Abstract
Previous research has clearly documented that risky decision making is different in young and older adults. Yet, there has been a relative dearth of research that seeks to understand such age-related changes in the neural activities associated with risk taking. To address this research issue, 21 men (12 young men, mean age 29.9 ± 6.2 years and 9 older men, mean age 65.2 ± 4.2 years) performed a risky-gains task while their brain activities were monitored by an fMRI scanner. The older adults, relative to their younger peers, presented with contralateral prefrontal activity, particularly at the orbitofrontal cortex. Furthermore, stronger activation of the right insula was observed for the older-aged participants compared to the younger-aged adults. The findings of this study are consistent with the a priori speculations established in accordance with the HAROLD model as well as previous findings. Findings of this study suggest that when making risky decisions, there may be possible neuropsychological mechanisms underlying the change in impulsive and risk-taking behaviors during the course of natural ageing.
Keywords: risk taking, ageing, insula, orbitofrontal cortex, prefrontal cortex, neuroimaging
INTRODUCTION
Seniors in our society are faced with many pragmatic predicaments in their everyday lives, including important issues related to healthcare and financial investments. Very often, they are required to make and act on risky decisions regarding these issues and the decisions have to be made on relatively little knowledge (Chou et al., in press). With increasing age, people seem to need more time for decision making; furthermore, their choices tend to be more conservative (Deakin et al., 2004; Leland and Paulus, 2005). This age-related change in the behavioral patterns of risk-taking suggests a possible age-associated difference in the neural activities that occur during risk taking. However, there has been a relative dearth of research seeking to understand the age-related changes in neural activities associated with risk taking.
The age-related structural changes in various brain areas (e.g. DeCarli et al., 1995; Murphy et al., 1996; Raz et al., 1997; Jack et al., 1998) can result in the alteration of neural activity during cognitive task performance, including making risky decision, in old age (see review by Grady, 2000). The alteration in neural activation is thought to relate to a change in cognitive strategies, which could be in the form of changing cognitive structures and cognitive processes (Cabeza, 2002). Previous neuroimaging studies have found that older adults, relative to their younger counterparts, generally show a bilateral pattern of prefrontal activity compared to younger adults during cognitive tasks such as verbal recall (Cabeza et al., 1997), cued recall (Backman et al., 1997), word and face recognition (Madden et al., 1999; Grady et al., 2002), episodic encoding and semantic retrieval (Stebbins et al., 2002; Logan et al., 2002), working memory (Reuter-Lorenz et al., 2000), perception (Grady et al., 2000) and inhibitory control (Nielson et al., 2002). Neural activations observed in the aforementioned studies followed a consistent pattern, as described in the model of hemispheric asymmetry reduction in older adults (HAROLD model), which indicates that prefrontal activity during cognitive performance tends to be less lateralized in older adults than it is in younger adults (Cabeza, 2002). The compensation view has been found to be the most convincing account of this age-related reduction (Daselaar and Cabeza, 2005). The basic idea is that collaboration between the two hemispheres is more advantageous than within-hemisphere processing when task demands are high (e.g. Banich and Belger, 1990; Brown and Jeeves, 1993; Weissman and Banich, 2000). Additionally, some studies have found an age-related contralateral prefrontal recruitment (see Cabeza, 2002 for a review). In other words, an increase in the recruitment of other brain regions appears to be a general response to the conditions of neural compromise or strain in older adults (Cabeza et al., 1997; Reuter-Lorenz et al., 2000; Logan et al., 2002).
Decision making in situations that involve risk taking depends on a series of cognitive and affective processes that aim to balance the potential losses and benefits of an action (Arce et al., 2006). Studies on the risk-taking behavior of healthy adults have reported activation of the orbitofrontal cortex (Krain et al., 2006), the inferior prefrontal cortex (Paulus et al., 2001), the ventrolateral and ventromedial frontal cortices (Elliott et al., 1999; Rogers et al., 1999; Elliott et al., 2000), the insula (Critchley et al., 2001), the anterior cingulate cortex (Elliott et al., 2000) and the parietal cortex (Paulus et al., 2001). Paulus et al. (2003) observed that right insula activation was significantly stronger when their subjects selected a risky response vs a safe response on the risky-gains task. They also reported that the degree of insula activation was related to the probability of selecting a safe response following a punished response, i.e. a response that would cause the participant to lose points, and to their subjects’ degree of harm avoidance and neuroticism. Their results seem to suggest that insula activation is task specific as a result of their subjects’ performance on the risky-gains task.
We employed the risky-gains task (Paulus et al., 2003) to examine the ageing effect on neural activity that is associated with risk-taking behaviors. In accordance with the HAROLD model, we speculated that, relative to their younger counterparts, older adults would show a bilateral pattern of activation or contralateral neural activity, particularly in the prefrontal regions. Also, based on the literature regarding the involvement of the orbitofrontal region in risky decision making (Krain et al., 2006), this study aimed to analyze the activity of the orbitofrontal region for hemispheric laterality. Furthermore, Paulus et al. (2003) reported a significantly stronger activation of the right insula when their subjects selected a risky response, relative to selecting a safe response. When compared to their younger peers, older adults are supposed to prefer to make safe as opposed to risky responses. Therefore, their selection of risky choices on the risky-gains task might activate stronger insula activity than it would in younger adults. Hence, we hypothesized that older adults, in comparison to younger adults, would activate the right insula and other brain regions that account for decision making and attentional control.
METHODS
Participants
Ethics approval was obtained from the institutional review board of the University of Texas. A total of 21 healthy male volunteers participated in this study. One group consisted of 12 young participants (mean age = 29.9 ± 6.2 years, mean education = 17.2 ± 1.8 years), and the other group consisted of 9 older participants (mean age = 65.2 ± 4.2 years, mean education = 16.2 ± 1.2 years). They were all strongly right handed (Snyder and Harris, 1993). None of the participants had a history of neurological or psychiatric illness. The study was explained to the participants prior to their informed consent being obtained.
Experimental task
Paulus et al. (2003) make reference to a risk-taking task, the risky-gains task. In each trial of the task, the participants are presented with the numbers 20, 40 and 80 in a fixed order. Each number appears on a screen for 1 s. If the participant presses a button when the number is on the screen, then s/he receives the number of points shown on the screen. Before the experiment, the participants are informed that for both 40 and 80 points, there is a possibility that the points are −40 and −80 instead of +40 and +80. The −40 and −80 appear in red and indicate that the participant loses 40 and 80 points, respectively. When 20 points appear on the screen, only a score of +20 can be obtained; there is no score of −20. Therefore, the participants have to take the risk of whether to wait for +40 after gaining 20 points or +80 after gaining 40 points, with a possibility of losing points, or they could simply take the +20 points. Details of the trial type are shown in Figure 1. The points accumulate from trial to trial. The participants received feedback immediately after making a response. The participants were given a final score after the last trial of the experiment was completed. The trial type is designed in such a way that the probability of the appearance of negative 40 or 80 trials would make a participant's final score identical were s/he select 20, 40 or 80 consistently (Paulus et al., 2003). Hence, there is no advantage for participants to only select the risky response (40 or 80) over the safe response (20). Altogether, there were 96 trials; each trial lasted for 4 s irrespective of the participant's choice. The 96 trials consisted of 54 trials of the non-punished trial type (i.e. +20, +40 and +80), 24 trials of the −40 punished trial type (i.e. −40) and 18 trials of the −80 punished trial type (i.e. −80); the trial types were presented in a random sequence. Before the experiment, the participants were given a set of practice trials so they could familiarize themselves with the task.
Fig. 1.
Schematic diagram of the risky-gains task.
Behavioral measures
The participant's performance on the risk-taking task is presented as a mean and s.d. for the selected options. The rate of selecting +20, +40 and +80 points was calculated for both the young and older participants. Given that only the selection of the +40 and the +80 points involves a risk-taking behavior, the selection of these two points are grouped together as a risky response in the analysis while the selection of +20 points represents a safe response. The analysis of the distribution of the participant's responses and reaction times across conditions and between the two age groups was performed using a repeated measures ANOVA.
Data acquisition
Event-related functional Magnetic Resonance Imaging (fMRI) was used in this study. The experimental paradigm consisted of four 24-trial blocks, each separated by 12 s of fixation, as shown in Figure 1. During the risk-taking task, the stimuli were shown through a back-projection display and the participants were required to make a response in each trial. The imaging was conducted on a 3 T Siemens MRI scanner (Siemens, Erlangen, Germany) at the Research Imaging Centre, University of Texas Health Science Center, San Antonio, Texas. The participants lay supine on the scanning table and were fitted with plastic ear-canal molds. Twenty-four contiguous gradient-echo planar images (EPIs), sensitive to blood oxygen level dependent (BOLD) contrast, were interleaved and acquired paralleled to the Ac–PC plane. The EPIs were acquired with TR = 2 s, TE = 30 ms, FOV = 256 mm × 256 mm, matrix size = 128 × 128, flip angle = 90° and slice thickness = 6 mm. For each slice, 222 images were acquired, with a total scan time of 7 min 24 s. The anatomical MRI was acquired using a T1-weighted, three-dimensional gradient-echo pulse sequence (TR = 20 ms, TE = 5.15 ms, FOV = 256 mm × 256 mm, slice thickness = 6 mm).
Imaging data processing and analysis
The functional images were analyzed using the Statistical Parametric Map (SPM2) software package (Wellcome Department of Cognitive Neurology, Institute of Neurology, Queen Square, London, UK), running under Matlab 6.5 (MathWorks, Natick, MA, USA). During the normalization process, the individual data were resliced into 4 mm isotopic voxels. The resulting images were then spatially smoothed by convolution with a three-dimensional Gaussian kernel (FWHM = 8 mm). These two regressors are referred to as: (i) making a safe response (i.e. +20 point trials) and (ii) making a risky response (i.e. +40 and +80 point trials). The response regressors are identified from the beginning of the trial to the time when the participant is making a response. The resulting time series data were high-pass filtered with the default threshold of 128 s to remove low frequency drift.
Subject-level statistical analyses were performed by setting up contrasts between the safe and risky responses at a threshold of P < 0.001 (uncorrected P-level). The safe response refers to selecting 20 points and the risky response refers to selecting both +40 and +80 points. Therefore, to determine the areas that are significantly activated with a risky vs a safe response, a contrast of a risky response minus a safe response was set up. A one-sample t-test was conducted to examine the neural activations of the young and older participants on the ‘risky vs safe’ contrast. A comparison of the activation between the young and older participants on these two contrasts was conducted using a two-sample t-test with a voxel-wise intensity threshold of P < 0.001 (uncorrected) and a spatial extent threshold (cluster size greater than 12 voxels) to control for multiple comparisons (Lee et al., 2006) in the generation of the t-maps. The activation maps were displayed using the MRIcro program (http://www.sph.sc.edu/comd/rorden/mricro.html) and the activation sites were labelled using the Automated Anatomical Labeling (AAL) software (Tzourio-Mazoyer et al., 2002), which were implemented into the toolbox available for SPM2. All these programs applied the standard MNI templates.
To verify if there were age-related differences in cerebral vasculature, which would affect the interpretations of the findings, a region of interest (ROI) analysis looking at the percentage signal changes in the primary motor cortex was performed. The primary motor cortex equivalent to Brodmann's area 4 defined by the AAL was selected. The ROI analysis was performed using the WFU PickAtlas (Maldjian et al., in press), a region of interest toolbox for SPM (www.rad.wfubmc.edu/fmri). The percentage signal change was calculated using MarsBar, a toolbox for SPM (http://marsbar.sourceforge.net) (Brett et al., 2002). The percentage signal change between the young and older adults in both the left and right primary motor cortex was compared using independent t-tests.
With regard to the prediction stipulated by the HAROLD model, we performed a ROI analysis to look at the percentage signal change in the prefrontal region. Specifically, we looked at the neural activities in both orbitofrontal cortices (OFC) because the OFC has been shown to be associated with risk-taking behavior (Paulus et al., 2001; Krain et al., 2006). For each subject, we selected the OFC based on the anatomical definition using the AAL, which was equivalent to Brodmann's areas 10, 11 and 47. The ROI analysis was performed using the WFU PickAltas (Maldjian et al., in press) and the percentage signal change was calculated using Marsbar (Brett et al., 2002). The percentage signal change was calculated for each of the left and right OFCs. The differences in the percentage signal change between the young and older adults in each of the left and right OFCs were compared using independent t-tests. The analysis of the neural activation of the OFC began with the ROI analysis. Whole brain analysis was performed subsequently in order to explore the activation patterns of the two age groups.
In addition to the OFC, we also examined percentage signal change at the right insula, which has been shown to be activated during the performance of the risky-gains task in a previous study conducted by Paulus et al. (2003). For each individual participant, a peak activating voxel of the right insula, identified in the old vs young contrast, was used as the ROI. The comparison between the young and older participant groups was carried out using an independent t-test.
RESULTS
Behavioral data
Table 1 shows the rates and response times of making a safe or risky response in the young and older adults. For the rate of response selection, a repeated measures ANOVA showed a significant interaction effect between age group and response selection [F(1,19) = 4.502, P = 0.047]. A post hoc analysis indicates that the older adults displayed a significantly higher rate of selecting a safe response compared to the young group (t19 = 2.096, P = 0.050), while the young group showed a significantly higher rate of selecting a risky response than the older adults (t19 = 2.138, P = 0.046). Additionally, the young adults made significantly more risky responses than safe responses (t11 = 2.817, P = 0.017). For the response time, a repeated measures ANOVA showed a significant main effect on response selection [F(1,19) = 21.629, P < 0.001]. A post hoc analysis showed that both the young and older adults responded faster when making a risky response compared to making a safe response (t11 = 5.916, P < 0.001 and t11 = 2.355, P = 0.046, respectively).
Table 1.
Mean (SD) Rate (%) and reaction time (ms) of selecting safe and risky responses by the younger and older men
| Rate (%) |
Reaction time (ms) |
|||
|---|---|---|---|---|
| Safe+ 20 | Risky+ 40 and + 80 | Safe+ 20 | Risky+ 40 and + 80 | |
| Younger men | 29.60 (11.28) | 46.53 (9.67) | 474.85 (62.62) | 386.18 (40.92) |
| Older men | 50.81 (32.81) | 31.94 (20.96) | 522.49 (132.90) | 421.05 (104.59) |
Imaging data
One male from the old group was excluded from the analysis of the neural activity, because he selected all +20 points (i.e. the safe response), with the exception of one +40 points (risky response) and one −40 points (punished trial), making the contrast invalid for interpretation. As the result, 12 young adults and 8 older adults were used in the analysis of the neural activity in this study.
Differences in primary motor activity
The ROI analysis in the primary motor cortex did not reveal any significant differences in percentage signal change in both primary motor cortices between the young and older adults (t18 = 0.776, P = 0.448 and t18 = −0.319, P = 0.754, respectively). This result suggests that the age-related differences in the cerebral vasculature were not significant and that comparison in neural activities between the two age groups could be reasonably interpreted in this study.
Activation of orbitofrontal cortex
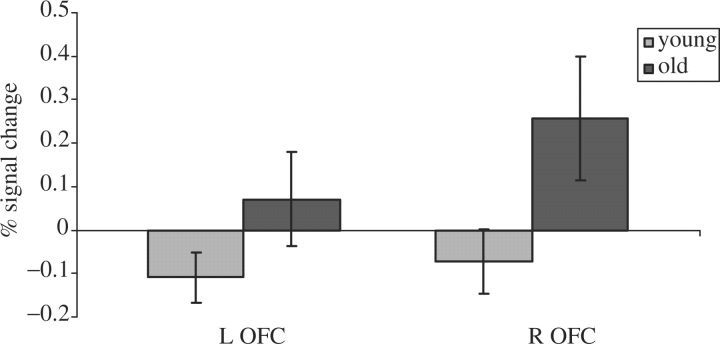
Figure 2 displays a plot of percentage signal change at the bilateral OFC. In both the left and right OFC, the older adults showed a positive percentage signal change while the young adults showed a negative percentage signal change. There were significant differences between the young and older adults in the right OFC (t18 = 2.246, P = 0.038).
Fig. 2.
Percent signal change (risky relative to safe response) to younger and older males in the OFC during the old > young contrast. Bars show standard errors.
Regions of activation
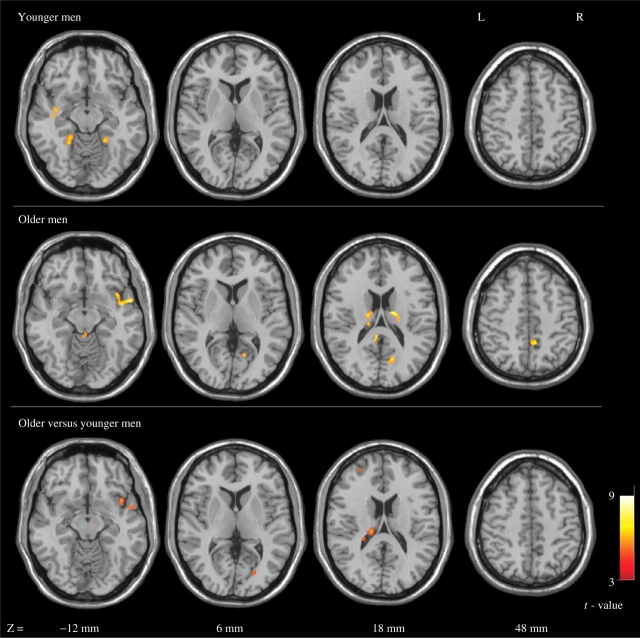
When comparing the risky vs safe response, the young and older males exhibited different patterns of brain activation. In the young male group, the left superior temporal gyrus (BA 38), the left parahippocmpal gyrus (BA 36) and the right cerebellum were significantly activated when making a risky response compared to when making a safe response. In the older male group, the brain activation was concentrated in the right hemisphere, including the right insula (BA 13), the right superior temporal gyrus (BA 38), the right calcarine (BA 18/31), the right precuneus (BA 7), the right thalamus and the left posterior cingulate gyrus (BA 30). Comparisons between the older and young males found that the older males showed significant activations of the right insula (BA 13), the right calcarine (BA 18), the left middle frontal gyrus (BA 10) and the left thalamus. The results are shown in Table 2 and Figure 3.
Table 2.
Results of the risk vs safe contrasts for the younger older, and older vs younger men
| Coordinate |
|||||||
|---|---|---|---|---|---|---|---|
| BA | Side | x | y | z | Volume (mm3) | T | |
| Younger men | |||||||
| Superior temporal gyrus | 38 | L | −40 | −6 | −14 | 104 | 5.73 |
| Parahippocampal gyrus | 36 | L | −26 | −28 | −20 | 552 | 5.68 |
| 36 | L | −28 | −44 | −8 | 744 | 5.71 | |
| Cerebellum | R | 16 | −26 | −24 | 320 | 5.68 | |
| Older men | |||||||
| Insula | 13 | R | 42 | 0 | −12 | 216 | 5.52 |
| Superior temporal gyrus | 38 | R | 52 | 4 | −14 | 216 | 7.56 |
| Calcarine | 18/31 | R | 12 | −62 | 10 | 208 | 6.14 |
| Precuneus | 7 | R | 4 | −48 | 50 | 128 | 5.69 |
| Posterior cingulate gyrus | 30 | L | −8 | −40 | 18 | 104 | 6.65 |
| Thalamus | R | 14 | −10 | 16 | 168 | 9.62 | |
| Older vs younger men | |||||||
| Middle frontal gyrus | 10 | L | −30 | 50 | 20 | 104 | 4.10 |
| Insula | 13 | R | 38 | 12 | −12 | 800 | 4.61 |
| Calcarine | 17/18 | R | 20 | −76 | 6 | 192 | 4.19 |
| Thalamus | L | −16 | −24 | 20 | 712 | 4.95 | |
BA, Brodmann's Area; L, left hemisphere; R, right hemisphere; x, y, z in MNI coordinates.
Fig. 3.
Axial t-maps of brain activation in the risky vs safe contrast (P < 0.001, minimum 12 clustering voxels). The images were superimposed on a standard SPM anatomical template with the z-coordinate for each slice shown in MNI space.
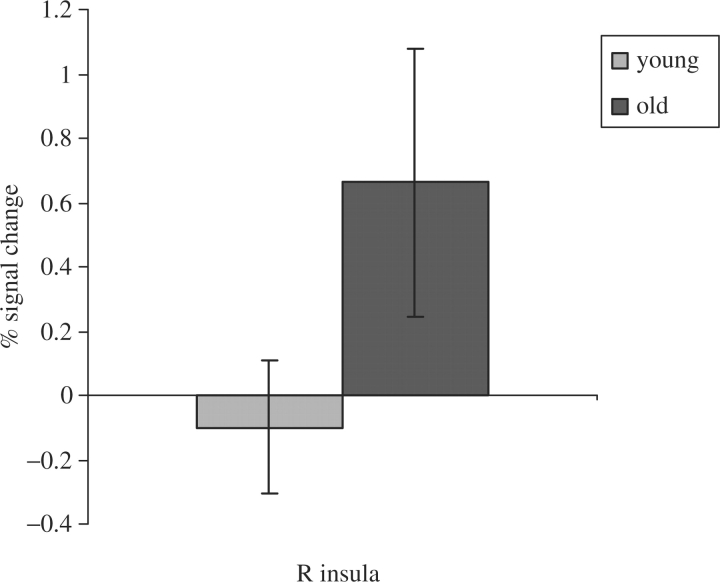
Figure 4 displays a plot of percentage signal change at the peak activating voxel of the right insula (BA 13). The difference between the young and older adults was marginally significant (P = 0.087). Further analysis revealed that the young adults showed a significant activation of the right insula at a threshold of P < 0.005 (uncorrected), which was consistent with the findings of Paulus et al. (2003) who showed that right insula (BA 13) activity was stronger when subjects selected a risky response vs a safe response.
Fig. 4.
Percent signal change (risky relative to safe response) to younger and older males in individual-subject functional regions defined by the peak activating voxel during the old > young contrast. Bars show standard errors.
DISCUSSION
Age-related change in neural activities that is associated with risk taking was examined by using the risky-gains task (Paulus et al., 2003). Consistent with the findings of Paulus et al. (2003), the younger participants in this study showed activation of the right insula (BA 13), confirming the validity of the paradigm employed in this study. The ageing effect on the neural activities while performing the task was examined. The findings indicate that the older adults, relative to their younger peers, presented with contralateral prefrontal activity, particularly at the orbitofrontal cortex. Furthermore, the present findings revealed that the older adults showed stronger activation of the right insula compared to the younger adults. These observations are consistent with our a priori speculations established in accordance with the HAROLD model as well as the previous findings of Paulus et al. (2003).
The involvement of the orbitofrontal cortex in risky decision making has been widely documented (Critchley et al., 2001; Ernest et al., 2002; O’Doherty et al., 2003; Cohen et al., 2005; Paulus et al., 2005; Eshel et al., 2007). The findings of this study indicate activity in the orbitofrontal region of both the younger and older participants, but percent signal change was positive for the older adults while negative for the young adults. Importantly, the older adults showed significantly more percent signal change than the young adults in the right OFC. It is therefore likely that the older adults, as compare to the younger adults, activated strongly in the right prefrontal area, in particular the OFC which is shown to be highly involved in risk taking (Krain et al., 2006). This pattern of activation is consistent with the predication set forth by the HAROLD model that ageing is associated with the recruitment of additional neural activities in regions contralateral to those seen in people of a relatively younger age (Cabeza, 2002). However, a bilateral pattern of prefrontal activation for the older participants was not observed in this study. Indeed, some studies have reported that older adults showed weaker prefrontal cortex activity in one hemisphere but stronger prefrontal activity in the contralateral hemisphere (Nagahama et al., 1997; Anderson et al., 2000; Rypma et al., 2001). This observation could also be related to the task-specific ageing effect in the prefrontal cortex (Cabeza, 2002). Backman et al. (1997), Grady et al. (2002) and Madden et al. (1999) also report contralateral activation patterns for older adults, relative to their younger age peers, when performing on the implicit and explicit retrieval tasks, face memory tasks or verbal recognition memory tasks.
Behaviorally, the older adult participants were less likely to take risks as reflected by their tendency to select the safe choices. When the neural activities during risk taking were examined, the older participants presented with stronger neural activities compared to the younger adults during risky decision making. Specifically, the right insula, whose activity is related to the degree of harm avoidance and neuroticism (Paulus et al., 2003), was strongly activated during the selection of risky over safe responses among the older adults. Therefore, the strong insula activity among the older adults, relative to the younger adults, may reflect an age-related trend of increased avoidance of risky situations and the anticipation of a greater negative impact in selecting the risky choices. The strong right insula activity found among the older adults may be related to their emotion processing in relation to the risky situation. Hastie and Dawes (2001) have shown that a high level of risk and uncertainty involves biases and emotions that act at an implicit level. Alternatively, the life experiences that are accumulated with age could contribute to the plastic change of the response system (Lee et al., 2007), allowing a greater selective control during decision making (Williams et al., 2006). In these circumstances, the increased insula activation among the older adults may signal a greater attempt to counteract the potentially aversive outcome when participating in the risk-taking task.
The findings suggest age-related differences in the pattern of neural activities that occurs during the performance on the experimental task. The young adults showed activations of the left parahippocampal gyrus, the left superior temporal gyrus and the cerebellum. The parahippocampal gyrus has been found to be activated by tasks that involve working memory and episodic retrieval (Wagner et al., 1998; Epstein et al., 1999). The cerebellum has been found to be activated in order to enhance performance on tasks that demand one's access to semantic and episodic knowledge, and in order to respond to a rapid and timed stimulus (Andreasen et al., 1999; Seidler et al., 2002). In line with these findings, activation of the parahippocampal and cerebellum was observed and may suggest that the young adults were mentally calculating the gains and losses of points during their selection of risky choices in order to try to maximize their net gain of points throughout the experiment. The activation of the superior temporal gyrus has been reported in many previous studies involving decision making. For example, Jung-Beeman et al. (2004) found that the anterior superior temporal gyrus was activated during problem solving. Paulus et al. (2001) reported that the superior temporal gyrus was associated with contingency-based decision making in the presence of random reinforcement. Furthermore, Paulus et al. (2005) observed a greater activation of the right insula and superior temporal gyrus when their subjects switched responses rather than staying with the same choice made on the previous trial. The older adults, on the other hand, showed activation of the right calcarine, the right precuneus, the thalamus and the left posterior cingulate gyrus, in addition to the activity in the right insula and the superior temporal gyrus, during the selection of risky choices over safe choices. These regions of activation have been reported during decision making (Ernst et al., 2002) and in tasks that demand visual perception and visual attention (Gains et al., 2004). Madden et al. (2004) also found that visual target detection activated occipital regions. The thalamus appears to play a further role in impulse regulation, as demonstrated in a study that examined gambling (Potenza et al., 2003). Moreover, the contrast between the younger and older adults reveals that the older adults appeared to have recruited the left middle frontal region during task performance. Activation of the middle frontal gyrus is associated with visual target detection and risky decision making (Madden et al., 2004; Krain et al., 2006). It is therefore likely that, compared to younger adults, older adults recruit more brain regions to modulate visual attention and facilitate visual target detection. Overall, the age-related difference in the pattern of neural activities during risk taking may be explained by the changes in strategy deployment during ageing. Consistent with our hypotheses, it appears that older adults utilize extra brain regions in an attempt to regulate their responses to non-aversive outcomes in risky situations and to counteract age-related neurocognitive decline.
Limitations
We fully acknowledge that the level of risk experienced by the individual might be lower during the selection of the 40 point than 80 point trials, hence the neural activity associated with these two risky choices may be different. However, in the analysis of the neural correlates of the risk-taking behavior, we had to group the 40 and 80 point trials together to represent the risky condition because the older adults were less likely to make risky responses, rendering insufficient trials for conducting separate analyses of the neural activities associated with two risky responses. In addition, the study found that the young adults selected more risky than safe choices. On the other hand, the older adults selected higher number of safe than risky choices. This behavioral pattern implies that the observed neural activities associated with risk taking by our subjects were derived from unevenly distributed risky and safe choices across the young and older adult groups. Although the different behavioral performance between the young and older adults could reflect genuine psychological differences between people in these two age groups, it might also cause differences in estimating reliability across the risk and safe conditions across the two groups. Furthermore, the specific risk-taking preference shown by our young (risk-seeking) and older (risk-aversion) participants suggest that the observed differences in the neural activities between people in these two age groups may also be related to possible differences in task strategies employed. To verify this speculation, we had planned for a subgroup analysis of neural activities for the young and older participants matched for behavioral performance. However, the sample sizes of the matched subgroups were too small for any meaningful analyses to be performed.
Because of the fewer attempts made to take risks by the older adults, the analysis of the punished vs risky contrast, as performed by Paulus et al. (2003), was technically not feasible in this study. In fact, significant prefrontal activation was observed in many other risk-taking studies (e.g. Ernst et al., 2002; Paulus et al., 2003; Krain et al., 2006; Van Leijenhorst et al., 2006). Furthermore, it is not possible to examine the linearity of the age-related changes observed with just two age groups.
The young and older participants of this study might perhaps differ not only in their ages but also on their socio-cultural backgrounds. These factors could influence their risk taking behavior and the associated neural activities. Future studies should consider including cohorts of different socio-cultural backgrounds for a better delineation of the relationship between ageing and the changes in neural activities that are associated with risk taking.
CONCLUSION
The present study expands on the previous research into risk-taking behaviors and examines the ageing effects on the neural activities that are associated with risky decision-making. The findings reveal that younger and older adults relied on different brain mechanisms in performing the risk-taking task; this may relate to the behavioral change of selecting fewer risky choices as a person ages. Our results suggest that possible neuropsychological reasons and mechanisms underlie the change in impulsive and risky behaviors during the course of natural ageing.
Acknowledgments
The project was supported by the University Development Fund (The University of Hong Kong).
Footnotes
Conflict of Interest
None declared.
REFERENCES
- Arce E, Miller DA, Feinstein JS, Stein MB, Paulus MP. Lorazepam dose-dependently decreases risk-taking related activation in limbic areas. Psychopharmacology. 2006;189:105–16. doi: 10.1007/s00213-006-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Iidaka T, McIntosh AR, Kapur S, Cabeza R, Craik FIM. The effects of divided attention on encoding- and retrieval-related brain activity: a PTE study of younger and older adults. Journal of Cognitive Neuroscience. 2000;12:775–92. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Paradiso S, Cizadlo T, Arndt S, Watkins GL, et al. The cerebellum plays a role in conscious episodic memory retrieval. Human Brain Mapping. 1999;8:226–34. doi: 10.1002/(SICI)1097-0193(1999)8:4<226::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman E, Almkvist O, Andersson J, Nordberg A, Winblad B, Reineck R, et al. Brain activation in young and older adults during implicit and explicit retrieval. Journal of Cognitive Neuroscience. 1997;9:376–91. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- Banich MT, Belger A. Interhemispheric interaction: how do the hemispheres divide and conquer a task? Cortex. 1990;26:77–94. doi: 10.1016/s0010-9452(13)80076-7. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Japan: Sendai; 2002. Jun–Jun. Region of interest analysis using an SPM toolbox [abstract] presented at the 8th international conference on functional mapping of the human brain. Available on CD-ROM in Neuroimage, 16(2) [Google Scholar]
- Brown WS, Jeeves MA. Bilateral visual field processing and evoked potential interhemispheric transmission time. Neuropsychologia. 1993;31:1267–81. doi: 10.1016/0028-3932(93)90097-j. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, Lee TMC, Ho AHY. Does mood state change risk taking tendency in older adults? Psychology and Aging. 2007;22:310–318. doi: 10.1037/0882-7974.22.2.310. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. Functional connectively with anterior cingulate and orbitofrontal cortices during decision-making. Cognitive Brain Research. 2005;23:61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Daselaar S, Cabeza R. Age-related changes in hemispheric organization. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2005. pp. 325–53. [Google Scholar]
- Deakin J, Aitken M, Robbins T, Sahakian BJ. Risk taking during decision-making in normal volunteers changes with age. Journal of the International Neuropsychological Society. 2004;10:590–8. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–84. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37:403–11. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron. 1999;23:115–25. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, et al. Decision-making in a risk-taking task: a pet study. Neuropsychopharmacology. 2002;26:682–91. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulated cortices. Neuropsychologia. 2007;45:1270–9. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gains G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Cognitive Brain Research. 2004;20:226–41. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Grady CL, Nernstein LJ, Beig S, Siegenthaler AL. The effects of encoding strategy on age-related changes in the functional neuroanatomy of face memory. Psychol Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and nondegraded face processing. Cognitive Neuropsychology. 2000;217:165–86. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biological Psychology. 2000;54:259–81. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Hastie R, Dawes RM. Rational Choice in an Uncertain World. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- Jack CR, Petersen RC, Xu Y, et al. The rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–9. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, et al. Neural activity when people solve verbal problems with insight. PLoS Biology. 2004;2:E97. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision making. Neuroimage. 2006;32:477–84. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Lee TMC, Zhang JX, Chan CCH, et al. Age-related differences in response regulation as revealed by functional MRI. Brain Research. 2006;1076:171–6. doi: 10.1016/j.brainres.2005.12.124. [DOI] [PubMed] [Google Scholar]
- Lee TMC, Leung AWS, Chan CCH. Regulation of human behaviors. Future Neurology. 2007;2:189–99. [Google Scholar]
- Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug and Alcohol Dependence. 2005;78:83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–40. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, et al. Adult age differences in functional neuroanatomy of verbal recognition memory. Human Brain Mapping. 1999;7:115–35. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel SA. Age-related changes in neural activity during visual target detection measured by fMRI. Cerebral Cortex. 2004;14:143–55. doi: 10.1093/cercor/bhg113. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Murphy DGM, DeCarli CS, McIntosh AR, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Archives of General Psychiatry. 1996;53:585–94. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama DJ, Yamaguchi H, et al. Age-related changes in cerebral blood flow activation during a card sorting test. Experimental Brain Research. 1997;114:571–7. doi: 10.1007/pl00005665. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan HP. Differences in the functional neuroanatomy of inhibitory control across the adult lifespan. Psychology Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23:7931–9. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, et al. Prefrontal, parietal, and temporal cortex networks underlie decision-making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–48. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–15. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, Skudlarski P, et al. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2003;60:828–36. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, et al. Selective aging of human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, Jonides J, Smith EE, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, et al. Choosing between small, likely rewards and large, unlikely rewards activates interior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19:9029–38. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakarn V, Desmond JD, Gabrieli JDE. Age differences in prefrontal activity in working memory. Psychology and Aging. 2001;16:371–84. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Purushothlm A, Kim S.-G, Ugurbil K, Willingham D, Ashe J. Cerebellum activation associated with performance change but not motor learning. Science. 2002;296:2043–6. doi: 10.1126/science.1068524. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Harris LJ. Handedness, sex, and familial sinistrality effects on spatial tasks. Cortex. 1993;29:115–134. doi: 10.1016/s0010-9452(13)80216-x. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, et al. Aging effects on memory encoding in the frontal lobes. Psychology and Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–70. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Glover GH, Gabrieli DE. Prefrontal cortex and recognition memory: functional-MRI evidence for context-dependent retrieval processes. Brain. 1998;121:1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Banich MT. The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology. 2000;14:41–59. doi: 10.1037//0894-4105.14.1.41. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, et al. The mellow years? Neural basis of improving emotional stability over age. Journal of Neuroscience. 2006;26:6422–30. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]