Abstract
BACKGROUND
In the early stages of human placentation, the decidua is invaded by fetal extravillous trophoblast (EVT) cells. Interactions between EVT cells and local decidual leukocytes are likely to contribute to immunological accommodation of the semi-allogeneic fetus.
METHODS AND RESULTS
Natural-killer group 2 member D (NKG2D) and 2B4 (CD244) are receptors ubiquitously expressed by the distinctive population of CD56 bright, uterine natural-killer cells, which dominate the decidua at the time of implantation. Here, we investigate the UL-16 binding protein (ULBP) and MHC class-I chain related molecule (MIC) ligands of NKG2D, the CD48 ligand of 2B4 and the non-classical HLA class-I molecule, HLA-F, at the maternal–fetal interface of normal pregnancies. For many of these molecules, significant mRNA expression was detected by RT-PCR in decidual and placental tissue throughout gestation. Flow cytometry of isolated cells or immunohistological staining of implantation site sections was then performed. No protein expression of NKG2D ligands or HLA-F could be detected in decidual leukocytes or fetal trophoblast cells from the first trimester. An NKG2D-Fc fusion protein identified no novel ligands for this promiscuous receptor at the maternal–fetal interface. Strong surface protein expression of CD48 by decidual leukocytes but not by trophoblast cells was detected by flow cytometry. Histological staining showed a clear aggregation of CD48+ cells around transformed spiral arteries of the implantation site.
CONCLUSIONS
We conclude that the role of NKG2D and 2B4 is not focussed on trophoblast recognition in normal pregnancy, but is more likely involved in cross-talk among maternal cells of the placental bed.
Keywords: UL-16 binding protein, HLA-F, trophoblast, decidual leukocytes, natural-killer cells
Introduction
Placentation in humans is a complex process in which invading extravillous trophoblast (EVT) cells move through the decidua to transform spiral arteries and establish an increased blood supply to the fetus (Moffett and Loke, 2006). EVT cells display an unusual array of HLA class-I (HLA-I) molecules: HLA-C, HLA-E and HLA-G (King et al., 2000; Apps et al., 2007, 2008). As they migrate through the uterine wall, EVT cells come into close contact with maternal decidual leukocytes composed mainly of natural-killer (NK) cells and macrophages with a small population of T cells. The dominant decidual leukocytes at the time of implantation are a distinctive population of uterine NK (uNK) cells that have a phenotype and function distinct from cells in peripheral blood (Moffett-King, 2002). There is growing evidence that uNK cells play a crucial role in regulating the degree of trophoblast invasion and therefore maternal blood flow to the placenta and developing fetus (Hiby et al., 2004; Hanna et al., 2006). uNK cells have killer cell immunoglobulin-like receptors (KIR), leukocyte immunoglobulin-like receptors (LILR) and CD94/NKG2 family receptors capable of recognizing all three trophoblast HLA-I molecules (King et al., 2000; Apps et al., 2007; Sharkey et al., 2008). uNK also express additional activating and inhibitory receptors including NK group 2 member D (NKG2D) and 2B4 (CD244) whose potential interactions with trophoblast ligands have not been explored (Hanna et al., 2006; Vacca et al., 2006; Zhang et al., 2007).
NKG2D is a C-type lectin-like molecule that is an activating receptor expressed by NK cells and T cell subsets (Raulet, 2003). Unlike many NK receptors, the ligands for NKG2D (NKG2DL) are diverse and numerous. In humans there are two families structurally related to HLA-I molecules: MHC class-I related (MIC) and UL-16 binding proteins (ULBP) (Eagle and Trowsdale, 2007). Certain NKG2DLs are constitutively expressed by cells at high risk of infection, such as the gut epithelium (Groh et al., 1996). NKG2DL expression can be induced in other tissues by stresses such as infection or transformation (Groh et al., 2001; Gasser et al., 2005). EVT cells are of extra-embryonic origin and express epithelial cell markers. By virtue of their endogenous retroviral expression and invasive behaviour, they also have similarities to infected and tumour cells. In addition, NKG2DLs have been found to be expressed by leukocytes such as activated antigen-presenting cells and provide a mechanism of interaction with NK cells (Draghi et al., 2007).
2B4 (CD244) is a receptor from the family of signalling lymphocyte-activation molecules (SLAM) which encode a ligand binding immunoglobulin (Ig)V domain distal to a single IgC2 domain (Ma et al., 2007). 2B4 is expressed by NK cells and T cell subsets (Valiante and Trinchieri, 1993) and binds CD48 (Brown et al., 1998), a glycosylphosphatidylinositol-linked CD2-related molecule expressed widely on haematopoietic cells but not investigated in the decidua (Korínek et al., 1991; Yokoyama et al., 1991). Although 2B4–CD48 interactions influence activation of leukocyte effector function, the outcomes are not clearly understood. In human NK cells, 2B4 can activate cytotoxicity and interferon (IFN)-γ production through SLAM- associated protein (SAP), an SH2 domain-containing adaptor molecule which results in severe immunodeficiency when defective (Tangye et al., 2000). In contrast, in the absence of SAP, 2B4 delivers inhibitory signals in murine and human NK cells (Parolini et al., 2000; Lee et al., 2004).
Trophoblast cells may express additional molecules, which are potential ligands for NK cell receptors. HLA-F is a non-classical HLA-I molecule with a restricted distribution of tissue expression (Lepin et al., 2000). Endoplasmic reticulum export trafficking is distinct from other HLA-I molecules but no specific functions are known (Boyle et al., 2006). Existing studies with the first widely available anti-HLA-F monoclonal antibody (mAb), 3D11, produced conflicting results regarding surface or intracellular HLA-F expression by trophoblast cells (Ishitani et al., 2003; Nagamatsu et al., 2006; Shobu et al., 2006).
Therefore, the NKG2D and 2B4 receptors expressed by uNK cells could bind ligands on EVT cells and influence NK cell function. We have now investigated the expression of NKG2DLs, CD48 and HLA-F on normal trophoblast and also on maternal cells present in the placental bed.
Materials and Methods
Cell lines and primary tissue
B lymphoblastoid 721 221 cells and the same line transfected with HLA-F were obtained from Dr N. Holmes (Lury et al., 1990). U373 cells and the same line transfected with MICB were obtained from Dr H. Reyburn (Valés-Gómez et al., 2006). HLA-I negative African Green Monkey CV-1 cells, human choriocarcinoma cell lines JEG-3 and JAR, as well as 293T, Jurkat, HT1080, H9 and Hela cell lines were all purchased from the American Type Culture Collection (ATCC reference numbers CCL-70, HTB-36, HTB-144, CRL-11268, TIB-152, CCL-121, HTB-176 and CCL-2, respectively). Using the primers shown (forward, reverse) extracellular regions of the ULBP1 (5′-TATAAGCTTGGCTGGTCCCGGGCA-3′, 5′-CTCGAATTCTCATCTGCCAGCTAG-3′), ULBP3 (5′-TATAAGCTTTTCGACTGGTCCGGG-3′, 5′-GATGAATTCTCAGATGCCAGGGAG-3′) and MICA (5′-TATAAGCTTCCCCACAGTCTTCGT-3′, 5′-TATGAATTCCTGGACCCTCTGCAG-3′) genes were each cloned into a mammalian expression vector p3xFLAG-CMV-9 (Sigma–Aldrich). A similar ULBP2 fragment in this vector was obtained from Bacon et al. (2004). CV-1 cells were individually transfected with these NKG2DL constructs using Lipofectamine 2000 (Invitrogen). Sequencing of the newly constructed plasmids used for these transfectants matched the Genebank sequences of accession numbers AB052907 (ULBP1), AB052908 (ULBP3) and L14848 (MICA) between the regions defined by the primers above.
Decidual and placental tissue was obtained at term or from elective terminations of normal pregnancies between 6 and 12 weeks gestation. Ethical approval for the use of these tissues was obtained from the Cambridge Local Research Ethics Committee. Decidual leukocytes and trophoblast cells were isolated as previously described (Trundley et al., 2006). Briefly, decidual tissue was disaggregated with collagenase and mononuclear cells purified on Lymphoprep. Trophoblast was released from chorionic villi by trypsin digestion and macrophages were depleted by adherence to plastic. Freshly isolated cells are predominantly of a villous trophoblast (VT) phenotype. After culture overnight on fibronectin, 50–80% of the cells become HLA-G+, characteristic of EVT cells.
mAbs and fusion proteins
mAbs to ULBP, MIC, HLA-F and CD48 molecules are detailed in Table I. A mAb to NKG2D (M585) was also obtained from Cosman et al. (2001) (Amgen Inc.). Well-established mAbs to HLA-DR (L243), HLA-I (W6/32), 2B4 (C1.7), cytokeratin (MNF116) and the Flag epitope (M2) were purchased from Becton Dickinson, Serotec, Beckman Coulter, Dako and Sigma–Aldrich, respectively. Isotype control IgG1, IgG2a, IgG2b, IgG3 and IgM mAbs were obtained from Oxford Biotechnology or BD Pharmingen. Binding of all these unlabelled mAbs was detected by phycoerythrin (PE)-conjugated secondary reagents to IgG (Sigma–Aldrich) or total mouse Ig (Dako). Conjugated mAbs used were CD3- fluorescein isothiocyanate (FITC) (SK7), CD14-FITC (MϕP9), HLA-DR-FITC (L243), CD56-Alexa 488 (B159), LILRB3-PE-Cy5 (ZM3.8), and control IgG1- or IgG2a-FITC (all BD Biosciences); HLA-G-FITC (clone G233 developed in our own laboratory (Loke et al., 1997) or clone MEM-G/9 from Abcam) and epidermal growth factor receptor (EGF-R)-FITC (EGFR1) from Insight Biotechnology. A sequenced NKG2D-IgG Fc fusion protein construct in the mammalian expression vector pcDNA3, obtained from Dr H. Reyburn (Valés-Gómez et al., 2003), was transiently transfected into 293T cells using Lipofectamine 2000 (Invitrogen). Fusion protein produced was quantified by Easy-Titer (Pierce) and in reducing sodium dodecyl sulphate polyacrylamide gel electrophoresis resolved as a single band at ∼70 kDa. Binding of this fusion protein was detected by a PE-conjugated anti-human IgG secondary antibody (Sigma–Aldrich).
Table I.
Monoclonal antibodies used to investigate expression of potential natural-killer cell ligands.
| Antigen | Clone name | Experimental conditions |
Source | Reference for production and characterization | |
|---|---|---|---|---|---|
| Flow cytometry | Immunohistochemistry | ||||
| ULBP1 | M295 | 12 µg/ml | 5 µg/ml | Dr D. Cosman (Amgen) | Cosman et al. (2001) |
| ULBP1 | AUMO1 | hybridoma supernatant diluted 1:3 | hybridoma supernatant diluted 1:4 | Dr A. Steinle | Welte et al. (2003) |
| ULBP2 | M311 | 12 µg/ml | 5 µg/ml | Dr D. Cosman (Amgen) | Cosman et al. (2001) |
| ULBP2 | BUMO1 | hybridoma supernatant diluted 1:3 | hybridoma supernatant diluted 1:4 | Dr A. Steinle | Welte et al. (2003) |
| ULBP3 | M550 | 16 µg/ml | 5 µg/ml | Dr D. Cosman (Amgen) | Cosman et al. (2001) |
| ULBP3 | 166510 | 8 µg/ml | 5 µg/ml | R&D Systems | R&D datasheet |
| MICA | M673 | 16 µg/ml | 5 µg/ml | Dr D. Cosman (Amgen) | Cosman et al. (2001) |
| MICA | AMO1 | 14 µg/ml | 5 µg/ml | Immatics Biotechnologies | Welte et al. (2003) |
| MICB | BMO1 | hybridoma supernatant diluted 1:3 | hybridoma supernatant diluted 1:4 | Dr A. Steinle | Welte et al. (2003) |
| MICB | BMO2 | hybridoma supernatant diluted 1:3 | hybridoma supernatant diluted 1:4 | Dr A. Steinle | Welte et al. (2003) |
| HLA-F | FG-1 | 15 µg/ml | 5 µg/ml | Dr E. Lepin | Lepin et al. (2000) |
| CD48 | Tü145 | 14 µg/ml | na | BD Pharmingen | Hadam (1989) |
| CD48 | 6.28 | 10 µg/ml | 5 µg/ml | Developmental Studies Hybridoma Bank | Yokoyama et al. (1991) |
ULBP, UL-16 binding protein; MIC, MHC class-I chain related.
RT-PCR
Fragments of decidual or placental tissue were stored at –80°C before total RNA isolation (Qiagen RNAeasy), DNA digestion (Qiagen DNase) and complementary DNA (cDNA) synthesis (Invitrogen Superscript II) following the manufacturers instructions. For each RT-PCR 50 ng of cDNA, 0.5 µM primers (Sigma–Genosys), 200 µM dNTP (Roche), between 2 and 3 mM magnesium chloride and 0.6 U of Amplitaq Gold DNA polymerase with its potassium chloride buffer (Applied Biosystems) were incubated on a Peltier Thermal Cycler. After an initial incubation at 95°C for 8 min, cycles of 95°C for 25 s, 50–70°C for 45 s and 72°C for 30 s were repeated for between 30 and 35 cycles. PCR products were resolved by agarose electrophoresis (1% agarose in TBE (10 mM Tris (pH 7.4) and 1 mM EDTA (pH 8) in dH20), with 0.5 µg/ml ethidium bromide (Sigma–Aldrich)) and a 1 kb reference ladder (Invitrogen). Gels were visualized using a FluorChem 9900 UV imaging system.
RT-PCR conditions were optimized to distinguish between NKG2DLs and HLA-I molecules of up to 97% nucleotide sequence identity. The primers and reaction conditions are detailed in Table II, those for MIC amplification were based on Welte et al. (2003). Primer pairs span intronic sequences to avoid amplification of genomic DNA. To confirm specific detection of the intended gene, several PCR products from selected amplification reactions were cloned into pCR4-TOPO vectors and DH5α E. Coli transformed using the TOPO cloning system (Invitrogen). Plasmids were extracted (Qiagen Miniprep) and sequencing using T7 (5′-TAATACGACTCACTATAGGG-3′) and M13R (5′-CAGGAAACAGCTATGAC-3′) primers. At least four PCR products from two or more tissues were sequenced for each of the NKG2DLs and HLA-I genes investigated. All of the 50 PCR products sequenced were the intended target.
Table II.
Primers and PCR conditions for gene expression analysis.
| Target transcript | Forward primer | Reverse primer | Amplicon size (bp) | Annealing temperature (°C) | [MgCl2] (mM) | Number of cycles | Positive control cell line |
|---|---|---|---|---|---|---|---|
| ULBP1 | 5′-GATGGGTCGACACACACTG-3′ | 5′-AGAGGGTGGTTTTGTTGGA-3′ | 560 | 64 | 2.5 | 30 | Jurkat |
| ULBP2 | 5′-GCTACCAAGATCCTTCTGT-3′ | 5′-GTCAAAGAGGAGGAAGAACTGC-3′ | 441 | 62 | 3.0 | 30 | Jurkat |
| ULBP3 | 5′-GCGATTCTTCCGTACCTGCTA-3′ | 5′-TGGTGGCTATGGCTTTGGGTT-3′ | 640 | 67 | 2.0 | 30 | HT1080 |
| ULBP4 | 5′-TATCCCTGACTTCTAGCCCT-3′ | 5′-GCCACTCACCATTTTGCCAC-3′ | 749 | 60 | 2.5 | 33 | HT1080 |
| RAET1G | 5′-AGCCCCGCGTTCCTTCTA-3′ | 5′-TGTATACAAGGCAAGAGGGC-3′ | 935 | 54 | 3.0 | 30 | HT1080 |
| RAET1L | 5′-CGCCATCCCAGCTTTG-3′ | 5′-TCAGATGCCAGGGAGGAT-3′ | 730 | 58 | 2.0 | 35 | Hela |
| MICA | 5′-CCTTGGCCATGAACGTCAGG-3′ | 5′-CCTCTGAGGCCTCRCTGCG-3′ | 177 | 60 | 2.0 | 33 | HT1080 |
| MICB | 5′-ACCTTGGCTATGAACGTCACA-3′ | 5′-CCCTCTGAGACCTCGCTGCA-3′ | 179 | 60 | 3.0 | 33 | Jurkat |
| HLA-G | 5′-GACTCGGCGTGTCCGAGGAT-3′ | 5′-GACCGCAGCTCCAGTGACT-3′ | 795 | 56 | 2.0 | 30 | JEG-3 |
| HLA-F | 5′-GTGGCCCTGAGGAACCTG-3′ | 5′-TGAGAGTAGCTCCCTCTGTTTCT-3′ | 740 | 60 | 2.5 | 33 | H9 |
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ | ∼450 | 56 | 2.5 | 30 | any |
R in a primer sequence indicates a mixture of A or G nucleotides in a ratio of approximately 1:1. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. RAET1, retinoic acid early transcript 1.
Flow cytometry
Flow cytometry conditions to detect NKG2DLs for which transcripts had been found were established using U373 cells stably expressing MICB, or ULBP1-3 and MICA expressed in HLA-I negative CV-1 cells (Table I). Staining of the flag epitope is included to indicate transfection efficiencies. For staining of primary cells, first Fcγ receptors were blocked by incubating with human IgG (Sigma–Aldrich). Unlabelled antibodies or fusion protein was added and detected with fluorochrome-conjugated secondary reagents. In blocking experiments, fusion protein was incubated with anti-NKG2D (M585) or isotype control antibody prior to staining. Free secondary antibody binding sites were blocked with the appropriate species Ig, before staining with directly conjugated mAb to identify each leukocyte or trophoblast cell population. VTs are HLA-I negative but are identified as the only cells in our trophoblast preparations to express EGF-R (Jokhi et al., 1994). EVT cells are the only cell type confirmed to express the non-classical HLA-G molecule, which is recognized specifically by our mAb G233 (Loke et al., 1997). Cells were analysed using a FACscan flow cytometer and CellQuest software (Becton Dickinson).
Immunohistochemistry
Acetone-fixed, frozen sections of human tissue or smears of cultured cells were blocked by incubating with serum of the species in which the secondary antibody was raised. Primary antibodies described above, were detected with biotinylated secondaries and streptavidin–horse–radish peroxidase (both Vector Laboratories) and developed with diaminobenzidine substrate (Sigma–Aldrich) as previously described (Jokhi et al., 1993). Sections were counterstained in Carazzi’s haematoxylin and mounted with glycerol/gelatin. For double staining, sections were additionally labelled with mAb to CD56 developed using FastRed substrate (Sigma–Aldrich).
Results
The receptors NKG2D and 2B4 are expressed by decidual leukocytes
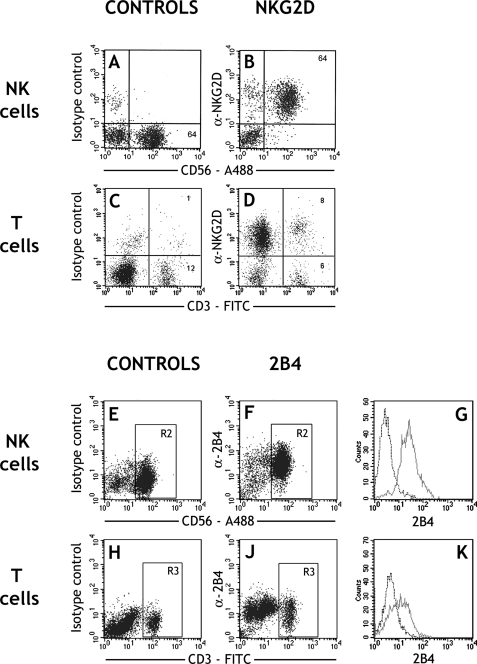
Flow cytometry of decidual leukocytes isolated from normal first trimester pregnancies confirmed expression of both NKG2D and 2B4 on all CD56+ and around 50% of CD3+ cells (Fig. 1). This is consistent with previous reports for decidual leukocytes (Hanna et al., 2006; Vacca et al., 2006; Zhang et al., 2007) and the pattern of expression for these receptors in peripheral blood (Valiante and Trinchieri, 1993; Raulet, 2003). Interestingly, NKG2D labelled with a higher intensity on uNK than peripheral blood NK cells from the same individual (data not shown (DNS)) consistent with reported mRNA level expression studies (Koopman et al., 2003).
Figure 1:
Expression of NKG2D and 2B4 receptors by decidual leukocytes.
Freshly isolated first-trimester decidual leukocytes from normal human pregnancies were stained for NKG2D (A–D) or 2B4 (CD244) (E–K), with CD56+ or CD3+ cells identified by directly conjugated monoclonal antibody (mAb). For each dot plot, leukocytes were gated by scatter properties (not shown). For traces G and K, the additional gates shown R2 (CD56+ cells) or R3 (CD3+ cells) were used to select NK and T lymphocytes, respectively. Broken traces represent isotype control and continuous traces the anti-2B4 mAb staining. These results are representative of decidual leukocytes isolated from at least four individuals. FITC, fluorescein isothiocyanate; A488, alexa fluor 488.
NKG2DL mRNA expression at the maternal–fetal interface
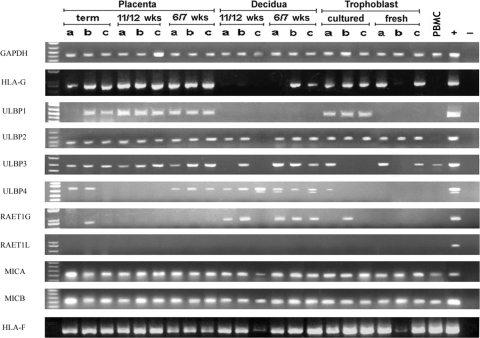
NKG2DL mRNA expression was investigated using RT-PCR capable of discriminating between the closely related MIC and ULBP family members. Samples of decidua and placental tissue from normal pregnancies in their first trimester and at term were screened, as well as freshly isolated trophoblast (which is predominantly villous) and isolated trophoblast cells cultured overnight (which differentiate to an HLA-G+ extravillous phenotype (Trundley et al., 2006)) (Fig. 2). Detection of glyceraldehyde 3-phosphate dehydrogenase and HLA-G transcripts verifies cDNA synthesis and confirms the presence of EVT cells, respectively. ULBP1 was detected in each of the three cultured EVT cell preparations and first trimester placental samples as well as two of the three term placental samples. In contrast, ULBP1 was not detected in freshly isolated VT, decidua or peripheral blood mononuclear cells (PBMC). ULBP2, MICA and MICB transcripts were widespread in decidual, placental and trophoblast preparations. ULBP3 was detected in all placental samples, but less frequently in trophoblast. ULBP4 and retinoic acid early transcript (RAET) 1G expression was detected mainly in decidual tissue. RAET1L was not detected in any primary tissue. Only low levels of ULBP3 and MIC transcripts were detected in peripheral blood. This rules out the possible detection of expression in decidual samples as a result of contamination by peripheral blood. ULBP1-3, MICA and MICB transcripts were also detected in the JEG-3 choriocarcinoma cell line (DNS). In summary, significant mRNA expression of ULBP1-3, MICA and MICB molecules was detected in trophoblast and decidual cells.
Figure 2:
mRNA expression of NKG2D ligands and non-classical HLA-I genes at the human implantation site.
Triplicate samples of placental and decidual tissues were obtained at term, 11–12 and 6–7 weeks gestation from normal human pregnancies. Trophoblast cells were also isolated from early placental samples (see Materials and Methods). cDNA from these tissues was screened by RT-PCR for each NKG2DL, HLA-G and HLA-F. PCR products from each reaction were cloned, sequenced and in every case the intended transcript had been amplified. ULBP, UL-16 binding protein; MIC, MHC class-I chain related; GAPDH, glyceraldehyde 3- phosphate dehydrogenase; RAET1, retinoic acid early transcript 1; PBMC, peripheral blood mononuclear cells.
Surface protein expression of NKG2DL investigated by flow cytometry
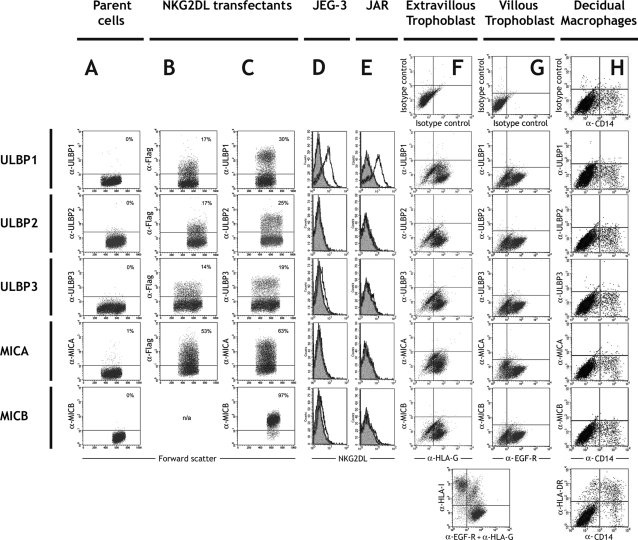
We next investigated protein expression of ULBP1-3, MICA and MICB on cells from the implantation site using flow cytometry. Individual transfectants were produced to establish the flow cytometry conditions for detection of each of these NKG2DLs (Fig. 3A–C). Using these flow cytometry conditions, the choriocarcinoma cell lines JEG-3 and JAR were initially tested. They only expressed ULBP1 surface protein (Fig. 3D and E). Next, freshly isolated first trimester trophoblast cells were analysed. Cells of villous and extravillous phenotype in our primary cell preparations were positively identified by labelling for EGF-R and HLA-G, respectively (Jokhi et al., 1994; Loke et al., 1997). Neither of these trophoblast sub-populations bound mAbs to ULBP1-3, MICA or MICB (Fig. 3F and G). As a positive control for HLA-I-like molecule staining, labelling was performed in parallel with the mAb W6/32 (Parham et al., 1979). Shown at the bottom of Fig. 3G, HLA-I negative VT remain in the lower right quadrant whereas EVT and stromal cells/leukocytes contaminating our primary cell preparations stain with W6/32 in the upper right and left quadrants, respectively.
Figure 3:
NKG2DL expression investigated by flow cytometry.
(A–C) A panel of transfected cells was stained with isotype control mAb (A), anti-flag mAb to indicate expression of tagged NKG2DL constructs in CV-1 cells (B) and anti-NKG2DL mAb (C). The proportion of cells staining (positive) is indicated by the percentage shown in each plot. (D and E) NKG2DL expression was investigated on the choriocarcinoma cell lines JEG-3 (D) and JAR (E). Binding of mAbs detecting each NKG2DL (open traces) and an isotype control (filled traces) is shown. (F and G) NKG2DL expression by fetal trophoblast cells isolated from normal, first trimester pregnancies. EVT cells are identified by labelling HLA-G (F), and VT cells by staining EGF-R (G). No NKG2DL mAbs bound either cell type, compared with an isotype control (top). Binding of the mAb W6/32 recognizing all HLA-I molecules is shown as a positive control (bottom). (H) NKG2DL expression by maternal decidual macrophages isolated from normal, first trimester pregnancies. Myelomonocytic cells are identified by CD14 labelling, but no NKG2DL mAbs bind compared with an isotype control (top). Binding of HLA-DR (bottom) represents a positive control. Primary cells from at least 10 individuals were independently analysed, with no NKG2DL expression detected throughout. Multiple mAbs for each NKG2DL are listed in Table I, the same binding patterns were observed with both mAbs to each NKG2DL throughout our study.
Our isolation of primary trophoblast cells includes a trypsin digestion to dissociate cells from chorionic villi (Trundley et al., 2006). Exposure of transfected cell lines to similar trypsin or collagenase incubations substantially decreased labelling of MIC and ULBP. This effect is not observed with HLA-I molecules (DNS). We therefore analysed trophoblast cells cultured overnight to check the possibility that surface NKG2DLs might be reconstituted and then detected. In these experiments, trophoblast cells were dissociated after culture by a brief incubation with EDTA. Neither VT nor EVT cells, identified by EGF-R and HLA-G labelling as before, bound mAb to ULBP1-3, MICA or MICB (DNS). In other tissues, surface expression of ULBP and MIC molecules is induced or up-regulated by stress stimuli such as infection or DNA damage (Groh et al., 2001; Gasser et al., 2005). We used the stress stimuli of irradiation or toll-like receptor (TLR) stimulation, but no induction of NKG2DL expression was observed (DNS).
Another possibility is that NKG2D on uNK cells interacts with its ligands on maternal decidual cells. For example, antigen presenting cells have been reported to express NKG2DLs when infected with influenza (Draghi et al., 2007). Anti-NKG2DL mAbs showed no labelling of decidual CD14+ HLA-DR+ myelomonocytic cells, or any other of the cells identified by a cocktail of leukocyte lineage markers either freshly isolated with collagenase or after overnight culture (Fig. 3H and DNS). Stress stimuli were also investigated as above but no inducible NKG2DL expression was detected (DNS).
NKG2DL protein expression investigated by immunohistochemistry
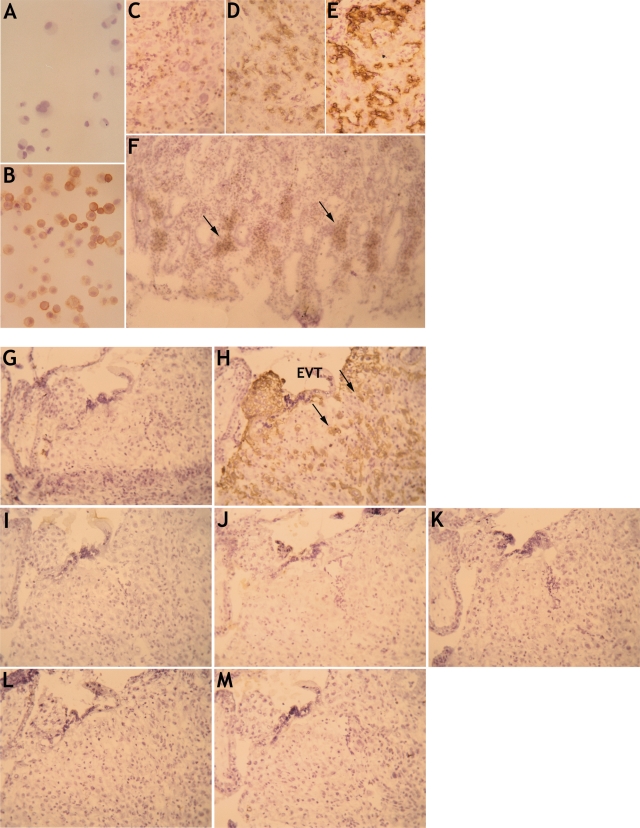
To avoid the difficulties of enzymic digestion stripping NKG2DLs from the cell surface and to detect any intracellular proteins present, we performed immunohistochemistry staining of acetone-fixed implantation site sections. The mAbs used were able to detect their antigens in cytospin smears of transfectants and on tissue sections of gastric mucosa and breast carcinoma (Fig. 4A–F) (Eagle and Trowsdale, 2007). Serial sections of the implantation site (identified by HLA-G+ EVT) from a normal human pregnancy at 8 weeks gestation were then screened for NKG2DL expression (Fig. 4G–N). No labelling with mAbs to the ULBP1-3, MICA or MICB molecules was observed by trophoblast or any other cells at the implantation site.
Figure 4:
NKG2DL expression investigated by immunohistochemistry.
(A and B) As positive controls, acetone fixed and frozen smears of cells individually transfected with ULBP1-3, and MICA and MICB were stained. Representative labelling with anti-MICB mAb to parent (A) and MICB-transfected U373 (B) cells is shown. (C–F) A section of breast carcinoma is shown stained with isotype control mAb (C), anti-MICA mAb (D) and for cytokeratin which is strongly positive (E). A section of gastric mucosa also stains with mAb to MICA (F). (G–M) Serial sections of a normal human implantation site at 8 weeks gestation labelled with isotype control mAb (G), anti-HLA-G mAb G233 to identify EVT cells (H), and mAb to ULBP1 (I), ULBP2 (J), ULBP3 (K), MICA (L) and MICB (M). No binding to any cell type was detected in sections from three independent donors. A and B are at ×400 magnification, C–M are ×200.
Investigation of novel NKG2DLs with an NKG2D-Fc fusion protein
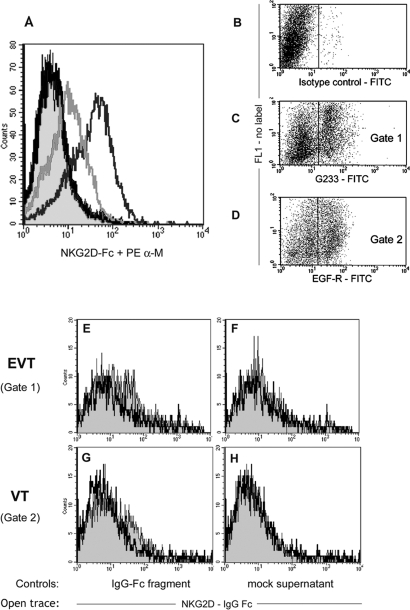
As NKG2D is a promiscuous receptor with polygenic and polymorphic ligands, it is possible that novel NKG2DLs are present at the maternal–fetal interface. An NKG2D-Fc fusion protein was used that specifically bound to MICB on transfected cells (Fig. 5A) (Sutherland et al., 2002). No binding of NKG2D-Fc was observed to freshly isolated or overnight cultured HLA-G+ or EGF-R+ trophoblast cells (Fig. 5B–H and DNS).
Figure 5:
No novel NKG2DLs are detected on human trophoblast cells using an NKG2D-Fc fusion protein.
(A) An NKG2D-Fc fusion protein was produced by transient transfection of 293T cells and bound MICB-transfected U373 cells (black trace), compared with control supernatant from 293T cells transfected with an empty plasmid (filled trace) or first incubation with a mAb to NKG2D (grey trace). (B–H) NKG2D-Fc binding to freshly isolated first trimester trophoblast cells was tested. Antibodies directly conjugated to FITC were used compared with an isotype control (B), to identify EVT cells by HLA-G labelling (C) or VT by EGF-R labelling (D). These positive cells in C and D were gated for analysis of NKG2D-Fc fusion protein binding (E–H). There was no binding to either trophoblast population of the fusion protein (black trace) compared with controls (filled traces) of an immunoglobulin G-Fc fragment (E and G) or the mock supernatant (F and H) for three independent donors.
HLA-F expression at the maternal–fetal interface
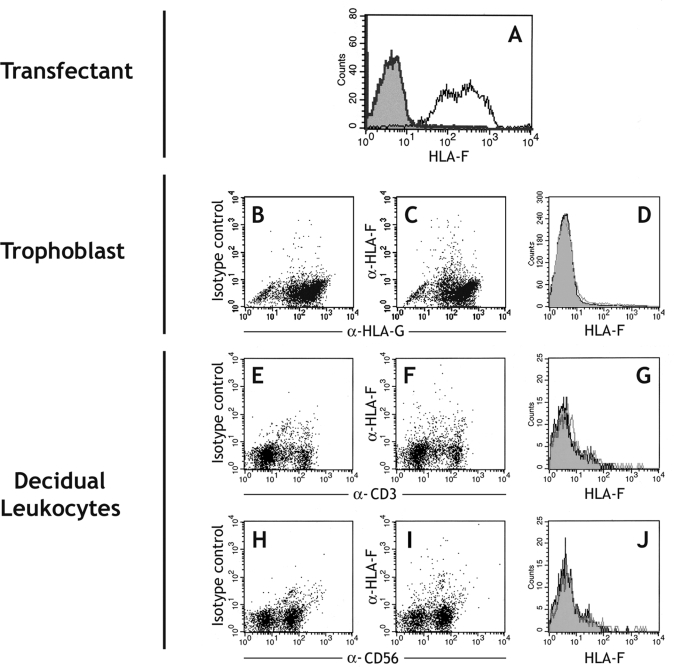
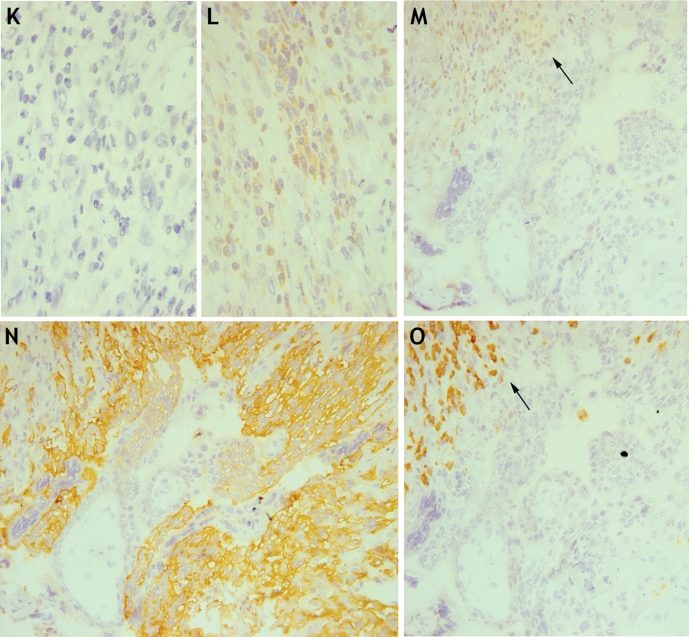
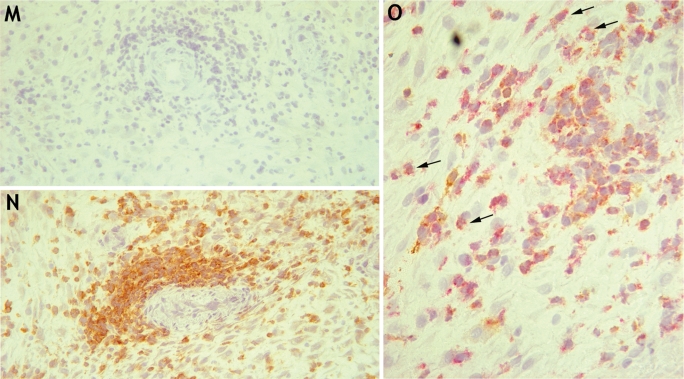
There are conflicting reports about the expression of HLA-F on trophoblast cells (Ishitani et al., 2003; Nagamatsu et al., 2006; Shobu et al., 2006). Using RT-PCR, we detected HLA-F transcripts in all decidua, placenta and trophoblast samples (Fig. 2). We next used a new well-characterized mAb FG-1 to screen for protein expression (Lepin et al., 2000). Using flow cytometry and immunohistochemistry, the mAb FG-1 was confirmed to detect HLA-F on transfected cells (Fig. 6A and DNS). No HLA-F expression was detected on freshly isolated trophoblast cells or decidual leukocytes by flow cytometry (Fig. 6B–J). By immunohistochemistry only very weak staining of FG-1 was seen (Fig. 6K and L). The cells staining were not EVT, but possibly uNK cells identified by labelling serial sections for FG-1, HLA-G and CD56 (Fig. 6M–O). The weak staining with mAb FG-1 was restricted to those uNK cells near the site of trophoblast invasion.
Figure 6:
Analysis of HLA-F expression at the first trimester implantation site.
(A) mAb FG-1 binds HLA-F transfected (open trace) compared with parent .221 cells (filled trace). (B–D) This anti-HLA-F mAb shows no binding to trophoblast cells isolated from normal first trimester placenta, with EVT cells identified by labelling of HLA-G. (E–J) The anti-HLA-F mAb FG-1 shows no binding to decidual leukocytes isolated from normal pregnancies. T lymphocytes (E–G) and NK cells (H–J) are identified by labelling of CD3 and CD56, respectively. Primary cells from at least 10 individuals were independently analysed by flow cytometry. (K–O) Serial sections of a normal human implantation site at 8 weeks gestation were labelled with isotype control mAb (K), or anti-HLA-F mAb FG-1 (L) at ×400 magnification. The same section is also shown stained (arrow) with FG-1 (M), anti-HLA-G mAb G233 (N) and anti-CD56 (O) at ×200 magnification. The immunohistochemistry figures shown are representative of staining observed in sections from three independent donors.
CD48 is expressed by decidual cells at the maternal–fetal interface
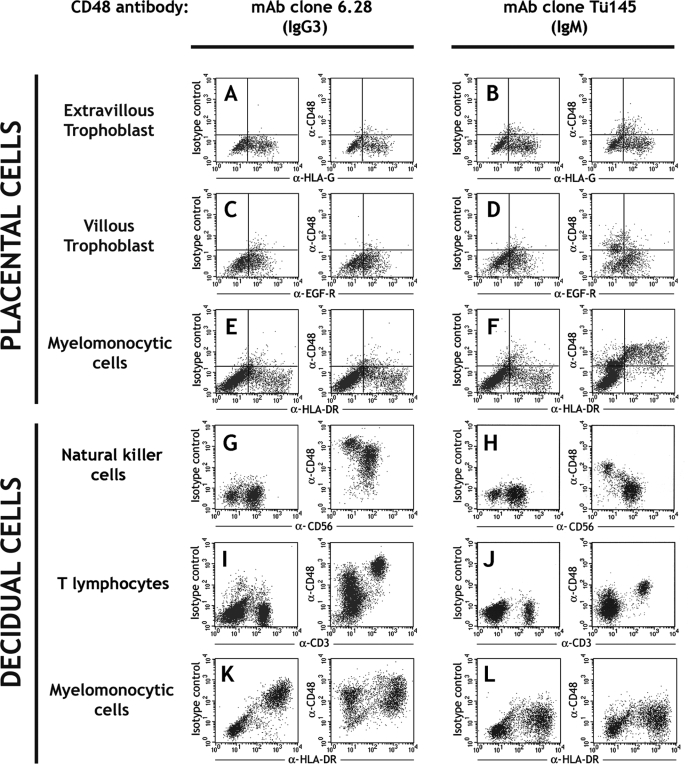
As all uNK cells express 2B4 (Fig. 1E–K), we investigated expression of its ligand CD48 at the maternal–fetal interface using two different mAbs, clones Tü145 and 6.28. In flow cytometry, fetal HLA-DR+ Hofbauer cells bound mAb Tü145 but not mAb 6.28, whereas HLA-G+ extravillous or EGF-R+ VT cells did not bind either of the anti-CD48 mAbs (Fig. 7A–F). In leukocytes isolated from the decidua, CD48 was strongly detected on all CD3+ T cells by both mAbs and CD56+ cells only by the mAb 6.28. HLA-DR+ or LILRB3+ myelomonocytic cells in the decidua did not bind either CD48 mAb relative to isotype controls (Fig. 7G–L and DNS). Histological staining of normal first trimester decidual sections with mAb 6.28 revealed striking aggregation of positive cells around modified spiral arteries at the implantation site, which showed some co-localization with CD56+ cells in double labelling (Fig. 7M–O).
Figure 7:
CD48 is expressed by decidual leukocytes but not fetal trophoblast cells.
(A–F) CD48 expression by fetal cells isolated from normal, first trimester pregnancies. EVT cells are identified by HLA-G labelling (A and B), VT cells by staining EGF-R (C and D) and myelomonocytic cells by HLA-DR expression (E and F). Binding of mAbs 6.28 and Tü145, both recognizing CD48, is shown relative to their respective controls. (G–L) CD48 expression by maternal decidual leukocytes isolated from normal, first trimester pregnancies. NK cells are identified by CD56 expression (G and H), T lymphocytes by CD3 staining (I and J) and myelomonocytic cells by HLA-DR labelling (K and L). Binding of both mAbs 6.28 and Tü145 recognizing CD48 is shown. Primary cells from at least 10 individuals were independently analysed by flow cytometry with each mAb. (M–O) Immunohistochemical staining of a normal human implantation site at 8 weeks gestation. Compared with an isotype control (M), anti-CD48 mAb 6.28 strongly stains decidual cells aggregated around transformed spiral arteries of the implantation site (N) (×200 magnification). A double labelled decidual section at ×400 magnification shows some co-localization (arrows) of mAb 6.28 staining (brown), with CD56+ cells (red). The immunohistochemistry figures shown are representative of staining observed in sections from three independent donors.
Discussion
In this study, we analysed the expression of possible ligands for NK receptors on cells at the maternal–fetal interface in the first trimester of pregnancy. The NKG2D receptor was strongly expressed by all uNK cells and 50% of T cells, consistent with increased NKG2D transcription in decidual compared with peripheral NK cells (Koopman et al., 2003). Although we found transcripts for several NKG2DLs in tissues from the implantation site, no protein expression was demonstrated by flow cytometry or immunohistochemistry on cells from normal pregnancy. Post-transcriptional control of NKG2DL expression is not unusual. Indeed, MIC transcripts are widely expressed but only restricted protein expression is observed and induction of surface ULBP protein expression by bronchial epithelial cells has been shown to occur without protein synthesis (Borchers et al., 2006). NKG2DLs are known to be up-regulated by various stress stimuli but after treatment with irradiation or pathogen-associated molecular pattern molecules no expression was found on any cell type. In contrast, and in keeping with their origin from malignant tumours, the choriocarcinoma cell lines JEG-3 and JAR did express ULBP1 surface protein. We also explored whether other, novel NKG2DLs might be found on trophoblast by screening with an NKG2D-Fc fusion protein but found no binding.
NKG2DLs may also be shed from infected or tumour cells and can be detected in the blood of cancer patients (Salih et al., 2002, 2006). Constitutive or soluble NKG2DL bind to NKG2D and down-regulate its expression on NK and T cells, abrogating NK activation towards the tumour or infected cell (Doubrovina et al., 2003; Oppenheim et al., 2005; Wiemann et al., 2005). A report that trophoblast cells produce soluble MIC and in this way mediate maternal tolerance to the fetus suggested a similar mechanism might operate in pregnancy (Mincheva-Nilsson et al., 2006). In agreement with our study, MIC mRNA was detected in trophoblast. MIC protein expression was demonstrated using a different mAb to that used in our study, E-16, but the specificity of this mAb has not been rigorously defined. Furthermore, functional experiments including blocking mAb to soluble MIC were performed without isotype controls. It would seem highly disadvantageous for a pregnant woman if the placenta was a source of soluble NKG2DL with resulting impairment of her systemic immune responses to tumours and infections. We also explored the possibility that NKG2DLs could be expressed by maternal cells in the decidua because following signalling via TLR, NKG2DLs can be up-regulated on antigen-presenting cells (Draghi et al., 2007). Macrophages and dendritic cells are abundant at the implantation site but were not found to express any NKG2DLs.
We can conclude that in normal pregnancies NKG2DL proteins are not found on any maternal or fetal cell at the implantation site. The situation might change in pathological pregnancies. Although we tested induction with classical stress stimuli such as TLR stimulation and irradiation, these are unlikely to occur very often in the uterus during pregnancy. Transplacental infections generally do not involve the decidua as the pathogens (e.g. human immunodeficiency virus) cross to the fetus via VT from maternal blood in the intervillous space. NKG2DLs could be up-regulated in response to stresses of pregnancy such as hypoxia or aneuploidy (Weier et al., 2005). This could be a mechanism for maternal NK cells to respond to abnormal concepti.
HLA-F is a non-classical HLA-I molecule with a restricted distribution of expression that is mainly not at the cell surface (Wainwright et al., 2000). The divergent trafficking of HLA-F suggests any functions of this molecule may be independent of peptide presentation to T cells (Boyle et al., 2006). HLA-F tetramers have been shown to bind LILRB1 and 2 which are both expressed by decidual leukocytes (Lepin et al., 2000). Previous studies using trophoblast cells are conflicting and have shown either intracellular or surface expression using the mAb 3D11 (Ishitani et al., 2003; Nagamatsu et al., 2006; Shobu et al., 2006). We used a newly available mAb, FG-1, which is highly specific and of high affinity (Lepin et al., 2000). With this reagent weak staining was only found for maternal lymphoid cells, probably CD56+ NK cells. We could demonstrate no surface expression of HLA-F by any cells at the implantation site by flow cytometry and all trophoblast populations were also negative by immunohistochemistry. Thus, at present, there is still only convincing evidence that three HLA-I molecules are displayed by human EVT cells: HLA-C, HLA-E and HLA-G (King et al., 2000; Apps et al., 2007, 2008).
The last NK receptor investigated was 2B4 which was detected on all NK cells and a subset of T cells, confirming previous reports using decidual and blood leukocytes (Vacca et al., 2006; Ma et al., 2007). CD48, the ligand for both 2B4 and CD2 receptors, was not found to be expressed by trophoblast cells. We used two mAbs to CD48 and found different patterns of binding for these reagents, a finding also observed with PBMC (Korínek et al., 1991; Yokoyama et al., 1991; Lo et al., 1998; Morandi et al., 2005; Hernández-Campo et al., 2006). In particular, all T cells bound both mAbs to CD48, but CD56+ NK cells bound only the mAb 6.28. In sections, the cells binding anti-CD48 mAb were aggregated around the spiral arteries that are the focus of trophoblast invasion. These results suggest that CD48–2B4 interactions may occur in the vicinity of spiral arteries at the implantation site, not with trophoblast cells but with other leukocytes. Interestingly, 2B4 ligation can inhibit cytoxicity and IFN-γ production by interleukin-2 stimulated uNK cells (Vacca et al., 2006).
Overall, we cannot find evidence for protein expression of ligands for the NK cell receptors NKG2D and 2B4 on normal first trimester trophoblast cells. Both of these receptors are highly expressed by uNK cells and may function in pathological pregnancies. For example, where concepti are affected by karyotypic or other abnormalities, NKG2DLs might be up-regulated and allow the mother to perceive and reject implantation. In cases of intrauterine infection, NKG2DLs may be induced on other maternal cells in the uterus such as myelomonocytic, glandular or stromal cells and modulate uNK cell function.
Funding
Our laboratory is funded by the Wellcome Trust.
Acknowledgements
We would like to thank all the donors and staff at Addenbrookes hospital, as well as Drs H Reyburn, L Bacon, R Eagle, A Steinle, E Lepin, N Lapaque and D Cosman (Amgen Inc.) for providing reagents.
References
- Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–1937. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Gardner L, Sharkey AM, Hiby SE, Moffett A. Conformation of human leucocyte antigen-C molecules at the surface of human trophoblast cells. Immunology. 2008 doi: 10.1111/j.1365-2567.2007.02789.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon L, Eagle RA, Meyer M, Easom N, Young NT, Trowsdale J. Two human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D. J Immunol. 2004;173:1078–1084. doi: 10.4049/jimmunol.173.2.1078. [DOI] [PubMed] [Google Scholar]
- Borchers MT, Harris NL, Wesselkamper SC, Vitucci M, Cosman D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:222–231. doi: 10.1152/ajplung.00327.2005. [DOI] [PubMed] [Google Scholar]
- Boyle LH, Gillingham AK, Munro S, Trowsdale J. Selective export of HLA-F by its cytoplasmic tail. J Immunol. 2006;176:6464–6472. doi: 10.4049/jimmunol.176.11.6464. [DOI] [PubMed] [Google Scholar]
- Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188:2083–2090. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D, Müllberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- Draghi M, Pashine A, Sanjanwala B, Gendzekhadze K, Cantoni C, Cosman D, Moretta A, Valiante NM, Parham P. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–744. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA. 1996;93:12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8 alpha beta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- Hadam M. Cluster Report: CD48. In: Knapp W, Dorken B, Gilks WR, Rieber P, Schmidt R, Stein H, von dem Borne A, editors. Leucocyte Typing IV. White Cell Differentiation Antigens. Oxford: Oxford University Press; 1989. pp. 661–664. [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hernández-Campo PM, Almeida J, Sánchez ML, Malvezzi M, Orfao A. Normal patterns of expression of glycosylphosphatidylinositol-anchored proteins on different subsets of peripheral blood cells: a frame of reference for the diagnosis of paroxysmal nocturnal hemoglobinuria. Cytometry B Clin Cytom. 2006;70:71–81. doi: 10.1002/cyto.b.20087. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Walker JJ, O’Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;171:1376–1384. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- Jokhi PP, Chumbley G, King A, Gardner L, Loke YW. Expression of the colony stimulating factor-1 receptor (c-fms product) by cells at the human uteroplacental interface. Lab Invest. 1993;68:308–320. [PubMed] [Google Scholar]
- Jokhi PP, King A, Loke YW. Reciprocal expression of epidermal growth factor receptor (EGF-R) and c-erbB2 by non-invasive and invasive human trophoblast populations. Cytokine. 1994;6:433–442. doi: 10.1016/1043-4666(94)90068-x. [DOI] [PubMed] [Google Scholar]
- King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, Hiby SE, McMichael AJ, Loke YW, Braud VM. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korínek V, Stefanová I, Angelisová P, Hilgert I, Horejsí V. The human leucocyte antigen CD48 (MEM-102) is closely related to the activation marker Blast-1. Immunogenetics. 1991;33:108–112. doi: 10.1007/BF00210823. [DOI] [PubMed] [Google Scholar]
- Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepin EJ, Bastin JM, Allan DS, Roncador G, Braud VM, Mason DY, van der Merwe PA, McMichael AJ, Bell JI, Powis SH, et al. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur J Immunol. 2000;30:3552–3561. doi: 10.1002/1521-4141(200012)30:12<3552::AID-IMMU3552>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Lo M, Mouhtouris E, Sandrin MS. CD48 Workshop Panel report. In: Kishimoto T, Kikutani H, von dem Borne A, Goyert SM, Mason D, Miyasaka M, Moretta L, Okumura K, Shaw S, et al., editors. Leucocyte Typing VI. White Cell Differentiation Antigens. USA: Garland Publishing Inc; 1998. pp. 509–511. [Google Scholar]
- Loke YW, King A, Burrows T, Gardner L, Bowen M, Hiby S, Howlett S, Holmes N, Jacobs D. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens. 1997;50:135–146. doi: 10.1111/j.1399-0039.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- Lury D, Epstein H, Holmes N. The human class I MHC gene HLA-F is expressed in lymphocytes. Int Immunol. 1990;2:531–537. doi: 10.1093/intimm/2.6.531. [DOI] [PubMed] [Google Scholar]
- Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Nagaeva O, Chen T, Stendahl U, Antsiferova J, Mogren I, Hernestål J, Baranov V. Placenta-derived soluble MHC class I chain- related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J Immunol. 2006;176:3585–3592. doi: 10.4049/jimmunol.176.6.3585. [DOI] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Morandi B, Costa R, Falco M, Parolini S, De Maria A, Ratto G, Mingari MC, Melioli G, Moretta A, Ferlazzo G. Distinctive lack of CD48 expression in subsets of human dendritic cells tunes NK cell activation. J Immunol. 2005;175:3690–3697. doi: 10.4049/jimmunol.175.6.3690. [DOI] [PubMed] [Google Scholar]
- Nagamatsu T, Fujii T, Matsumoto J, Yamashita T, Kozuma S, Taketani Y. Human leukocyte antigen F protein is expressed in the extra-villous trophoblasts but not on the cell surface of them. Am J Reprod Immunol. 2006;56:172–177. doi: 10.1111/j.1600-0897.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- Parham P, Barnstable CJ, Bodmer WF. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C antigens. J. Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- Parolini S, Bottino C, Falco M, Augugliaro R, Giliani S, Franceschini R, Ochs HD, Wolf H, Bonnefoy JY, Biassoni R, et al. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med. 2000;192:337–346. doi: 10.1084/jem.192.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol. 2006;67:188–195. doi: 10.1016/j.humimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Sharkey AM, Gardner L, Hiby S, Farrell L, Apps R, Masters L, Goodridge J, Lathbury L, Stewart CA, Verma S, et al. KIR expression in uterine NK cells is biased towards recognition of HLA-C and alters with gestaional age. J Immunol. 2008 doi: 10.4049/jimmunol.181.1.39. in press. [DOI] [PubMed] [Google Scholar]
- Shobu T, Sageshima N, Tokui H, Omura M, Saito K, Nagatsuka Y, Nakanishi M, Hayashi Y, Hatake K, Ishitani A. The surface expression of HLA-F on decidual trophoblasts increases from mid to term gestation. J Reprod Immunol. 2006;72:18–32. doi: 10.1016/j.jri.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Sutherland CL, Chalupny NJ, Schooley K, VandenBos T, Kubin M, Cosman D. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J Immunol. 2002;168:671–679. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Phillips JH, Lanier LL, Nichols KE. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J Immunol. 2000;165:2932–2936. doi: 10.4049/jimmunol.165.6.2932. [DOI] [PubMed] [Google Scholar]
- Trundley A, Gardner L, Northfield J, Chang C, Moffett A. Methods for isolation of cells from the human fetal-maternal interface. Methods Mol Med. 2006;122:109–122. doi: 10.1385/1-59259-989-3:109. [DOI] [PubMed] [Google Scholar]
- Vacca P, Pietra G, Falco M, Romeo E, Bottino C, Bellora F, Prefumo F, Fulcheri E, Venturini PL, Costa M, et al. Analysis of natural killer cells isolated from human decidua: evidence that 2B4 (CD244) functions as an inhibitory receptor and blocks NK-cell function. Blood. 2006;108:4078–4085. doi: 10.1182/blood-2006-04-017343. [DOI] [PubMed] [Google Scholar]
- Valés-Gómez M, Browne H, Reyburn HT. Expression of the UL16 glycoprotein of Human Cytomegalovirus protects the virus-infected cell from attack by natural killer cells. BMC Immunol. 2003;4:4. doi: 10.1186/1471-2172-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valés-Gómez M, Winterhalter A, Roda-Navarro P, Zimmermann A, Boyle L, Hengel H, Brooks A, Reyburn HT. The human cytomegalovirus glycoprotein UL16 traffics through the plasma membrane and the nuclear envelope. Cell Microbiol. 2006;8:581–590. doi: 10.1111/j.1462-5822.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397–1406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright SD, Biro PA, Holmes CH. HLA-F is a predominantly empty, intracellular, TAP-associated MHC class Ib protein with a restricted expression pattern. J Immunol. 2000;164:319–328. doi: 10.4049/jimmunol.164.1.319. [DOI] [PubMed] [Google Scholar]
- Weier JF, Weier HU, Jung CJ, Gormley M, Zhou Y, Chu LW, Genbacev O, Wright AA, Fisher SJ. Human cytotrophoblasts acquire aneuploidies as they differentiate to an invasive phenotype. Dev Biol. 2005;279:420–432. doi: 10.1016/j.ydbio.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Welte SA, Sinzger C, Lutz SZ, Singh-Jasuja H, Sampaio KL, Eknigk U, Rammensee HG, Steinle A. Selective intracellular retention of virally induced NKG2D ligands by the human cytomegalovirus UL16 glycoprotein. Eur J Immunol. 2003;33:194–203. doi: 10.1002/immu.200390022. [DOI] [PubMed] [Google Scholar]
- Wiemann K, Mittrücker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Staunton D, Fisher R, Amiot M, Fortin JJ, Thorley-Lawson DA. Expression of the Blast-1 activation/adhesion molecule and its identification as CD48. J Immunol. 1991;146:2192–2200. [PubMed] [Google Scholar]
- Zhang Y, Zhao A, Wang X, Shi G, Jin H, Lin Q. Expressions of natural cytotoxicity receptors and NKG2D on decidual natural killer cells in patients having spontaneous abortions. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.08.009. in press. [DOI] [PubMed] [Google Scholar]