFC (BA 11/47), the bilateral temporal poles (BA 21/38) and the left PSTS (BA 39; Table 3, Figure 1).
Table 3.
Anatomical locations and co-ordinates of activations
| Anatomical region | L/R | BA | Number of voxels in cluster | Z score of local maximum | MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Main effect of task | |||||||
| Socio-normative judgments > grammatical judgments | |||||||
| Medial frontal gyrus, VMPFC | L | 10/11 | 172 | 3.61 | −8 | 42 | −18 |
| Inferior frontal gyrus/temporal gyrus | L | 3142 | |||||
| Inferior frontal gyrus, OFC | 11/47 | 4.05 | −40 | 28 | −22 | ||
| Middle temporal gyrus, temporal pole | 21/38 | 4.65 | −42 | 12 | −38 | ||
| Inferior temporal gyrus, temporal pole | 20 | 4.19 | −64 | −12 | −28 | ||
| Middle temporal gyrus, temporal pole | R | 21/38 | 1609 | 4.85 | 50 | 10 | −34 |
| Posterior superior temporal sulcus (PSTS) | L | 39 | 787 | 4.01 | −50 | −68 | 34 |
| Grammatical judgments > socio-normative judgments | |||||||
| Medial frontal gyrus, VMPFC | R | 10 | 32 | 3.45 | 32 | 70 | 0 |
| Superior parietal lobes | L/R | 7 | 2548 | ||||
| Precuneus | L | 7 | 3.67 | −14 | −74 | 50 | |
| Precuneus | R | 7 | 4.27 | 20 | −68 | 52 | |
| Inferior parietal lobe | R | 40 | 96 | 3.49 | 46 | −38 | 48 |
| Middle occipital gyrus | R | 19 | 34 | 3.44 | 42 | −82 | 8 |
| Main effect of correctness | |||||||
| Violations of social norms and grammatical rules > non-violations | |||||||
| Posterior superior temporal sulcus (PSTS)/Inferior parietal lobule (IPL) | L | 39/40 | 30 | 3.38 | −62 | −56 | 42 |
| Task by correctness interaction | |||||||
| Precuneus | L | 7 | 70 | 3.26 | −36 | −68 | 42 |
Mixed effects analysis (n = 23) of main effects and interaction. See text for a detailed description.
Note: Clusters of maximally activated voxels that (i) survived statistical thresholding at Z = 3.09 (P < 0.001) and (ii) had a cluster size of at least 221 mm3 (=28 voxels), resulting in a corrected mapwise P < 0.05.
BA, Brodmann area; MNI coordinates, coordinates referring to the standard brain of the Montreal Neurological Institute; OFC, orbitofrontal cortex; VMPFC, ventromedial prefrontal cortex.
Fig. 1.
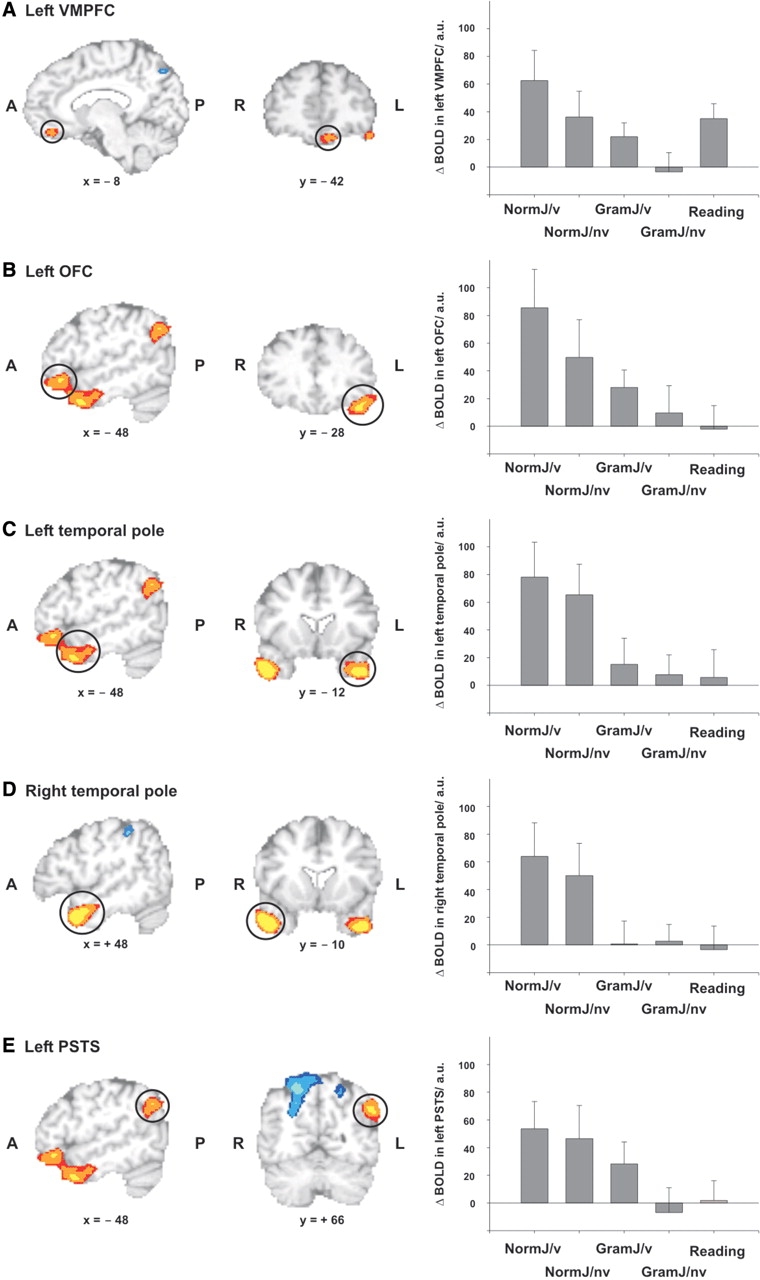
Main effect of task. Left panel: Brain regions showing a main effect of task (socio-normative judgment vs grammatical judgment). Yellow-red regions responded more during socio-normative than during grammatical judgments, blue regions showed the reverse pattern (greater responses during grammatical than during socio-normative judgments). Results of group analysis (mixed effects analysis, n = 23) thresholded at Z = 3.09. Right panel: BOLD responses [mean and standard error of the mean in arbitrary units (a.u.), n = 23] during the five conditions (NormJ/v = violations of social norms, NormJ/nv = non-violations of social norms, GramJ/v = violations of grammatical rules, GramJ/nv = non-violations of grammatical rules, Reading = reading control task) in these regions. Analyses of BOLD responses in left VMPFC, right temporal pole and left PSTS were based on functional ROIs thresholded at Z = 3.09. Analyses in left OFC and left temporal pole were based on functional ROIs thresholded at Z = 3.89. All ROIs were clusters with a size of at least 28 voxels (cluster size corresponding to a corrected cluster threshold of P < 0.05).
The comparison of grammatical vs socio-normative judgments revealed activations in the right VMPFC (BA 10), the parietal lobes (BA 7/40), especially in the bilateral precuneus (BA 7), and in the right middle occipital gyrus (BA 19, Table 3).
A main effect of correctness (comparison of violations vs non-violations of social norms and grammatical rules) was only found in the left PSTS extending to the inferior parietal lobule (IPL, BA 39/40; Table 3). In that region, greater BOLD responses were found when participants identified violations of social norms and grammatical rules, in contrast to non-violations. Note that the left PSTS also showed a main effect of task (more activation during socio-normative than during grammatical judgments).
No brain region showed more activation during identification of non-violations compared to identification of violations at the threshold levels described earlier.
The left precuneus (BA 7; Table 3) was the only brain region that showed an interaction of the two factors task (socio-normative judgments vs grammatical judgments) and correctness (violations vs non-violations of a social norm or grammatical rule). This region also showed a main effect of task (grammatical vs socio-normative judgments) and responded most during identification of violations in the grammatical judgment task.
Covariation of BOLD responses with C-scores
C-scores covaried significantly with changes in BOLD activity in right DLPFC during socio-normative relative to grammatical judgments [n = 23; BA 45/46; Montreal Neurological Institute (MNI) coordinates: x = 50, y = 18, z = 20; Z-score of local maximum = 3.60; 403 voxels; cf. Figure 2A], that is, participants with lower moral judgment competence recruited the right DLPFC more (during socio-normative relative to grammatical judgments) than those with greater competence in this domain.
Fig. 2.
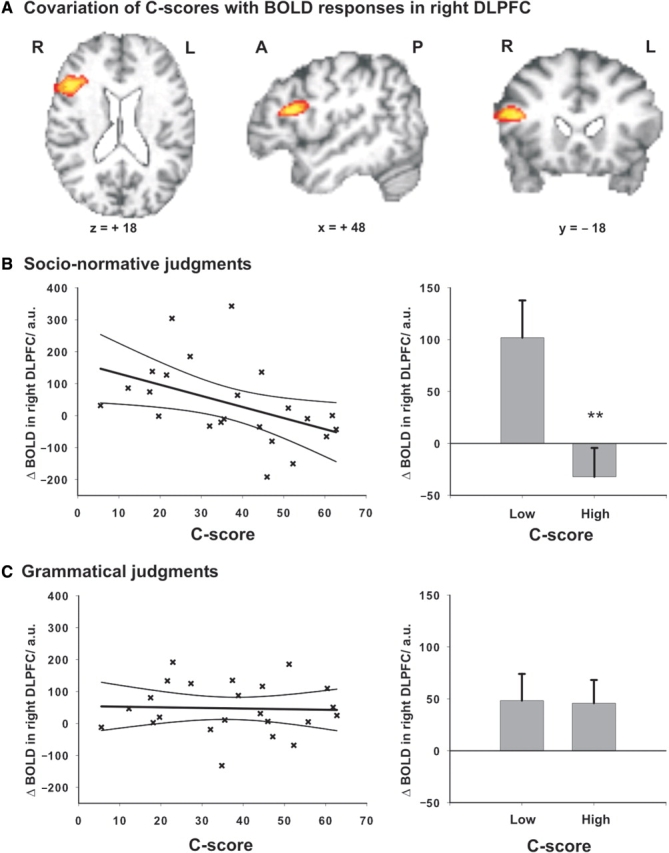
Moral judgment competence reflected in BOLD responses in right DLPFC. (A) Covariation of C-scores with BOLD responses in right DLPFC during socio-normative vs grammatical judgments [activation from higher level analysis thresholded at Z = 3.09 (P < 0.05, corrected)]. (B) Left panel: Negative correlation of C-scores and BOLD responses in right DLPFC during socio-normative judgments [r = −0.45; P = 0.03; C-scores plotted against BOLD responses in arbitrary units (a.u.) with regression line and 95% confidence limits]. Right panel: The subgroup with lower moral judgment competence (median split, n = 12) showed significantly greater activation in right DLPFC (mean and standard error of the mean) during socio-normative judgments than the subgroup with greater moral judgment competence (n = 11). (C) Left panel: No correlation of C-scores and BOLD responses in right DLPFC during grammatical judgments [r = −0.04; P = 0.64; C-scores plotted against BOLD responses in arbitrary units (a.u.) with regression line and 95% confidence limits]. Right panel: No difference in BOLD responses (mean and standard error of the mean) between the two subgroups during grammatical judgments.
An ROI analysis confirmed that C-scores correlated negatively with BOLD responses in the right DLPFC during the socio-normative (r = −0.453; P = 0.03), but did not correlate during the grammatical judgment task (r = −0.04; P = 0.644, cf. Figure 2B). Notably, individual moral judgment competence accounted for 20.5% of the variance in right prefrontal BOLD responses during socio-normative judgments.
Furthermore, participants with lower moral judgment competence (median split sample; n = 12) showed significantly greater BOLD responses in the right DLPFC than participants with higher moral judgment competence (n = 11) during socio-normative judgments [comparison of subgroups: t(21) = 2.91; P = 0.008]. During grammatical judgments there was no significant difference between the two subgroups [t(21) = 0.07; P = 0.94; Figure 2C]. There was no covariation of C-scores with BOLD signal changes elicited in other conditions or contrasts.
As reported earlier, C-scores were not correlated with RTs, error rates, skin conductance level or post hoc ratings of social norm violations and thus only covaried with changes in BOLD activity in right DLPFC. Conversely, activity in right DLPFC during socio-normative judgment was also not correlated with RTs (r = 0.13; P = 0.57), error rates (r = −0.25; P = 0.25), skin conductance level (r = −0.09; P = 0.70) or post hoc ratings of social norm violations (immorality: r = 0.18; P = 0.43; emotional arousal: r = −0.12; P = 0.60; emotional valence: r = 0.03; P = 0.88; familiarity: r = −0.18; P = 0.42; imagery: r = 0.22; P = 0.34). Thus, individual differences in BOLD activity in right DLPFC cannot be explained by individual differences in task difficulty, emotional arousal or personal experience of social norm violations.
As described earlier, the whole-brain analysis revealed that BOLD activity only covaried with C-scores in right DLPFC during socio-normative but not during grammatical judgments. Moreover, we investigated whether individual differences in moral judgment competence also modulate BOLD activity in the cerebral network engaged in socio-normative relative to grammatical judgments (main effect of task). We found no significant correlation of C-scores and BOLD responses in functional ROIs (brain regions found to be associated with socio-normative judgments, main effect of task) during the socio-normative judgment task (left VMPFC: r = −0.13; P = 0.57; left OFC: r = −0.19; P = 0.39; left temporal pole: r = −0.05; P = 0.82; right temporal pole: r = −0.18; P = 0.41; left PSTS: r = −0.20; P = 0.35) as well as during identification of social norm violations specifically (left VMPFC: r = −0.23; P = 0.29; left OFC: r = −0.17; P = 0.43; left temporal pole: r = −0.01; P = 0.98; right temporal pole: r = −0.12; P = 0.58; left PSTS: r = −0.19; P = 0.39). However, using a median-split approach an additional ROI analysis revealed a main effect of the factor group (‘low’ vs ‘high’ moral judgment competence) on activity in the left VMPFC [F(1,22) = 5.98; P = 0.023] and the left PSTS [F(1,22) = 5.92; P = 0.024] specifically during identification of social norm violations. That is, participants with lower moral judgment competence showed greater activity in these regions (Figure 3).
Fig. 3.
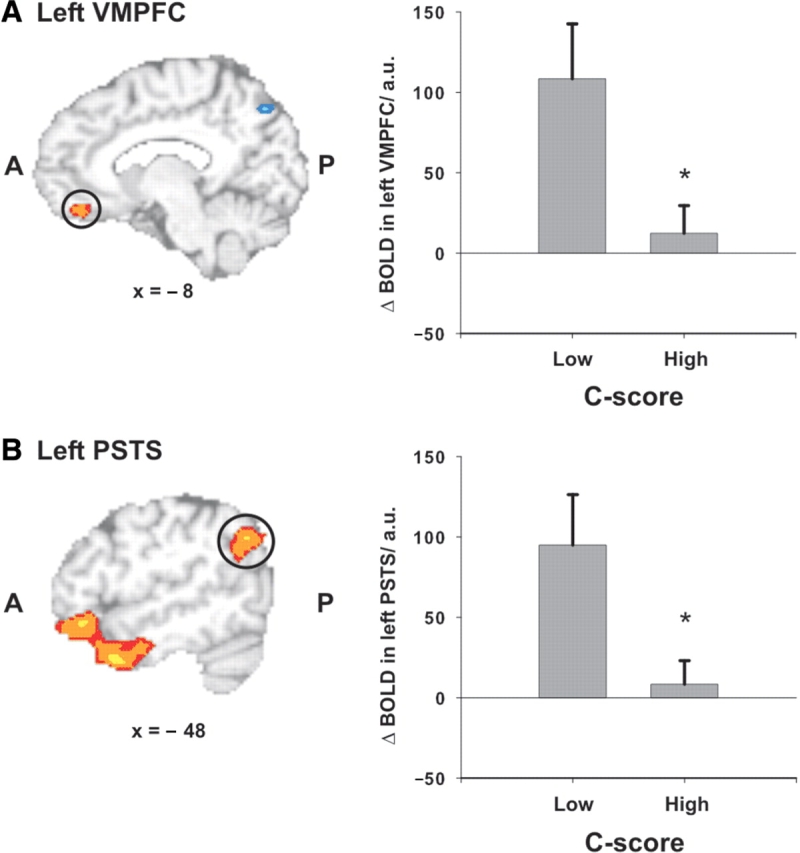
Moral judgment competence modulates BOLD responses in regions involved in socio-normative judgments. BOLD responses in (A) left VMPFC and (B) left PSTS during identification of social norm violations [activation from higher level analysis thresholded at Z = 3.09 (P < 0.05, corrected) based on functional ROIs thresholded at Z = 3.09]. Participants with lower moral judgment competence (median split, n = 12) showed significantly greater activation (mean and standard error of the mean) than participants with higher moral judgment competence (n = 11).
DISCUSSION
In the present study, we investigated how individual differences in moral judgment competence are reflected in the brain during a simple socio-normative judgment task. We replicated previous findings on a cerebral network involved in moral cognition. Activity in this network was modulated by individual differences in moral judgment competence. In particular, participants with lower moral judgment competence showed greater BOLD responses in the left VMPFC and the left PSTS during identification of social norm violations than participants with higher moral judgment competence. Moreover, we found that moral judgment competence was inversely correlated with neural activity in the right DLPFC during socio-normative relative to grammatical judgments.
A cortical network activated during moral judgments
Contrasting activity during socio-normative judgments with grammatical judgments revealed activation in the left VMPFC, the left OFC, the temporal poles and the left PSTS. These results are in line with previous fMRI studies, which identified a similar functional cerebral network contributing to various moral or socio-normative judgment tasks, including simple moral decisions as well as complex dilemmatic moral judgments (see Greene and Haidt, 2002; Casebeer, 2003; Casebeer and Churchland, 2003; Moll et al., 2003, 2005; Goodenough and Prehn, 2004; Lieberman, 2007 for reviews).
Activity in the VMPFC, the OFC, the temporal poles and the PSTS has often been associated with social cognition, especially during mentalizing or theory of mind tasks (Gallagher and Frith, 2003; Frith and Frith, 2006; Saxe, 2006). In the present experiment, participants had to decide whether an action described in the sentence represented a social norm violation or not. The social norm violations used were unambiguous and neither perspective taking nor reading the mental states or intentions of the agent was required. However, it is very likely that participants routinely reflect on their subjective experiences and put themselves into the position of the agent when judging a behavior as good or bad (Amodio and Frith, 2006). In particular, activity in the temporal poles has recently been associated with providing abstract concepts describing a social behavior (like virtuous or guilty; Zahn et al., 2007).
Other studies also report on activations in some more regions involved in emotion processing such as the PCC (Greene et al., 2001, 2004; Moll et al., 2002a, b; Heekeren et al., 2005; Harenski and Hamann, 2006) and the amygdala (Moll et al., 2002a, b; Berthoz et al., 2006; Harenski and Hamann, 2006) during moral judgment tasks. Supposedly, the lack of activation in those regions associated with emotional processing in our study is related to the fact that our material was less emotionally charged than the material used in other studies (i.e. sentences devoid of bodily harm vs complex dilemmas, often involving harm or even death). Social norm violations were rated more emotionally arousing and more unpleasant than non-violations. However, regarding the main effect of task (social-normative vs grammatical judgments) skin conductance level data simultaneously recorded in the fMRI scanner confirmed that there was no significant difference at least in physical arousal during experimental blocks of socio-normative judgment compared to blocks of grammatical judgment.
Neural activity during grammatical judgments
The right VMPFC, the superior parietal lobes (namely the precuneus), the IPL, and the middle occipital gyrus showed greater responses during grammatical judgments as compared to socio-normative judgments. Enhanced activity in these brain regions might reflect the attention demanding visual search for features representing grammatical errors (cf. Coull et al., 1998; Hopfinger et al., 2000; Liu et al., 2003 for the involvement of a right fronto-parietal network in sustained selective attention). Higher processing demands during the grammatical judgment task are also indicated by longer RTs during grammatical judgments as compared to socio-normative judgments. Interestingly, the left precuneus responded most during identification of violations during the grammatical judgment task, the condition in which RTs were shorter while more errors occurred (indicating a speed-accuracy tradeoff).
Neural correlates of individual differences in moral judgment competence
Individual differences modulated neural activity in the left VMPFC and the left PSTS specifically during the identification of social norm violations (median split analysis) and in the right DLPFC during socio-normative judgments in general (violations and non-violations of social norms) but not during grammatical judgments (covariance analysis). Participants with lower moral judgment competence showed significantly more activity in these brain regions than participants with greater moral judgment competence.
In the literature, greater neural activity in participants with lower competence in a certain cognitive task has been associated with compensation (Kosslyn et al., 1996; Rypma et al., 2006). In detail, efficiency theories suggest that individuals differ in the efficiency with which fundamental cognitive operations are performed. According to these theories, efficient individuals are able to perform fundamental cognitive operations faster than inefficient individuals and with minimized resource allocation (Vernon, 1983). Increased activation in individuals with lower competence thus may be due to the increased recruitment of cognitive resources. In the present study, C-scores were not correlated with RTs or error rates and the median split samples did not differ with regard to these parameters. This may be due to the fact that the task used in the present experiment was very easy and unambiguous and RTs were generally shorter than in other studies using more complex and dilemmatic moral judgment tasks (Greene et al., 2001, 2004). Additionally, although the range of moral judgment scores in our sample was reasonably wide, participants in our sample had a well-pronounced moral judgment competence compared to participants in other studies (Lind, 2007), and the label ‘low moral judgment competence’ was rather used in a statistical sense than to indicate deficits in moral judgment competence.
Studies on patients with brain lesions provided first evidence that damage to the prefrontal cortex (especially its ventromedial and orbitofrontal portions) leads to deficits in social behavior and moral decision making (Damasio et al., 1994; Dimitrov et al., 1999). The VMPFC is recruited when we have to understand other people's behavior in terms of their intentions or mental states (Berthoz et al., 2002; Frith and Frith, 2006). The orbital part of the VMPFC in particular (as well as the orbitofrontal cortex in general) has been associated with representing the expected value of possible outcomes of a behavior with respect to rewards and punishments (Camille et al., 2004; Walton et al., 2004; Amodio and Frith, 2006; Luo et al., 2006). Luo et al. (2006), for instance, manipulated the intensity of moral transgressions and showed increased neural activity in VMPFC and amygdala in response to high relative to low legal and illegal stimuli. Activity in VMPFC, thus, is apparently associated with both positively and negatively valenced information on the expected reinforcement (Luo et al., 2006). Additionally, the VMPFC seems to play an important role in the generation of social emotions such as compassion, shame, and guilt that are closely related with moral values when confronted with social norm violations (Koenigs and Tranel, 2007; Koenigs et al., 2007). With respect to moral judgment competence, it should be noted that patients with VMPFC lesions acquired in adulthood display irresponsible and inappropriate social behavior but normal basic cognitive abilities and a preserved knowledge about the accepted standards of moral behavior (Saver and Damasio, 1991; Anderson et al., 1999). This might indicate a deficit in the ability to consistently apply moral orientations; however, none of these studies explicitly investigated moral judgment competence.
Activity in the PSTS has been reported in almost all functional imaging studies investigating moral judgment and decision making (Greene et al., 2001, 2004; Moll et al., 2001, 2002a, b; Berthoz et al., 2002; Heekeren et al., 2003, 2005; Borg et al., 2006; Finger et al., 2006; Harenski and Hamann, 2006). Although the precise function of the PSTS remains unclear, this region seems to play a central role in representing socially significant information from different domains/modalities. For instance, the PSTS contributes to multisensory integration (Beauchamp et al., 2004), to the processing of biological motion cues (Beauchamp et al., 2002, 2003; Schultz et al., 2005) and to the detection and analysis of goals and intentions of another person's behavior (Schultz et al., 2004; Young et al., 2007). The important role of PSTS in social cognition and the processing of another person's beliefs or mental states is also supported by lesion studies (Samson et al., 2004).
In our study, activity in the left PSTS (extending to the left IPL) also showed a main effect of correctness (comparison of violations vs non-violations of social norms and grammatical rules, respectively). Activity in PSTS has also been found when participants were presented with salient or task-relevant and attention-attracting events (Downar et al., 2002; Kincade et al., 2005) and thus activity in left PSTS may reflect the detection of violations of socio-normative as well as grammatical rules (violations of any kind).
Moral judgment competence correlated with changes in BOLD activity in right DLPFC during socio-normative judgments but not during grammatical judgments. During socio-normative judgments, participants with comparably low moral judgment competence recruited the right DLPFC more than those with greater competence in this domain. Notably, individual moral judgment competence accounted for 20.5% of the variance in right prefrontal activity.
As described in detail earlier, moral judgment competence represents the ability to apply a moral orientation in a consistent and differentiated manner in varying social situations (Lind, 2007). Increased activity in right DLPFC thus can be interpreted as higher processing demand due to the application of knowledge about social norms and rules during the decision-making process. More evidence for a role of the DLPFC in moral judgment and the implementation of moral behavior comes from a study using repetitive transcranial magnetic stimulation (rTMS). Here, a disruption of the right, but not the left DLPFC reduces the subject's willingness to reject their partner's intentionally unfair monetary offers. Importantly, subjects are still able to judge the unfair offers as unfair, which indicates that the right DLPFC plays a key role especially in the implementation of fairness-related behaviors (Knoch et al., 2006). That rTMS study thus provides complementary evidence to our study in showing that the right DLPFC is crucial for the execution of normatively appropriate behavior.
Greater responses in right DLPFC may also be interpreted in the context of two current theoretical models describing how the PFC controls complex behavior that are not mutually exclusive. First, the PFC is eminent in the implementation of control processes, task monitoring, and inhibitory control during rule-based response selection (Miller, 2000; Bunge, 2004). In line with this functional account of PFC function, an fMRI study investigating dilemmatic moral judgments showed activation of the DLPFC when control processes were needed to override prepotent emotional responses to make a utilitarian decision (e.g. smothering a crying baby to save more lives; Greene et al., 2004) or to resist temptations (Knoch and Fehr, 2007). An fMRI study investigating the impact of interracial contact on executive functions, moreover, showed that participants with high scores on subtle measures of racial bias showed increased activity in right DLPFC when presented with black faces (Richeson et al., 2003). Increased activity in the right DLPFC in these participants was interpreted as additional effort to suppress an automatic activation of negative stereotypes because these participants also endorse egalitarian values and following contemporary societal norms it is unacceptable to show racial prejudices against black people. Second, the structured-event-complex framework model explains activity in the PFC as reflecting content-specific representations rather than task- or function-specific processes. According to this representational account, a structured-event-complex (such as going to a restaurant or giving a dinner party) represents knowledge abstracted across a number of similar events (including temporal organizations, social rules or special features). This event-sequence knowledge is assumed to be stored in long-term memory and guides the perception and execution of goal-oriented behavior (Grafman, 1995; Wood and Grafman, 2003). Activity in right DLPFC may thus indicate an increased recruitment of the rule-based event knowledge in participants with lower moral judgment competence.
It should be noted that it is presently unclear, how exactly moral judgment competence as measured by the MJT maps on other cognitive abilities such as general intelligence. Future studies will have to address this question.
CONCLUSION
By using an easy and unambiguous socio-normative judgment task, we replicated the findings on the functional network related to moral cognition and provide the first evidence that neural activity in this network is modulated by individual differences in moral judgment competence. Participants with lower moral judgment competence recruited the left VMPFC and the left PSTS more than participants with greater competence when identifying social norm violations. Because increased activation in individuals with lower moral judgment competence may be due to the increased recruitment of mental resources, we interpret increased activity in VMPFC and PSTS as increased involvement of social cognitive and emotional processes such as mentalizing or estimating the value of possible outcomes of a behavior and the experience of moral emotions during moral judgment. Moreover, we found that moral judgment competence score was inversely correlated with activity in the right DLPFC during socio-normative relative to grammatical judgments, indicating higher processing demand due to the controlled application of rule-based knowledge during the decision-making process.
Thus, our data are in line with current models of the neurocognition of morality according to which both emotional and cognitive components play an important role. However, the question is not only which processes are involved in moral judgment, but also how well a decision maker is able to integrate these different processes (e.g. emotional responses with rational reasoning processes) sensitive to the context of the particular social situation he or she faces (Talmi and Frith, 2007). This view highlights the role of individual differences, for instance, in moral judgment competence, which is defined as the ability to apply a certain moral orientation in a consistent and differentiated manner in varying social situations (Lind, 2007).
Acknowledgments
This study was financially supported by grants from the Gruter Institute of Law and Behavioral Research, the Graduate Program Berlin (Scholarship Nachwuchsfoerderung), the BMBF (Berlin NeuroImaging Center, BNIC) and the DFG [Emmy-Noether-Program (HE 3347/1-2)]. We gratefully acknowledge the help of S. Prehn with programming the experiment. We thank C. Frith, E. Fehr, D. Knoch and G. Lind for comments on the article.
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Rijn H. The CELEX Lexical Database [CD-ROM] (Release 1) Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1993. [Google Scholar]
- Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004;41:809–23. doi: 10.1016/s0896-6273(04)00070-4. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:149–59. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Grezes J, Armony JL, Passingham RE, Dolan RJ. Affective response to one's own moral violations. Neuroimage. 2006;31:945–50. doi: 10.1016/j.neuroimage.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Blair RJR. A cognitive developmental-approach to morality – investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Borg JS, Hynes C, Van HJ, Grafton S, Sinnott-Armstrong W. Consequences, action, and intention as factors in moral judgments: an FMRI investigation. Journal of Cognitive Neuroscience. 2006;18:803–17. doi: 10.1162/jocn.2006.18.5.803. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal Activity. New York: Plenum Press; 1992. [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cognitive Affective & Behavioral Neuroscience. 2004;4:564–79. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–70. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Casebeer WD. Moral cognition and its neural constituents. Nature Reviews Neuroscience. 2003;4:840–6. doi: 10.1038/nrn1223. [DOI] [PubMed] [Google Scholar]
- Casebeer WD, Churchland PS. The neural mechanisms of moral cognition: a multiple-aspect approach to moral judgment and decision-making. Biology & Philosophy. 2003;18:169–94. [Google Scholar]
- Colby A, Kohlberg L, Speicher B, et al. The Measurement of Moral Judgment. New York: Cambridge University Press; 1987. [Google Scholar]
- Coull JT, Frackowiak RSJ, Frith CD. Monitoring for target objects: activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–34. doi: 10.1016/s0028-3932(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cushman F, Young L, Hauser M. The role of conscious reasoning and intuition in moral judgment: testing three principles of harm. Psychological Science. 2006;17:1082–9. doi: 10.1111/j.1467-9280.2006.01834.x. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage – clues about the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Dimitrov M, Phipps M, Zahn TP, Grafman J. A thoroughly modern gage. Neurocase. 1999;5:345–54. [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology. 2002;87:615–20. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Kamel N, Mitchell DG, Blair JR. Caught in the act: the impact of audience on the neural response to morally and socially inappropriate behavior. Neuroimage. 2006;33:414–21. doi: 10.1016/j.neuroimage.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gibbs JC, Basinger KS, Fuller R. Moral Maturity: Measuring the Development of Sociomoral Reflection. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Goodenough OR, Prehn K. A neuroscientific approach to normative judgment in law and justice. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2004;359:1709–26. doi: 10.1098/rstb.2004.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J. Similarities and distinctions among current models of prefrontal cortical functions. Structure and Functions of the Human Prefrontal Cortex. 1995;769:337–68. doi: 10.1111/j.1749-6632.1995.tb38149.x. [DOI] [PubMed] [Google Scholar]
- Greene J, Haidt J. How (and where) does moral judgment work? Trends in Cognitive Sciences. 2002;6:517–23. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Gross ML. Ethics and Activism: The Theory and Practice of Political Morality. Cambridge, MA: Cambridge University Press; 1997. [Google Scholar]
- Haidt J. The emotional dog and its rational tail: a social intuitionist approach to moral judgment. Psychological Reviews. 2001;108:814–34. doi: 10.1037/0033-295x.108.4.814. [DOI] [PubMed] [Google Scholar]
- Haidt J. The new synthesis in moral psychology. Science. 2007;316:998–1002. doi: 10.1126/science.1137651. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–24. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Hauser M, Cushman F, Young L, Jin RKX, Mikhail J. A dissociation between moral judgments and justications. Mind & Language. 2007;22:1–21. [Google Scholar]
- Hauser MD. The liver and the moral organ. Social Cognitive and Affective Neuroscience. 2006;1:214–20. doi: 10.1093/scan/nsl026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, Villringer A. Influence of bodily harm on neural correlates of semantic and moral decision-making. Neuroimage. 2005;24:887–97. doi: 10.1016/j.neuroimage.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A. An fMRI study of simple ethical decision-making. Neuroreport. 2003;14:1215–9. doi: 10.1097/00001756-200307010-00005. [DOI] [PubMed] [Google Scholar]
- Heidbrink H. Moral judgment competence and political learning. In: Lind G, Hartmann HA, Wakehut RH, editors. Moral Development and Social Environment. Studies in the Philosophy and Psychology of Moral Judgment and Education. Chicago: Precedent Publishing; 1985. pp. 259–71. [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–91. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. Journal of Neuroscience. 2005;25:4593–604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Fehr E. Annals of the New York Academy of Sciences. Vol. 1104. 2007. Resisting the power of temptations: the right prefrontal cortex and self-control; pp. 123–34. [DOI] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the ultimatum game. Journal of Neuroscience. 2007;27:951–6. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, et al. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–11. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlberg L. Stage and sequence: the cognitive-developmental approach to socialization. In: Goslin DA, editor. Handbook of Socialization Theory and Research. Chicago: Ran McNally; 1969. pp. 347–480. [Google Scholar]
- Kohlberg L. Development of moral character and moral ideology. In: Haffman ML, Haffman LW, editors. Review of Child Development Research. New York: Russel Sage Foundation; 1964. pp. 381–431. [Google Scholar]
- Kosslyn SM, Thompson WL, Kim IJ, Rauch SL, Alpert NM. Individual differences in cerebral blood flow in area 17 predict the time to evaluate visualized letters. Journal of Cognitive Neuroscience. 1996;8:78–82. doi: 10.1162/jocn.1996.8.1.78. [DOI] [PubMed] [Google Scholar]
- Lerkiatbundit S, Utaipan P, Laohawiriyanon C, Teo A. Impact of the Konstanz method of dilemma discussion on moral judgment in allied health students: a randomized controlled study. Journal of Allied Health. 2006;35:101–8. [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lind G. Experimental questionnaires: a new approach to personality research. In: Kossakowski A, Obuchowski K, editors. Progress in Psychology of Personality. Amsterdam: North-Holland; 1982. pp. 132–44. [Google Scholar]
- Lind G. An introduction to the Moral Judgment Test (MJT). Konstanz: University of Konstanz; 1998. [Retrieved 16 July 2007]. Unpublished manuscript. from http://www.uni-konstanz.de/ag-moral/mut/mjt-intro.htm. [Google Scholar]
- Lind G. The moral judgment test: comments on Villegas de Posada's critique. Psychological Reports. 2006;98:580–4. doi: 10.2466/pr0.98.2.580-584. [DOI] [PubMed] [Google Scholar]
- Lind G. The meaning and measurement of moral judgment revisited – A dual aspect model. In: Fasko D, Willis W, editors. Contemporary P-hilosophical Perspectives on Moral Development and Education. Creskill, NJ: Hampton; 2007. (in press) [Google Scholar]
- Lind G, Wakenhut RH. The assessment of moral judgment competence with a standardized questionnare. Diagnostica. 1980;26:312–34. [Google Scholar]
- Lind G, Wakenhut RH. Testing for moral judgment competence. In: Lind G, Hartmann HA, Wakenhut RH, editors. Moral Development and Social Environment. Studies in the Philosophy and Psychology of Moral Judgment and Education. Chicago: Precedent Publishing; 1985. pp. 79–105. [Google Scholar]
- Liu TS, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cerebral Cortex. 2003;13:1334–43. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- Luo QA, Nakic M, Wheatley T, Richell R, Martin A, Blair RJR. The neural basis of implicit moral attitude – an IAT study using event-related fMRI. Neuroimage. 2006;30:1449–57. doi: 10.1016/j.neuroimage.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Mériau K, Wartenburger I, Kazzer P, et al. A neural network reflecting individual differences in cognitive processing of emotions during perceptual decision making. Neuroimage. 2006;33:1016–27. doi: 10.1016/j.neuroimage.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Mikhail J. Universal moral grammar: theory, evidence and the future. Trends in Cognitive Sciences. 2007;11:143–52. doi: 10.1016/j.tics.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Moll J, Eslinger PJ, Oliveira-Souza R. Frontopolar and anterior temporal cortex activation in a moral judgment task: preliminary functional MRI results in normal subjects. Arquivos de Neuropsiquiatria. 2001;59:657–64. doi: 10.1590/s0004-282x2001000500001. [DOI] [PubMed] [Google Scholar]
- Moll J, Oliveira-Souza R, Bramati IE, Grafman J. Functional networks in emotional moral and nonmoral social judgments. Neuroimage. 2002a;16:696–703. doi: 10.1006/nimg.2002.1118. [DOI] [PubMed] [Google Scholar]
- Moll J, Oliveira-Souza R, Eslinger PJ. Morals and the human brain: a working model. Neuroreport. 2003;14:299–305. doi: 10.1097/00001756-200303030-00001. [DOI] [PubMed] [Google Scholar]
- Moll J, Oliveira-Souza R, Eslinger PJ, et al. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002b;22:2730–6. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Human Brain Mapping. 2005;24:313–24. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro DA, Bloom P. The intelligence of the moral intuitions: comment on Haidt (2001) Psychological Reviews. 2003;110:193–6. doi: 10.1037/0033-295x.110.1.193. [DOI] [PubMed] [Google Scholar]
- Rest J. Mannual for the Defining Issue Test: An Objective Test for Moral Judgment Development. Minneapolis: University of Minneapolis Press; 1974. [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, et al. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6:1323–8. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, et al. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–79. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else's belief. Nature Neuroscience. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–9. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16:235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schultz J, Friston KJ, O’Doherty J, Wolpert DM, Frith CD. Activation in posterior superior temporal sulcus parallels parameter inducing the percept of animacy. Neuron. 2005;45:625–35. doi: 10.1016/j.neuron.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Schultz J, Imamizu H, Kawato M, Frith CD. Activation of the human superior temporal gyrus during observation of goal attribution by intentional objects. Journal of Cognitive Neuroscience. 2004;16:1695–705. doi: 10.1162/0898929042947874. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sprinthall N, Sprinthall RC, Oja SN. Educational Psychology. A Developmental Approach. New York: McGraw-Hill; 1994. [Google Scholar]
- Stöber J. The Social Desirability Scale-17 (SDS-17): convergent validity, discriminant validity, and relationship with age. European Journal of Psychological Assessment. 2001;17:222–32. [Google Scholar]
- Talmi D, Frith C. Neurobiology – feeling right about doing right. Nature. 2007;446:865–6. doi: 10.1038/446865a. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Braver TS, Jonides J. Individual differences. Cognitive Affective & Behavioral Neuroscience. 2005;5:115–6. doi: 10.3758/cabn.5.2.115. [DOI] [PubMed] [Google Scholar]
- Vernon PA. Speed of information-processing and general intelligence. Intelligence. 1983;7:53–70. [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nature Neuroscience. 2004;7:1259–65. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4:139–47. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proceedings of the National Academy of Sciences. 2007;104:8235–40. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences. 2007;104:6430–5. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]


