Abstract
Following exposure to intermittent hypoxia, respiratory motor activity and sympathetic nervous system activity may persist above baseline levels for over an hour. The present investigation was designed to determine whether sustained increases in minute ventilation and sympathovagal (S/V) balance, in addition to sustained depression of parasympathetic nervous system activity (PNSA), were greater in men compared with women following exposure to intermittent hypoxia. Fifteen healthy men and women matched for age, race, and body mass index were exposed to eight 4-min episodes of hypoxia during sustained hypercapnia followed by a 15-min end-recovery period. The magnitude of the increase in minute ventilation during the end-recovery period, compared with baseline, was similar in men and women (men, 1.52 ± 0.03; women, 1.57 ± 0.02 fraction of baseline; P < 0.0001). In contrast, depression of PNSA and increases in S/V balance were evident during the end-recovery period, compared with baseline, in men (PNSA, 0.66 ± 0.06 fraction of baseline, P < 0.0001; S/V balance, 2.8 ± 0.7 fraction of baseline, P < 0.03) but not in women (PNSA, 1.27 ± 0.19 fraction of baseline, P = 0.3; S/V balance, 1.8 ± 0.6 fraction of baseline, P = 0.2). We conclude that a sustained increase in minute ventilation, which is indicative of long-term facilitation, is evident in both men and women following exposure to intermittent hypoxia and that this response is independent of sex. In contrast, sustained alterations in autonomic nervous system activity were evident in men but not in women.
Keywords: carbon dioxide, parasympathetic nervous system, sympathovagal balance
Long-term facilitation (LTF) of minute ventilation and/or its components (i.e., tidal volume and breathing frequency) may be elicited during and following short-term exposure to intermittent hypoxia (i.e., 20 –30 min) (38, 40, 44). LTF is characterized by a gradual increase in respiratory motor activity during successive periods of normoxia that separate hypoxic episodes and by respiratory activity that remains elevated for up to 90 min following exposure to intermittent hypoxia (40). LTF of minute ventilation or phrenic nerve activity (3, 10, 22, 48, 49) and genioglossus muscle or hypoglossal nerve activity (2, 18, 34, 37) have been observed in goats (49), dogs (10), cats (34), ducks (41), rats (3, 18), and mice (48). Similarly, LTF of minute ventilation and genioglossus muscle activity have been observed in healthy humans (21) during wakefulness when carbon dioxide levels are sustained slightly above baseline values during and following exposure to intermittent hypoxia. However, LTF is absent in healthy individuals (14, 24, 35, 36, 43) and individuals with obstructive sleep apnea (26) when carbon dioxide is maintained at normocapnic or hypocapnic levels.
In addition to carbon dioxide levels, the manifestation of LTF may also be impacted by a variety of other factors, one factor being sex (5, 39). Studies completed in animals have shown that LTF is greater in young male rats (52) compared with female rats in either the estrous or diestrous phase (51). Conversely, in middle-aged rats, LTF during the estrous and diestrous cycle is greater in females (51) compared with males (52). The possibility that sex hormones have a role in the manifestation of LTF is supported by studies that have shown that gonadectomy attenuates LTF in male rats and that the restoration of testosterone levels restores LTF (53). Additionally, the amplitude of LTF is different in the diestrous phase compared with the estrous in both young and middle-aged female rats (51). On the basis of the reported findings in rats, it is possible that differences in LTF exist between human men and women. Thus the first purpose of our study was to determine whether the manifestation of LTF of minute ventilation and its components (i.e., tidal volume and breathing frequency) differs between men and women matched for age, race, and body mass index. We hypothesized that the magnitude of LTF would be greater in men compared with women.
In addition to its impact on ventilation, studies have shown that sympathetic nervous system activity (SNSA) persists above baseline values for up to 3 h following exposure to hypoxia (12, 29, 50). Although no study to our knowledge has systematically examined whether LTF of SNSA induced by intermittent hypoxia is sex dependent, Jones and colleagues (23) did investigate whether the response of SNSA to a short period of sustained hypoxia was different in young healthy men and women (23). These investigators reported that the response of the SNSA to hypoxia occurred at a faster rate in women compared with men (23). Moreover, once the hypoxic stimulus was removed, the latency for the return of SNSA to baseline levels was short in women, whereas SNSA remained elevated in men throughout the recovery period (23). This latter finding suggests that long-term alterations in autonomic nervous system activity following exposure to intermittent hypoxia might vary between men and women. Thus the second purpose of our study was to determine, using the noninvasive technique of heart rate variability, whether sustained depression in parasympathetic nervous system activity (PNSA) and sustained facilitation of sympathovagal (S/V) balance was evident in young healthy men and women during and following exposure to intermittent hypoxia. We hypothesized that the magnitude of sustained depression in PNSA and sustained facilitation of S/V balance would be greater in men compared with women.
MATERIALS AND METHODS
The Human Investigation Committees of Wayne State University School of Medicine and John D. Dingell Veterans Affairs Medical Center approved the experimental protocol. Fifteen healthy men and 15 healthy premenopausal women in the follicular phase of their menstrual cycle visited the laboratory on two occasions after informed consent was obtained. The men and women were matched on the basis of age, body mass index, and race. During the first visit to the laboratory, all subjects underwent a physical examination, and women also completed a pregnancy test. Once the inclusion criteria were satisfied, subjects were exposed to two 4-min episodes of hypoxia to ensure familiarization with the experimental protocol and apparatus.
Before the second visit, subjects were advised to fast for a minimum of 3 h, to avoid alcohol and caffeine for 8 h, and to have a minimum of 7 h of sleep to preclude any influence that these factors might have on the measured responses. On the second occasion, subjects arrived at the same time of the day to account for the influence of circadian rhythms on the measured variables. Additionally, 20 ml of venous blood were drawn into Vacutainer tubes with a clot activator (BD Diagnostics, Franklin Lakes, NJ) from 11 of the 15 women to measure sex hormone levels to confirm that each subject was in the follicular phase of her menstrual cycle. The sample collected was tested for the steroid hormones progesterone, estrogen, follicle-stimulating hormone, and luteinizing hormone. For the remaining participants (n = 4), we assumed they were in the follicular phase of their menstrual cycle based on self-report. Subsequently, subjects assumed a supine position, and the required monitoring equipment was attached (see below for details regarding monitoring equipment). Thereafter, subjects breathed room air for 10 min so that baseline values of minute ventilation, breathing frequency, tidal volume, the end-tidal partial pressure of oxygen (PetO2), and the end-tidal partial pressure of carbon dioxide (PETCO2) could be determined. The PetCO2 was then increased 4 Torr above normocapnic baseline values, and measurements of minute ventilation, breathing frequency, tidal volume, PetO2, and PetCO2 were obtained for an additional 15 min before exposure to hypoxia. This level of carbon dioxide was maintained throughout the remainder of the protocol. Subjects were exposed to eight 4-min episodes of hypoxia separated by 5 min of normoxia. During the hypoxic episodes, PetO2 was maintained at 50 Torr. At the completion of each episode, hypoxia was abruptly terminated with a single breath of 100% oxygen to rapidly bring the PetO2 to the normoxic range. Following the last exposure to hypoxia (eighth episode), respiration was monitored continuously for 15 min during the poststimuli recovery period.
Instrumentation
During completion of the intermittent hypoxia protocol, subjects breathed through a face mask, which allowed end-tidal oxygen (model no. 17518; Vacumed, Ventura, CA) and carbon dioxide (model no. 17515, Vacumed) to be sampled from two separate mask ports. The face mask was connected to a pneumotachograph (model no. RSS100-HR; Hans Rudolph, Kansas City, MO), which monitored breath-by-breath changes in ventilation. The pneumotachograph was attached to a two-way valve. The inspiratory port of the valve was connected to a stopcock. Subjects inspired either room air or the contents from one of two bags attached to the stopcock. One bag contained 8% oxygen-92% nitrogen to rapidly reduce PetO2 to 50 Torr at the onset of each hypoxic episode, and the other bag contained 100% oxygen to rapidly induce normoxia at the end of each hypoxic episode. Additionally, the output from a flowmeter was attached to a stopcock port connected to the inspiratory port of the valve. Gas from two cylinders containing 100% oxygen and 100% carbon dioxide were connected to the flowmeter. Thus supplemental oxygen and carbon dioxide could be added to the 8% oxygen-92% nitrogen to maintain desired levels of PetO2 (50 Torr) and PetCO2 (i.e., 4 Torr above baseline). An ECG was monitored and a pulse oximeter was used to monitor oxygen saturation (Biox 3700; Ohmeda, Boulder, CO). A 16-bit analog-to-digital converter (AT-MIO-16XE-50; National Instruments, Austin, TX) digitized the analog signals for online computer analysis using software specifically designed for this purpose. The software calculated minute ventilation, tidal volume, breathing frequency, PetO2, and PetCO2 on a breath-by-breath basis.
Data analysis
Ventilatory data (minute ventilation, tidal volume, and breathing frequency) were obtained from all men and women. In two women, R-waves in the ECG could not be clearly differentiated because of signal noise. Consequently, heart rate variability measures were obtained from 13 of the 15 matched pairs.
Average values of minute ventilation, tidal volume, breathing frequency, PetCO2, and PetO2 were determined for the last 10 min of the 15-min baseline period recorded immediately before intermittent hypoxia. Average values were also obtained for the last 2 min of each hypoxic episode. Additionally, average values were obtained from the last 2 min of the normoxic recovery periods that followed each hypoxic episode and for the initial, middle, and last 5 min of the poststimuli recovery period that followed the eighth hypoxic episode. These average values are reported both in absolute values and as a fraction of baseline values. Using the average absolute values of minute ventilation from both the hypoxic episodes and the normoxic recovery periods, we measured the ventilatory response to each hypoxic episode. This response was measured by subtracting the minute ventilation measured during the normoxic period that preceded each hypoxic episode from the average value of minute ventilation measured during the last 2 min of the hypoxic episode. The resulting change in ventilation was divided by the change (i.e., decrease) in PetO2, that occurred during the transition from the preceding normoxic period to the hypoxic episode to ultimately obtain a measure of the ventilatory response to each hypoxic episode (ΔV̇e/ΔPETO2). Note that the oxygen level during each hypoxic episode was maintained at 50 Torr. Thus differences in ΔPETO2 measured between episodes were solely due to discrepancies in PetO2 that existed between normoxic periods that preceded each hypoxic episode (see RESULTS and Fig. 1).
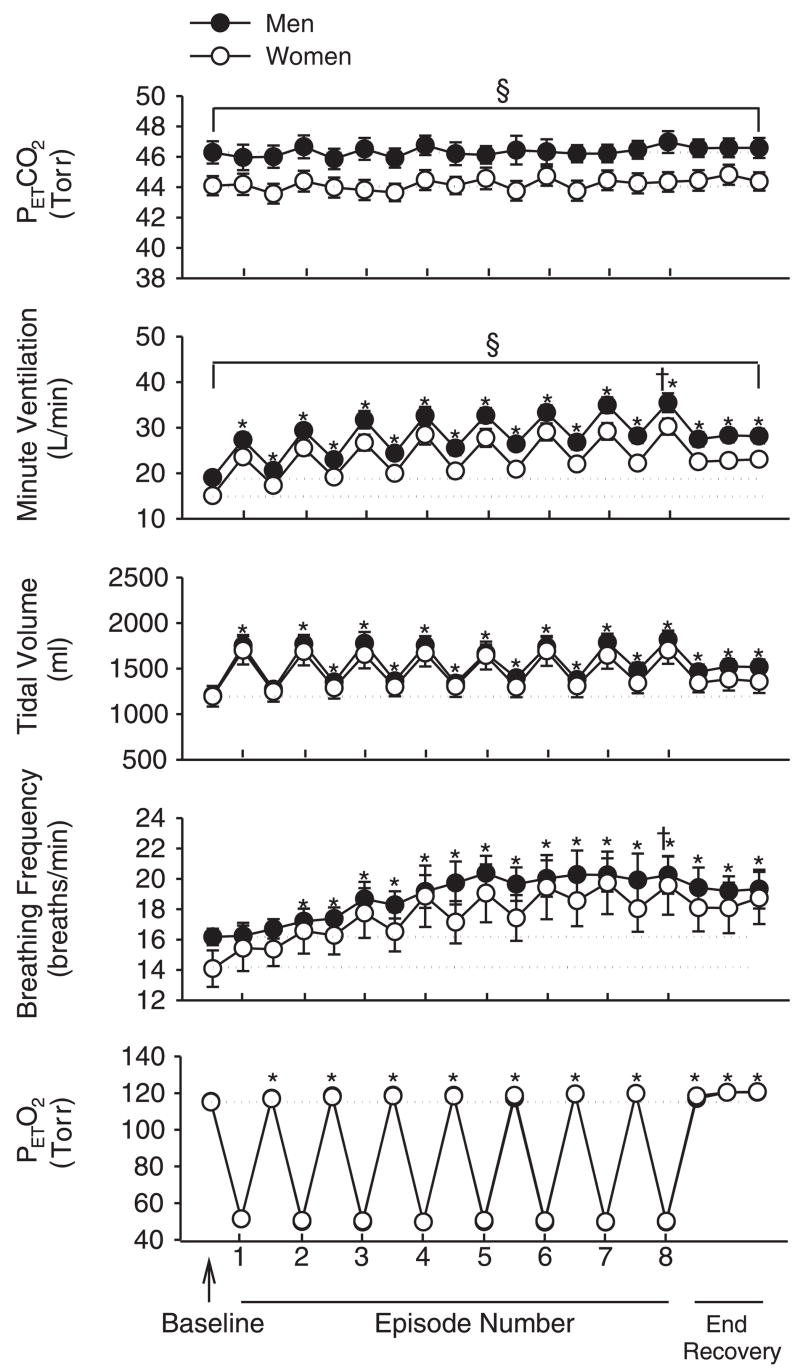
Fig. 1.
Average values for end-tidal carbon dioxide partial pressure (PetCO2), minute ventilation, tidal volume, breathing frequency, and end-tidal oxygen partial pressure (PetO2) recorded from the last 10 min of baseline, the last 2 min of each episode of hypoxia (indicated by vertical tick mark on x-axis), the last 2 min of each normoxic period that separated the hypoxic episodes, and the initial, middle, and final 5 min of the 15-min poststimuli recovery period for men (●) and women (○). Note that a progressive increase in minute ventilation and breathing frequency occurred during and following exposure to intermittent hypoxia in both men and women. Also note that tidal volume was significantly greater than baseline during recovery. The gradual increase in minute ventilation observed during the recovery periods was accompanied by increases in PetO2. Additionally, notice that the dashed lines denote baseline measures for the men and women and that the female symbols are obscuring the male symbols on the PetO2 graph. *Significantly different from baseline; §significantly different from women; †significantly different from the initial hypoxic episode.
To obtain noninvasive measures of autonomic nervous system activity, the time between each detected R-wave [i.e., interbeat interval (IBI)] was determined for the final 9 min of the baseline period (three 3-min segments) before the initial hypoxic episode, the last 3 min of each episode, and the last 3 min of each recovery period interspersed between the episodes. Additionally, the final 15-min recovery period was divided into five 3-min segments, and the IBIs for each segment were determined. The sequence of IBIs for each segment was interpolated, and a power density spectrum was created via a discrete Fourier transform of the interpolated IBI data. The resulting spectrum was integrated, and areas associated with discrete frequency bands were determined. Power spectra within the >0.15-to-0.40-Hz range were defined as high-frequency (HF) components. HF power of R-R variability is considered a function of cardiac PNSA to the heart (47b). Power spectra within the 0.04-to-0.15-Hz range were defined as low-frequency (LF) components. The LF component of R-R variability is considered to be modulated by both SNSA and PNSA (27, 47b). The LF-to-HF ratio (LF/HF) is assumed to be an index of S/V input to sinoatrial node activity and has been found to be representative of sympathetic and parasympathetic balance in both physiological and pathophysiological conditions (33, 42, 47b).
Statistical analysis
A paired t-test was used to determine whether anthropometric variables differed between the men and women. A two-way analysis of variance with repeated measures in conjunction with Student-Newman-Keuls post hoc test was used to determine whether minute ventilation, tidal volume, breathing frequency, PetO2, and PetCO2 during the recovery periods were significantly different from baseline. The factors in the design were group (i.e., men vs. women) and period (recovery period 1–7 and end recovery). The two-way analysis of variance was also used to determine whether minute ventilation, tidal volume, breathing frequency, and the ΔV̇e/ΔPetO2 was greater during the final hypoxic episode compared with the initial episode. The heart rate variability data were not normally distributed, so a Friedman analysis of variance on ranks was used to determine whether heart rate, HF, and LF/HF during the recovery periods were significantly different from baseline. Additionally, this analysis was used to determine whether progressive decreases in HF and increases in LF/HF and heart rate occurred from the initial hypoxic episode to the final hypoxic episode. A value of P ≤ 0.05 was considered significant.
RESULTS
Table 1 shows that the men and women were matched for age, body mass index, and race. Conversely, the height and weight of the men was greater compared with the women. The hormone levels shown in Table 1 confirm that the women were in the follicular phase of their menstrual cycle when exposed to intermittent hypoxia. During baseline, when subjects were breathing room air, minute ventilation and PetCO2 was significantly greater in men compared with the women (minute ventilation, 10.4 ± 0.4 vs. 8.9 ± 0.5 l/min, P = 0.04; PetCO2, 41.9 ± 0.7 vs. 39.6 ± 0.4 Torr, P ≤ 0.01). As expected, these differences remained once the PetCO2 was increased 4 Torr above baseline levels (P < 0.05) (Fig. 1). During the intermittent hypoxia protocol, minute ventilation and breathing frequency were greater during the final episode compared with the initial episode in both the men and women (minute ventilation, P < 0.001; breathing frequency, P < 0.001) (Fig. 1). In contrast, tidal volume during the final and initial hypoxic episodes was similar (Fig. 1). In addition to the alterations in minute ventilation and breathing frequency observed during hypoxia, minute ventilation, tidal volume, and breathing frequency gradually increased during the recovery periods that were interspersed between hypoxic episodes. Consequently, these variables during the end-recovery period were significantly greater than baseline in both men and women (P ≤ 0.001) (Fig. 1). The increase in minute ventilation was accompanied by a gradual increase in PetO2 that remained above baseline values during the end-recovery period (P ≤ 0.001) (Fig. 1). Figure 2 shows that, although a gradual increase in minute ventilation was observed during both the hypoxic episodes and recovery periods, the ΔV̇e/ΔPetO2 measured for the initial hypoxic episode was greater than that of the final hypoxic episode in both men and women (P < 0.03).
Table 1.
Average anthropometric values for men and women
| Men | Women | |
|---|---|---|
| Age, yr | 24.4 ± 1.4 | 24.0 ± 1.2 |
| Height, cm | 176.0 ± 1.5 | 166.4 ± 1.8* |
| Weight, kg | 75.6 ± 4.0 | 67.0 ± 2.6* |
| BMI, kg/m2 | 24.4 ± 0.73 | 24.1 ± 0.83 |
| Race | 9 C, 2 AA, 3 AS | 9 C, 2 AA, 3 AS |
| Estrogen, pg/ml | 224.1 ± 30.2 | |
| Progesterone, ng/ml | 1.1 ± 0.39 | |
| FSH, mIU/ml | 5.2 ± 0.44 | |
| LH, mIU/ml | 7.5 ± 1.7 |
Values are means ± SE. Average hormone levels for women are also shown. BMI, body mass index; C, Caucasian; AA, African-American; AS, Asian; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Significantly different from men; P ≤ 0.05.
Fig. 2.
Average change in ventilation divided by change in PetO2 (ΔV̇e/ΔPetO2) measured from the men and women for the initial and final hypoxic episode. Note that ΔV̇e/ΔPetO2 for the final episode was less than the value measured for the initial episode. †Significantly different from the initial hypoxic episode.
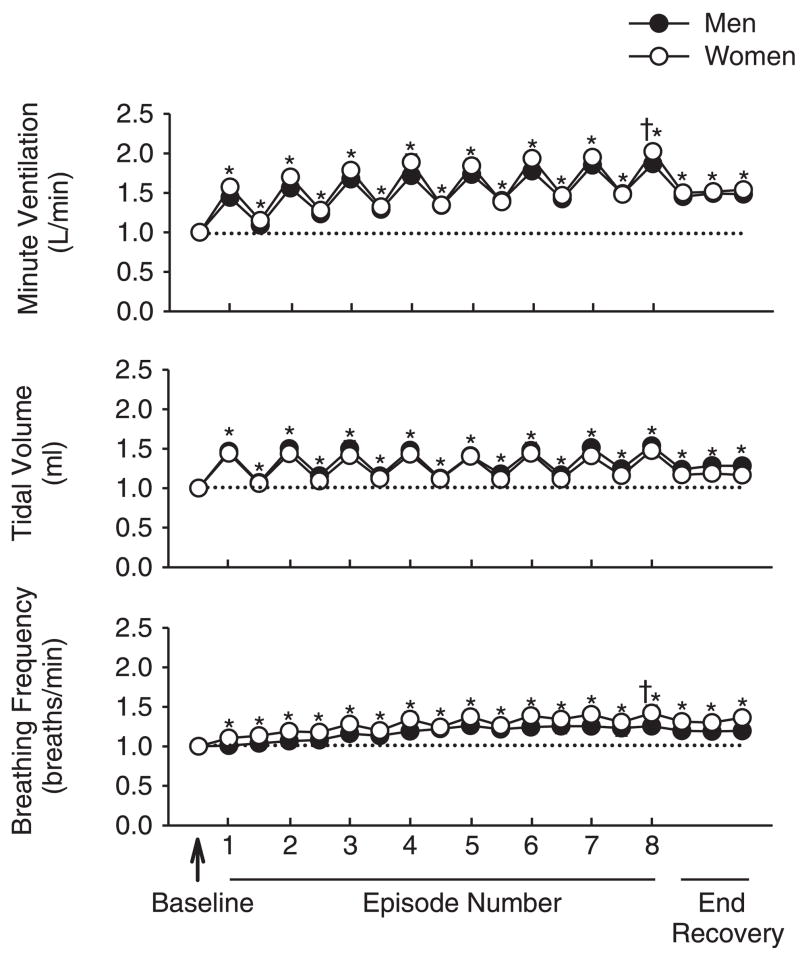
Although the change in minute ventilation both during and after exposure to intermittent hypoxia followed a similar pattern within the men and women (Fig. 1), minute ventilation overall was greater in the men compared with the women for each hypoxic episode and recovery period (P < 0.05). This difference was eliminated once the data were standardized to baseline values (Fig. 3). Thus the enhancement of minute ventilation and breathing frequency during the last episode compared with the initial hypoxic episode (P ≤ 0.001) was similar between groups (Fig. 3). Moreover, the increase in minute ventilation (P < 0.01), tidal volume (P < 0.02), and breathing frequency (P < 0.02) during recovery compared with baseline was similar between groups (Fig. 3).
Fig. 3.
Average values for minute ventilation, tidal volume, and breathing frequency standardized relative to baseline. The data were obtained from the last 10 min of baseline, the last 2 min of each episode of hypoxia (indicated by vertical tick mark on x-axis), the last 2 min of each normoxic period that separated the hypoxic episodes, and the initial, middle, and final 5 min of the 15-min poststimuli recovery period for men (●) and women (○). Note that the responses described for Fig. 1 are evident here. However, note that once the data were standardized relative to baseline, no differences exist between the male and female groups. *Significantly different from baseline; †significantly different from the initial hypoxic episode.
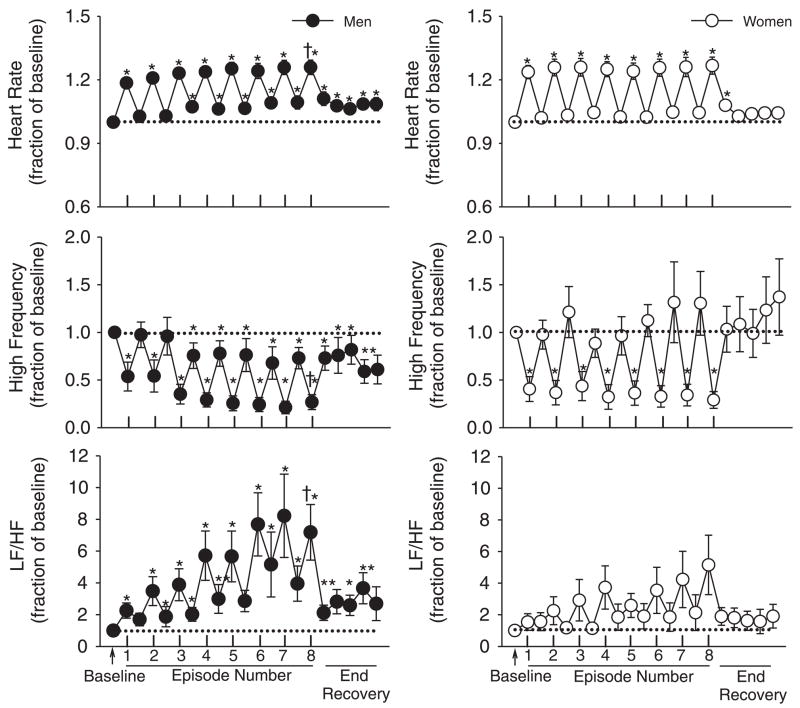
Heart rate, HF, and LF/HF measured from the men and women during the baseline period when carbon dioxide was elevated 4 Torr above room air values were not significantly different (heart rate, 68.3 ± 2.6 vs. 69.0 ± 2.7 beats/min, P = 0.80; HF, 4,598.5 ± 1,440.0 vs. 6,412.6 ± 1,720.5 ms2/Hz, P = 0.39; LF/HF, 0.7 ± 0.2 vs. 2.0 ± 1.2, P = 0.31). Figure 4 shows the heart rate, HF, and LF/HF measures obtained for each episode and recovery period standardized as a fraction of baseline measures. The results show that in the men heart rate increased during intermittent hypoxia compared with baseline (P < 0.001) and that a progressive increase occurred from the initial episode to the final episode (P < 0.01) (Fig. 4, top, left). The increase in heart rate that was observed during intermittent hypoxia persisted into the recovery periods. Consequently, heart rate during the final recovery period was increased compared with baseline (P < 0.03). The progressive increase in heart rate was accompanied by a decline in HF (P < 0.02) (Fig. 4, middle, left), and by an increase in LF/HF (P < 0.03) from the initial hypoxic episode to the final hypoxic episode (Fig. 4, bottom, left). Moreover, during the recovery periods that followed the hypoxic episodes HF, remained reduced and LF/HF remained increased compared with baseline. Heart rate in the women also increased during the hypoxic episodes compared with baseline (P < 0.001) (Fig. 4, top, right). However, measures obtained during the initial and last hypoxic episodes were similar. The increase in heart rate was accompanied by a decrease in HF activity (P < 0.01), which was similar during the initial and final hypoxic episodes (Fig. 4, middle, right). In contrast, no statistically significant increase in LF/HF during hypoxia was evident in the women, although a trend toward an increase was evident during the latter hypoxic episodes (Fig. 4, bottom, right). Moreover, in contrast to the men, during the recovery periods HF and LF/HF were similar compared with baseline.
Fig. 4.
Average values for heart rate, high-frequency (HF) measures of heart rate variability, and low-frequency (LF)/HF ratio standardized relative to baseline for men (●) and women (○). The data were obtained from the last 9 min (3 3-min intervals) of baseline, the last 3 min of each episode of hypoxia (indicated by vertical tick mark on x axis), the last 3 min of each normoxic period that separated the hypoxic episodes, and from 5 3-min intervals during the poststimuli recovery period. Note that heart rate was elevated during recovery compared with baseline in the men and that this increase was accompanied by a decrease in HF measures of heart rate variability and increases in the LF/HF ratio relative to baseline in men. In contrast, note that heart rate, high frequency measures of heart rate variability, and the LF/HF ratio during recovery were not significantly different compared with baseline in women. *Significantly different from baseline; †significantly different from the initial hypoxic episode.
DISCUSSION
The primary findings of this study are that LTF of minute ventilation occurs in young healthy men and women during wakefulness and that the magnitude of this phenomenon is independent of sex. Moreover, we found that along with changes in minute ventilation, S/V balance was enhanced and PNSA depressed following exposure to intermittent hypoxia in men but not in women.
Methodological issues
The advantage of utilizing heart rate variability to monitor changes in autonomic nervous system activity is that continuous measures of PNSA and S/V balance can be obtained for prolonged periods of time. Moreover, the technique is noninvasive, so in contrast to more invasive techniques it did not impact on the respiratory and cardiovascular parameters that we measured throughout and following exposure to intermittent hypoxia.
HF measures obtained from the power spectrum derived from R-R interval variability are quantitative reflections of respiratory sinus arrhythmia modulated by the vagus nerve. Thus the HF amplitude is thought to reflect cardiac PNSA to the heart, and this assumption has been confirmed by numerous studies (47b). However, vagal modulation of the heart period can be affected by respiratory parameters (7). The magnitude of the respiratory sinus arrhythmia is known to decrease with reductions in tidal volume and increases in respiratory rate. In our study the decrease in HF measures, relative to baseline, that we observed occurred in the presence of either an unchanging tidal volume or an increase in tidal volume. Thus changes in tidal volume were not responsible for the decrease in HF that we observed. Conversely, because breathing frequency gradually increased throughout the intermittent hypoxia protocol, it is possible that it impacted our measures of HF. However, because the increase in breathing frequency was similar in both the men and women, a similar depression in HF activity would be expected in both sexes following exposure to intermittent hypoxia. This was not the case. Despite similar increases in breathing frequency following exposure to intermittent hypoxia in both men and women, the depression of HF measures was only evident in the men and not the women. Moreover, no significant correlation was found to exist when the HF measures recorded during the end-recovery period were correlated to breathing frequency measures.
Respiratory and autonomic responses during exposure to intermittent hypoxia
Our results showed that minute ventilation progressively increased from the first to the last hypoxic episode in both men and women, which is in agreement with our previous finding (21). The amplitude of the progressive increase was similar between sexes and was due principally to a progressive increase in the breathing frequency response to hypoxia. This is in contrast to the tidal volume response to hypoxia, which was similar during the first and last hypoxic episode. The gradual increase in minute ventilation from the first to the last hypoxic episode likely manifested itself because carbon dioxide was sustained slightly above baseline levels (i.e., 4 Torr) since we observed previously in humans that this phenomenon was not evident when carbon dioxide was sustained at or below baseline values (21, 26, 35, 43).
Given that the absolute values in minute ventilation gradually increased from the first to the last hypoxic episode, a similar increase in the ΔV̇e/ΔPetO2 might be expected. However, this was not the case principally because the change in minute ventilation from baseline immediately preceding each hypoxic episode gradually decreased. Our observations are similar to previous findings in cats and ducks (17, 41). These studies reported that gradual increases in internal intercostal nerve activity or tidal volume were observed from the initial to the final episode of carotid sinus nerve stimulation or hypoxia. However, the change in internal intercostal nerve activity or tidal volume relative to the preceding baseline period either remained constant or decreased.
The ΔV̇e/ΔPetO2 is considered by many investigators to be indicative of peripheral chemoreflex sensitivity (8, 9, 44). Accordingly, our findings might suggest that chemoreflex sensitivity was decreasing from the initial to the final hypoxic episode. Then again, there are no published data to support that this event occurs during brief exposures to intermittent hypoxia. The decrease in ΔV̇e/ΔPetO2 we observed might also have occurred because the peripheral chemoreflex threshold gradually decreased from the first to the last hypoxic episode. Although increases in the peripheral chemoreflex threshold have not been documented during exposure to intermittent hypoxia, increases have been observed during exposure to short periods of sustained hypoxia (31). Nonetheless, we believe that it is unlikely that decreases in peripheral chemoreflex sensitivity and/or increases in the chemoreflex threshold are responsible for the decreases in ΔV̇e/ΔPetO2 observed. We and others (1, 16, 19, 28, 35) have shown that peripheral chemoreflex sensitivity is increased and the threshold is decreased or remains unchanged following exposure to acute or chronic intermittent hypoxia. Instead, we speculate that the neural mechanism potentially responsible for both the gradual increase in minute ventilation during the baseline and hypoxic periods (i.e., see LTF of minute ventilation) was gradually approaching a point of saturation. This possibility is supported by work completed in cats, which has shown that the neural mechanism responsible for either short-term potentiation or LTF of phrenic nerve activity appears to saturate completely at a level of inspiratory activity that is lower than maximal phrenic nerve activity (15, 17).
In addition to minute ventilation, heart rate gradually increased from the initial episode to the final episode in men. This progressive increase has also been observed previously in men exposed to intermittent hypoxia (16). In contrast, a similar increase in heart rate occurred during the initial and final episodes in the women. Although the pattern of the heart rate response from the initial to final hypoxic episode varied between sexes, both groups ultimately achieved a similar heart rate during the final hypoxic period. In conjunction with the increase in heart rate during hypoxia, HF measures of heart rate variability, which we assumed were indicative of PNSA, were depressed during exposure to intermittent hypoxia. The degree of depression from baseline was similar in the men and women by the final episode of hypoxia. However, a similar level of depression was achieved in each episode for the women, whereas, in contrast, depression of HF measures from the initial to the final episode occurred in a more progressive fashion in the men. Moreover, a progressive increase in S/V balance was observed in the men so that the S/V balance during the last hypoxic episode was greater than the initial hypoxic episode. A similar trend was observed in some women; however, overall S/V balance was not different during the hypoxic episodes and baseline.
LTF of minute ventilation
A gradual manifestation of LTF of minute ventilation or phrenic nerve activity occurs during normoxic recovery periods that separate episodes of hypoxia or carotid sinus nerve stimulation in a variety of species (3, 10, 41, 48, 49). The results from the present study have also demonstrated that a gradual increase in minute ventilation occurs from the initial to the final recovery period in healthy young men and women. Our finding in men replicates our results from a previous study (21), whereas the finding in women establishes that LTF, initially reported in a small number of women (n = 4) (21), can be evoked consistently in larger numbers. However, in contrast to animal studies, LTF following exposure to intermittent hypoxia may only be evident in humans during wakefulness if carbon dioxide is sustained slightly above baseline levels during and following exposure to intermittent hypoxia (21). We showed that LTF was clearly evident when carbon dioxide was sustained slightly above baseline levels during and following exposure to intermittent hypoxia. In contrast, we and others (14, 24, 35, 36, 43) have shown that LTF of minute ventilation was absent in humans during wakefulness when carbon dioxide was sustained at hypocapnic or normocapnic levels.
Given that sustained levels of carbon dioxide are required for the manifestation of LTF, it may be argued that this stimulus rather than intermittent hypoxia was primarily responsible for the LTF of minute ventilation we observed. However, support in the literature for this possibility is equivocal (20, 25, 46). In previous studies that examined the ventilatory response to sustained hypercapnia, a gradual increase in ventilation was not universally observed in all subjects, leading to nonsignificant findings (20, 25). More importantly, if any increase in ventilation was observed beyond 15 min of exposure to sustained hypercapnia (i.e., the length of the baseline period in the present investigation) at the level employed in our study (i.e., 4 Torr above baseline), the increases were significantly less [e.g., less than 11% above baseline levels (20, 46)] than the elevated levels in minute ventilation that we observed during the end-recovery period in the present study (47–53% greater than baseline). More convincingly, in a previous investigation we showed that exposure to sustained hypercapnia in the absence of intermittent hypoxia did not lead to significant increases in ventilation relative to baseline (21). Conversely, using a protocol similar to one employed in the present investigation (i.e., intermittent hypoxia in the presence of sustained hypercapnia), LTF of minute ventilation was clearly induced (21).
Besides the potential impact of carbon dioxide on the manifestation of LTF, studies completed in rats indicate that sex may impact the manifestation of LTF (5, 39). More specifically, the magnitude of LTF of phrenic nerve activity for a given age appears to be sex dependent. LTF of phrenic nerve activity is most prominent in young male rats and diminishes as they age (52), whereas LTF of phrenic nerve activity increases in female rats from youth to middle age (51). Collectively, results from these studies suggest that LTF of phrenic nerve activity is greater in young male rats compared with female rats and is less in middle-aged male rats compared with female rats (51, 52). The possibility that sex might have an impact on the manifestation of LTF indicates that sex hormones might have a key role in the manifestation of LTF. This potential link between sex hormones and the manifestation of LTF is supported by studies that have shown that gonadectomy attenuates LTF in rats and that the restoration of testosterone levels restores LTF (53). Additionally, the amplitude of LTF is different in the diestrous phase compared with the estrous in both young and middle-aged female rats (51).
Given the findings in rats, we hypothesized that LTF would be greater in young men compared with women matched for age, race, and body mass index. However, in contrast to our hypothesis, our results showed that the magnitude of LTF was similar in men and women once the data were standardized to baseline values. Besides species differences, there are a number of potential reasons why our results are dissimilar to those previously found in animals. It is possible that minute ventilation needed to be recorded for a longer period of time following the last hypoxic episode to disseminate sex differences in LTF of minute ventilation. Previous studies have shown that LTF may persist for up to 60 min and may gradually increase within that time period (see Fig. 1 in Ref. 39). If the rate at which LTF gradually increases within the 60-min time frame varies between sexes, this difference could become clearer if minute ventilation was recorded for a longer period of time. Moreover, our studies were completed during wakefulness, whereas animal studies that have investigated sex differences were completed under conditions of anesthesia. Anesthesia could expose different capabilities within the respiratory control system of male and female rats to respond to intermittent hypoxia (see “LTF in different experimental preparations” in Ref. 39 and “Intermittent hypoxia causes respiratory LTF ” in Ref. 32 for additional discussion). Lastly, although we did not observe sex differences in LTF of minute ventilation, it is possible that the magnitude of LTF of genioglossus muscle activity is sex dependent since variations in the expression of LTF have been shown to exist between different groups of motoneurons (see “Long-term facilitation is also expressed in upper airway muscle activity” in Ref. 32 and “Hypoglossal and phrenic motor output may be differentially influenced by neuromodulators” in Ref. 6 for additional discussion).
Heart rate variability following exposure to intermittent hypoxia
In addition to LTF of minute ventilation, recent animal (13) and human studies (30) have indicated that LTF of SNSA can also be activated concurrently following exposure to intermittent hypoxia. Our results support these findings in that both minute ventilation and heart rate gradually increased during the recovery periods that interspersed the hypoxia episodes and remained elevated during the final recovery period that followed the last hypoxic episode. The increase in heart rate was accompanied by a suppression of HF activity and an elevation in LF/HF during the final recovery period. However, the heart rate and autonomic responses were only clearly evident in the men. On the basis of our assumptions (see Data analysis for assumptions), our results indicate that suppression of PNSA and elevations in S/V balance may be observed typically in men following exposure to intermittent hypoxia.
There is only one other study (16) to our knowledge that has investigated heart rate variability alterations in men using a paradigm that was similar to our intermittent hypoxia protocol. Foster and colleagues (16) investigated whether exposure to six 5-min episodes of hypoxia, separated by 5 min of normoxia, for 1 h over a 12-day period impacted heart rate variability. The authors reported that heart rate variability remained unaltered after 12 days of exposure. However, it was difficult to determine whether measures of HF and LF/HF were altered on a given day because the heart rate variability data were not presented (16). Other studies have also investigated autonomic nervous system responses following exposure to hypoxia, but these studies differed from our investigation either because the pattern and duration of exposure to hypoxia were dissimilar (i.e., exposure to intermittent hypoxia or continuous hypoxia over a longer time duration) and/or a different technique was used to measure autonomic nervous system activity (i.e., recordings from the peroneal nerve). Nonetheless, a suppression of HF and an increase in S/V balance has been observed in cats in response to long-term exposure to intermittent hypoxia (45). Likewise, the findings of Sevre and colleagues (47a) indicate that sustained increases in normalized LF and sustained suppression of normalized HF may occur in humans following exposure to chronic continuous hypoxia (see Fig. 3 of Ref. 47a). Moreover, an increase in SNSA derived from peroneal nerve recordings has been reported following exposure to acute intermittent hypoxia (12, 29).
In contrast to the findings in the men, our results showed that heart rate in the women returned to resting levels during each recovery period. The lack of a sustained increase in heart rate was accompanied by HF and LF/HF measures during recovery that were similar to baseline. To our knowledge, no other studies have systematically examined whether autonomic responses following intermittent hypoxia are sex dependent. In two studies that did compare autonomic responses in men and women following exposure to hypoxia, the number of men was greater than the number of women so that close matching for a variety of anthropometric factors (i.e., age, race, and body mass index) did not occur (23, 29). Moreover, the potential impact of the menstrual cycle on autonomic responses following hypoxia was either not considered (29) or could not be adequately determined because of sample size (23). The studies also differed from our investigation because SNSA was determined by obtaining recordings from the peroneal nerve (23, 29). Likewise the pattern and duration of the hypoxic stimulus differed from that employed in the present study, with one investigation exposing participants to one bout of 10% hypoxia for 15 min while carbon dioxide was sustained at baseline levels (23) and the other investigation subjecting participants to 20-s apneas per minute for 30 min while carbon dioxide levels were allowed to decrease below baseline values during recovery (29). Thus similarities or differences in our findings with previous results must be interpreted with caution. Nonetheless, one study reported that muscle SNSA after repetitive hypoxia was elevated in both healthy men and women and that the response was similar (29). In contrast to this finding, and in agreement with our results, Jones and colleagues (23) reported that following exposure to sustained hypoxia SNSA decreased close to baseline levels in women but remained elevated in men during the recovery period.
Physiological significance
It has been hypothesized that repetitive episodes of hypoxemia that occur in conjunction with apneic events in individuals with sleep apnea may lead to the initiation of LTF of minute ventilation. If this occurs, then our results suggest that the expression of LTF may be similar in young men and women. Our results also indicate that LTF of minute ventilation may be accompanied by sustained depression in PNSA and elevation in S/V balance following exposure to acute intermittent hypoxia. These acute responses to intermittent hypoxia indicate that chronic exposure could result in a more pronounced depression in PNSA and a more pronounced elevation in SNSA. The consequence of these alterations in autonomic nervous system activity could be sustained increases in blood pressure and reduced opposition to sympathetic stimulation of the heart in individuals with sleep apnea. This suggestion is supported by evidence that has shown that individuals diagnosed with sleep apnea have a greater level of SNSA compared with healthy individuals (11). Moreover, the elimination of sleep apnea with continuous positive airway pressure results in a decrease in sympathetic tone in individuals with sleep apnea. It is interesting to note, however, that the depression of PNSA and the increase in S/V balance were observed principally in the men following exposure to intermittent hypoxia and not women in our investigation. If these findings ultimately extend to individuals with sleep apnea, then it is possible that alterations in autonomic nervous system activity may not be as great in women compared with men with sleep apnea exposed to a similar intensity of hypoxemia.
Acknowledgments
GRANTS
This work was supported by a Department of Veterans Affairs Merit Award and National Heart, Lung, and Blood Institute Grant R01-HL-085537.
References
- 1.Ainslie PN, Kolb JC, Ide K, Poulin MJ. Effects of five nights of normobaric hypoxia on the ventilatory responses to acute hypoxia and hypercapnia. Respir Physiol Neurobiol. 2003;138:193–204. doi: 10.1016/s1569-9048(03)00190-3. [DOI] [PubMed] [Google Scholar]
- 2.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 3.Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol. 2002;131:65–77. doi: 10.1016/s1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 6.Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 7.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623– 648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 8.Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey J, Pack A, editors. Regulation of Breathing. New York: Dekker; 1995. pp. 617–668. [Google Scholar]
- 9.Bisgard GE, Busch MA, Forster HV. Ventilatory acclimatization to hypoxia is not dependent on cerebral hypocapnic alkalosis. J Appl Physiol. 1986;60:1011–1015. doi: 10.1152/jappl.1986.60.3.1011. [DOI] [PubMed] [Google Scholar]
- 10.Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- 11.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 12.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol. 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- 13.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 14.Diep TT, Khan TR, Zhang R, Duffin J. Long-term facilitation of breathing is absent after episodes of hypercapnic hypoxia in awake humans. Respir Physiol Neurobiol. 2007;156:132–136. doi: 10.1016/j.resp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Eldridge FL, Millhorn DE, Waldrop TG. Input-output relationships of the central respiratory controller during peripheral muscle stimulation in cats. J Physiol. 1982;324:285–295. doi: 10.1113/jphysiol.1982.sp014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster GE, McKenzie DC, Milsom WK, Sheel AW. Effects of two protocols of intermittent hypoxia on human ventilatory, cardiovascular and cerebral responses to hypoxia. J Physiol. 2005;567:689– 699. doi: 10.1113/jphysiol.2005.091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477:469– 479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol. 2005;98:1761–1767. doi: 10.1152/japplphysiol.01142.2004. [DOI] [PubMed] [Google Scholar]
- 19.Garcia N, Hopkins SR, Powell FL. Effects of intermittent hypoxia on the isocapnic hypoxic ventilatory response and erythropoiesis in humans. Respir Physiol. 2000;123:39– 49. doi: 10.1016/s0034-5687(00)00145-6. [DOI] [PubMed] [Google Scholar]
- 20.Georgopoulos D, Berezanski D, Anthonisen NR. Effects of CO2 breathing on ventilatory response to sustained hypoxia in normal adults. J Appl Physiol. 1989;66:1071–1078. doi: 10.1152/jappl.1989.66.3.1071. [DOI] [PubMed] [Google Scholar]
- 21.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–R1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- 23.Jones PP, Davy KP, Seals DR. Influence of gender on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. Clin Physiol. 1999;19:153–160. doi: 10.1046/j.1365-2281.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 24.Jordan AS, Catcheside PG, O’Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- 25.Khamnei S, Robbins PA. Hypoxic depression of ventilation in humans: alternative models for the chemoreflexes. Respir Physiol. 1990;81:117–134. doi: 10.1016/0034-5687(90)90074-9. [DOI] [PubMed] [Google Scholar]
- 26.Khodadadeh B, Badr MS, Mateika JH. The ventilatory response to carbon dioxide and sustained hypoxia is enhanced after episodic hypoxia in OSA patients. Respir Physiol Neurobiol. 2006;150:122–134. doi: 10.1016/j.resp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- 28.Koehle MS, Sheel AW, Milsom WK, McKenzie DC. Two patterns of daily hypoxic exposure and their effects on measures of chemosensitivity in humans. J Appl Physiol. 2007;103:1973–1978. doi: 10.1152/japplphysiol.00545.2007. [DOI] [PubMed] [Google Scholar]
- 29.Leuenberger UA, Hogeman CS, Quraishi S, Linton-Frazier L, Gray KS. Short-term intermittent hypoxia enhances sympathetic responses to continuous hypoxia in humans. J Appl Physiol. 2007;103:835– 842. doi: 10.1152/japplphysiol.00036.2007. [DOI] [PubMed] [Google Scholar]
- 30.Lusina SJ, Kennedy PM, Inglis JT, McKenzie DC, Ayas NT, Sheel AW. Long-term intermittent hypoxia increases sympathetic activity and chemosensitivity during acute hypoxia in humans. J Physiol. 2006;575:961–970. doi: 10.1113/jphysiol.2006.114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahamed S, Duffin J. Repeated hypoxic exposures change respiratory chemoreflex control in humans. J Physiol. 2001;534:595– 603. doi: 10.1111/j.1469-7793.2001.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- 33.Malfatto G, Facchini M, Sala L, Branzi G, Bragato R, Leonetti G. Effects of cardiac rehabilitation and beta-blocker therapy on heart rate variability after first acute myocardial infarction. Am J Cardiol. 1998;81:834– 840. doi: 10.1016/s0002-9149(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 34.Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol. 1997;82:419– 425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- 35.Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J Appl Physiol. 2004;96:1197–1205. doi: 10.1152/japplphysiol.00573.2003. [DOI] [PubMed] [Google Scholar]
- 36.McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol. 1996;81:866– 875. doi: 10.1152/jappl.1996.81.2.866. [DOI] [PubMed] [Google Scholar]
- 37.McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol. 2001;124:117–128. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- 42.Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–1831. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- 43.Morelli C, Badr MS, Mateika JH. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J Appl Physiol. 2004;97:1673–1680. doi: 10.1152/japplphysiol.00541.2004. [DOI] [PubMed] [Google Scholar]
- 44.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 45.Rey S, Del RR, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds WJ, Milhorn HT, Jr, Holloman GH., Jr Transient ventilatory response to graded hypercapnia in man. J Appl Physiol. 1972;33:47–54. doi: 10.1152/jappl.1972.33.1.47. [DOI] [PubMed] [Google Scholar]
- 47a.Sevre K, Bendz B, Hanko E, Nakstad AR, Hauge A, Kasin JI, Lefrandt JD, Smit AJ, Eide I, Rostrup M. Reduced autonomic activity during stepwise exposure to high altitude. Acta Physiol Scand. 2001;173:409– 417. doi: 10.1046/j.1365-201X.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 47b.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 48.Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed both during sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- 49.Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol. 1997;499:543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. 2001;91:1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- 51.Zabka AG, Behan M, Mitchell GS. Selected contribution: time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- 52.Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001;531:509–514. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zabka AG, Mitchell GS, Behan M. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol. 2006;576:903–912. doi: 10.1113/jphysiol.2006.114850. [DOI] [PMC free article] [PubMed] [Google Scholar]