Abstract
BACKGROUND
The role of the immune system in the pathogenesis of endometriosis remains elusive. It has been shown that patients have an altered peritoneal environment with increased levels of inflammatory cytokines, activated macrophages and reduced clearance of retrogradely transported endometrial fragments. However, it is not known if this unique inflammatory situation is cause or consequence of endometriosis. This study investigates the impact of a pre-existing peritoneal inflammation on endometriosis establishment in a mouse model.
METHODS
Endometriosis was induced by intraperitoneal injection of enhanced green fluorescent protein (EGFP)-expressing endometrium in mice. In parallel, a peritonitis model was established via intraperitoneal injection of thioglycolate medium (TM). Finally, endometriosis was induced in the inflamed peritoneal cavity and lesion establishment as well as morphological and histological characteristics were analysed.
RESULTS
Induction of endometriosis in an inflamed peritoneal cavity resulted in fewer lesions and significantly lower sum of lesion surface area per mouse in the TM-treated group. Additionally, a higher amount of non-attached debris could be detected in the peritoneal cavity of TM-treated mice.
CONCLUSIONS
An intraperitoneal inflammation decreases endometriosis establishment in this mouse model. Thus, a pre-existing peritoneal inflammation might not be a factor favouring the development of endometriosis.
Keywords: EGFP, endometriosis, immune system, inflammation, mouse model
Introduction
Endometriosis is a common benign gynaecological disorder with around 10–22% of all women in reproductive age being affected (Wheeler, 1989; Moen and Muus, 1991). The disease is characterized by the presence of endometrial tissue outside the uterine cavity. Endometriotic lesions are frequently found in the pelvis minor, e.g. on the ovaries, peritoneum, pouch of Douglas and rectovaginal septum. Common symptoms are severe pelvic pain, dysmenorrhoea and dyspareunia as well as sub- and infertility (Mahutte and Arici, 2002; D’Hooghe et al., 2003).
The aetiology of endometriosis is not fully understood yet. According to the widely accepted theory by Sampson, lesion development is caused by retrograde menstruation (Sampson, 1927). But it has been shown that despite retrograde menstruation occurring in around 80% of all menstruating women (Halme et al., 1984), only ∼10% of them develop endometriosis. Thus, it remains to be elucidated which mechanisms are responsible for adhesion and invasion of the shed fragments.
Over the last years, the potential role of the immune system in the pathophysiology of endometriosis has increasingly gained attention and intense research efforts have been made. Various studies have been conducted to show that peritoneal fluid (PF) and peritoneal immune cells of women with endometriosis differ from that of healthy women. Examination of the PF of patients revealed significantly increased amounts of cytokines like interleukin (IL)-6, IL-8 and the monocyte chemotactic protein-1, (MCP-1) (Pizzo et al., 2002; Kalu et al., 2007). MCP-1 is a chemo-attractant that recruits macrophages into the peritoneal cavity which are able to secret further pro-inflammatory cytokines like Regulated upon Activation, Normal T cell Expressed and Secreted (RANTES) (Lebovic et al., 2004), and angiogenic IL-8. Moreover, peritoneal macrophages (PMs) are increased in the PF of patients. Additionally, these macrophages produce higher levels of IL-1ß, IL-6, IL-8, IL-10 and tumour necrosis factor-alpha (TNF-α) under basal and stimulated conditions in vitro compared with PMs of healthy women (Rana et al., 1996; Montagna et al., 2007). PMs from women with endometriosis also stimulate eutopic and ectopic endometrial cell proliferation in vitro (Loh et al., 1999; Braun et al., 2002) and macrophage-conditioned medium acts as a growth factor on murine endometrial stromal cells (Olive et al., 1991). Macrophage secretory products, IL-8 and TNF-α, are known to enhance proliferation and adhesion of endometrial cells (Arici et al., 1998; Garcia-Velasco and Arici, 1999; Harada et al., 1999). Profiles of other immune cells are altered as well: natural killer cell cytotoxicity (Vinatier et al., 1996) and T-cell cytotoxicity is decreased (Harada et al., 2001) in peritoneal cells from endometriosis patients.
Taken together, these findings suggest that the immune system in the peritoneal environment of women with endometriosis is altered and seems to be highly activated. So far, it is not known whether this unique inflammatory situation is caused by endometriosis establishment or results from a pre-existing inflammation.
The present study addresses one aspect of this ‘chicken or egg’ question, analysing the influence of a pre-existing peritoneal inflammation on endometriosis establishment in a mouse model. In this novel approach, endometriosis was induced in an inflamed peritoneal cavity and the effect on lesion establishment was investigated.
Materials and Methods
Mice
Transgenic mice (C57BL/6-Tg(ACTB-EGFP)1Osb/J) ubiquitously expressing enhanced green fluorescent protein (EGFP) were obtained from Jackson Laboratories (Maine, USA) and were bred at the Bayer Schering Pharma AG on-site animal care facility. Mice were fed on mouse diet and water ad libitum and kept on a light/dark cycle of 12/12 h under controlled conditions. Female, heterozygous mice, 6–8 months old, were used as endometrium donors and 8–12 weeks old wild-type C57BL/6 mice (Charles River, Berlin, Germany) as recipient mice.
All procedures involving animals were performed in accordance with institutional, state and federal guidelines.
Induction of peritonitis
A non-septic non-chronic peritoneal inflammation was induced by injecting thioglycolate medium (TM) as described before (Argyris, 1967). Briefly, peritoneal exudate cells (PECs) were elicited by injecting 1 ml of 3% sterile aged TM Brewer modified (BD, Sparks, USA) into the peritoneal cavity. Three days later, mice were sacrificed and PECs were harvested by peritoneal lavage with 5 ml ice-cold, sterile phosphate-buffered saline (PBS; PAA, Pasching, Austria). After centrifugation, cells were counted using a cell counter (CASY, Schaerfe System GmbH, Reutlingen, Germany). To exclude potentially contaminating erythrocytes, only cells ≥6 µm in diameter were counted. Finally, cells were frozen as 1 × 106 cells/100 µl in 10% dimethyl sulfoxide-containing fetal calf serum (FCS) (Gibco–Invitrogen, Karlsruhe, Germany) until further examination.
Fluorescence-activated cell sorting
Cells were thawed on ice, washed twice with PBS, resuspended in fluorescence-activated cell sorting (FACS)-blocking buffer (1% bovine serum albumin in PBS containing anti-CD16/CD32 antibody, Pharmingen/BD Bioscience, San Diego, USA) and incubated for 15 min at 4°C. Subsequently, cells were labelled with the following anti-mouse antibodies: anti-CD11b-FITC, anti-F4/80-PE, anti-GR1-APC and anti-NK1.1-PE (all BD Bioscience) for 20 min at 4°C. Appropriate isotype antibodies were used as negative control in all cases. To exclude dead cells, aggregates and debris, cells were gated using the forward–sideward scatter plot. The data were analysed using the software CellQuest Pro (BD Bioscience).
In vitro lipopolysaccharide stimulation and cytokine enzyme-linked immunosorbent assay
Peritoneal cells from six TM-stimulated and six untreated control mice (5 × 105 cells in 100 µl per well) were cultured in a 96-well-plate (Corning, USA) using RPMI medium (10% FCS and 1% penicillin/streptavidin; PAA) for 12 h. Subsequently, plates were washed with PBS and adhering macrophages were stimulated with 0.1–1000 ng/ml lipopolysaccharide (LPS) (Sigma–Aldrich, Munich, Germany) for 20 h. Following incubation, supernatants were collected and IL-12 and TNF-α levels were measured by enzyme-linked immunosorbent assay (ELISA) using maxisorp plates (Nunc, Wiesbaden, Germany) and ELISA sets (ready-set-go! reagent, eBioscience, San Diego, USA).
A standard curve was established using serial dilutions of purified IL-12 starting at a concentration of 500 pg/ml (1000 pg/ml for TNF-α). The IL-12 and TNF-α ELISAs were sensitive down to 2 and 8 pg/ml, respectively.
Induction of endometriosis
Induction of endometriosis was performed according to a method reported by Hirata et al. (2005) with slight modifications. In contrast to their work, no estrogen supplementation was given, since it has been shown that estrogens have an influence on the immune system (Straub, 2007). To avoid any impact of estrogens, intact donor mice were used and recipient mice were not supplemented with estrogen. Briefly, EGFP-transgenic mice in estrus state were anaesthetized with CO2 and killed by cervical dislocation. The uterus horns were removed and stored in 37°C warm Dulbecco’s modified eagle medium (DMEM w/o phenol red, Gibco–Invitrogen). Endometrium was peeled off with tweezers and chopped into 400 × 400 µm wide pieces using a tissue chopper (McIwain tissue chopper, Agar Scientific, Essex, UK). The endometrium fragments of two uteri were pooled and every recipient mouse received 40 mg tissue in 400 µl warm DMEM. The suspended tissue was injected into the peritoneal cavity through the abdominal wall with a 20-gauge needle on the midline just below the umbilicus. During this procedure, recipient mice were anaesthetized with dimethyl ether. Ten days after the injection of endometrium fragments, the mice were sacrificed.
Fluorescence imaging of EGFP-expressing tissues
Ten days after the injection of the endometrial fragments, mice were euthanized with an overdose of dimethyl ether. Autopsy was performed by a longitudinal midline incision. A stereo-microscope (Stemi SV6, Carl Zeiss AG, Göttingen, Germany) with a fluorescence device and a camera (AxioCam HRm, Zeiss) was used to illuminate the peritoneal cavity. EGFP-expressing lesions and non-attached tissue fragments were removed and snap-frozen in optimal cutting temperature compound Tissue-Tek (Sakura Finetek, Zoeterwoude, The Netherlands) in liquid nitrogen or fixed with 4% paraformaldehyde (PFA) in PBS for 24 h.
Induction of endometriosis in inflamed peritoneal cavity
First, peritonitis was induced as described above in wild-type C57BL/6 mice (n = 20). The control animals (n = 20) were not treated with TM. Three days later, TM-treated and control mice were injected each with 40 mg of EGFP-expressing endometrium fragments under anaesthesia as described above. Ten days after injection, mice were sacrificed and the peritoneal cavity was examined under fluorescent light. Lesions were excised, photographed and measured using the software Axiovision. Non-attached debris was taken out and counted. Finally, lesions and debris were snap frozen or fixed in PFA for further analysis.
Histological and immunohistochemical examinations
Frozen tissues were cut at 10 µm on a cryostat. PFA-fixed lesions were embedded in paraffin and cut at 5 µm thickness. For gross examination, sections were stained with haematoxylin–eosin. Weigert-Elastica-van Gieson (W-E-vG) staining was performed using resorcin–fuchsin red (Merck, Darmstadt, Germany), iron haematoxylin (Merck) and picrin acid (Fluka Biochemika, Buchs, Switzerland) combined with thiazin red (Chroma, Muenster, Germany) leading to black–brown coloured nuclei, black coloured elastic fibres, red coloured collagen connective tissue and yellow coloured muscle tissue.
Immunohistochemical examinations were carried out using either the DAKO EnVision protocol for rabbit antibodies (Carpinteria, USA), a Mouse-to-Mouse detection kit protocol for mouse monoclonal antibodies (Chemicon-Millipore, Temecula, USA) or the ABC streptavidin–biotin method. Binding of primary antibody was carried out overnight at 4°C using the following antibodies: monoclonal rat anti-mouse Ki67 (DAKO), monoclonal mouse anti-mouse cytokeratin (CK), multi Ab-1 (clone C11, Dianova, Hamburg, Germany), polyclonal rabbit anti-mouse estrogen receptor alpha, (clone MC-20, Santa Cruz Biotechnology, Santa Cruz, USA), polyclonal rabbit anti-mouse smooth muscle actin (Spring Bioscience, Fremont, USA). Negative controls were only incubated with antibody diluent (DAKO). Counterstaining was performed with haematoxylin (Merck). The results were analysed and documented with the microscope AxioPlan 2 and an AxioCam camera (Zeiss) using the software AxioVision Rel. 4.5.
Statistical analysis
The peritoneal cell counts of all animals were averaged and are presented as mean ± standard deviation (SD). Statistical analysis of cell counts was performed with Mann–Whitney rank sum test by using SigmaStat 3.0 (Systat, San Jose, USA).
The lesions were measured and the cross-sectional areas (CSAs) for every lesion were calculated according to the formula for an ovoid: diameter 1 × diameter 2 × π/4 (Becker et al., 2005; Efstathiou et al., 2005). All CSAs were taken for further analysis and only animals with lesions were included. Log-transformed values were used for the statistical comparisons of CSA between control and TM-treated animals. A general mixed linear model was fitted, using the treatment group as fixed effects and animal as random experimental unit. To model the covariance structure between the measurements of each animal, a compound symmetry structure was assumed. Different covariance structures were used between the treatment groups. The Kenward–Rogers approach was applied for the calculation of the denominator degrees of freedom for the F-tests in a two-sided test.
Additionally, the sum of the CSA of the lesions per mouse was used as a measure for the overall burden of the animal. In this analysis, all 20 animals in both groups were considered. To compare the overall burden, an exact two-sided Wilcoxon rank-sum test was used on the sum of the CSAs per animal. Animals without lesions were included with value zero.
The analyses were performed using PROC MIXED in SAS®, Version 9.1.3 (Heidelberg, Germany). Probability P < 0.05 was considered as statistically significant.
Results
Evaluation of thioglycolate-induced acute peritoneal inflammation
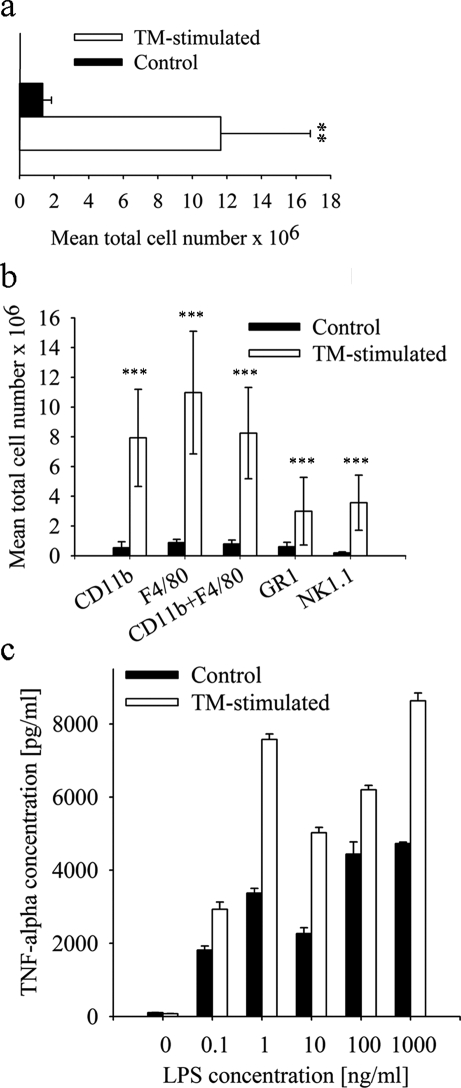
A well-known method to induce a non-chronic inflammatory response in the murine peritoneal cavity is the intraperitoneal injection of TM (Li et al., 1997). To characterize the elicited inflammatory response, PECs were harvested by peritoneal lavage 3–5 days after TM-injection. In response to TM treatment, an increase in peritoneal cell counts could be detected. Highest cell numbers were found on Day 3 showing a 9-fold increase within the PF of TM-treated mice compared with PBS-treated controls (11.6 ± 5.3 × 106 versus 1.3 ± 0.5 × 106; P < 0.01; Fig. 1a). Cell counts gradually declined thereafter on Day 4 and 5 (data not shown) indicating an acute, non-chronic inflammation.
Figure 1:
Differences in peritoneal cell number and profiles after TM-stimulation compared with control.
(a) Total cell amount after peritoneal lavage in control mice (n = 12; 1.3 ± 0.5 × 106) and TM-treated mice (n = 12; 11.6 ± 5.2 × 106) 3 days after TM-injection (**P < 0.01). (b) After analysing the PECs by FACS, higher total amounts of innate immune cells like neutrophils (CD11b; 7.9 ± 3.3 × 106 versus 0.5 ± 0.4 × 106), macrophages (CD11b and F4/80 positive; 8.3 ± 3.1 × 106 versus 0.8 ± 0.3 × 106), granulocytes (GR1; 3.0 ± 2.3 × 106 versus 0.6 ± 0.3 × 106) and natural killer cells (NK1.1; 3.6 ± 1.9 × 106 versus 0.2 ± 0.08 × 106) were detected in TM-treated mice (***P < 0.001). (c) A concentration-dependent increase of TNF-α was detected after LPS stimulation in the supernatant of pooled PM from TM-stimulated mice (n = 6) and resident macrophages from control mice (n = 6). All results are expressed as mean ± SD. Statistical analysis was performed using Mann–Whitney rank sum test.
FACS analysis of PEC revealed an increase in different innate immune cells in the TM-treated mice compared with controls. Numbers of macrophages (F4/80- and CD11b-positive) were significantly elevated in the treatment group (8.3 ± 3.1 × 106 versus 0.8 ± 0.3 × 106; P < 0.001, Fig. 1b) representing ∼70% of the total cell number, thus demonstrating that macrophages are the predominant cell type in the inflamed peritoneal cavity. In addition, populations of granulocytes (GR1-positive) and natural killer cells (NK1.1-positive) were significantly increased (Fig. 1b). PM of TM-stimulated and control mice were isolated from the PF and cultured in vitro to examine their cytokine secretion. Cytokine levels of TNF-α and IL-12 (data not shown) were increased in the TM-group compared with controls after 20 h LPS stimulation (Fig. 1c) although this was not statistically significant. In the PF, no inflammatory cytokines could be detected (data not shown).
Taken together, these findings demonstrate that i.p. injection of TM into mice leads to an acute non-septic peritonitis with increased levels of innate immune cells. Furthermore, the cytokine levels in vitro suggest a higher activity of macrophages after TM treatment, which thus resemble macrophages from endometriosis patients.
Induction of endometriosis
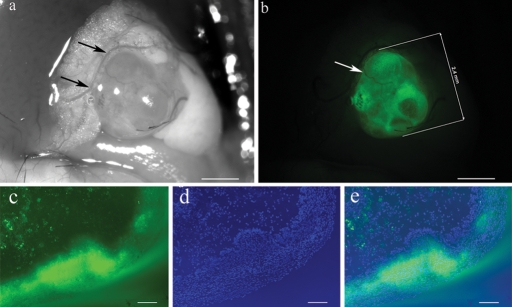
EGFP-expressing endometrium fragments were injected into the peritoneal cavity of C57BL/6 mice. On average, 95% of the animals developed around 2–3 endometriosis-like lesions. Macroscopically, lesions consisted of white–light yellow ellipsoid nodules that were 0.5–4 mm in diameter. They were formed on the omentum, peritoneum, pancreas and its surrounding fatty tissue. Lesions could also be detected behind the bifurcation of the uterus as well as embedded in the fatty tissue around the uterine horns (Fig. 2a). As exemplified, all lesions emitted green light under fluorescence light (488 nm, Fig. 2b).
Figure 2:
Macroscopic and histological appearance of an enhanced green fluorescent protein expressing lesion embedded in fat tissue 10 days after injection of endometrium fragments.
(a) Lesion under impinging light shows the embedding in blood supplied fat tissue. Supporting blood vessels are indicated by arrows. (b) Same lesion examined under fluorescent light. A supporting blood vessel is indicated by the arrow. (c–e) Picture of a frozen lesion section taken with 800 ms exposure time showing (c) EGFP-expressing cells under fluorescent light, (d) all nuclei (DAPI-staining) and (e) the merged image. Scale bar a–b = 1 mm, c–e = 100 µm. Original magnification c–e: ×100.
The frozen section in Fig. 2a–c illustrates the presence of EGFP-expressing cells under fluorescent light. Some parts of epithelium and stromal structures emit strong green fluorescence, whereas others display only weak or no fluorescence.
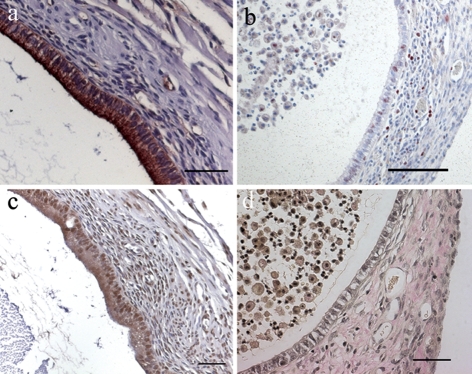
Typical histological characteristics of endometriotic lesions could be demonstrated by immunohistochemistry. Staining against specific CKs (CK8, CK18) confirms the presence of glandular epithelium (Fig. 3a). Proliferation marker Ki67 staining reveals cell proliferation within the epithelium and surrounding stromal cells. Moreover, proliferating cells can be seen in the content of the cystic lumen (Fig. 3b). Estrogen receptor alpha positive cells, displayed by brown nuclei, can be detected in the lesion epithelium as well as in stromal structures (Fig. 3c). Finally, the W-E-vG staining demonstrates the presence of collagen connective tissue which appears red after the trichrom staining (Fig. 3d).
Figure 3:
Histological and immunohistochemical examination of EGFP-expressing endometriotic lesions.
(a) Anti-CK staining shows glandular epithelial cells. (b) Proliferating cells positive for Ki67 in cyst epithelium, stroma and in cyst content display red stained nuclei. (c) Estrogen receptor alpha positive cells in epithelium and stroma are indicated by brown stained nuclei. (d) W-E-vG staining of an endometriotic lesion reveals collagen connective tissue in red and nuclei in black. Scale bar: a, c, d = 50 µm, b = 100 µm. Original magnification: a, c, d = ×200, b = ×100.
In conclusion, the injection of EGFP-expressing endometrium leads to development of endometriotic lesions, displaying typical characteristics of endometriosis as seen in histological analysis. Thus, this model is a valuable tool to examine endometriosis in vivo.
Induction of endometriosis in inflamed peritoneal cavity
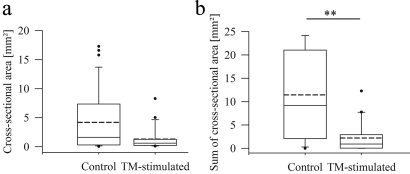
Following induction of peritonitis, endometrial tissue fragments were injected into the peritoneal cavity of TM-treated mice. Lesions formed under these conditions were examined after autopsy and compared with lesions from endometriosis control mice. On gross examination, fewer mice with lesions were found in the TM-treated group (Table I). Five of twenty mice (25%) developed no endometriosis compared with one of 20 (5%) in the control group. Furthermore, peritoneal cavities of TM-treated mice contained more non-attached debris than those of control animals. Ninety percent of TM-treated mice had non-attached tissue fragments in their peritoneal cavities compared with 40% in the control group (Table I), indicating a defect in adhesion processes, whether among the endometrial fragments and/or on the peritoneal surfaces. This finding is supported by a reduced average lesion number of 1.65 per mouse (75% take rate) in the TM-treated group and 2.04 in the control group (95% take rate). Moreover, lesions from TM-treated mice were smaller compared with controls (median CSA: 0.60 ± 1.86 versus 1.60 ± 5.99 mm2; Fig. 4a). The CSAs in the TM-treated group tended to be ∼48% of those in the control group, with a 95% confidence limit of [20.3%, 114%] (P = 0.0943). In this analysis, only mice with lesions were taken into account.
Table I.
Comparison of different parameters analysed for lesion establishment and appearance in control mice and TM-stimulated mice (n = 20 per group).
| Parameter | Control | TM-group | P-value |
|---|---|---|---|
| Mice with no lesions | 1 (5%) | 5 (25%) | |
| Mice with debris | 8 (40%) | 18 (90%) | |
| Mean lesion number per mouse | 2.40 ± 1.4 | 1.65 ± 1.4 | |
| Lesion number range | 0–6 | 0–4 | |
| Mean of CSA/mouse (mm2) | 4.86 ± 4.52 | 1.41 ± 1.63 | |
| Median of CSA (mm2) | 1.60 ± 5.99 | 0.60 ± 1.86 | |
| Geometric mean of CSA (mm2) | 1.33 ± 6.28 | 0.60 ± 3.48 | |
| Sum CSA of all lesions (mm2) | 217.43 | 44.18 | |
| Median of sum CSA (mm2) | 8.8 ± 9.93 | 0.92 ± 3.26 | 0.0013 |
Statistical analysis: F test (CSA), Wilcoxon rank-sum test (Sum CSA). CSAs, cross-sectional areas.
Figure 4:
CSA and sum of the CSAs of endometriotic lesions in controls compared with TM-stimulated mice shown in box plots.
(a) Ten days after injection of EGFP-expressing endometrium fragments in the inflamed peritoneal cavity, the CSA of lesions was determined in controls and TM-treated animals with lesions. Comparison displayed a tendency towards smaller CSA (P = 0.0943). (b) The sum of CSAs per mouse was compared between all controls and all TM-treated mice showing a significantly lower disease burden in treated animals (**P = 0.0013). The solid line indicates the median, the dashed line the mean. Statistical analysis was performed using a two-tailed F-test for CSA comparison and a two-tailed Wilcoxon rank-sum test for analysis of sum CSA.
Another examined parameter was the sum of the CSA of the lesions per mouse, used as a measure for the overall disease burden of the animal. Statistical analysis revealed that the TM-treated mice had a significantly reduced overall disease burden. The box plot (Fig. 4b) of the sum of the CSA per mouse shows the statistically significant difference between the two groups. The median overall burden in the TM-treated group was 0.92 mm2, which was significantly lower than the overall burden of 8.8 mm2 in the control group (P = 0.0013; n = 20 per group).
The histological examination of lesions was carried out by morphological identification following haematoxylin–eosin staining and immunohistochemistry. In both groups, the lesions displayed endometriotic characteristics like glandular epithelium and stromal cells. They had either a compact content (Fig. 5a) or a cyst-like appearance (Fig. 5b). The supporting blood vessels embedded in fat can be seen on Fig. 5a. Furthermore, haematoxylin–eosin staining revealed several eosinophile patches within the lesion, which could be identified as collagen connective tissue by W-E-vG staining (data not shown). Moreover, mineralization of tissue could be seen in TM-treated mice and controls in both lesion types (cystic and compact) indicating necrosis.
Figure 5:
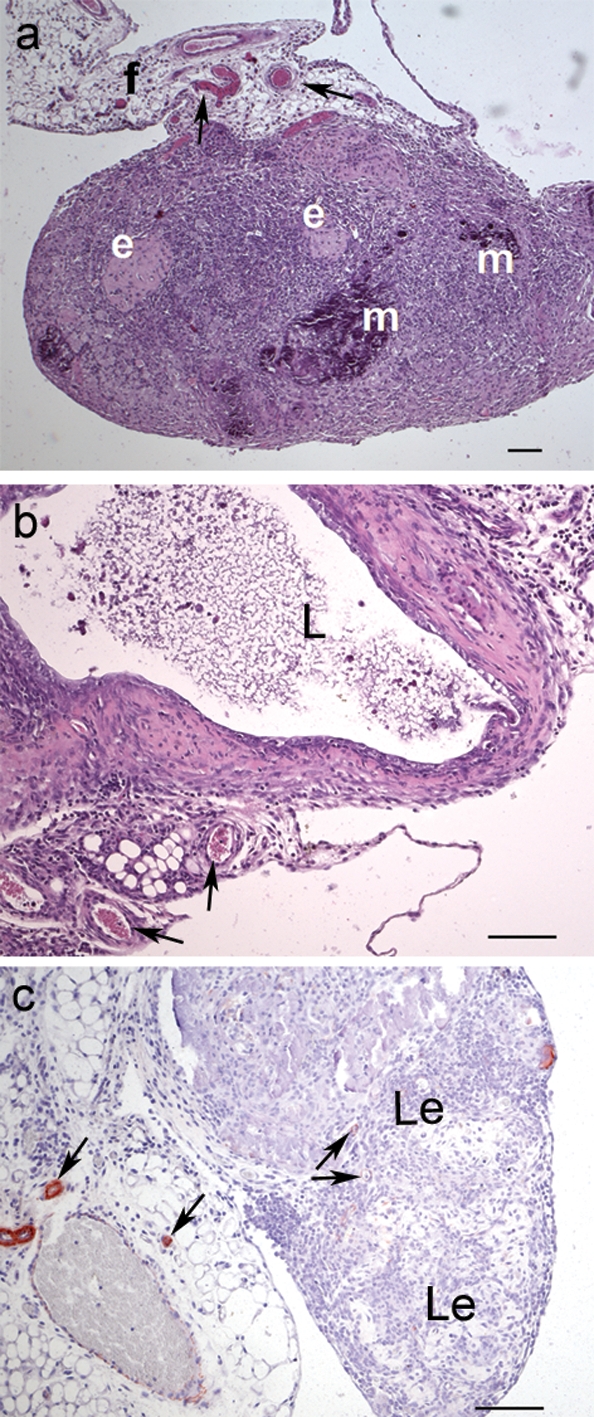
Histological examination of lesions.
(a) Haematoxylin–eosin stained lesion from a TM-treated mouse with compact content, showing supporting vessels (indicated by arrows) embedded in fat (f), several sites of mineralization (m) and eosinophile patches (e). (b) Lesion from a control mouse with cystic lumen (L) surrounded by stromal cells and supporting vessels. (c) Immunohistochemical smooth muscle actin staining reveals small vessels in the supporting fat tissue and a few pericytes in the lesions (Le), indicated by the arrows. No further actin staining can be detected. Scale bars: 100 µm. Original magnification: a = ×50, b–c = ×100.
Immunohistochemical examination with alpha-smooth muscle actin antibodies displayed only a low number of positive cells in the lesions from both groups, but strong signals in surrounding vessels (Fig. 5c), indicating that there are no muscle fibres (e.g. myometrium) and only a few pericytes in the lesions. Additionally, this staining illustrated the rich blood supply of the lesions.
Another difference between TM-treated mice and controls besides the lesion number was the incidence of non-attached debris. Ninety percent of TM-treated mice and 40% of control mice displayed intraperitoneally non-attached fragments. Fig. 6a shows the high amount of loose fragments taken out of a TM-treated mouse, on average 12–15 pieces. Control mice had only around one to three pieces per mouse. Histological examination of debris sections revealed a heterogenic, diffuse structure. No glandular epithelium could be detected and a cystic lumen could only be seen in one single piece of a control mouse (Fig. 6c). Eosinophil patches occurred in different places which could be further characterized as collagen connective tissue as shown by W-E-vG staining. Additionally, in the debris sections no CD31 positive cells could be detected (data not shown) and several necrotic areas could be seen.
Figure 6:
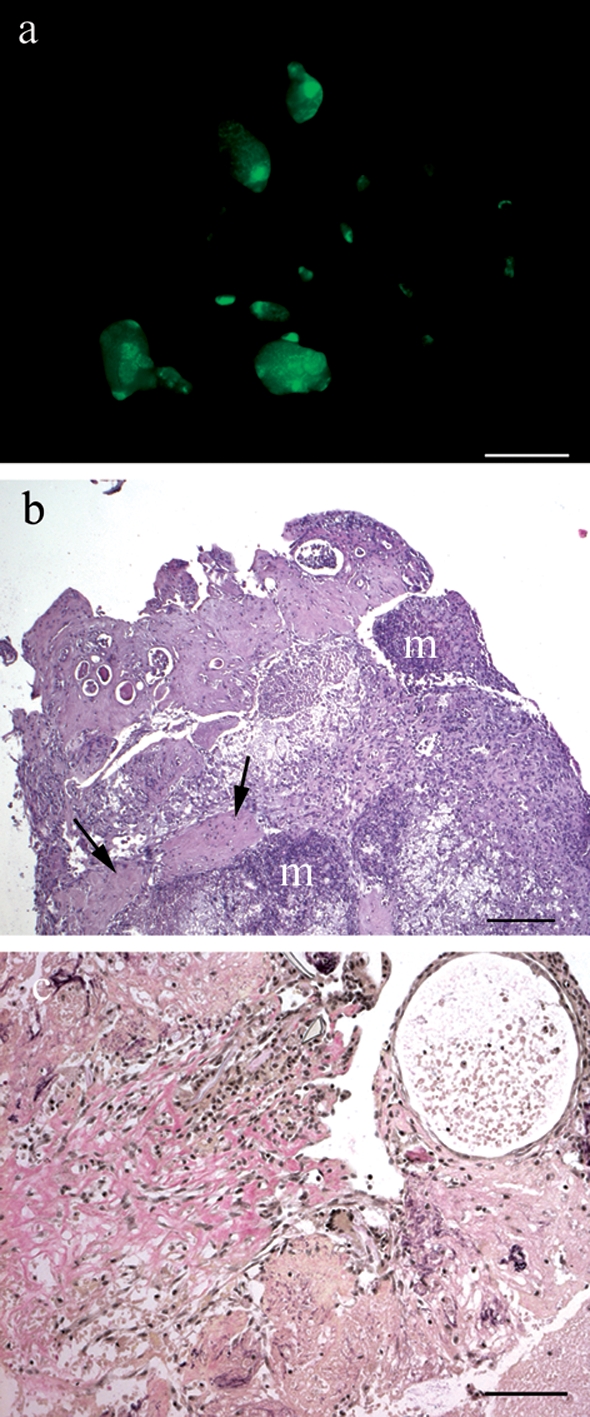
Non-attached debris of a TM-treated mouse removed after autopsy.
a) Debris under fluorescent light in a TM-treated mouse. (b) Haematoxylin–eosin staining of a paraffin-embedded debris section. Eosinophil patches are indicated by arrows. Mineralized tissue (m) demonstrates ongoing necrosis. (c) W-E-vG staining of a debris section. Collagen connective tissue stains red, muscle yellow and nuclei black. Scale bar: a = 2 mm, b = 200 µm, c = 100 µm. Original magnification: b = ×50, c = ×100.
Taken together, the induction of endometriosis in an inflamed peritoneal cavity resulted in fewer lesions per mouse and a smaller lesion size as well as lower disease burden compared with non-treated controls. Furthermore, more mice with debris in the peritoneal cavity were detected in the TM group with a higher amount of debris per mouse.
Discussion
In the pathogenesis of endometriosis, the role of the immune system still remains enigmatic. It has been shown that endometriosis patients have an altered peritoneal environment and their peritoneal immune cells differ from cells of healthy subjects. However, there is an impaired clearance of regurgitated endometrial tissue pieces in the peritoneal cavity. The present study focused on the influence of inflammation on endometriosis establishment by combining a peritonitis mouse model with an endometriosis model.
Peritoneal inflammation was induced by intraperitoneal injection of TM. This method has been routinely used for several decades (Argyris, 1967). Induction of peritonitis can also be achieved by injection of other agents, such as casein, bacillus Calmette-Guérin vaccine or methylated bovine serum albumin (mBSA) (Cook et al., 2003; Hrabak et al., 2006), each leading to different effects on peritoneal cells. Comparison between mBSA and TM revealed that TM injection recruited more immune cells into the peritoneal cavity than mBSA treatment (Cook et al., 2003). In addition, macrophages were larger, more vacuolated and displayed different surface marker distribution compared with resident and mBSA-elicited macrophages (Cook et al., 2003). Furthermore, the TM-excited macrophages resemble activated macrophages in many respects (Den Otter et al., 1982). In addition, isolated TM-elicited macrophages revealed increased TNF-α and IL-12 secretion, a characteristic also seen in endometriosis patients (Rana et al., 1996; Wu et al., 1999). Thus, the injection of TM was used as a model for an acute pre-inflamed peritoneal cavity with features resembling the detected inflammatory situation in patients.
Examining endometriosis evokes the need of a model system. The only animals that are known to develop endometriosis spontaneously are primates, two baboon species (Papio anubis and Papio cynocephalus) and the rhesus monkey, Macaca mulatta (MacKenzie and Casey, 1975; D’Hooghe et al., 1991). The long period until endometriosis occurs and the infrequent rate make this model hardly susceptible to systematic evaluation. Alternatively, rodent models can be used. Somigliana et al. (1999) showed very successfully the establishment of endometriosis-like lesions in the mouse after endometrium injection. In the present study, a slightly modified method was used by injecting EGFP-expressing endometrium into intact wild-type mice. Endometrial fragments expressing EGFP were used to facilitate the discovery of small, hidden lesions which are not detectable under impinging light. Compared with a transplantation model, which involves sutured uterus biopsies on mouse gut mesenterium (Cummings and Metcalf, 1995), the tissue fragments in this model adhere spontaneously after injection onto specific locations like the omentum and peritoneum. These observations correspond to previous results reported by Hirata et al. (2005). Interestingly, approaches from Vernon and Wilson to inject endometrial scrapes into the peritoneal cavity of a rat did not show lesion establishment. According to the authors, this might have been due to a very short treatment period of 14 days (Vernon and Wilson, 1985).
Another frequent site of adherence is the fatty tissue around the uterus and pancreas, presumably due to estrogen production in fat tissue (Brodie, 1979). The predisposition of lesions for fatty tissues is a phenomenon also described by other authors investigating endometriosis establishment in murine models (Somigliana et al., 1999; Hirata et al., 2005). Adipose tissues produce estrogens and thus might support the lesion’s growth. However, other pro-angiogenic factors derived from these tissues might have an impact as well. The cytokine leptin, for example, is secreted by adipocytes and has strong angiogenic features (Sierra-Honigmann et al., 1998). Furthermore, it has been shown that its absence or ablation disrupts the establishment of endometriosis-like lesions in a mouse models (Styer et al., 2008). The role of fatty tissue as favourite attachment site is an interesting finding and seems to be important beyond estrogen production in this model. In patients, high concentrations of leptin have been found in the PF (Matarese et al., 2000), but to our knowledge, fatty tissues are not favoured attachment sites for endometriotic lesions in humans.
Our observations of lesion surface area in the endometriosis model revealed that despite the injected tissue fragment size of 400 × 400 µm, lesions with a size up to 4 mm in diameter could be detected. This may be due to an adherence of pieces with each other and the subsequent growth of the conglomerate. The immunohistochemical stainings for Ki67 in Fig. 3b show that epithelial cells as well as stromal cells of lesions continue to proliferate, thus confirming lesion growth. Further immunohistochemical evaluations demonstrated that glandular epithelium as well as estrogen receptor alpha are present in this EGFP-endometrium derived lesions, which has not been shown before. Examination of frozen lesion sections under fluorescent light revealed heterogenic distribution of EGFP, with some stroma and epithelial cells not emitting green light (exposure time: 800 ms). Interestingly, analysis of EGFP uterus sections also displayed varying EGFP fluorescence in stroma and epithelial cells (data not shown). Indeed, using an extended exposure time, these cells in the endometrium as well as in the lesions show a green fluorescence. Hence, we conclude that the cells in Fig. 2 not displaying green fluorescence still derive from EGFP-endometrium. Non-EGFP expressing host cells are mainly found at the border of lesions in the attachment zone, like it also has been shown by Hirata et al. (2005).
The very low detection of alpha-smooth muscle actin in lesions after immunohistochemical staining suggests that there are only a few pericytes present in the tissue sections. Pericytes usually surround endothelial cells and build together with the basal membrane the mature microvessel. In contrast, immature microvessels consist only of endothelial cells. Endometriosis is strongly associated with neovascularization (Groothuis et al., 2005; Becker and D’amato, 2007; Laschke and Menger, 2007) and immature vessel formation (Hull et al., 2003). Thus, the lesions developed after the injection of EGFP expressing endometrium somehow display the characteristic neovascular situation detected in human endometriotic lesions.
To investigate the impact of inflammation on endometriosis establishment, the peritonitis model was combined with the endometriosis model. The induction of a broad acute inflammation followed by endometriosis induction has not been published before. Peritonitis was evoked by single treatment with TM and, 3 days later, endometriosis was induced by injection of EGFP-expressing endometrial fragments. When analysing the amount and size of lesions established in the peritoneal cavity a significantly lower overall disease burden expressed as sum of the CSA per mouse was detected in the TM-treated mice.
Additionally, although not statistically significant, the peritoneal cavity of mice treated with TM contained more non-attached debris. This might be due to impaired adherence of the tissue fragments with each other and with peritoneal surfaces, leading to the high amount of debris detected in TM-injected mice. At the time of endometrium injection, the peritoneal cavity of TM-treated mice contained around 10-fold more macrophages than that of control mice. Macrophages can execute diverse functional activities including phagocytosis and oxidative burst, matrix dissolution and tissue remodelling (Janeway et al., 2005). Additionally, Lin et al. could show that macrophages and neutrophils infiltrate into ectopic endometrial tissue and the peritoneal cavity. Their local secretion of cytokines and chemokines possibly influences the development of lesions, too (Lin et al., 2006). Hence, we hypothesize that elevated numbers of macrophages lead to increased phagocytosis, cell destruction through radicals released at respiratory burst and, finally, to a pronounced tissue degradation, impairing the adherence abilities of the endometrium fragments.
Moreover, a higher clearance of tissue fragments due to an increased phagocytosis rate could be an explanation for fewer lesions in the TM-treated mice. Endometrium of EGFP-expressing mice was peeled off the myometrium, chopped and suspended in medium. Consequently, tissue integrity was broken and damaged cells including their former content were injected into the peritoneal cavity. This process attracts macrophages and phagocytosis starts. Thus, a 10-fold increase of cells capable of phagocytosis detected after TM injection might lead to a faster clearance of the tissue than it would occur with an unstimulated immune system.
This in vivo study demonstrates that peritoneal inflammation decreases the establishment of endometriosis-like lesions in this mouse model. To our knowledge, this is the first study investigating the impact of an inflamed peritoneal environment on the establishment of endometriotic lesion in the EGFP mouse model. Our results correspond to another study with a pro-inflammatory compound published by Somigliana et al. (1999). They demonstrated that treatment with the recombinant cytokine IL-12 (2 days prior endometriosis induction until 2 days after) effectively reduced ectopic implantation of endometrial fragments in a mouse model. IL-12 influenced the immune system specifically by favouring the generation of T-helper 1 response. Furthermore, the authors proposed 'IL-12 may enhance the growth and augment the cytolytic activity of both NK/LAK (lymphokine-activated killer) and T cells' and thus lead to reduced endometriosis establishment. Hence, in contrast to TM, which elicits a broad inflammation, IL-12 activates a particular pathway of immune response.
Interestingly, Efstathiou et al. have found that a treatment with non-steroidal anti-inflammatory drugs (NSAIDs) also reduces disease burden in an endometriosis mouse model. According to the authors, the NSAIDs used in their experiments display characteristics beyond anti-inflammatory features. They suggested that celecoxib, for example, might be associated with induction of apoptosis in the endometriotic explants. Additionally, another cause for lesion reduction could be the inhibiting effects of NSAIDs on angiogenesis (Efstathiou et al., 2005). A potential explanation for this controversy might be, that TM-induced inflammation, IL-12 and NSAIDs all act through different mechanisms and pathways leading finally to the same outcome, the reduced endometriosis establishment. Taken together, all these data suggest that not only one pathway but several factors are involved in the development of the disease and underline the importance of the immune system in this condition.
Regarding the altered immune system in the peritoneal cavity of endometriosis patients, the stimulus responsible for this inflammatory situation has not been demonstrated yet. A possible reason could be that the regurgitating menstrual tissue fragments evoke this inflammatory environment. Approaches have been made to investigate this hypothesis. Haney et al. examined if menstrual debris might be the stimulus responsible for PF inflammation and concluded that these tissue fragments are probably not a major factor. After examining the correlation of endometriosis extent with PF volume and cells, they did not find a significant relationship (Haney et al., 1991). Alternatively, an already existing peritoneal inflammation before endometriosis establishment might be an explanation for the detected inflammatory events in the peritoneal cavity of patients. However, in our mouse model, we could show that an acute peritonitis does not increase the establishment of lesions.
As for the human, the induced peritoneal inflammation in this study represents only certain aspects of the inflammatory events observed in endometriosis. A primary defect like abnormal macrophage function in endometriosis patients is an obvious possibility as a leading factor underlying lesion establishment. Additionally, a lower phagocyting capacity caused by lesion derived proteins is postulated for PM from endometriosis patients (Sharpe-Timms et al., 2002).
In conclusion, the initiation of an inflammatory response in the peritoneal cavity followed by endometriosis induction seems to be a valuable technique to investigate the role of the immune system in this disease. The study could show that pre-existing inflammation does not increase disease burden in a mouse model and thus might not be the main factor favouring the development of endometriosis. In human patients other factors like primary macrophage defects rather than inflammation may be responsible for endometriosis establishment. Thus, further experiments should be conducted to examine the impact of immunologic alterations on endometriosis establishment to gain deeper knowledge of involvement of the immune system in this disease.
Acknowledgements
The authors thank Ronald Weidmann, Thomas Degenhardt and especially Kerstin Folgnand for excellent technical assistance as well as Dr Peter Hauff for technical equipment supply. The helpful discussions about histopathology with Dr Anna-Lena Frisk are gratefully acknowledged. Additionally, we thank Dr Richardus Vonk, who kindly assisted with statistical analyses. The authors thank Prof Carsten Niemitz for helpful comments on the manuscript.
References
- Argyris BF. Role of macrophages in antibody production. Immune response to sheep red blood cells. J Immunol. 1967;99:744–750. [PubMed] [Google Scholar]
- Arici A, Seli E, Zeyneloglu HB, Senturk LM, Oral E, Olive DL. Interleukin-8 induces proliferation of endometrial stromal cells: a potential autocrine growth factor. J Clin Endocrinol Metab. 1998;83:1201–1205. doi: 10.1210/jcem.83.4.4743. [DOI] [PubMed] [Google Scholar]
- Becker CM, D’Amato RJ. Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc Res. 2007;74:121–130. doi: 10.1016/j.mvr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Becker CM, Sampson DA, Rupnick MA, Rohan RM, Efstathiou JA, Short SM, Taylor GA, Folkman J, D’Amato RJ. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005;84(Suppl 2):1144–1155. doi: 10.1016/j.fertnstert.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Braun DP, Ding J, Dmowski WP. Peritoneal fluid-mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil Steril. 2002;78:727–732. doi: 10.1016/s0015-0282(02)03318-6. [DOI] [PubMed] [Google Scholar]
- Brodie AM. Recent advances in studies on estrogen biosynthesis. J Endocrinol Invest. 1979;2:445–460. doi: 10.1007/BF03349349. [DOI] [PubMed] [Google Scholar]
- Cook AD, Braine EL, Hamilton JA. The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response. J Immunol. 2003;171:4816–4823. doi: 10.4049/jimmunol.171.9.4816. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol. 1995;9:233–238. doi: 10.1016/0890-6238(95)00004-t. [DOI] [PubMed] [Google Scholar]
- D’Hooghe TM, Bambra CS, Cornillie FJ, Isahakia M, Koninckx PR. Prevalence and laparoscopic appearance of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) Biol Reprod. 1991;45:411–416. doi: 10.1095/biolreprod45.3.411. [DOI] [PubMed] [Google Scholar]
- D’Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21:243–254. doi: 10.1055/s-2003-41330. [DOI] [PubMed] [Google Scholar]
- Den Otter W, De Groot JW, Van Basten CD, Rademakers LH, De Weger RA, Pels E. Brewer thioglycollate medium induces different exudates in guinea pigs and mice. Exp Mol Pathol. 1982;36:403–413. doi: 10.1016/0014-4800(82)90069-7. [DOI] [PubMed] [Google Scholar]
- Efstathiou JA, Sampson DA, Levine Z, Rohan RM, Zurakowski D, Folkman J, D’Amato RJ, Rupnick MA. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil Steril. 2005;83:171–181. doi: 10.1016/j.fertnstert.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Arici A. Interleukin-8 stimulates the adhesion of endometrial stromal cells to fibronectin. Fertil Steril. 1999;72:336–340. doi: 10.1016/s0015-0282(99)00223-x. [DOI] [PubMed] [Google Scholar]
- Groothuis PG, Nap AW, Winterhager E, Grummer R. Vascular development in endometriosis. Angiogenesis. 2005;8:147–156. doi: 10.1007/s10456-005-9005-x. [DOI] [PubMed] [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- Haney AF, Jenkins S, Weinberg JB. The stimulus responsible for the peritoneal fluid inflammation observed in infertile women with endometriosis. Fertil Steril. 1991;56:408–413. doi: 10.1016/s0015-0282(16)54532-4. [DOI] [PubMed] [Google Scholar]
- Harada T, Enatsu A, Mitsunari M, Nagano Y, Ito M, Tsudo T, Taniguchi F, Iwabe T, Tanikawa M, Terakawa N. Role of cytokines in progression of endometriosis. Gynecol Obstet Invest. 1999;47(Suppl 1):34–39. doi: 10.1159/000052857. (Discussion 39–40) [DOI] [PubMed] [Google Scholar]
- Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1–10. doi: 10.1016/s0015-0282(01)01816-7. [DOI] [PubMed] [Google Scholar]
- Hirata T, Osuga Y, Yoshino O, Hirota Y, Harada M, Takemura Y, Morimoto C, Koga K, Yano T, Tsutsumi O, et al. Development of an experimental model of endometriosis using mice that ubiquitously express green fluorescent protein. Hum Reprod. 2005;20:2092–2096. doi: 10.1093/humrep/dei012. [DOI] [PubMed] [Google Scholar]
- Hrabak A, Bajor T, Csuka I. The effect of various inflammatory agents on the alternative metabolic pathways of arginine in mouse and rat macrophages. Inflamm Res. 2006;55:23–31. doi: 10.1007/s00011-005-0004-6. [DOI] [PubMed] [Google Scholar]
- Hull ML, Charnock-Jones DS, Chan CL, Bruner-Tran KL, Osteen KG, Tom BD, Fan TP, Smith SK. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88:2889–2899. doi: 10.1210/jc.2002-021912. [DOI] [PubMed] [Google Scholar]
- Janeway JA, Travers P, Walport M, Shlomchik MJ. Immunobiology. 6th edn. New York, USA: Garland Science Publishing; 2005. [Google Scholar]
- Kalu E, Sumar N, Giannopoulos T, Patel P, Croucher C, Sherriff E, Bansal A. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–495. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update. 2007;13:331–342. doi: 10.1093/humupd/dmm006. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Chao VA, Taylor RN. Peritoneal macrophages induce RANTES (regulated on activation, normal T cell expressed and secreted) chemokine gene transcription in endometrial stromal cells. J Clin Endocrinol Metab. 2004;89:1397–1401. doi: 10.1210/jc.2003-031010. [DOI] [PubMed] [Google Scholar]
- Li YM, Baviello G, Vlassara H, Mitsuhashi T. Glycation products in aged thioglycollate medium enhance the elicitation of peritoneal macrophages. J Immunol Methods. 1997;201:183–188. doi: 10.1016/s0022-1759(96)00224-4. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147:1278–1286. doi: 10.1210/en.2005-0790. [DOI] [PubMed] [Google Scholar]
- Loh FH, Bongso A, Fong CY, Koh DR, Lee SH, Zhao HQ. Effects of peritoneal macrophages from women with endometriosis on endometrial cellular proliferation in an in vitro coculture model. Fertil Steril. 1999;72:533–538. doi: 10.1016/s0015-0282(99)00292-7. [DOI] [PubMed] [Google Scholar]
- MacKenzie WF, Casey HW. Animal model of human disease. Endometriosis. Animal model: endometriosis in rhesus monkeys. Am J Pathol. 1975;80:341–344. [PMC free article] [PubMed] [Google Scholar]
- Mahutte NG, Arici A. New advances in the understanding of endometriosis related infertility. J Reprod Immunol. 2002;55:73–83. doi: 10.1016/s0165-0378(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Matarese G, Alviggi C, Sanna V, Howard JK, Lord GM, Carravetta C, Fontana S, Lechler RI, Bloom SR, De Placido G. Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. J Clin Endocrinol Metab. 2000;85:2483–2487. doi: 10.1210/jcem.85.7.6703. [DOI] [PubMed] [Google Scholar]
- Moen MH, Muus KM. Endometriosis in pregnant and non-pregnant women at tubal sterilization. Hum Reprod. 1991;6:699–702. doi: 10.1093/oxfordjournals.humrep.a137411. [DOI] [PubMed] [Google Scholar]
- Montagna P, Capellino S, Villaggio B, Remorgida V, Ragni N, Cutolo M, Ferrero S. Peritoneal fluid macrophages in endometriosis: correlation between the expression of estrogen receptors and inflammation. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2006.11.200. June 2 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Olive DL, Montoya I, Riehl RM, Schenken RS. Macrophage-conditioned media enhance endometrial stromal cell proliferation in vitro. Am J Obstet Gynecol. 1991;164:953–958. [PubMed] [Google Scholar]
- Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54:82–87. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65:925–930. [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynyecol. 1927;14:422–429. [Google Scholar]
- Sharpe-Timms KL, Zimmer RL, Ricke EA, Piva M, Horowitz GM. Endometriotic haptoglobin binds to peritoneal macrophages and alters their function in women with endometriosis. Fertil Steril. 2002;78:810–819. doi: 10.1016/s0015-0282(02)03317-4. [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, Panina-Bordignon P. Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum Reprod. 1999;14:2944–2950. doi: 10.1093/humrep/14.12.2944. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Styer AK, Sullivan BT, Puder M, Arsenault D, Petrozza JC, Serikawa T, Chang S, Hasan T, Gonzalez RR, Rueda BR. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology. 2008;149:506–514. doi: 10.1210/en.2007-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- Vinatier D, Dufour P, Oosterlynck D. Immunological aspects of endometriosis. Hum Reprod Update. 1996;2:371–384. doi: 10.1093/humupd/2.5.371. [DOI] [PubMed] [Google Scholar]
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41–46. [PubMed] [Google Scholar]
- Wu MY, Ho HN, Chen SU, Chao KH, Chen CD, Yang YS. Increase in the production of interleukin-6, interleukin-10, and interleukin-12 by lipopolysaccharide-stimulated peritoneal macrophages from women with endometriosis. Am J Reprod Immunol. 1999;41:106–111. doi: 10.1111/j.1600-0897.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]