Abstract
Postnatal day (P)11–20 (+)-methamphetamine (MA) treatment impairs spatial learning and reference memory in the Morris water maze, but has marginal effects on path integration learning in a labyrinthine maze. A subsequent experiment showed that MA treatment on P11–15, but not P16–20, is sufficient to induce Morris maze deficits. Here we tested the effects of P11–15 MA treatment under two different rearing conditions on Morris maze performance and path integration learning in the Cincinnati water maze in which distal cues were unavailable by using infrared illumination. Littermates were treated with 0, 10, 15, 20, or 25 mg/kg x 4 per day (2 h intervals). Half the litters were reared under standard housing conditions and half under partial enrichment by adding stainless steel enclosures. All MA groups showed impaired Cincinnati water maze performance with no significant effects of rearing condition. In the Morris maze, the MA-25 group showed impaired spatial acquisition, reversal, and small platform learning. Enrichment significantly improved Morris maze acquisition in all groups but did not interact with treatment. The male MA-25 group was also impaired on probe trial performance after acquisition and on small platform trials. A narrow window of MA treatment (P11–15) induces impaired path integration learning irrespective of dose within the range tested but impairments in spatial learning are dependent on dose. The results demonstrate that a narrower exposure window (5 days) changes the long-term effects of MA treatment compared to longer exposures (10 days).
Keywords: Methamphetamine, psychostimulants, brain development, learning and memory, spatial learning, path integration learning
1. Introduction
(+)-Methamphetamine (MA) is widely abused among adolescents and young adults with annual prevalence rates from 2.4–4.7% (Johnston et al., 2006a; Johnston et al., 2006b). Approximately half of these users are women, some of whom will become pregnant and use the drug during pregnancy. Although this problem is widely recognized, little is known about the long-term effects of MA after intrauterine exposure.
Infants exposed to MA (Little et al., 1988) or a combination of MA and cocaine show reduced birth weight, increased prematurity, and intraventricular hemorrhage (Dixon and Bejar, 1989; Oro and Dixon, 1987) as well as signs of withdrawal using the Finnegan rating scale (Dixon, 1989). Flash-evoked visual potentials were abnormal in 78% of MA-cocaine exposed infants and reduced visual recognition memory on the Fagan Test of Infant Intelligence was seen in 6–12 month old exposed infants (Struthers and Hansen, 1992). Reduced creatine and glutamate/glutamine ratios by magnetic resonance spectroscopy (Smith et al., 2001), and volumetric reductions in hippocampus and forebrain were found by MRI in prenatally MA-exposed children (Chang et al., 2004). In a prospective study of 1,618 pregnant women, 84 of whom abused MA, exposed infants had reduced birth weight and increased rates of SGA (small for gestational age) (Smith et al., 2006). Fifty-six percent of the MA abusing women used the drug during the first and second trimesters and 44% throughout pregnancy (Smith et al., 2006). In another study, 47 MA-exposed infants had reduced birth weight and head circumference, higher rates of SGA, increased agitation, vomiting, and tachypnea compared to 49 unexposed infants (Chomchai et al., 2004).
We developed rat models of MA exposure during early (Acuff-Smith et al., 1995) and later stages of brain development (Vorhees et al., 1994a; Vorhees et al., 1994b). These models are analogous to the first and second half of human intrauterine brain development based on interspecies comparisons (Bayer et al., 1993; Clancy et al., 2007a; Clancy et al., 2007b; Herlenius and Lagercrantz, 2004; Rice and Barone, Jr., 2000; West and Pierce, 1987). Sprague-Dawley rats exposed to MA from P11–20 exhibited spatial learning and memory deficits in the Morris water maze (MWM), while cued MWM performance was unaffected. Multiple T-maze learning in the Cincinnati water maze (CWM) showed a non-significant trend toward impaired learning (Vorhees et al., 1994a; Vorhees et al., 2000; Williams et al., 2003c), while no changes in MWM matching-to-sample performance were seen (Williams et al., 2003c). The effects of P11–20 MA treatment on spatial MWM performance were replicated in ACI (Vorhees et al., 1998) and Dark Agouti rats (Vorhees et al., 1999), and have recently been reported to occur in mice (Acevedo et al., 2007) and in rats after prenatal exposure (Slamberova et al., 2005). Follow-up experiments showed that doses as low as 0.625 mg/kg x 4 doses/day fromP11–20 induce MWM spatial deficits as well (Williams et al., 2004). In addition, treatment of offspring on fewer days (P11–15) caused similar MWM deficits (Williams et al., 2003a).
As noted, the MWM effects could be induced by exposure on P11–20 or only P11–15, suggesting a critical period for this effect. As also noted, MA-related CWM effects after P11–20 treatment showed trends towards impaired learning in three experiments (p < 0.10). The CWM test in each of these experiments was done under ambient lighting. These lighting conditions leave distal cues visible. Recently, we have used the CWM as a test of path integration learning (for reviews see (Etienne, 1992; Etienne and Jeffery, 2004)). Therefore, the first major objective of this experiment was to examine path integration ability following neonatal MA exposure. In order to emphasize use of path integration, we eliminated distal cues that provide landmarks that can be used for spatial navigation. To do this, we tested animals in the CWM in complete darkness using an infrared light emitter and camera.
A second major objective was to determine the effects of an environmental rearing manipulation on the effects stemming from MA exposure. The Association for the Accreditation and Assessment of Laboratory Animal Care (AAALAC) mandates environmental enrichment for laboratory rodents. We designed an enrichment that could be standardized and sterilized, and that rats could not chew or otherwise damage. It consisted of a curved piece of stainless steel ~18 cm in length and ~ 10 cm in height placed in the cage throughout life with the open side facing down to make a dome-like enclosure or “hut” (see Fig. 1). Rats readily used these to explore, climb on, and sleep in or on. Accordingly, the experimental design consisted of two conditions: half the litters were reared with huts and half were reared without. Multiple doses of the drug were given to one male-female pair of animals within each litter in a split-litter design.
Fig. 1.
Partial enrichment. Rats were raised in one of two environments: box cages with hardwood bedding without stainless steel huts, or box cages with hardwood bedding with stainless steel huts as partial enrichment (pE). Although the photograph shows only one rat in the cage, in the experiment rats were house in same-sex pairs.
2. Methods and materials
2.1 Subjects
Nulliparous female Sprague-Dawley CD, IGS rats (Charles River, Raleigh, NC) were bred in house to males of the same strain and supplier. Evidence of pregnancy was designated as E0; most females delivered on E22. Birth was designated P0 and litters were culled non-systematically to 5 males and 5 females on P1. Animals were maintained in polycarbonate shoebox cages (46 × 24 × 20 cm) on a 14 h light/10 h dark cycle (lights on at 600 h) with food and water available ad lib in a vivarium maintained at 21 ± 1°C with 50 ± 10% humidity and AAALAC accredited. The research protocol was approved by the Cincinnati Children’s Research Foundation Animal Care and Use Committee. Within each litter, 1 male and 1 female pair was uniquely identified by ear-punch and designated to receive one of 5 treatment groups. All groups received 4 subcutaneous injections every 2 h each day on P11–15 with one of the following doses of (+)-MA: 0, 10, 15, 20 or 25 mg/kg/dose dissolved in saline and given in a dosing volume of 3 ml/kg. These groups are hereafter referred to as Saline, MA-10, MA-15, MA-20, and MA-25. All doses were of (+)-methamphetamine HCl (calculated as the free base and greater than 95% pure, provided by the National Institute on Drug Abuse). All doses were given in the dorsum and injection sites varied to prevent irritation. Offspring were weighed daily during treatment. Forty litters were treated creating a total of 400 offspring enrolled in the experiment. Twenty litters were reared in standard housing conditions (here after referred to as “standard”, i.e., in box cages with wood chips during lactation and in same-sex pairs after P28) and another 20 litters in partial enrichment (pE) conditions (partial enrichment, i.e., in box cages with wood chips with a stainless steel semicircular ‘hut’ added during lactation and in same-sex pairs after P28). The stainless steel enclosures are 17.8 cm long curved metal pieces bent to form a half-circle with the open side being 18.4 cm across (the diameter were they complete circles), and 10.2 cm high when positioned with the open side face down (Fig. 1). The enclosures are made of 16 gauge stainless steel (type 304) with a #4 brushed finish, rounded corners, and smoothed edges.
Litters were weaned on P28 and separated into same-sex pairs for the remainder of the experiment. Offspring were weighed prior to each dose and on P1, P7, P11–15, P21 and weekly thereafter. Behavioral testing began between P60–66. In the list of tests below, P60 is used as the reference age. Mortality was also recorded.
2.2 Straight Channel
On P60 animals were tested in a 15 × 244 cm straight swimming channel with a wire escape ladder mounted at one end. Each rat received 4 consecutive trials on the test day. On each trial, the rat was placed at one end facing the end wall and timed until it grasped the escape at the opposite end (maximum time = 2 min/trial).
2.3 Cincinnati Water Maze
Beginning on P63 animals were tested in the Cincinnati water maze (CWM). The CWM design is as previously described (Vorhees, 1987). The maze consists of 9 closed T-shaped cul-de-sacs that branch from a central channel extending from the starting point to the goal where an escape ladder is located. The arms of the T’s and the channels are 15 cm wide and the walls are 51 cm high. The water was 25 ± 1 cm deep and maintained at room temperature (21 ± 1°C). Testing was performed under an infrared light emitter to enhance image quality from the overhead camera. Testing under infrared was designed to eliminate extramaze (distal spatial) cues. To begin each trial, an animal was placed in the maze at the start position and allowed to find the goal. Two trials per day were administered with a 5-min limit per trial and a minimum 5-min intertrial interval. Errors, latency to escape, and number of returns to the start were scored. An error is defined as a head and shoulder entry into one of the dead-end arms of a T. Entries into the goal arm, even if the animal failed to reach the escape ladder and swam away, was not scored as an error, but such occurrences are very rare. Animals were tested for 12 consecutive days.
2.4 Morris Water Maze
The MWM used was 210 cm in diameter, made of stainless steel, and painted flat black (Vorhees and Williams, 2006). The maze was surrounded on all sides by white curtains that could be opened or closed to reveal or obscure room cues. In addition to the indigenous room cues, the 3 walls nearest the maze (representing arbitrarily N, E, and W relative to where the experimenter stood at position S) had large geometric figures mounted on them above the edge of the pool. Testing was conducted in 3 phases: acquisition, reversal, and shifted-reduced. Each phase consisted of 4 trials per day for 5 consecutive days with the curtains open; this was followed by one additional day when a single probe trial was given. Probe trials were 30 s. The time limit on learning trials was 2 min and the intertrial interval (ITI) was 15 s spent on the platform. Animals not finding the platform within 2 min were placed on the platform. Goal platforms (either 10 × 10 cm or 5 × 5 cm) were made of clear acrylic with thin nylon screening attached to the surface. During testing, the platform was positioned 1–3 cm below the surface of the water and was camouflaged by virtue of being transparent against a black background. Water temperature was 21 ± 1°C.
The acquisition phase began on P77. During acquisition, the 10 × 10 cm platform was used and located in the SW quadrant halfway between the center and the sidewall. Rats were started at one of four positions located distal to the quadrant containing the platform in a random order with the restraint that they received one trial from each of the four starting positions per day. The start positions used were: NW, N, E, and SE. These positions were used to eliminate short paths to the goal, such as those that are possible if S or W start positions were used. The day after the last acquisition trial, each rat was given a 30 s probe trial. For the probe trial, rats were started from a position they had never been started from before (NE).
Beginning on P84 rats were tested in the MWM in the reversal phase again with a 10 × 10 cm platform placed in the NE quadrant. The same procedure used for acquisition was used for reversal (5 days, 4 trials/day). Start positions were SE, S, W, and NW. On the sixth day, the platform was removed and a 30 s reversal probe trial was administered with the start position at SW.
Beginning on P91 rats were tested in the MWM in the shifted-reduced phase. During reduced testing a smaller 5 × 5 cm platform was used and was re-positioned to the NW quadrant. Since platform positions of SW and NE had been used, this final phase is not a reversal, but rather a quadrant shift combined with a smaller platform in order to increase the accuracy required of the animal to find the platform and thereby make the spatial demands of the task more difficult.
Performance was recorded using Smart video tracking software (San Diego Instruments, San Diego, CA). For the learning trials, path length, cumulative distance from the platform, latency and speed were analyzed. For the probe trials, dependent measures were time and distance in the target quadrant, number of target site crossings, average distance from the platform site, and swim speed. The tracker recorded the animal’s position every 0.2 s.
2.5 Statistical Procedures
Because the experiment used a split-litter design, offspring were matched on multiple factors by virtue of being littermates. Litter was used as a random factor (block) in a completely randomized block analysis of variance (ANOVA). In this model, treatment group (dose) and sex were between factors within each block with litter as the block factor. Rearing condition was a between subjects factor in the model. Measures taken repetitively on the same animal, such as day, were repeated measure (within) factors. Hence the design was a 3-between (treatment group, sex, rearing condition) x 1-within (day) randomized block ANOVA. Data were analyzed using SAS Proc Mixed (SAS Institute, Cary, NC). Each model was checked for best fit against covariance matrix models provided by Proc Mixed. In most cases best fit parameters indicated that the autoregressive-1 (AR(1)) covariance structure was optimal, but in a few cases compound symmetry (CS) was the better fit. Proc Mixed provides adjusted degrees of freedom that do not match those obtained in general linear model ANOVAs and can be fractional in some cases. The Kenward-Rogers method of calculating degrees of freedom was used (SAS Institute). Significant interactions were analyzed using simple-effect slice ANOVAs at each level of the repeated measure factor. Because our previous experiment showed that on both mazes, MA-treated groups performed worse than SAL, pairwise group comparisons were conducted using directional Dunnett-Hsu tests comparing each MA group to SAL. All data are expressed as group mean ± SEM.
3. Results
3.1 General Characteristics
There were no differences in mortality as a function of rearing condition therefore, the two conditions were combined. There were no deaths in the saline group, 1 in the MA-10, 4 in the MA-15, 6 in the MA-20, and 14 in the MA-25 group.
During the treatment interval (P11–15), MA-treatment caused a significant main effect on body weight (F(4,338) = 92.30, p < 0.0001), in which MA-treated animals gained less weight than saline controls. The main effects of sex (F(1,338) = 58.93, p < 0.0001) and day (F(4,1012) = 78.22, p < 0.0001) and the interactions of drug treatment x sex x rearing condition (F(4,338) = 3.27, p < 0.01) and drug treatment x day (F(16,1260) = 56.38, p < 0.0001) were also significant. The drug treatment x sex x rearing condition interaction was analyzed further using slice ANOVAs for each sex and enrichment condition. Drug treatment was significant (p < 0.0001) for both sexes under both rearing conditions. The pups reared under partial enrichment (pE) weighed significantly more than those reared in standard conditions; also males weighed more than females. The drug treatment effect reflected that MA-treated groups weighed less than saline-treated controls, and further comparisons showed that none of the MA-treated groups differed among themselves (Table 1). When analyzed for the drug treatment x day interaction, there were no significant group differences that existed on P11 prior to the first injection. Group differences became significant on P12 and remained through P15 with all MA groups differing significantly from saline controls (not shown).
Table 1.
The body weight of offspring during the MA dosing period. Data are LS means (± SEM) offspring (g) averaged across days P11–15 to show the main effect of MA treatment
| Group | ||||||
|---|---|---|---|---|---|---|
| Saline | MA-10 | MA-15 | MA-20 | MA-25 | ||
| Sex | Rearing | |||||
| Males | Std | 31.9 ± 0.7 | 27.2 ± 0.7* | 26.5 ± 0.7* | 26.1 ± 0.7* | 26.3 ± 0.7* |
| pE | 31.4 ± 0.7 | 28.0 ± 0.7* | 27.8 ± 0.7* | 27.4 ± 0.7* | 27.6 ± 0.7* | |
| N (Std/pE) | 20/20 | 20/19 | 18/18 | 19/20 | 19/18 | |
| Females | Std | 28.8 ± 0.7 | 26.5 ± 0.7* | 25.5 ± 0.7 | 25.4 ± 0.7 | 24.5 ± 0.7 |
| pE | 30.9 ± 0.7 | 26.3 ± 0.7* | 26.2 ± 0.7* | 26.8 ± 0.7* | 25.0 ± 0.7* | |
| N (Std/pE) | 20/20 | 20/20 | 20/19 | 20/19 | 19/15 | |
P < 0.001 compared to Saline group of the same sex and rearing condition.
After the end of drug treatment, offspring were weighed on P21 and P28 prior to separation of the litters. Again, drug treatment caused a significant main effect (F(4,302) = 133.15, p < 0.0001), but by this age, no main effect of rearing condition was present. Other effects were sex (p < 0.0001), drug treatment x sex (p < 0.02), age (p < 0.0001), sex x age (p < 0.0001), and rearing condition x age (p < 0.03). Slice ANOVAs for the drug treatment x sex interaction showed that drug treatment was significant for both males and females (p < 0.0001) and all MA groups differed significantly from saline at both of these ages (see Table 2).
Table 2.
The body weight of offspring (LS mean ± SEM) by age with the number of animals in each condition underneath. P21 was chosen to show body weights shortly after the end of treatment. P35 was chosen to show body weight a week after weaning. P56 was chosen to show body weight shortly before the start of behavioral testing. P91 was chosen to show body weight after the conclusion of behavioral testing
| Group | |||||
|---|---|---|---|---|---|
| Age (P) | Saline | MA-10 | MA-15 | MA-20 | MA-25 |
| Males | |||||
| 21 | 61.6 ± 1.8 36† |
50.0 ± 1.8* 34 |
47.2 ± 1.8* 34 |
45.5 ± 1.8* 33 |
44.4 ± 1.9* 30 |
| 35 | 170.7 ± 4.8 38 |
156.4 ± 4.9* 36 |
150.3 ± 5.0* 34 |
146.5 ± 4.9 35 |
145.1 ± 5.3* 30 |
| 56 | 370.1 ± 4.8 36 |
356.7 ± 4.9* 34 |
347.3 ± 5.1* 31 |
336.8 ± 4.9* 33 |
347.3 ± 5.4* 27 |
| 91 | 522.7 ± 4.9 32 |
513.4 ± 5.1* 30 |
491.3 ± 5.2* 29 |
484.7 ± 5.1* 30 |
492.9 ± 5.6* 24 |
| Females | |||||
| 21 | 57.7 ± 1.8 36 |
47.8 ± 1.8* 36 |
45.5 ± 1.8* 35 |
45.3 ± 1.8* 34 |
41.8 ± 1.9* 29 |
| 35 | 141.8 ± 4.8 38 |
134.0 ± 4.8 38 |
125.7 ± 4.9 36 |
130.7 ± 4.9 36 |
126.4 ± 5.3 30 |
| 56 | 241.1 ± 4.8 36 |
234.3 ± 4.8 36 |
226.8 ± 4.9 33 |
233.0 ± 4.9 33 |
228.2 ± 5.2 29 |
| 91 | 301.8 ± 4.9 32 |
295.8 ± 4.9 32 |
295.3 ± 5.1 29 |
299.9 ± 5.1 29 |
299.3 ± 5.4 26 |
P < 0.001 compared to Saline for all ages in males and on P21 and P28 only for females.
N = group size.
Analyses of body weights from P35–91 showed that drug treatment continued to significantly reduce weight in the MA groups (F(4,310) = 13.24, p < 0.0001). Other significant factors in the ANOVA were sex (F(1,310) = 3579.60, p < 0.0001) and age (F(8,2380) = 7445.10, p < 0.0001). Three interactions were also significant. These were the drug treatment x sex (p < 0.01), sex x age (p < 0.0001), and rearing condition x age (p < 0.01) interactions. Slice ANOVAs on each sex showed that the effect of drug treatment was significant for males (p < 0.0001) but not for females. For males, each MA-treated group differed from saline controls (MA-10 group p < 0.02, all other MA groups p < 0.0001; see Table 2).
3.2 Straight Channel
Straight channel swimming times were assessed to ensure that animals were acclimated to swimming before entering the water mazes and that all groups had comparable motoric abilities and motivation to escape from the water and to teach the animals that there was an escape ladder. Analyses of straight channel swimming times showed no drug treatment, sex, or drug treatment x sex effects. Two main effects were significant: rearing condition (F(1,347) = 34.62, p < 0.0001) and trial (F(3,1041) = 163.80, p < 0.0001). There were no significant interactions. The mean swimming latency averaged across trials, sexes, and treatment groups for the 2 rearing conditions were: Standard = 18.5 ± 0.5 s and pE = 14.8 ± 0.3 s.
3.3 Cincinnati Water Maze
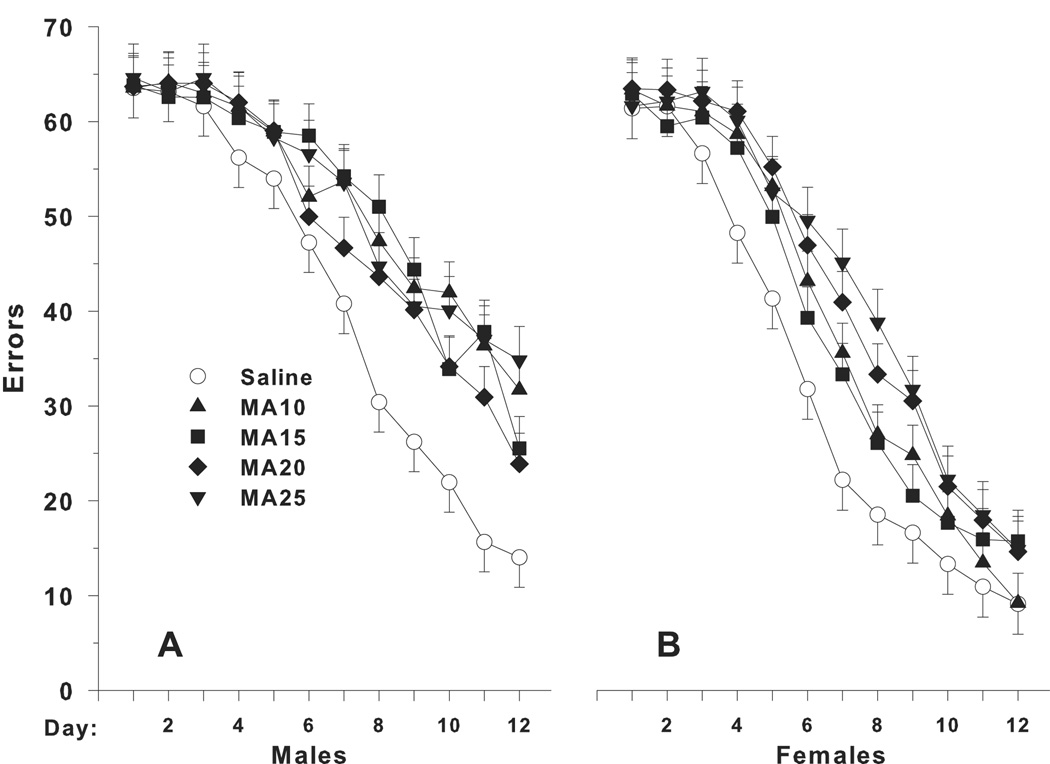
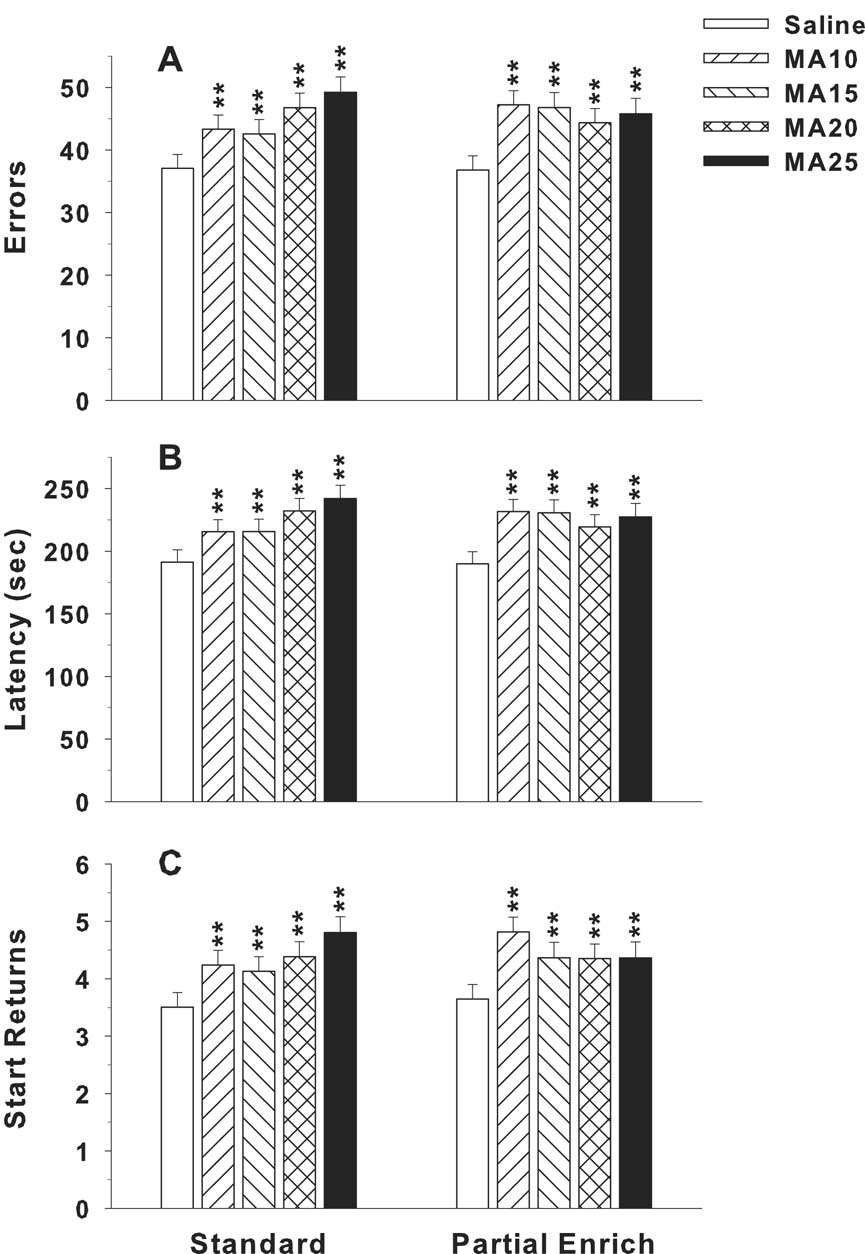
Three variables were analyzed for the CWM: latency to reach the escape, errors of commission, and returns to start. For errors, the main effect of drug treatment group was significant (F(4,312) = 7.69, p < 0.0001). Other significant main effects were sex (p < 0.0001) and test day (p < 0.0001). Significant interactions were for drug treatment x day (F(44,3872) = 1.90, p < 0.0001), drug treatment x sex x day (F(44,3872) = 1.77, p < 0.002), and sex x day (p < 0.0001). Each MA-treatment group differed from saline on multiple days with the groups separating significantly on day 4 of testing and remaining different until the last or next to last day of testing. Although the MA-25 group appeared slightly more affected, the MA groups did not differ significantly among themselves. The error data, by day and sex, are shown in Fig. 2. The drug treatment x sex x day effect was sorted for each sex on each day. For males, there were significant treatment effects on days 7–12, whereas for females the significant treatment effects began earlier (days 4–9). The shift in the temporal pattern of the MA effect for males and females was attributable to the fact that drug treatment group differences were most pronounced on the days when the learning curve was steepest and this occurred earlier in females than males. There were also some fluctuations as to which MA group made more errors than SAL on which specific days that were dependent of sex. For males (Fig. 2A), the pattern was: MA-10 > SAL on days 7–12; MA-15 > SAL on days 7–9 and 11–12, with a trend (p < 0.10) on day-10; MA-20 > SAL on day 8–9 and 11, with trends (p < 0.10) on days 10 and 12; and MA-25 > SAL on days 7–8 and 10–12, with a trend (p < 0.06) on day 9. For females (Fig. 2B), the MA groups made more errors than SAL as follows: MA-10 > SAL on days 4–5 and 7, with a trend (p < 0.07) on day-6; MA-15 > SAL on day 5; MA-20 > SAL on days 4–9; and MA-25 > SAL also on days 4–9. The overall drug treatment difference for errors between SAL and the MA groups is shown in Fig. 3A. The main effect of rearing condition was not significant nor was there any significant interaction involving rearing condition, but because rearing condition was a part of the experimental design, it is included in Fig. 3.
Fig. 2.
Mean (± SEM) performance in the Cincinnati Water Maze (CWM). A, average daily number of errors (2 trials/d). B, average daily latency (s) to reach the escape ladder. C, average daily number of returns to the start. Since enrichment was not significant, the standard and pE (partial enrichment) groups were combined; accordingly, group sizes are Males/Females: Saline = 40/39, MA-10 = 38/40, MA-15 = 35/37, MA-20 = 38/38, and MA-25 = 31/32.
Fig. 3.
Mean (± SEM) performance in the Cincinnati water maze averaged across days and shown as a function of rearing condition. ‘Standard; refers to animals reared prior to and after weaning in box cages (after weaning in same sex pairs); ‘Partial Enrich’ (pE) refers to rearing under conditions identical to standard conditions except with the addition of a stainless steel hut (see Methods for details). A, Errors; B, latency, and C, returns (2 trials/day). **P < 0.01 vs. SAL. Group sizes are as in Fig. 2.
For latency, drug treatment also produced a significant main effect (F(4,481) = 7.24, p < 0.0001). Other significant factors were sex (p < 0.0001), day (p < 0.0001), and sex x day (p < 0.0001). However, the drug treatment x sex interaction and drug treatment x day interactions were not significant. The drug treatment main effect showed that all four MA-treated groups had longer latencies than SAL (Fig. 3B). The sex and sex x day effects were caused by the fact that females performed better than males. The main effect of rearing condition was not significant nor was there any significant interaction involving rearing condition.
An important measure of path integration learning is returns to start because when an animal is uncertain of its trajectory or vector, it reorients itself by returning to the start in order to begin again from the origin. Rats with fimbria-fornix lesions make such start returns more than controls (Whishaw and Maaswinkel, 1998). An analysis of returns showed a significant main effect of drug treatment (F(4,632) = 7.61, p < 0.0001). Other significant main effects were sex (p < 0.0001) and day (p < 0.0001). The only other significant effect was the sex x day interaction (p < 0.0001). The main effect of rearing condition was not significant nor was there any significant interaction involving rearing condition. The pattern of effects on returns averaged across days is illustrated in Fig. 3C. Although the group differences are not as large as for errors or latency, they nonetheless show that the MA groups consistently returned to the start more than the saline group. The sex x day interaction revealed that the MA groups returned to the start more after the first 2 days of testing and continued this pattern throughout the remainder of the test.
3.4 Morris Water Maze--Acquisition
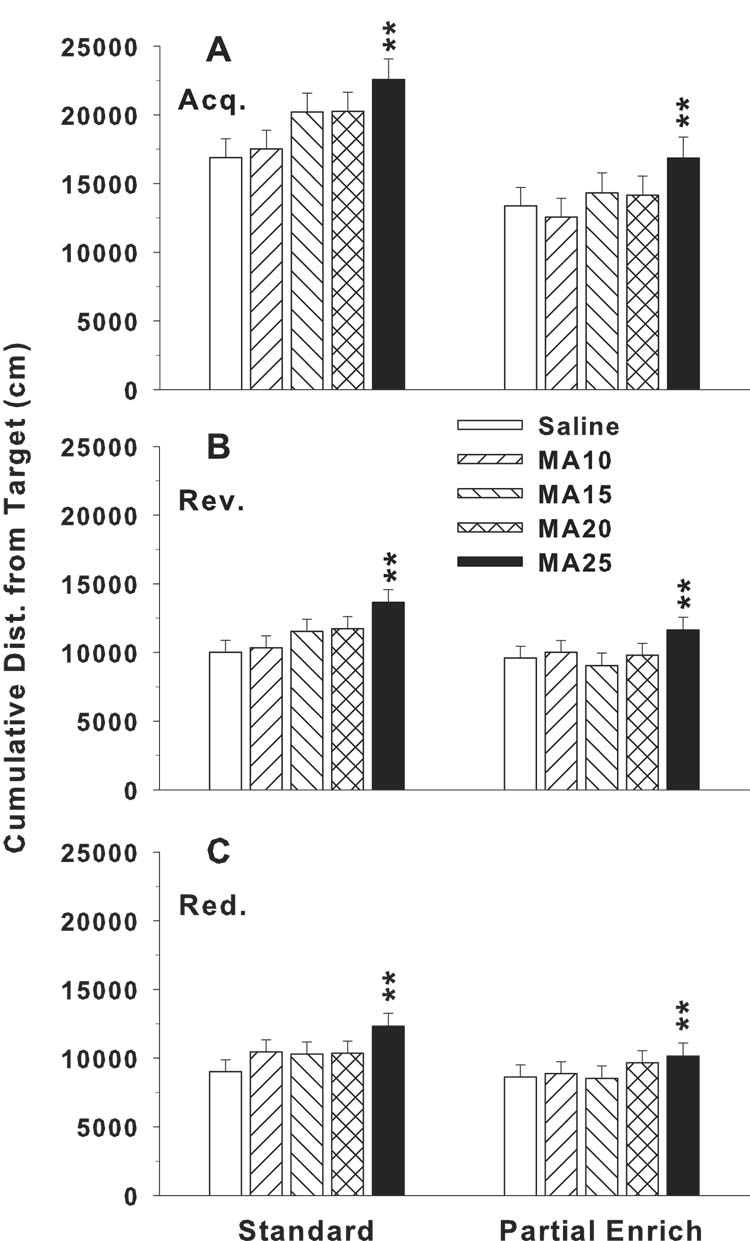
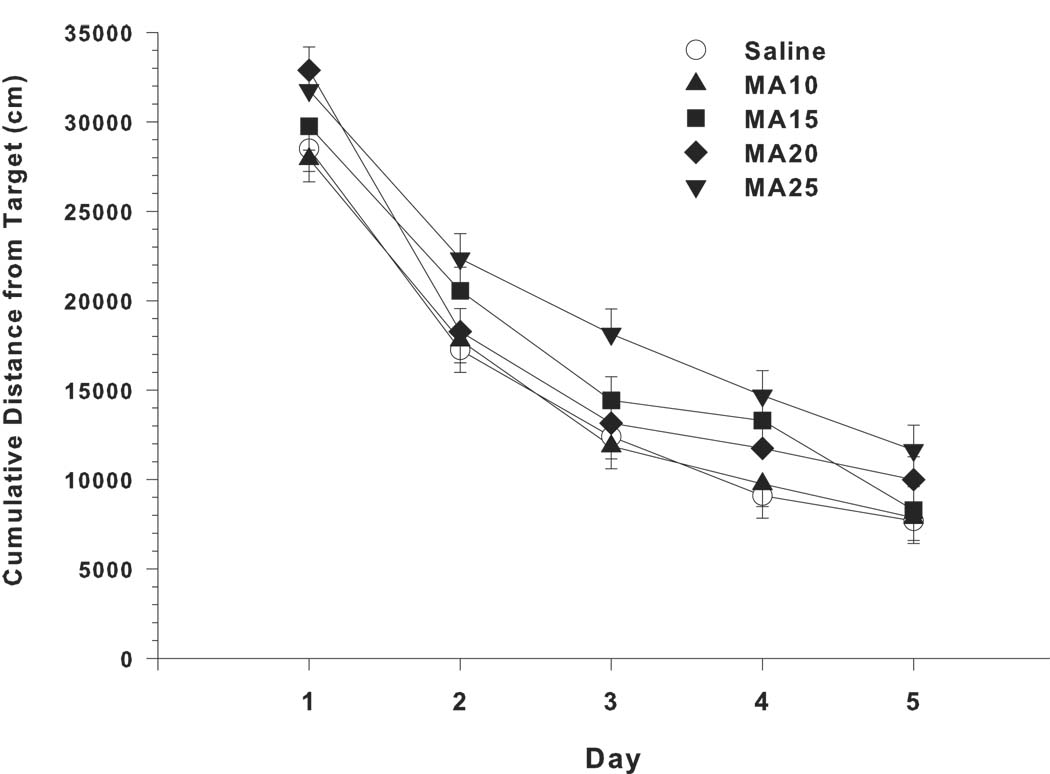
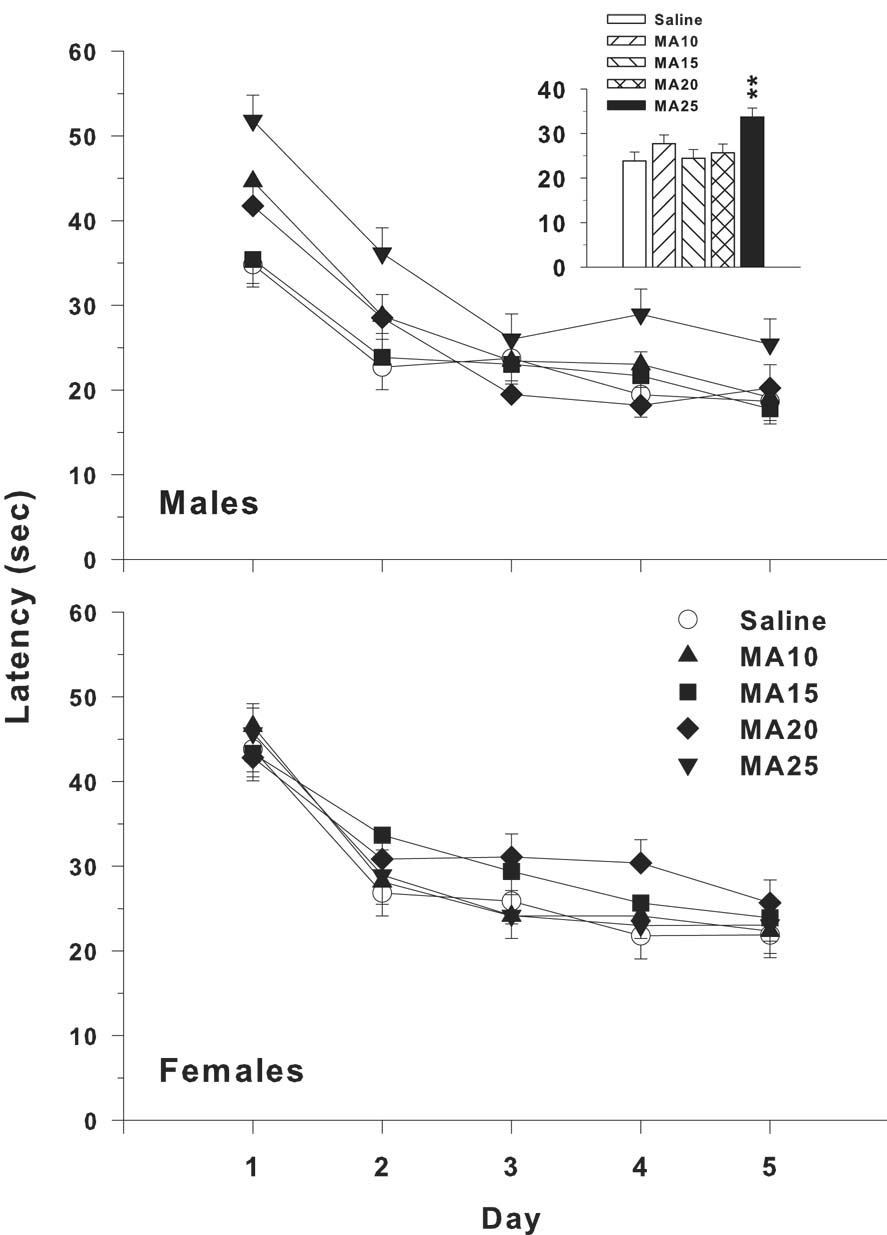
Analyses of latency, showed significant main effects of drug treatment (F(4,317) = 4.02, p < 0.01) and rearing condition (F(1,36.3) = 23.48, p < 0.0001). The only other effect was day (p < 0.0001). Several interactions not involving drug treatment were also significant. These were sex x rearing condition (p < 0.04), sex x day (p < 0.001), and rearing condition x day (p < 0.001). A nearly identical pattern was found for path length, i.e., there was a significant drug treatment main effect (F(4,316) = 3.47, p < 0.01), along with effects for rearing condition (p < 0.001), day (p < 0.0001), sex x day (p < 0.0001), and rearing condition x day (p < 0.001). The same pattern was again seen on cumulative distance from the platform: main effect of drug treatment (F(4,320) = 4.25, p < 0.003) (Fig. 4A), with other effects being rearing condition (p < 0.0001), day (p < 0.0001), and rearing condition x day (p < 0.001). On all 3 measures, the main effect of drug treatment was further analyzed using the Dunnett-Hsu test for each drug group compared to control. In each case, the only group that differed from saline was the MA-25 (p < 0.01). Cumulative distance was chosen (Fig. 4A) to illustrate the main effect of drug treatment as a function of rearing condition. The main effect of rearing condition showed that all groups reared in the pE condition had lower cumulative distances from the platform than those reared in standard conditions. Moreover, the drug treatment effect found in the MA-25 group remained constant across rearing conditions and no interaction between rearing condition and drug treatment group was found. The learning curves during acquisition are shown in Fig. 5.
Fig. 4.
Morris water maze performance during the 3 phases of hidden platform learning shown separately by rearing conditions (standard vs. pE (partial enrichment)). Data are (mean ± SEM) for cumulative distance from the platform measured every 0.1 s and averaged across trials, days, and sexes. A: acquisition (10 × 10 cm platform; B: reversal (10 × 10 cm platform), C: shifted-reduced platform trials (5 × 5 cm platform). For each phase, there were 4 trials/d for 5 consecutive days. **P < 0.01 vs. SAL. Rearing condition was a significant main effect but did not interact with drug treatment during acquisition. With males and females combined, groups sizes are for standard/pE rearing: Saline = 39/40, MA-10 = 39/39, MA-15 = 38/34, MA-20 = 37/38, MA-25 = 32/31.
Fig. 5.
Morris water maze (hidden platform) performance during acquisition shown as the mean ± SEM for each day (sexes combined) to illustrate the learning curve for cumulative distance. The MA-25 group was the only group significantly impaired (see Fig. 4). Group sizes are as in Fig. 4.
3.5 Morris Water Maze--Reversal
Analyses on the data for reversal may be seen for cumulative distance in Fig. 4B. As for acquisition, reversal data showed a significant drug treatment main effect on latency (F(4,313) = 2.97, p < 0.02). However, unlike acquisition, there was no effect of rearing condition (F(1,37.8) = 2.02, p = 0.16). Day was again significant (p < 0.0001) as was the drug treatment x day interaction (F(16,1242) = 2.38, p < 0.002). For path length, the main effect of drug treatment fell short of significance (p = 0.12), but the drug treatment x day interaction was significant (F(16,1239) = 2.10, p < 0.01). Sex (p < 0.01) and day (p < 0.0001) were also significant. For cumulative distance, there was a main effect of drug treatment (F(4,395) = 3.89, p < 0.01) and drug treatment x day interaction (F(16,1418) = 2.12, p < 0.01), but no effect of rearing condition or sex. Day was again significant (p < 0.0001), as was rearing condition x day (p < 0.03). As during acquisition, the cumulative distance data were consistent in showing that the MA-25 group was impaired in performance compared to the saline controls.
3.6 Morris Water Maze--Reduced
In the third phase of MWM learning, the platform was moved to a quadrant adjacent to the reversal quadrant and a smaller platform was used (75% smaller, i.e., 5 × 5 cm). For latency, the drug treatment main effect fell short of significance (F(4,312) = 2.25, p = 0.063). However, there was a significant drug treatment x sex interaction (F(4,313) = 3.25, p < 0.02) which is illustrated in Fig. 6. The males in the MA-25 group had increased latencies (Fig. 6 inset), whereas females did not. Sex (p < 0.02) and day (p < 0.0001) were also significant. For path length, only the main effects of sex (p < 0.0003) and day (p < 0.0001) were significant. For cumulative distance, the main effect of drug treatment was significant (F(4,313) = 2.43, p < 0.05) as was the drug treatment x sex interaction (F(4,313) = 2.77, p < 0.03). The main effect of drug treatment is shown in Fig. 4C and shows that the MA-25 group was impaired in learning the new position of the platform compared to saline controls. There was no effect of rearing condition. Sex (p < 0.01; males performed better than females) and day (p < 0.0001; all groups improved over days) were also significant.
Fig. 6.
Morris water maze shifted-reduced hidden platform phase. Shown are the mean (± SEM) latency to find the platform for males and females on each day. Inset: Mean (± SEM) averaged across days to show the effect of the MA-25 group vs. SAL in males. **P < 0.01 vs. SAL. Group sizes are: standard rearing, male, Saline = 20, MA-10 = 19, MA-15 = 18, MA-20 = 18, MA-25 = 15; standard rearing, female, Saline = 19, MA-10 = 20, MA-15 = 20, MA-20 = 19, MA-25= 17. For pE rearing, male, Saline = 20, MA-10 = 19, MA-15 = 17, MA-20 = 19, MA-25 = 16, and pE rearing, female Saline = 20, MA-10 = 20, MA-15 = 17, MA-20 = 19, MA-25 = 16.
Latency has sometimes been questioned as an index of learning because it could be confounded by differences in swimming speed. Because of this, cumulative distance has been suggested as a better index (Gallagher et al., 1993). However, it is theoretically possible for 2 animals to swim identical paths to the platform and differ in speed and the slower animal will have a larger cumulative distance score since this index is sampled as a function of time (every 0.2 s). A slower animal obtains a larger number of 0.2 s samples than a faster animal traveling the same path to the goal. Path length should, by the same reasoning, be immune from this effect. Yet the main effect of MA on reversal and reduced platform phases were not significant for path length. In order to check whether latency and cumulative distance were being affected by swimming speed, we analyzed swimming velocity directly. On acquisition, reversal and reduced test phases, no treatment effects or interactions were found, nor did they approach significance (not shown).
3.7 Morris Water Maze—Probe trials
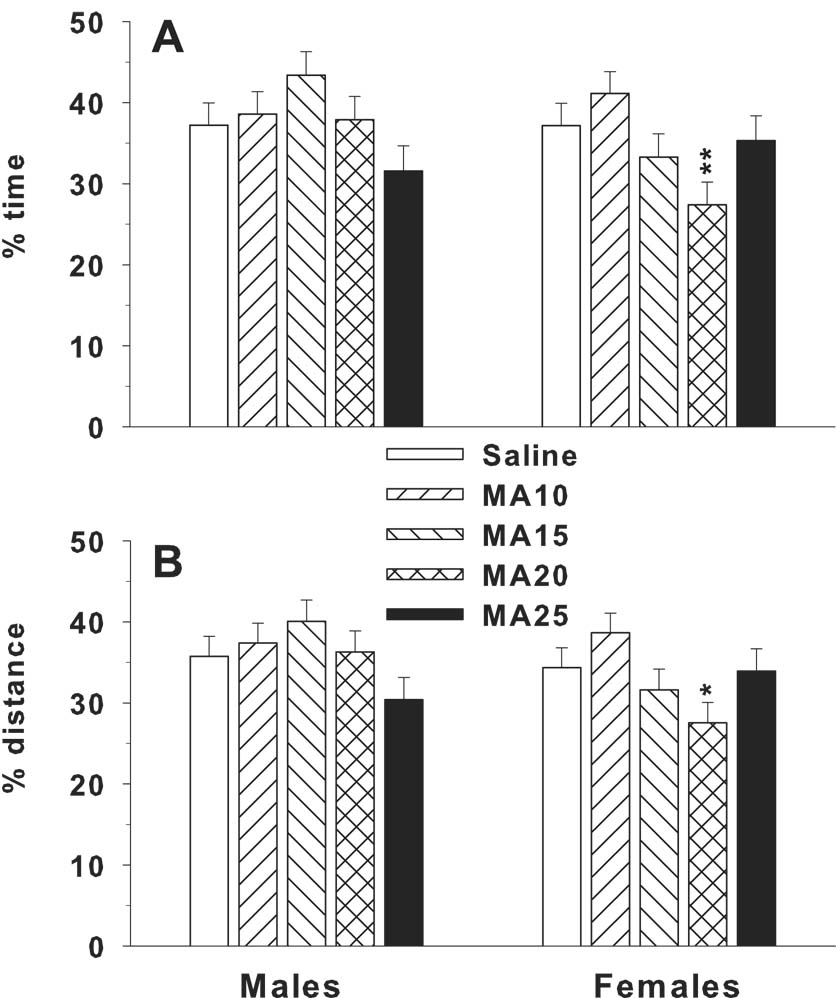
On the acquisition probe trial, there were no drug treatment effects on average distance to the platform site, although there was a trend found for the drug treatment x sex interaction (p = 0.068). For platform site crossings, drug treatment showed no significant effect, but the drug treatment x sex (F(4,283) = 2.40, p < 0.05) and drug treatment x sex x rearing condition (F(4,283) = 2.93, p < 0.05) interactions were significant. Rearing condition was also a significant main effect (p < 0.01). The 3-way drug treatment x sex x rearing condition interaction showed a significant effect only for the male pE condition (p < 0.01). A priori pairwise comparisons using Dunnett-Hsu’s test comparing each drug treatment group to control showed that no drug group differed significantly from saline controls, although a trend (p < 0.10) toward the MA-25 male group having fewer crossings than the male SAL group was seen (SAL = 1.2 ± 0.3 vs. MA-25 = 0.6 ± 0.1). For percent time in the target quadrant, there was a significant drug treatment x sex interaction (F(4,283) = 3.09, p < 0.02; Fig 7A), and a significant drug treatment main effect on percent time in the target quadrant (F(4,283) = 2.60, p < 0.04). Percent distance in the target quadrant was similar with a drug treatment x sex (F(4,283) = 2.51, p < 0.05) and nearly significant drug treatment main effect (F(4,283) = 2.23, p = 0.066). Follow up analyses on the percent time drug treatment x sex interaction showed the drug treatment effect was significant for females (p < 0.01) and a trend for males (p < 0.08). Dunnett-Hsu pairwise comparisons showed that MA-20 females spent less time in the target quadrant (Fig. 7A) and traveled less distance (Fig. 7B) than control females. There were no significant drug treatment effects of rearing condition on any of the probe trial measures.
Fig. 7.
Morris water maze probe trial performance 24 h after the last trial of acquisition. Shown are mean (± SEM) percent time (top panel) and distance (bottom panel) spent in the target quadrant with the platform removed during the 30 s probe trial. *P < 0.05, **P < 0.01 vs. SAL. Group sizes are as in Fig. 6.
Analyses of the probe trial data on reversal for average distance, crossings, and percent distance and time in the target quadrant showed no effects of drug treatment, rearing condition or interactions.
Analyses of probe trial data on reduced platform trials showed a significant drug treatment x sex interaction on crossings (F(4,307) = 2.49, p < 0.05). Further analyses for each sex showed no significant drug treatment effects, but there was a trend toward an effect among the males (p < 0.07) which was not analyzed further. No effect of rearing condition on crossings was found. For average distance, percent distance, and percent time in the target quadrant, no significant drug treatment, rearing condition or interactions were obtained.
Speed was also analyzed on probe trials in all 3 phases of the MWM and no significant effects or trends were obtained.
3.8 Sex Differences in Morris water maze versus Cincinnati water maze performance
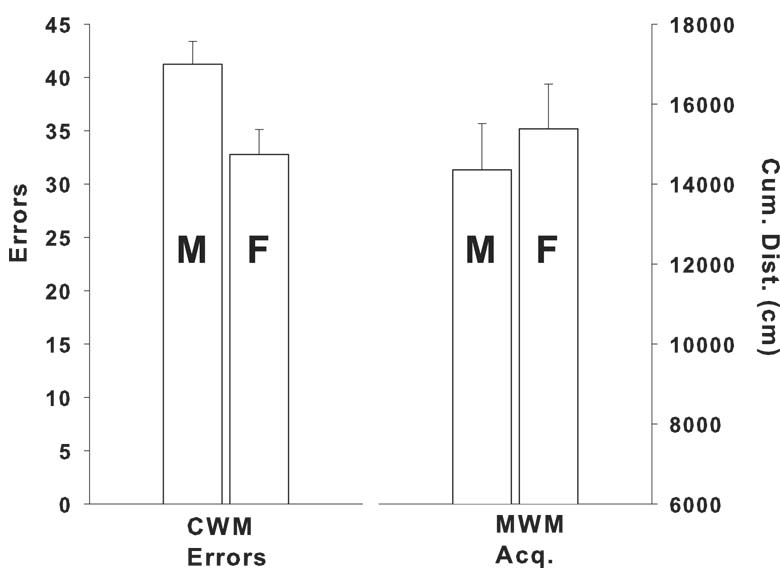
In the analyses of the CWM and MWM data, it was noticed that in the MWM males across all groups consistently perform better than females, an effect that has been described in the literature (Beiko et al., 2004; D'Hooge and De Deyn, 2001; Means et al., 1992; Perrot-Sinal et al., 1996), including previous data from this lab (Williams et al., 2003b; Williams et al., 2003c; Williams et al., 2003a; Williams et al., 2004). In examining the CWM data, we observed the opposite pattern, i.e., that females across all groups consistently perform better than males, an effect we have seen in our data before, although we have not made a special note of it. In order to illustrate this difference, Fig. 8 shows the mean (±SEM) of CWM errors for males and females (left) and for MWM acquisition cumulative distance (right). As can be seen, in the CWM females make fewer errors than males. By contrast, in the MWM females have longer cumulative distances from the platform than males. While these are both sexually dimorphic effects and both are in water mazes, the opposite direction of the effects between sexes further supports the notion that these mazes are measuring different central learning processes.
Fig. 8.
Comparisons of sex differences in the CWM versus MWM. Left, mean (±SEM) errors in the CWM averaged across days, trials and treatment groups are shown for males (M) and females (F). Right, mean (±SEM) cumulative distance (cm) from the platform in the MWM averaged across days, trials, and treatment groups during the acquisition phase (similar male/females differences were seen on reversal and reduced platform trials and on all probe trials) are shown for males (M) and females (F).
4. Discussion
The present experiment tested the effects of P11–15 treatment with MA at different doses on later learning using two different kinds of tests of learning: the Morris and Cincinnati water mazes under two rearing conditions. The principal findings were that using this shorter exposure period, MA impairs both path integration learning in the CWM and spatial learning in the MWM, however, the effects in the CWM was seen at all doses whereas the effects in the MWM were seen only at the highest dose tested. The results also showed that even a modest environmental enrichment (adding a stainless steel enclosure as partial enrichment) significantly improved initial MWM performance. Interestingly, it had no significant effect on CWM performance. Moreover, the partial enrichment rearing did not interact with the effects of MA at any dose on either test of learning.
The two maze tests we used rely on different learning strategies. The Morris water maze is a well-established test of spatial learning and reference memory in the hidden platform version. Spatial navigation is mediated primarily by the hippocampus (Brandeis et al., 1989; Burgess, 2002; D'Hooge and De Deyn, 2001; McNamara and Skelton, 1993; Morris et al., 2003), although other brain regions are also involved. We have previously shown that P11–20 treatment with MA results in MWM spatial learning and reference memory deficits (Skelton et al., 2007; Vorhees et al., 1994a; Vorhees et al., 1998; Vorhees et al., 1999; Vorhees et al., 2000; Vorhees et al., 2007; Williams et al., 2002; Williams et al., 2003c; Williams et al., 2004) indicating that hippocampal-dependent learning and memory are affected by this drug following exposure during this specific developmental window. Spatial navigation depends on the availability of distal cues in order to use an allocentric strategy to find the hidden platform in the MWM efficiently across a series of trials (Morris, 1981). It has been established that disrupting distal cues in the MWM, disrupts performance, e.g. (Maurer and Derivaz, 2000). Hence distal cues are essential to this form of learning through the use of cues located outside the swimming tank. Neonatal MA exposure, especially when treatment occurs between P11–20, reliably disrupts this type of learning while leaving learning using proximal cues, in the cued platform version of the maze, intact.
Learning to find the hidden platform in the MWM requires secondary or subordinate skills in addition to allocentric ability. For example, the animal must not only learn to orient to distal cues, it must simultaneously learn that the platform is not located around the perimeter of the maze, nor in the center, but in the region in between. It must also learn that there is a platform and that they must climb upon and remain on the platform in order to escape the water and to ultimately be removed from the maze by the experimenter. Cain and colleagues (Cain et al., 1996; Cain et al., 2006; Cain and Saucier, 1996; Hoh and Cain, 1997; Saucier et al., 1996; Saucier and Cain, 1995) and Morris and colleagues (Bannerman et al., 1995) have shown that pretraining animals for these subordinate skills prior to exposure to the typical conditions, eliminates some types of drug-induced spatial impairments, suggesting that sensorimotor factors sometimes play a role. In the case of neonatal MA treatment, we tested for just such effects and showed that MA induces both spatial and subordinate skill MWM learning impairments and these can be distinguished from one another (Williams et al., 2002), such that pretraining eliminated acquisition deficits, but not those seen during reversal training. Since reversal training occurs after subordinate skills are learned, these deficits represent a reliable spatial impairment.
Together with our previous data, the learning effects observed in the present experiment are unlikely to be attributable to sensorimotor deficits for three reasons: (1) no differences were seen on straight channel swimming times. This test requires no learning after the first trial in which animals determine that they need only swim to the opposite end in order to find the escape ladder. Once this is determined, animals may be observed to swim from one end to the other as rapidly as possible. This provides an index of swimming ability and motivation to escape. The data showed no differences among the MA-treated groups compared to saline controls. (2) We assessed swimming speed in the MWM and found no differences among groups in speed on learning or memory trials. (3) If MA-treated animals had significant sensorimotor impairments, one would expect differences on all phases of MWM testing, but in fact differences were selective, with no differences on reversal or shifted-reduced probe trials and no effects below the high dose on learning trials even though these lower dose groups showed impaired learning in the CWM. This pattern argues against any generalized sensorimotor impairment-based explanation for the learning effects obtained.
In the present experiment we focused on a shorter MA exposure period than used previously. We did this based on a prior experiment in which we found that MA treatment on P11–15, but not on P16–20, induced MWM learning deficits (Williams et al., 2003a). In that experiment, a single dose of MA was tested. We treated neonatal rats with 10 mg/kg x 4 doses/d of MA on P11–15. In the present experiment, we treated rats on these same days with doses of 10, 15, 20, or 25 mg/kg x 4 doses/d. Surprisingly, we found MWM maze deficits only in the high dose group (25 mg/kg), but not in any of the lower dose groups, including the dose at which we had found effects previously. However, there is a significant procedural difference between the two experiments. In an attempt to make the task more sensitive in our previous experiment, we tested the rats in the MWM using the small (5 × 5 cm) platform during acquisition. An examination of the learning curves in that experiment (Williams et al., 2002), shows that while it succeeded in revealing group differences, the rate of learning was very slow, even among controls, compared to experiments that have used a 10 × 10 cm platform in the same 210 cm diameter tank. Because of the poor learning in that experiment, we decided not to use this procedure in future experiments, but rather to use the smaller platform only after the animals had prior experience (acquisition and/or reversal) with the larger 10 × 10 cm platform. This approach improved the learning rate, yet still succeeded in showing the value of using the small platform to uncover learning differences that were not apparent using only the larger platform, e.g. (Vorhees et al., 2000; Williams et al., 2003c). Accordingly, in the present experiment we used this multiphasic MWM test procedure to evaluate animals, i.e., using a larger platform during acquisition and reversal and the smaller platform during shifted platform trials (i.e., with platform in the quadrant adjacent to that used during reversal). We predicted a dose-dependent, stepwise impairment in MWM performance at progressively higher doses. Unexpectedly, only the MA-25 group showed clear deficits, leading us to conclude that the P11–15 exposure period, while sensitive, is not the maximally sensitive period for spatial learning deficits. Rather, the data suggest that a 10-day (P11–20) exposure likely has an additive effect that produces a more pronounced effect such that lower doses, such as 10 mg/kg x 4/d, or even 0.625 mg/kg x 4/d, will induce MWM deficits (Williams et al., 2004). Alternatively it could be argued that the higher dose group was more comparable to the total drug exposure seen with a 10 day dosing regimen of 10 mg/kg/dose, although no effects in the MWM were observed with the 20 mg/kg/dose group in this experiment. That this requirement for increased days of exposure was not apparent in the experiment comparing P11–15 vs. P16–20 exposure periods we now interpret as being attributable to the use of the small platform during acquisition training in our previous experiment. We suggest that the small platform made the task sufficiently difficult that it may have obscured differences present in the P16–20 MA-treated group, or that the P11–15 exposure period is required for spatial learning deficits, but these deficits are more pronounced when MA is delivered over a longer period of time.
In three previous experiments we tested the effects of P11–20 MA treatment on learning in a 9-unit multiple T-maze (Cincinnati water maze), and each time found trends toward impairment that fell short of significance (Vorhees et al., 1994a; Vorhees et al., 2000; Williams et al., 2003c). At that time, we tested animals under standard house lighting. Subsequently, we changed the procedure to one that eliminated the use of distal cues. We did this because we already knew from the MWM findings that MA-treated neonates had a spatial impairment and wondered if the trend in the CWM might be the result of animals using combined or redundant allocentric and egocentric strategies to solve the maze, thereby showing no significant effect. To test this hypothesis we eliminated access to distal cues by testing in complete darkness and used an infrared camera to score performance. Not surprisingly, under these conditions the task becomes very difficult and the rats learn at a much slower rate. However, given enough trials, they do master the maze and ultimately perform quite well (see Fig. 2). As the present results show, this change in procedure revealed that MA-treated rats at all doses showed impaired performance. The effects were dramatic and affected all aspects of performance, i.e., the MA-treated animals had longer latencies to reach the escape ladder, made more errors by entering more dead-end cul-de-sacs, and returned to the start more frequently than saline controls.
Path integration is conserved in organisms ranging from ants (Wittlinger et al., 2006) and rodents, to humans (Etienne and Jeffery, 2004). It is a form of egocentric learning that relies upon self-movement cues to locate places in an environment based on direction and rate of movement, i.e., a trajectory or vector through space rather than distal cue navigation (Etienne and Jeffery, 2004). Unlike spatial or allocentric (landmark-based) learning, path integration is dependent on movement cues (primarily internal) rather than objects located at a distance (Etienne, 1992; Etienne and Jeffery, 2004). The neural circuits underlying path integration in rats partially overlap with those of spatial navigation inasmuch as some place cells in the hippocampus are activated during path integration, however, path integration depends upon head-direction cells in the presubiculum and grid cells in the entorhinal cortex (Fuhs and Touretzky, 2006; McNaughton et al., 2006; Rondi-Reig et al., 2006; Sargolini et al., 2006; Whishaw et al., 1997). Path integration is critically dependent on connections between head direction and grid cells, especially those in layers II and III of the medial entorhinal cortex (Sargolini et al., 2006). Although it remains to be proven that entorhinal cortex lesions selectively impair CWM learning compared to MWM learning, the fact that rats are severely impaired in the CWM when visual cues are removed but are only marginally affected when they are present, argues in favor of the view that egocentric learning is more sensitive to the effects of neonatal MA-treatment after exposure on P11–15. As such, this represents a potentially useful finding about the long-term effects of developmental MA treatment.
We tested the effects of a partial environmental enrichment provided to half the litters during development and throughout the experiment compared to the other half of the litters that were reared and housed as adults in standard box cages in order to determine whether such partial enrichment alters the effects of neonatal MA treatment. Even in conditions which are more enriched than in the past (i.e., box cages vs. older wire bottom cages, housing animals in pairs vs. single housing, and not weaning litters until P28; all practices designed to improve laboratory housing conditions for rodents), adding a simple item such as a stainless steel enclosure to each cage produced significant main effects on initial spatial learning. In all treatment groups, the animals raised with enrichment performed significantly better on all measures of MWM performance than those raised in standard box cages. Importantly, however, from the perspective of interest in the effects of MA, drug treatment did not interact significantly with rearing partial enrichment hence, alleviating concern over whether this change in housing practice might alter the basic effects of MA on brain development and behavior. Interestingly, however, this same enrichment did not produce a main effect (or interaction) on performance in the CWM. This suggests that this task is not sensitive to the added effects of placing an enclosure into box cages throughout the animals’ lives and continuing when they are pair housed after P28. This is not to suggest that more elaborate enrichment if compared to single, wire-bottom, P21 weaned rats might not reveal enrichment effects on CWM performance, but rather the incremental effect of the enclosure did not result in a significant effect on this task.
The mechanism(s) underlying the developmental effects of MA on learning are not known. P11–20 MA treatment causes increased release of corticosterone (Williams et al., 2000), showing a U-shaped response function in which corticosterone release is larger 30 and 105 min after MA treatment on P1 or P3 than on P5, 7, 9, 11, or 13, and was higher again on P15, 17 and 19 (Williams et al., 2006). This resembles what is termed the stress hyporesponsive period (SHRP) (Sapolsky and Meaney, 1986), which is described as beginning shortly after birth and extending to P14. When the drug is given longer, for 4 days with one additional dose on the fifth day, 30 min corticosterone release was no longer U-shaped but progressively increasing with age, and 105 min levels were lower on all days than after the dose measured at 30 min. Moreover, these corticosterone effects remain evident 18 h after the final dose (Schaefer et al., 2006; Schaefer et al., 2007). We are currently testing the role of MA-induced corticosterone release on later learning.
Rats treated with 10 mg/kg x 4/day of MA on P11–20 and assessed at P90, also show 10–15% reductions in neostriatal 3H-spiperone binding to D2-like receptors with no changes in affinity. They also show 20% reductions in neostriatal PKA activity, and 15% reductions in neostriatal dopamine and DOPAC concentrations (Crawford et al., 2003). Rats given MA on P11, P11–15, or P11–20 and examined 24 h later, show no differences in dopamine levels (Schaefer et al., 2006; Schaefer et al., 2007), indicating that the long-term changes in dopamine in adulthood are not caused by neonatal reductions during treatment. As with the effects on corticosterone, it is not yet know if any of these neurotransmitter, receptor, or enzymes mediate the learning effects and future studies will be require to determine the relationship among these changes.
Acknowledgment
This research was supported by the following grants from the U.S. National Institutes of Health: DA006733 (CVV), and DA014269 (MTW). We thank Mary S. Moran for assistance with data analyses and Susan K. Miranowski for assistance with behavioral testing during her Summer Undergraduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Acevedo SF, de EI, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychopharmacology. 2007;32:665–672. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- Acuff-Smith KD, Schilling MA, Fisher JE, Vorhees CV. Stage-specific effects of prenatal D-methamphetamine exposure on behavioral and eye development in rats. Neurotoxicol.Teratol. 1995;18:199–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RGM. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav. Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of memory and learning. Int.J.Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Burgess N. The hippocampus, space, and viewpoints in episodic memory. Q.J.Exp.Psychol. 2002;A55:1057–1080. doi: 10.1080/02724980244000224. [DOI] [PubMed] [Google Scholar]
- Cain DP, Boon F, Corcoran ME. Thalamic and hippocampal mechanisms in spatial navigation: a dissociation between brain mechanisms for learning how versus learning where to navigate. Behav.Brain Res. 2006;170:241–256. doi: 10.1016/j.bbr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D. The neuroscience of spatial navigation: Focus on behavior yields advances. Rev.Neurosci. 1996;7:215–231. doi: 10.1515/revneuro.1996.7.3.215. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: Contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behav.Neurosci. 1996;110:86–102. doi: 10.1037//0735-7044.110.1.86. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatr.Res.: Neuroimaging. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chomchai C, Na MN, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian. J.Trop.Med.Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2003;48:131–137. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res.Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dixon SD. Effects of transplacental exposure to cocaine and methamphetamine on the neonate. Western J.Med. 1989;150:436–442. [PMC free article] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: Incidence and clinical correlates. J.Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Etienne AS. Navigation of a small mammal by dead reckoning and local cues. Curr.Dir.Psychol.Sci. 1992;1:48–52. [Google Scholar]
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Fuhs MC, Touretzky DS. A spin glass model of path integration in rat medial entorhinal cortex. J.Neurosci. 2006;26:4266–4276. doi: 10.1523/JNEUROSCI.4353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav.Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp.Neurol. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hoh TE, Cain DP. Fractionating the nonspatial pretraining effect in the water maze task. Behav.Neurosci. 1997;111:1285–1291. doi: 10.1037//0735-7044.111.6.1285. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. National Institute on Drug Abuse. Bethesda, MD: U.S. Department of Health and Human Services; 2006a. Monitoring the Future: National Survey Results on Drug Use, 1975–2005. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. National Institute on Drug Abuse. Bethesda, MD: U.S. Department of Health and Human Services; 2006b. Monitoring the Future: National Survey Results on Drug Use, 1975–2005. [Google Scholar]
- Little BB, Snell LM, Gilstrap LC. Methamphetamine abuse during pregnancy: Outcome and fetal effects. Obstet. Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- Maurer R, Derivaz V. Rats in a transparent morris water maze use elemental and configural geometry of landmarks as well as distance to the pool wall. Spatial Cogn.Comput. 2000;2:135–156. [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res.Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the 'cognitive map'. Nat.Rev.Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Means LW, Alexander SR, O'Neal MF. Those cheating rats: Male and female rats use oder trails in a water-escape "working memory" task. Behav.Neural Biol. 1992;58:144–151. doi: 10.1016/0163-1047(92)90387-j. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos.Trans.R.Soc.Lond B Biol.Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learn.Motiv. 1981;12:239–260. [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: Maternal and neonatal correlates. J.Pediatr. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav.Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ.Hlth.Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J.Neurosci. 2006;26:4071–4081. doi: 10.1523/JNEUROSCI.3408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Saucier D, Cain DP. Spatial learning without NMDA receptor-dependent long-term potentiation. Nature. 1995;378:186–189. doi: 10.1038/378186a0. [DOI] [PubMed] [Google Scholar]
- Saucier D, Hargreaves EL, Boon F, Vanderwolf CH, Cain DP. Detailed behavioral analysis of water maze acquisition under systemic NMDA or muscarinic antagonism: Nonspatial pretraining eliminates spatial learning deficits. Behav.Neurosci. 1996;110:103–116. [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−)methylphenidate administration in the neonatal rat. J.Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short- and long-term effects of (+)-methamphetamine and (+/−)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J.Neurochem. 2007;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R, Pometlova M, Syllabova L, Mancuskova M. Learning in the Place navigation task, not the New-learning task, is altered by prenatal methamphetamine exposure. Brain Res.Dev.Brain Res. 2005;157:217–219. doi: 10.1016/j.devbrainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della GS, Liu J, Lester BM. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. Dev.Behav.Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: A comparison of performance in two Water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicology and Teratology. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentation and spatial learning deficits. Psychopharmacology. 1994a;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats: II. Hypoactivity and altered responses to pharmacological challenge. Psychopharmacology. 1194b;114:402–408. doi: 10.1007/BF02249329. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: Selective effects on spatial navigation and memory. J.Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Morford LL, Inman SL, Reed TM, Schilling MA, Cappon GD, Moran MS, Nebert DW. Genetic differences in spatial learning between Dark Agouti and Sprague-Dawley strains: Possible correlation with CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics. 1999;9:171–181. [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Schilling MA, Fisher JE, Moran MS, Cappon GD, Nebert DW. CYP2D1 polymorphism in methamphetamine-treated rats: Genetic differences in neonatal mortality and a comparison of spatial learning and acoustic startle. Neurotoxicol.Teratol. 1998;20:265–273. doi: 10.1016/s0892-0362(97)00129-3. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav.Pharmacol. 2007;18:549–562. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat.Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Pierce DR. Perinatal alcohol exposure and neuronal damage. In: West JR, editor. Alcohol and Brain Development. New York: Oxford University Press; 1987. pp. 120–157. [Google Scholar]
- Whishaw IQ, Maaswinkel H. Rats with fimbria-fornix lesions are impaired in path integration: A role for the hippocampus in "sense of direction". J.Neurosci. 1998;18:3050–3058. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, McKenna JE, Maaswinkel H. Hippocampal lesions and path integration. Curr.Opin.Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol.Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003a;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Behavioral and growth effects induced by low dose methamphetamine administration during the neonatal period in rats. Int.J.Dev.Neurosci. 2004;22:273–283. doi: 10.1016/j.ijdevneu.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects, or injection stress. Brain Research. 2003b;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood, SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003c;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Furay AR, Ehrman LA, Vorhees CV. Ontogeny of the adrenal response to (+)-methamphetamine in neonatal rats: The effect of prior drug exposure. Stress. 2006;9:153–163. doi: 10.1080/10253890600902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal days 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Research. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Wittlinger M, Wehner R, Wolf H. The ant odometer: stepping on stilts and stumps. Science. 2006;312:1965–1967. doi: 10.1126/science.1126912. [DOI] [PubMed] [Google Scholar]