Abstract
BACKGROUND
Letrozole is the third-generation aromatase inhibitor (AI) most widely used in assisted reproduction. AIs induce ovulation by inhibiting estrogen production; the consequent hypoestrogenic state increases GnRH release and pituitary follicle-stimulating hormone (FSH) synthesis.
METHODS
A systematic search of the literature was performed for both prospective and retrospective studies. Meta-analyses of randomized clinical trials (RCTs) were performed for three comparisons: letrozole versus clomiphene citrate (CC), letrozole + FSH versus FSH in intrauterine insemination (IUI) and letrozole + FSH versus FSH in IVF. In the absence of RCTs, non-randomized studies were pooled.
RESULTS
Nine studies were included in the meta-analysis. Four RCTs compared the overall effect of letrozole with CC in patients with polycystic ovary syndrome. The pooled result was not significant for ovulatory cycles (OR = 1.17; 95% CI 0.66–2.09), or for pregnancy rate per cycle (OR = 1.47; 95% CI 0.73–2.96) or for pregnancy rate per patient (OR = 1.37; 95% CI 0.70–2.71). In three retrospective studies which compared L + FSH with FSH in ovarian stimulation for IUI, the pooled OR was 1.15 (95% CI 0.78−1.71). A final meta-analysis included one RCT and one cohort study that compared letrozole + gonadotrophin versus gonadotrophin alone: the pooled pregnancy rate per patient was not significantly different (OR = 1.40; 95% CI 0.67–2.91).
CONCLUSIONS
Letrozole is as effective as other methods of ovulation induction. Further randomized-controlled studies are warranted to define more clearly the efficacy and safety of letrozole in human reproduction.
Keywords: androgens, FSH, IVF
Introduction
Ovulation induction regimens are widely used in assisted reproduction techniques to treat infertility. Pulsatile administration of GnRH was established as an effective and safe means of treating hypogonadotrophic hypogonadal women (WHO class I) (The ESHRE Capri Workshop Group, 1995). However, in patients with chronic anovulation who have adequate serum estrogen levels, and follicle-stimulating hormone (FSH) and prolactin (PRL) within normal limits, with or without and clinical or biochemical hyperandrogenism (WHO class II), one therapeutic option would be to block estrogen action at the central level.
Clomiphene citrate (CC) is a non-steroidal selective estrogen receptor modulator, which acts primarily by binding with estrogen receptors at the hypothalamus (Kurl and Morris, 1978). This competitive inhibition results in a perceived drop of circulating estrogen to the hypothalamus, eventually leading to increased gonadotrophin secretion and subsequent induction of ovulation (Kerin et al., 1985). Augmenting endogenous FSH with CC treatment is associated with a risk of ovarian hyperstimulation syndrome and multiple gestations (Fisher et al., 2002). Although CC results in ovulation in most patients, the pregnancy rates are disappointing. This has been attributed to its peripheral antiestrogenic effects, mainly on the quality or quantity of cervical mucus, and endometrial growth and maturation (Fritz et al., 1991) that could prevent pregnancy in the face of successfully induced ovulation. Long-lasting estrogen receptor depletion has been involved in the antiestrogenic mechanism of action of CC. It also appears that CC accumulates in the body because of its long half-life. Because of these problems, the concept of aromatase inhibition was proposed as a new method of ovulation induction that could avoid many of the adverse effects of CC (Mitwally and Casper, 2000).
Aromatase is a microsomal member of the cytochrome P450 hemoprotein-containing enzyme complex superfamily (P450arom, the product of the CYP19 gene) that synthesizes estrogens by catalyzing three consecutive hydroxylation reactions converting C19 androgens to aromatic C18 estrogenic steroids. Aromatase converts androstenedione to estrone and testosterone to estradiol. Its activity can be demonstrated in several tissues, including the ovaries, brain, placenta, adipose tissue, muscle, liver, breast and estrogen-dependent breast cancer. Aromatase is expressed in a tissue-specific manner. This enzyme is mainly expressed in the ovaries of premenopausal women. A very high level of aromatase is expressed in placenta in pregnant women. In post-menopausal women, the main source of estrogens is the adipose tissue (Cole and Robinson, 1990).
Estradiol is produced by the ovarian granulosa cells and exerts a negative feedback effect on FSH release from the pituitary gland. When aromatization of androgens to estrogens is inhibited, a reduction of circulating estrogens causes modifications in the hypothalamic–pituitary–ovary axis, including:
release of the hypothalamic–pituitary axis from estrogenic negative feedback and FSH secretion is increased, with the resultant stimulating effect on the growth of ovarian follicles.
increase of intraovarian androgens secondary to aromatase inhibition. A transient androgenic environment due to relatively short half-life (∼45 h) seems to augment follicular sensitivity to FSH. The concept that androgens actually enhance early follicular growth is becoming increasingly important (Weil et al., 1998).
aromatase inhibitors (AIs) do not antagonized estrogen receptors in the brain and, therefore, feedback central mechanisms remain intact. The initiation of follicle growth accompanied by increasing concentrations of estrogens results in normal negative feedback loop that limits FSH response and atresia of small follicles, generally leading to mono-ovulatory cycles (Casper and Mitwally, 2006).
The third-generation AIs include two non-steroidal inhibitors, anastrozole and letrozole, and a steroidal agent, exemestane. Anastrozole and letrozole are selective AIs. They are reversible and highly potent. These agents have been used as an adjunct treatment for breast cancer in post-menopausal women. First-generation (aminoglutethimide) and second-generation (fadrozol and formestane) AIs are no longer used because of problems caused by their low potency, lack of specificity and side effects, especially with aminoglutethimide (Holzer et al., 2006). Conclusive data regarding the optimal doses of AIs in reproductive medicine are lacking. In most studies, letrozole has been administered at once-daily doses of 2.5–5 mg for 5 days. Higher doses are associated with a persistent inhibition of aromatase and a very low estrogen levels to ensure an adequate endometrial growth at the time of ovulation. In the case of anastrozole, sufficient data to determine the optimal dose are not available, although the recommended daily dose of 1 mg in patients with hormone-sensitive breast cancer may be adequate to achieve correct follicular growth (Miller et al., 2000; Holzer et al., 2006).
In in vitro studies, letrozole showed the lowest IC50 and the greatest relative potency, which indicates a higher in vitro inhibitory effect on the enzyme aromatase (Bhatnagar et al., 1990). Human pharmacodynamic studies demonstrated that letrozole reduced specifically and markedly plasma concentrations of estradiol, estrone and estrone sulfate. The administration of this drug, however, had no effect on plasma levels of other steroidal hormones, so that concomitant treatment with corticosteroids or mineralocorticoids is not needed. In none of the studies, treatment with letrozole caused accumulation of androgens, androgen precursors, luteinizing hormone (LH), FSH, thyroid-stimulating hormone (TSH) or renin. Letrozole showed a higher potency in the inhibition of aromatization and distribution of estrogen plasma levels than anastrozole (Bhatnagar et al., 1990).
Following the administration of a single oral dose of letrozole 2.5 mg to healthy subjects, the drug was completely and rapidly absorbed from the gastrointestinal tract, reaching maximum plasma concentrations about 1 h after dosing. The extent of absorption is not significantly affected by food; therefore, letrozole may be taken with or without food. Letrozole showed rapidly and extensively distribution into peripheral tissues. A daily dose of 2.5 mg achieved an apparent stable distribution volume of 1.9 l/kg. In plasma, 60% of letrozole was weakly bound to proteins, mostly albumin. The major route of elimination is via hepatic metabolism to a pharmacologically inactive carbinol metabolite. The drug is excreted mainly via the kidneys. After administration of 2.5 mg 14C-labeled letrozole, ∼90% of radiolabeled letrozole is recovered in urine and only 4% in feces. Of letrozole recovered in urine, >65% corresponds to the glucuronide conjugate of carbinol, 9% to two unidentified metabolites and 5% to unchanged letrozole. Letrozole terminal elimination half-life is about 2 days and steady-state plasma concentration after daily 2.5 mg dosing is reached in 2–6 weeks (Bhatnagar et al., 1990).
In the populations studied (adults between 35 and 80 years of age), changes of pharmacokinetic parameters according to age were not observed. In patients with renal insufficiency, with renal clearance >10 ml/min, or in patients with mild to moderate liver dysfunction, dose adjustments of letrozole were not required (Bhatnagar et al., 1990).
Side effects from letrozole are uncommon and related to suppression of the production of estrogens as a result of aromatase inhibition induced by the drug. Side effects include hot flashes (11%), nausea (7%), fatigue (5%), alopecia and vaginal bleeding, which occur more frequently in breast cancer patients than in women treated for ovulation induction due to differences in the duration of treatment. Finally, administration of cimetidine had no effect on pharmacokinetics of letrozole, and letrozole had no effect on pharmacokinetics of warfarin. However, co-administration with taxoxifen leads to a significant decrease in letrozole plasma levels.
It was postulated that it may be possible to mimic the action of CC without depletion of estrogen receptors by administration of an AI in the early part of the menstrual cycle. Aromatase P450 is an enzyme that catalyzes the production of estrogens (i.e. the conversion of androstenedione and testosterone to estrone and estradiol, respectively). Aromatase is a good target for selective inhibition because estrogen production is a terminal step in the biosynthetic sequence. Inhibition of aromatization will block estrogen production from all sources and release the hypothalamic/pituitary axis from estrogenic negative feedback. The resultant increase in gonadotrophin secretion will stimulate growth of ovarian follicles. Because AIs do not deplete estrogen receptors, as does CC, normal central feedback mechanisms remain intact. As the dominant follicle grows and estrogen levels rise, normal negative feedback occurs centrally, resulting in suppression of FSH and atresia of the smaller growing follicles. A single dominant follicle, and mono-ovulation, should occur in most cases (Casper and Mitwally, 2006).
This interesting therapeutic conception based on the mechanism of action of AIs may be refuted by arguing that selective inhibition of aromatase could result in temporary accumulation of intraovarian androgens because conversion of androgen substrate to estrogen is blocked by aromatase inhibition, which in turn may be particularly deleterious for women with polycystic ovary syndrome (PCOS). However, studies of ovarian follicular development in primates support a stimulatory role for androgens in early follicular growth (Weil et al., 1998). Testosterone was found to augment follicular FSH receptor expression, suggesting that androgens promote follicular growth and estrogen biosynthesis indirectly by amplifying FSH effects (Weil et al., 1999).
It is likely that women with PCOS already have a relative aromatase deficiency in the ovary, leading to increased intraovarian androgens, which leads to the development of multiple small follicles responsible for the polycystic morphology of the ovaries. The androgens may also increase FSH receptors making women with PCOS exquisitely sensitive to an increase in FSH through exogenous administration of gonadotrophins, and hence the high risk of ovarian hyperstimulation syndrome and multiple ovulation (Vendola et al., 1998).
As a result of the mechanisms of action described above, AIs appear as new drugs to induce ovulation in women with normal or increased levels of endogenous estrogens, such as those with PCOS which constitute the largest group of anovulatory patients. The lack of antiestrogenic effect is another interesting characteristic of the mechanism of action of AIs, thus avoiding cervical mucus and endometrial morphology interaction. AIs do not have androgenic, progestagenic or estrogenic activity (Fatemi et al., 2003).The use of AIs represents an important conceptual change in the area of reproductive medicine and offers an interesting therapeutic strategy based on the physiology of the normal ovulatory cycle, which has always been a primary aim for researchers (Guzick, 2007; Wu et al., 2007). Moreover, clinical studies of AIs for ovarian stimulation in IVF have shown that AIs could be a low-cost alternative to natural-cycle IVF in patients who are poor responders to FSH (Goswami et al., 2004; Schoolcraft et al., 2004; Verpoest et al., 2006).
Although AIs offer a reasonably promising, effective and safe option for ovulation induction as single agents or in combination with FSH for assisted reproduction procedures, results of clinical series should be assessed with caution due to limitations related to the small sample sizes and heterogeneity of diseases, which do not allow to draw firm conclusions of the efficacy of these agents. Data of a recent study suggest that the alert of a higher risk of congenital cardiac and skeletal malformations in the newborns conceived after infertility treatment with the AI, letrozole, seems unfounded (Tulandi et al., 2006). Challenging aspects of this new concept of oral ovulation induction open a new era of treatment of infertility in reproductive medicine.
The objective of this review is to provide current data of clinical interest in the following areas: (i) use of letrozole in PCOS, (ii) letrozole plus gonadotrophins in ovarian stimulation for intrauterine insemination (IUI), (iii) letrozole for IVF, (iv) use of letrozole for fertility preservation in oncological patients and (v) safety profile of AIs in ovulation induction. Besides a narrative description of clinically relevant data, a systematic review methodology was adopted and three meta-analyses (letrozole versus clomiphene in PCOS; letrozole combined with FSH versus FSH alone in ovarian stimulation for IUI and letrozole combined with FSH versus FSH alone for IVF) were performed.
Materials and Methods
We identified all English language medical papers published by means of the PubMed electronic database using the following search terms: letrozole, aromatase inhibitors, clomiphene citrate, controlled ovarian stimulation, ovulation induction, intrauterine insemination, in vitro fertilization, ART and PCOS. Cross-references picked-up during the review search were also selected if they were not included initially. Both prospective and retrospective studies were considered. Studies presented at meetings or congresses, with only abstracts available, were not included. Variables included were ovulatory cycles, pregnancy cycle rate and pregnancy patient rate in the letrozole versus CC in patients with PCOS. In the other groups, the only variable included was the pregnancy rate. Electronic versions of the retrieved documents were printed. Relevant studies for the analysis of AIs in PCOS should fulfill the following inclusion criteria: (i) letrozole as the AI study drug, (ii) randomized-controlled clinical trials with clomiphene as the comparator drug and (iii) pregnancy rate as one of the end-points of the trial. Relevant studies for the analysis of AIs in ovarian stimulation for IUI as well as for IVF should meet the following inclusion criteria: (i) letrozole as the AI study drug co-administered with FSH, (ii) randomized and non-randomized designs, (iii) use FSH alone in the comparator arm and (iv) pregnancy rate as one of the end-points of the trial.
A pair of two of the authors (M.A.C. and M.F.) independently assessed every study selected. Doubts were solved by consensus after re-review of the publications. Data entry and statistical analysis was performed with the use of Review Manager software (RevMan 4.2, Cochrane Collaboration, Oxford, UK). Meta-analyses of randomized clinical trials (RCTs) were performed for three comparisons: letrozole versus CC, letrozole + FSH versus FSH in IUI and letrozole + FSH versus FSH in IVF. In the absence of RCTs, non-randomized studies were pooled. Heterogeneity was explored by the chi-squared test and was calculated with the I2 statistics, a transformation of the Q statistics that estimates the percentage of the variation in effect sizes that is due to heterogeneity. When heterogeneity was present, the effects were examined using a random effects model. However, when heterogeneity was not present, the random model was also used because it was considered a more conservative approach, particularly if the number of studies was small. The common odds ratios with 95% confidence intervals (CI) were estimated.
Results
Of a total of 37 citations identified in the initial search, 14 studies were considered potentially eligible to be included in the review for one or both authors. During the second phase of the inclusion process, five studies were excluded because of lack of fulfillment of the inclusion criteria (3 studies) or considered irrelevant for the purpose of the review. Finally, nine studies were included with a total of 2573 women.
Aromatase inhibitors in PCOS
Details of relevant clinical studies of the use of letrozole in women with PCOS are shown in Table I. In the first clinical study of AIs for ovulation induction, 22 women who had failed to respond to CC were treated with letrozole. Twelve women with PCOS received letrozole 2.5 mg daily for 5 days. Ovulation occurred in 75% of patients and pregnancy was achieved in 25% (Mitwally and Casper, 2001). In another study of CC-resistant women with PCOS, letrozole induction of ovulation was associated with an ovulation rate of 54.6% and pregnancy rate of 25% (Elnashar et al., 2006).
Table I.
Data of clinical studies of the use of AIs in women with PCOS.
| First author, year | Study design | Drug, daily dose | Women no. | Cycles no. | Mature follicles or >15 mm no. | Endometrial growth (mm) | Ovulatory cycles (%) | Pregnancy cycle rate (%) | Pregnancy patient rate (%) | Miscarriage (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mitwally, 2001 | Prospective | Letrozole 2.5 mg | 12 | 12 | 2.1 | 8.1 | 75 | 25 | 35 | |
| Al-Omari, 2004 | Prospective, randomized, double-blind | Letrozole 2.5 mg | 22 | 22 | 1.7 | 8.2 | 84.4 | 18.8 | 27 | |
| Anastrazole 1 mg | 18 | 18 | 2.3 | 6.5 | 60.0 | 9.7 | 16.6 | |||
| Atay, 2006 | Prospective, randomized | Letrozole 2.5 mg | 51 | 51 | 1.2 | 8.4 | 82.4 | 21.6 | 21.6 | |
| Clomiphene 100 mg | 55 | 55 | 2.4 | 5.2 | 63.6 | 9.1 | 9.1 | |||
| Bayar, 2006 | Prospective, randomized, double-blind | Letrozole 2.5 mg | 38 | 99 | 1 | 8 | 65.7 | 9.1 | 21.6 | 2.6 |
| Clomiphene 100 mg | 36 | 95 | 1 | 8 | 74.7 | 7.4 | 19.4 | 0 | ||
| Sohrabvand, 2006 | Prospective, randomized, single-blind | Letrozole 2.5 mg + metformin | 29 | 53 | 1.9 | 8.2 | 90.6 | 19 | 34.5 | 0 |
| Clomiphene 100 mg + metformin | 30 | 67 | 1.8 | 5.5 | 80.6 | 7 | 16.7 | 40 | ||
| Badawy, 2007 | Prospective, randomized | Letrozole 5 mg | 218 | 540 | 2.3 | 8.1 | 67.5 | 15.1 | 37.6 | 12.1 |
| Clomiphene 100 mg | 220 | 523 | 3.1 | 9.2 | 70.9 | 17.9 | 42.7 | 9.7 | ||
| Elnashar, 2006 | Prospective | Letrozole 2.5 mg | 44 | 44 | 1.2 | 10.2 | 54.6 | 13.6 | 25 | 0 |
Different studies have assessed the efficacy of AIs for ovulation induction compared with CC. Findings of four prospective randomized studies deserve to be commented on (Atay et al., 2006; Bayar et al., 2006; Sohrabvand et al., 2006; Badawy et al., 2007). In all studies, 2.5 mg letrozole (Atay et al., 2006; Bayar et al., 2006; Sohrabvand et al., 2006) or 5 mg letrozole (Badawy et al., 2007) was administered daily for 5 days. Human chorionic gonadotrophin (hCG) at a dose of 10 000 IU was administered when at least one follicle with a mean diameter ≥18 mm was observed using transvaginal ultrasound. Differences among these studies are mainly related to the selection of patients. In the study of Atay et al. (2006), 106 women with oligoamenorrhea and PCOS were enrolled (55 received CC and 51 letrozole). Results were more favorable in the letrozole group than in the CC group regarding the percentage of ovulatory cycles (82.4% versus 63.6%), pregnancy (21.6% versus 9.1%), monofollicular cycles (1.2 versus 2.4 follicles ≥18 mm on the day of hCG administration) and endometrial thickness (8.4 mm versus 5.2 mm). In the study of Bayar et al. (2006), 36 patients (95 cycles) were given CC and 38 patients (95 cycles) were given letrozole. Differences regarding ovulation rates (74.5% versus 65.7%) or pregnancy rates (7.4% versus 9.1%) were not found, although the percentage of monofollicular cycles was higher in letrozole-treated women in relation to significantly lower estradiol levels on the day of hCG. In the study of Sohrabvand et al. (2006), 59 women with PCOS resistant to CC were treated with the combination of letrozole and meftormin (53 cycles) or CC and metformin (67 cycles). Differences between the study groups included higher endometrial thickness in women treated with letrozole and metformin (8.2 versus 5.5 mm) and higher total estradiol level on day of hCG administration and mean estradiol level per mature follicle in the CC group. Recently, Badawy et al. (2007) studied 438 infertile women (1063 cycles) with PCOS. Patients were randomized to treatment with 5 mg of letrozole daily (218 patients, 540 cycles) or 100 mg of CC daily (220 patients, 523 cycles). In this study, advantage to the use of letrozole over CC as a first-line treatment for induction of ovulation in women with PCOS was not observed as significant differences in ovulatory cycles, pregnancy rates or miscarriage rates were not found. In contrast to previous studies, endometrial thickness at the time of hCG administration was significantly greater in the CC group (9.2 versus 8.1 mm).
One further prospective randomized study used letrozole versus anastrozole for infertility treatment in women with PCOS. In this study, 22 women with PCOS were assigned to letrozole (2.5 mg/day for 5 days) and 18 to anastrozole (1 mg/day for 5 days); in all patients, hCG was administered to trigger ovulation. The ovulation rate was significantly higher in the letrozole group than in the anastrozole group (84.4% versus 60%). Differences in pregnancy were also significant (27% of women in the letrozole group and 16.6% in the anastrozole group) (Al-Omari et al., 2004).
It is remarkable that in none of the aforementioned studies, hyperstimulation syndrome or multiple gestations were reported.
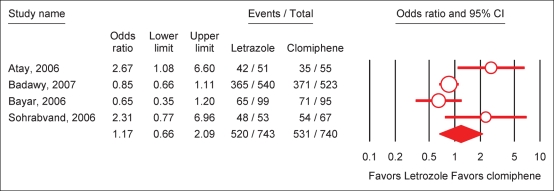
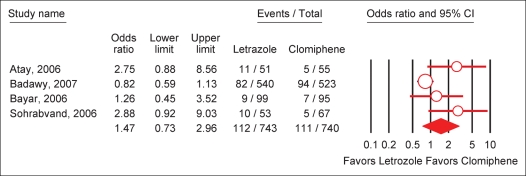
A meta-analysis including the four randomized-controlled studies comparing letrozole and clomiphene was done (Atay et al., 2006; Bayar et al., 2006; Sohrabvand et al., 2006; Badawy et al., 2007). The overall effects of letrozole in comparison with CC in PCOS was neither significant for ovulatory cycles (OR = 1.17; 95% CI 0.66–2.09), nor for pregnancy cycle rate (OR = 1.47; 95% CI 0.73–2.96) and for pregnancy patient rate (OR = 1.37; 95% CI 0.70–2.71) (Figs 1 and 2). For all three outcomes, the I2 was above 50% indicating that the studies were not statistically homogeneous.
Figure 1:
Effect of letrozole on ovulation rate per cycle in PCOS.
Test for heterogeneity: χ2 = 9.54, df = 3 (P = 0.02), I2 = 68.6%. Test for overall effect: Z = 0.53 (P = 0.59).
Figure 2:
Effect of letrozole on pregnancy rate per cycle in PCOS.
Test for heterogeneity: χ2 = 8.03, df = 3 (P = 0.02), I2 = 62.7%. Test for overall effect: 1.07 (P = 0.28).
Aromatase inhibitors plus gonadotrophins in ovarian stimulation for IUI
In studies published in the literature, there are some discrepancies regarding the optimal dose of letrozole, timing of gonadotrophin administration and various aspects related to ovarian and endometrial response.
Al-Fozan et al. (2004) compared the effects of the letrozole (7.5 mg/day) and CC (100 mg/day) in women undergoing ovulation induction and IUI. The pregnancy rate per cycle was similar in both groups, but the number of follicles of ≥14 mm and of >18 mm was higher in women treated with letrozole. In the study of Fatemi et al. (2003), 15 patients undergoing IUI received from Day 3 to Day 7 of the cycle either letrozole 2.5 mg/day (n = 7) or CC 100 mg/day (n = 8). Significantly more follicles (≥17 mm) developed in patients in the CC group compared with those in the letrozole group. In a retrospective analysis, Healey et al. (2003) compared FSH alone or a combination of FSH and letrozole 5 mg/day and also showed that women co-treated with letrozole developed more follicles >14 mm. However, the dose of 2.5 mg/day of letrozole associated with gonadotrophins resulted in a significantly lower number of mature follicles when compared with CC combined with human menopausal gonadotrophin (hMG) (Jee et al., 2006) and FSH alone (Bedaiwy et al., 2007). These data suggest that to obtain more moderate ovarian responses, the optimal dose of letrozole is 2.5 mg/day on Days 3–7 of the menstrual cycle combined with gonadotrophins (preferably FSH).
A summary of the results obtained in women with anovulatory infertility treated with gonadotrophins and those treated with gonadotrophins plus letrozole is shown in Table II. Significant differences in pregnancy outcome between the combined regimen of IAs and FSH versus FSH alone were not observed, although the use of FSH alone was associated with a higher rate of multiple gestations, particularly in anovulatory women (Mitwally et al., 2005a; Bedaiwy et al., 2007). In all three studies, a significantly lower FSH dose was used in the letrozole arm. The pooled mean difference was 691 IU (95% CI 619–764).
Table II.
Comparison of patients (ovulatory infertility) treated with gonadotrophins and those treated with gonadotrophins plus letrozole in ovarian stimulation cycles for IUI.
| Data | Mitwally and Casper (2003) |
Healey et al. (2003) |
Bedaiwy et al. (2007) |
|||
|---|---|---|---|---|---|---|
| Letrozole + FSH | FSH | Letrozole + FSH | FSH | Letrozole + FSH | FSH | |
| Cycles, no. | 36 | 56 | 60 | 145 | 483 | 125 |
| Days of stimulation | 12.5 (1.9) | 11.4 (1.4) | 7.6 (2) | 9.5 (3) | 8.4 (1.7) | 7.7 (2.2) |
| FSH dose, IU | 465 (309) | 1114 (393)* | 600 (405) | 940 (464)* | 394 (355) | 1317 (943)* |
| Mature follicles on hCG day | 3 (1.2) | 2.7 (1.5) | 3.2 (1.2) | 2.2 (1.5) | 2.61 (1.3) | 3.45 (1.7) |
| Estradiol on hCG day, pmol/l | 1540 (877) | 3213 (1483)* | Not stated | Not stated | 1604 (1715) | 2585 (1792)* |
| Endometrial thickness on hCG day, mm | 9.1 (2) | 10 (2) | 8.5 (2.6)* | 9.4 (1.9) | 8.5 (2) | 9 (1) |
| Pregnancy rate, % | 22.2 | 21.4 | 21.6 | 20.9 | 19 | 16 |
| Multiple pregnancy rate, % | Not stated | Not stated | 0 | 5 | 22.5 | 31.2 |
Data as mean (SD) unless otherwise stated.
*P < 0.05 between the groups of letrozole + FSH and FSH alone.
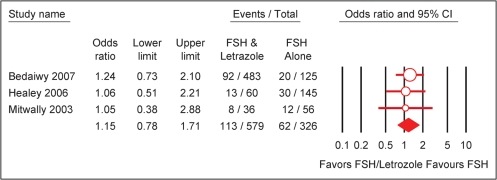
A meta-analysis including the above three retrospective studies comparing letrozole + FSH versus FSH in ovarian stimulation for IUI was done (Healey et al., 2003; Mitwally and Casper, 2003; Bedaiwy et al., 2007). The only variable included was pregnancy rate per cycle. Meta-analysis procedures were similar to those described in the PCOS section. The results showed no significant differences in the outcome variable between letrozole + FSH versus FSH (OR = 1.15; 95% CI 0.78–1.71) (Fig. 3).
Figure 3:
Effect of letrozole on pregnancy rate per cycle in intrauterine insemination.
Test for heterogeneity: χ2 = 0.15, df = 2 (P = 0.93), I2 = 0%. Test for overall effect: Z = 0.70 (P = 0.48).
In a recent study, prospective randomized study in 50 couples with unexplained infertility that failed to conceive after three cycles of CC combined to IUI, Gregoriou et al. (2007) compared ovulation induction either with letrozole (5 mg on Days 3−7 of the cycle) or recombinant FSH (150 IU every 2 days) combined to IUI. In this study, significant differences in pregnancy rate per cycle (8.9% in the letrozole group versus 14% in the gonadotrophin IUI group), cumulative pregnancy rate per couple (24% versus 36%) and the take home baby rate (20% versus 28%) were not observed.
Aromatase inhibitors for IVF
There is little information about the use of AIs for IVF because the number of series published in the literature is small and the majority of studies are non-randomized. In all studies published in this area, letrozole was the AI used.
In a prospective randomized pilot study, Verpoest et al. (2006) showed that response to controlled ovarian stimulation improved with the use of AIs. In normoresponders undergoing an ovarian stimulation protocol with recombinant FSH 150 IU and GnRH antagonist, randomized to receiving letrozole 2.5 mg/day during the first 5 days of stimulation versus no letrozole, a higher number of oocytes retrieved per cycle was documented in the letrozole group (14.8 versus 9.6), although differences were not statistically significant. Pregnancy outcome was similar in both study groups. The use of AIs would allow to correct endometrial asynchrony produced by controlled ovarian stimulation and to improve embryo quality by decreasing the incidence of chromosome aneuploidies in the oocytes retrieved.
A comparison of data reported in the studies of Goswami et al. (2004), Garcia-Velasco et al. (2005) and Schoolcraft et al. (2008) is presented in Table III.
Table III.
Comparison of patients (ovulatory infertility) treated with gonadotrophins and those treated with gonadotrophins plus letrozole in IVF treatment.
| Data |
Goswami et al. (2004) |
Garcia-Velasco et al. (2005) |
Schoolcraft et al. (2008) |
|||
|---|---|---|---|---|---|---|
| Letrozole + FSH | FSH | Letrozole + FSH | FSH | Letrozole + FSH | FSH | |
| Cycles, no. | 13 | 25 | 71 | 76 | 179 | 355 |
| Days of stimulation | Not stated | Not stated | 9.3 (0.3) | 8.9 (0.2) | 9.9 (1.3) | 10.1 (1.6) |
| FSH dose, IU | 150 (0)* | 2865 (/228)* | 3627 (116) | 3804 (127) | 4222 (742) | 3937 (975) |
| Oocytes retrieved | 1.6 (0.8) | 2.1 (/0.7) | 6.1 (0.4) | 4.3 (0.3) | 12 (6) | 13 (5.3) |
| Estradiol on hCG day, pg/ml | 227 (45)* | 380 (46)* | 770 (67) | 813 (60) | 1403 (965)** | 3147 (1189)** |
| Pregnancy rate, % | 23 | 24 | 22.4 | 15.2 | Not stated | Not stated |
| Implantation rate, % | Not stated | Not stated | 25** | 9.4** | 15 | 21 |
| Ongoing pregnancy rate, % | Not stated | Not stated | Not stated | Not stated | 37** | 52** |
Data as mean (SD) unless otherwise stated.
*P < 0.01 and **P < 0.05 between the groups of letrozole + FSH and FSH alone.
Goswami et al. (2004) reported the first RCT to assess whether incorporation of letrozole could be an effective low-cost IVF protocol for poor responders. Women over 35 years of age, who had failed one to three IVF attempts due to poor ovarian response to conventional long GnRH agonist stimulation protocol, were selected for this study. A total of 13 participants were randomized to letrozole 2.5 mg/day from Day 3 to Day 7 of the menstrual cycle and subcutaneous rFSH at a dose of 75 IU/day on Days 3 and 8, while the remaining 25 underwent long GnRH agonist protocol and stimulated with rFSH (300–450 IU/day) (controls). Compared with the control group, the letrozole–FSH group received a significantly lower total dose of FSH and had significantly decreased levels of terminal E2. The two groups did not differ with respect to the numbers of matured follicles, retrieved oocytes, transferable embryos and endometrial thickness. The pregnancy rate/stimulated cycle was also similar. The authors concluded that adjunctive use of letrozole may form an effective means of low-cost IVF protocol in poorly responding women.
Garcia-Velasco et al. (2005) evaluated the use of letrozole as an adjuvant to FSH treatment in IVF cycles of poor responders. To be included in the study, patients had to have at least one previous canceled IVF attempt in which four or fewer follicles 16 mm in diameter were obtained. Women were divided into a control group of 76 patients treated with high-dose gonadotrophins in a GnRH-antagonist regimen, whereas the experimental group of 71 patients received letrozole 2.5 mg plus gonadotrophins for the first 5 days of stimulation followed by the same gonadotrophin/antagonist regimen. The number of oocytes retrieved, androgens intrafollicular levels and implantation rates were significantly higher among letrozole-treated patients. Pregnancy rates were also higher, but differences were not statistically significant.
Schoolcraft et al. (2008) have recently reported the results of a comparison of the efficacy of a microdose GnRH agonist flare with a GnRH antagonist/letrozole protocol before IVF–embryo transfer in poor responders. There were no differences in duration or doses of gonadotrophins required, stimulation days, numbers of oocytes, percentage of mature oocytes obtained, fecundation rate and embryo quality. However, ongoing pregnancy rates were significantly lower in the letrozole protocol than in the microdose GnRH agonist flare protocol (37% versus 52%).
A meta-analysis including two controlled studies (one randomized and one non-randomized) comparing letrozole and FSH was done (Goswami et al., 2004; Garcia-Velasco et al., 2005). The only variable included was pregnancy rate per patient. Significant differences in the pregnancy rate per patients between both treatment modalities were not found (OR = 1.40; 95% CI 0.67−2.91).
Use of letrozole for fertility preservation in oncological patients
The effect of AIs on the production of estrogens has led to their use in combination with low-dose gonadotrophins to decrease estrogens levels (Holzer et al., 2006). The combined protocol of letrozole and low-dose FSH was evaluated in the study of Oktay et al. (2005). A total of 29 patients underwent 33 ovarian stimulation cycles with either tamoxifen 60 mg/day alone or in combination with low-dose FSH or letrozole 5 mg in combination with FSH. After IVF, all resultant embryos were cryopreserved to preserve fertility. Recurrence rates were compared with 31 who elected not to undergo IVF. A significantly greater number of follicles >17 mm, mature oocytes and embryos were observed in both groups of low-dose FSH combined with either tamoxifen or letrozole when compared with tamoxifen alone. However, peak estradiol levels were lower with letrozole + FSH compared with tamoxifen + FSH. Recurrence-free survival analysis did not show differences between the IVF and the control groups.
In a study of the same group published 1 year later (Oktay et al., 2006), Stages I–IIIA breast cancer patients (n = 47) received 5 mg/day letrozole and 150–300 IU FSH to cryopreserve embryos or oocytes. Age-matched retrospective controls (n = 56) were selected from women who underwent IVF for tubal disease. Whereas letrozole and FSH stimulation resulted in significantly lower peak estradiol levels [mean (SD) 483.4 (278.9) versus 1464.6 (644.9) pg/ml] and 44% reduction in gonadotrophin requirement, compared with controls, the number of embryos obtained and fertilization rates were similar. A similar protocol of letrozole started 2 days before gonadotrophin administration and then given concomitantly has been successfully applied in four young patients with endometroid carcinoma undergoing IVF cycles for immediate or delayed embryo transfer to gestational carriers before or after staging and definitive surgery (Azim and Oktay, 2007).
Safety profile of aromatase inhibitors in ovulation induction
Some studies have shown that anastrozole did not produce teratogenic effects in animals embryo development; however, there is a lot of concern related to the inadvertent exposure to letrozole during pregnancy (Tiboni, 2004). The study of Hu et al. (2002) examined how profound changes in androgen/estrogen ratio would affect mouse in vitro follicular development. Arimidex, a potent follicular AI, was used for this purpose. It was found that a pronounced estrogenic environment is not essential for in vitro folliculogenesis. Drastic changes in the intrafollicular steroid concentrations do not disrupt meiotic maturation nor compromise early preimplantation development.
The short half-life of AIs and the administration of these drugs during early follicular phase from Day 3 to Day 7 of the cycle leave a sufficient interval for complete washout to occur before fertilization and implantation. In a phase I study performed to evaluate the safety, pharmacokinetics and pharmacodynamics of anastrozole, 20 women with regular ovulatory cycles received single dose of 5, 10, 15 or 20 mg, and 6 received five daily doses of 10 or 15 mg (Tredway et al., 2004). The pharmacokinetics of anastrozole in this study were linear, predictable and consistent with previously published data from healthy volunteers (Plourde et al., 1994; Boeddinghaus and Dowsett, 2001). In the single-dose groups, anastrozole was well and rapidly absorbed, with Cmax occurring within 2 h. In the multiple-dose groups, the Cmin versus time profiles were consistent with linear kinetics and with a compound with a plasma t1/2 > 30 h. The experiment duration was insufficient to definitively evaluate t1/2 and AUC0–∞; however, the interim parameters AUC0–last and Cmax, and Cmin and Tmax were all considered sufficient to meet the study objective and to confirm the published profile for anastrozole in post-menopausal women (Boeddinghaus and Dowsett, 2001; Plourde et al., 2004).
On the other hand, in the single-dose groups, E2 levels reached their nadir 3–6 h after administration, decreasing by an average of 39% from baseline. FSH levels rose by 13%, 52%, 49% and 75% in the 5, 10, 15 and 20 mg groups, respectively, at ∼24 h after dosing. Most subjects recruited just one mature follicle, with no apparent effect on endometrial maturation. No safety concerns were noted (Tredway et al., 2004). The development of program of the drug remains active.
According to the relatively short half-life of AIs (∼45 h), biological plausibility of the teratogenic effects of AIs when these drugs are used in the early follicular phase can be rejected.
Nevertheless, if AIs are going to be used for ovulation induction, measurement of beta-hCG may be recommended to ensure that candidates to AIs treatment are not pregnant. It is amply demonstrated that neither CC nor AIs including letrozole should be administered in pregnant women. Prospective, randomized studies assessing the potential teratogenic effects of AIs as the primary end-point have not been conducted. In a cohort study comparing the outcome of pregnancies achieved after letrozole and other ovarian stimulation treatments with a control group of pregnancies spontaneously conceived without ovarian stimulation, there were 394 pregnancy cycles in 345 infertile couples (63 pregnancies with 2.5 mg of letrozole alone or with gonadotrophins, 70 pregnancies with 5.0 mg of letrozole, 113 pregnancies with clomiphene alone or with gonadotrophins, 110 pregnancies with gonadotrophins alone and 38 pregnancies achieved without ovarian stimulation). Pregnancies conceived after letrozole treatments were associated with similar miscarriage and ectopic pregnancy rates compared with all other groups (Mitwally et al., 2005a).
A small study presented at the 2005 American Society for Reproductive Medicine meeting suggested that the AI, letrozole, could cause serious fetal anomalies when used off-label for ovulation induction. In that study, Marinko Biljan, MD, director of the Montreal Fertility Center, found a malformation rate of 4.7% among 150 babies born after the use of letrozole, compared with a rate of just 1.8% in a database of 36 050 normal conceptions. An identical number of birth defects in each group was reported, but the incidence of cardiac malformations and malformation of the musculoskeletal system was significantly higher in letrozole-treated group. As a result, the pharmaceutical company (Novartis), which markets the drug as Femara for the treatment of breast cancer, issued global warnings to healthcare professionals about the potential for letrozole to cause embryo and fetus toxicity in premenopausal women, and that the drug should only be used for its primary indication—as breast cancer therapy for post-menopausal women. That warning was probably premature and based on a study with several methodological problems. The main criticism is that the controls were normal deliveries, which are known to have a lower risk of malformations than babies born to women needing assistance to ovulate. The mean (standard deviation, SD) age of women in the letrozole group was 35.2 (4.7) years compared with 30.5 (1.2) in the control cohort. Moreover, cardiac and possibly skeletal abnormalities are likely to be diagnosed before birth, and the mothers transferred to a tertiary care hospital for delivery. Therefore, it is possible that such abnormalities were underrepresented in the control cohort. In addition, only 110 women treated with letrozole gave birth to singleton infants, and it is well known that congenital malformations are more common in twin births than in singletons.
In a large retrospective study conducted in five fertility centers in Canada (Tulandi et al., 2006), the incidence of congenital malformations among offspring of mothers who conceived with CC (n = 397) or with letrozole (n = 514) treatment for infertility was assessed. Overall, congenital malformations and chromosomal abnormalities were found in 14 of 514 newborns in the letrozole group (2.4%) and in 19 of 397 newborns in the CC group (4.8%). The major malformation rate in the letrozole group was 1.2% (6/514) and in the CC group was 3.0% (12/397). In addition, the rate of all congenital cardiac anomalies was significantly higher in the CC group (1.8%) compared with the letrozole group (0.2%) (P = 0.02). On the basis of these data, the concern that letrozole use for ovulation induction could be teratogenic is unfounded.
Discussion
Medical induction of ovulation using CC is currently a first-line treatment modality in women with WHO type II anovulation (Eijkemans et al., 2003). The mechanism of action of CC is related to a negative feedback to the endogenous estrogen, resulting in a higher amplitude of gonadotrophin surges, i.e. LH and FSH. Although CC therapy is associated with a high ovulation rate (60–80% of patients), less than half women become pregnant, with a fecundation rate per cycle of 15% in those in which spontaneous ovulation has been achieved (Garcia et al., 1977). Moreover, the percentage of ovarian hyperstimulation syndrome and multiple gestations is low (Hughes et al., 2000). Discrepancies between ovulation and pregnancy rates as well as the high rate of miscarriage have been attributed to the negative effect of CC on the oocyte, the endometrium and the cervical mucus (Kousta et al., 1997). In the form available for clinical use, CC is a racemic mixture of two stereoisomers, enclomiphene (enC) and zuclomiphene (zuC), they also have vastly different biological half-lives in vivo; enC disappears rapidly from the circulation, whereas zuC is cleared slowly and may accumulate across consecutive cycles of treatment. Unintended, adverse, antiestrogenic effects of CC on the quality and quantity of cervical mucus production or endometrial proliferation and maturation have been related to accumulation of zuC (Young et al., 1999). Morphometric analysis of the endometrium from women with CC-treated cycles revealed abnormal endometrial development as demonstrated by a reduction in glandular density and an increase in the number of vacuolated cells (Sereepapong et al., 2000).
The concept of using AIs for ovulation induction as a new method that could avoid many of the adverse effects of CC has recently been explored. Special emphasis has been placed on the mechanism of action of third-generation AIs compared with CC. Among the AI group, letrozole is the drug most frequently used in all published studies so far. Besides the lack of antiestrogenic effect on the endometrium and cervical mucus, estrogen negative-feedback is not affected by aromatase inhibition, limited FSH response can be a protective factor for multiple ovulation and hyperstimulation syndrome, and accumulation of intraovarian androgens and up-regulation of estrogen receptors augment follicular sensitivity and favor rapid endometrial growth (Casper and Mitwally, 2006). Moreover, given the short half-life compared with CC and the absence of long-lasting antiestrogenic negative effects, clinical use of AIs may be expected to be associated with higher pregnancy rates and/or lower miscarriage rates.
The currently available evidence precludes to draw definite conclusions about outcome of the use of AIs for ovulation induction in PCOS. Methodological limitations are primarily related to the study design (only four studies were prospective and randomized and only two of them were masked for observers and/or patients), small sample sizes, differences in eligibility criteria or sites in which studies were conducted. All studies agree regarding a higher percentage of monofollicular cycles in women treated with AIs compared with CC and, consequently, lower preovulatory estradiol concentrations. The studies are consistent in their findings of the negative impact of CC on endometrial thickness compared with IAs. Surprisingly, in the study of Badawy et al. (2007), endometrial thickness was significantly greater in the CC group than in women given letrozole, which was explained by the authors to more growing follicles and the higher levels of estrogen and progesterone in the CC group, although endometrial thickness in both study groups was >5 or 6 mm. An endometrium that is thinner than 5–6 mm is usually associated with significant likelihood of failure to conceive (Gonen and Casper, 1990). On the other hand, in one study which examined cervical mucus, letrozole, unlike CC, had no adverse antiestrogenic effect (Elnashar et al., 2006). Despite all differences between IAs and CC, comparable ovulation, pregnancy and miscarriage rates were reported in the majority of clinical series, suggesting that success obtained with the use of IAs for ovulation induction in women with PCOS has been lower than expected. A further consideration of the costs of each product (IAs are 10 times more expensive) would probably make CC a more efficient modality of treatment. Finally, the impact of the administration of 10 000 IU hCG when at least one follicle with a mean diameter ≥18 mm is observed in women treated with antiestrogens is unknown, as well as the effect of 2.5 or 5 mg doses of letrozole on the differences in the results obtained. Moreover, whether variations in the established protocol, starting the administration of IAs earlier and/or during a different number of days, may result in better clinical outcomes are unclear.
Although a major advantage of AIs for ovulation induction in women with PCOS is mono-ovulation (Casper, 2003), to ensure multiple ovulation in IUI cycles, addition of a low dose of FSH to the AI is required. The use of an AI in conjunction with gonadotrophins reduces the dose of FSH required to achieve optimum-controlled ovarian stimulation, and this combination has been found to be more cost-effective than FSH alone because of the difference of FSH dose and cost (Bedaiwy et al., 2006; Casper and Mitwally, 2006). In addition, co-treatment with an AI reduces hyperestrogenism derived from ovarian stimulation.
On the basis of the pharmacokinetic characteristics of third-generation AIs, rapidly absorbed after oral administration, terminated elimination half-life about 48 h, and the absence of accumulation of metabolites, the use a single dose of letrozole has been proposed with the aim of achieving maximum estrogenic suppression during the initial phase of the cycle and absence of drug metabolites during fertilization and embryogenesis. In a comparison of a single dose of letrozole of 20 mg with the standard dose of 2.5 mg/day for 5 days, similar results were obtained (Mitwally and Casper, 2001), although more studies are needed to assess the efficacy of this strategy.
Another interesting aspect of the co-administration of letrozole and FSH refers to the day of starting FSH injections. It seems that starting FSH at Day 7 after onset on menses (letrozole is given from Day 3 to Day 7) is an optimal schedule for a cycle sufficiently large to attain total plasma clearance of letrozole, thus minimizing the effects of the drug on the endometrium (Casper, 2003; Mitwally and Casper, 2004). Commencement of FSH treatment before ending letrozole regimen may cause opposite effects (Bedaiwy et al., 2007).
There is no evidence of the negative effect of letrozole on endometrial thickness at the end of the stimulation cycle except for cases in which the dose of letrozole is higher than 2.5 mg and gonadotrophins are initiated before termination of AI treatment.
The ideal regimen of the clinical use of AIs in ovarian stimulation for IUI is the administration of letrozole 2.5 mg/day from Day 3 to Day 7 plus FSH (100 IU/day) starting on Day 8 after onset of menses. However, the amount of FSH added to the letrozole cycle should be flexible (100 IU/day may be too high in some young patients and good responders and too little for poor responders). This schedule favors lower consumption of FSH injections, a more moderate ovarian responses are obtained (lesser mature follicles and lower levels of estradiol), minimizing the effect of letrozole on the endometrium (Healey et al., 2003; Mitwally and Casper, 2003; Bedaiwy et al., 2007). A recent study has shown that ovarian stimulation with letrozole was equally effective to stimulation with gonadotrophins for couples who had failed to conceive after treatment with CC combined with IUI (Gregoriou et al., 2007). The increased risk for ovarian hyperstimulation syndrome and the risk for multiple gestations, combined to the significant cost of the medication, the inconvenience and discomfort experienced by women taking gonadotrophins therapy, makes the use of letrozole an attractive alternative before they proceed with IVF.
The role of AIs in ovarian stimulation regimens in assisted reproduction cycles is controversial. According to lower estradiol levels associated with the use of AIs, these agents may be indicated to reduce the risk for severe ovarian hyperstimulation syndrome. It has been argued that low estradiol concentrations may be effective to suppress premature LH surge (Mitwally et al., 2005b). Secondarily, stimulation of the endogenous production of gonadotrophins through negative feedback mechanisms reduces the use of exogenous gonadotrophins and, consequently, the cost of an IVF treatment cycle (Goswami et al., 2004). In addition, AIs do not deplete estrogenic receptors present in the hypothalamus–pituitary axis and the endometrium (Casper, 2003; Casper and Mitwally, 2006), and besides stimulation of endogeneous gonadotrophins, an intrafollicular androgenic environment induced by AIs (Garcia-Velasco et al., 2005) may enhance follicular response to FSH by overexpression and sensitization of FSH follicle receptors (Weil et al., 1998).
All studies done in IVF protocols have been carried out in patients with poor response to ovarian stimulation. It has been shown that women with low response to gonadotrophin stimulation exhibited a lower expression of FSH receptor on human granulosa cells (Thiruppathi et al., 2001). In contrast, women with ovarian hyperresponse, such as polycystic ovary, show overexpression of FSH follicular receptors. Given that women treated with IAs had temporary accumulation of intraovarian androgens (Webber et al., 2003), it has been postulated that this androgenic intrafollicular environment may improve response to ovarian stimulation in low responders. In a prospective pilot study of low responder patients with a previous canceled IVF cycle (Garcia-Velasco et al., 2005), adding letrozole for the first 5 days of ovarian stimulation in IVF patients, higher levels of follicular fluid testosterone and androstenedione were observed in patients who exhibited a significantly higher number of oocytes retrieved as well as a higher implantation rate, hypothesizing that letrozole may improve the prognosis of these patients. However, no enhancement of pregnancy rates was noted (Garcia-Velasco et al., 2005). In another pilot study, Schoolcraft et al. (2008) compared the efficacy of a microdose GnRH agonist flare with a GnRH antagonist/letrozole protocol before IVF–ET in poor responders. The authors concluded that higher ongoing pregnancy rates and trend toward superior implantation rates would suggest that microdose GnRH agonist flare represents a preferred approach for the poor responder, and attributed the poorer outcome of the GnRH antagonist/letrozole group to the fact that letrozole increases follicular fluid androgen levels, which may have a deleterious effect on oocyte quality. However, methodological shortcomings of the study, including the inclusion of heterogeneous patients and large differences in the treatment protocols may question that differences encountered may solely be attributed to the use of letrozole.
Therefore, the effect of letrozole on the low response may be attributed to two mechanisms: (i) the positive effect that hyperandrogenism at the follicular level may have on the FSH receptors and (ii) the effect of the endogenous secretion of FSH resulting from aromatase inhibition combined with exogenous administration of FSH. In this respect, adjunctive use of letrozole may form an effective means of low-cost IVF protocol in poorly responding women (Goswami et al., 2004).
In summary, prospective, randomized, controlled studies are required to determine the role of letrozole for ovarian stimulation in IVF both in poor responders as in the framework of low-cost IVF protocols.
Another additional indication of the use of letrozole has been ovarian stimulation in patients with cancer to preserve fertility. Co-adjuvant oncological treatments may have a deleterious effects on the patient's fertility, but the incidence of infertility or subfertility in women undergoing cancer treatment varies and depends on many factors (i.e. the effects of chemotherapy and radiation therapy depend on the drug or size/location of the radiation field, dose, dose-intensity oral or intravenous method of administration, disease, age and pretreatment fertility of the patient). With the improvement of life expectancy of cancer-treated patients, fertility preservation is often possible (Lee et al., 2006).
Fertility preservation options for female patients with malignancy include oocyte or embryo cryopreservation before starting chemotherapy or radiation therapy (Oktay, 2006; Seli and Tangir, 2005). Success of treatment partially depends on the number of oocytes retrieved after ovarian stimulation. However, it should be noted that hypothetically, increasing estradiol levels may have adverse effects in patients with estrogen-dependent tumors. Ovarian stimulation with letrozole and FSH appears to reduce estrogen exposure compared with standard IVF, without affecting oocyte quality, fecundation rate and the number of embryos obtained. Even in the case of elevated estrogen levels after oocyte retrieval, the use of letrozole could be maintained for some days to obtain reduction of estrogen levels.
The American Society of Clinical Oncology has recently issued a special article on recommendations for fertility preservation in cancer patients (Lee et al., 2006). It is emphasized that oncologists should refer interested and appropriate patients to reproductive specialists working with institutional review board-approved consents as soon as possible. Some methods of fertility preservation in females require timing with the menstrual cycle, so expeditious referrals are suggested to avoid missing opportunities. When referring patients, however, oncologists should remember that many methods are still investigational.
Conclusions
At the present time, there is insufficient evidence to establish definite recommendations on the use of AIs for ovulation induction. Data of studies already published in the literature should be interpreted considering methodological limitations related to a few prospective randomized designs, small study populations, differences in the doses of letrozole, etc. Therefore, large prospective, randomized, controlled trials are necessary to assess the real benefits offered by letrozole in the treatment of infertility.
In women with PCOS, the percentage of monofollicular cycles obtained in patients treated with AIs is higher than in those treated with CC, as a result of which a lower rate of multiple pregnancies may be expected. When endometrial thickness was examined, most studies agree with the negative impact of CC compared with AIs, except in the study of Badawy et al. (2007) in which endometrial thickness was significantly greater in the CC group. Although intra- and interobserver variability of the technique may account for the differences, findings reported by Badawy et al. should be considered because this study comprises the largest population to date. However, results of the meta-analysis showed that letrozole was not significantly superior to CC in the following variables: ovulatory cycles, pregnancy cycle rate and pregnancy patient rate.
The recommended regimen in ovarian stimulation for IUI includes the use of letrozole 2.5 mg/day (from Day 3 to Day 7 of the cycle) plus FSH (usually 100 IU/day, although doses can vary depending on the characteristics of the patients) starting on Day 8. This schedule favors lower consumption of FSH injections and more moderate ovarian responses are obtained (lesser mature follicles and lower levels of estradiol), minimizing the effect of letrozole on the endometrium.
In IVF treatment, letrozole may reduce the requirements of exogenous gonadotrophins and, consequently, the cost of an IVF treatment cycle. This is particularly important in poor responders. In this group of patients, AIs may have an amplifying effect of the ovarian response. Data of the meta-analysis showed that letrozole increased pregnancy rates, but there was insufficient evidence of a difference with FSH alone. Further prospective, randomized, controlled studies are advisable to determine the role of letrozole for ovarian stimulation in IVF.
Chemotherapy and radiation therapy, with a deleterious effect on the patient's fertility, are frequent modalities of treatment in oncological patients. The use of letrozole has been proposed to decrease estradiol levels in cancer-treated women who want to become pregnant. In patients with estrogen-dependent breast cancer, the addition of letrozole 5 mg/day to gonadotrophins in ovarian stimulation protocols decreases significantly the levels of estradiol without affecting oocyte quality, fecundation rate and number of embryos obtained.
Cohort studies do not show an increase of congenital malformations among offspring of mothers who conceived with letrozole treatment for infertility. Because of the short half-life of AIs, the biological plausibility of the teratogenic effects when these drugs are used in the early follicular phase can be discarded.
Author's Role
Authorship credit should be equally distributed among the authors independently of the order.
Funding
The authors are grateful to Organon Spain for their support of our research. The authors have no other relevant affiliations or financial involvement with other organization or entity.
Acknowledgement
The authors thank Marta Pulido, MD, for editing the manuscript and for editorial assistant.
Conflict of interest: not declared.
References
- Al-Fozan H, Al-Khadouri M, Tan SL, Tulandi T. A randomized trial of letrozole versus clomiphene citrate in women undergoing superovulation. Fertil Steril. 2004;82:1561–1563. doi: 10.1016/j.fertnstert.2004.04.070. [DOI] [PubMed] [Google Scholar]
- Al-Omari WR, Sulaiman WR, Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2004;85:289–291. doi: 10.1016/j.ijgo.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Atay V, Cam C, Muhcu M, Cam M, Karateke A. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34:73–76. doi: 10.1177/147323000603400109. [DOI] [PubMed] [Google Scholar]
- Azim A, Oktay K. Letrozole for ovulation induction and fertility preservation by embryo cryopreservation in young women with endometrial carcinoma. Fertil Steril. 2007;88:657–664. doi: 10.1016/j.fertnstert.2006.12.068. [DOI] [PubMed] [Google Scholar]
- Badawy A, Abdel Aal I, Abulatta M. Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.02.062. doi:10.1016/j.fertnstert.2007.02.062. [DOI] [PubMed] [Google Scholar]
- Bayar U, Tanriverdi HA, Barut A, Ayoğlu F, Ozcan O, Kaya E. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85:1045–1048. doi: 10.1016/j.fertnstert.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Forman R, Mousa NA, Al Inany HG, Casper RF. Cost-effectiveness of aromatase inhibitor co-treatment for controlled ovarian stimulation. Hum Reprod. 2006;21:2838–2844. doi: 10.1093/humrep/del273. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Mousa NA, Esfandiari N, Forman R, Casper RF. Follicular phase dynamics with combined aromatase inhibitor and follicle stimulating hormone treatment. J Clin Endocrinol Metab. 2007;92:825–833. doi: 10.1210/jc.2006-1673. [DOI] [PubMed] [Google Scholar]
- Bhatnagar AS, Häusler A, Schieweck K, Lang M, Bowman R. Highly selective inhibition of estrogen biosynthesis by CGS 20267, a new non-steroidal aromatase inhibitor. J Steroid Biochem Mol Biol. 1990;37:1021–1027. doi: 10.1016/0960-0760(90)90460-3. [DOI] [PubMed] [Google Scholar]
- Boeddinghaus IM, Dowsett M. Comparative clinical pharmacology and pharmacokinetic interactions of aromatase inhibitors. J Steroid Biochem Mol Biol. 2001;79:85–91. doi: 10.1016/s0960-0760(01)00126-1. [DOI] [PubMed] [Google Scholar]
- Casper RF. Letrozole: ovulation or superovulation? Fertil Steril. 2003;80:1335–1339. doi: 10.1016/j.fertnstert.2003.05.004. [DOI] [PubMed] [Google Scholar]
- Casper RF, Mitwally MFM. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91:760–771. doi: 10.1210/jc.2005-1923. [DOI] [PubMed] [Google Scholar]
- Cole PA, Robinson CH. Mechanism and inhibition of cytochromes P-450 aromatase. J Med Chem. 1990;33:2933–2944. doi: 10.1021/jm00173a001. [DOI] [PubMed] [Google Scholar]
- Eijkemans MJ, Imani B, Mulders AG, Habbema JD, Fauser BC. High singleton live birth rate following classical ovulation induction in normogonadotrophic anovulatory infertility (WHO 2) Hum Reprod. 2003;18:2357–2362. doi: 10.1093/humrep/deg459. [DOI] [PubMed] [Google Scholar]
- Elnashar A, Fouad H, Eldosoky M, Saeid N. Letrozole induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome may not depend on the period of infertility, the body mass index, or the luteinizing hormone/follicle-stimulating hormone ratio. Fertil Steril. 2006;85:511–513. doi: 10.1016/j.fertnstert.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Kolibianakis E, Tournaye H, Camus M, Van Steirteghem AC, Devroey P. Clomiphene citrate versus letrozole for ovarian stimulation: a pilot study. Reprod Biomed Online. 2003;7:543–546. doi: 10.1016/s1472-6483(10)62070-6. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Reid RL, Van Vugt DA, Casper RF. A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on ovulatory function in normal women. Fertil Steril. 2002;78:280–285. doi: 10.1016/s0015-0282(02)03241-7. [DOI] [PubMed] [Google Scholar]
- Fritz MA, Holmes RT, Keenan EJ. Effect of clomiphene citrate treatment on endometrial estrogen and progesterone receptor induction in women. Am J Obstet Gynecol. 1991;165:177–185. doi: 10.1016/0002-9378(91)90247-o. [DOI] [PubMed] [Google Scholar]
- Garcia J, Jones GS, Wentz AC. The use of clomiphene citrate. Fertil Steril. 1977;28:707–717. doi: 10.1016/s0015-0282(16)42670-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Moreno L, Pacheco A, Guillén A, Duque L, Requena A, Pellicer A. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82–87. doi: 10.1016/j.fertnstert.2005.01.117. [DOI] [PubMed] [Google Scholar]
- Gonen Y, Casper RF. Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum Reprod. 1990;5:670–674. doi: 10.1093/oxfordjournals.humrep.a137165. [DOI] [PubMed] [Google Scholar]
- Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, Chakravarty BN, Kabir SN. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19:2031–2035. doi: 10.1093/humrep/deh359. [DOI] [PubMed] [Google Scholar]
- Gregoriou O, Vlahos NF, Konidaris S, Papadias K, Dimitrios B, Creatsas GK. Randomized controlled trial comparing superovulation with letrozole versus recombinant follicle-stimulating hormone combined to intrauterine insemination for couples with unexplained infertility who had failed clomiphene citrate stimulation and intrauterine insemination. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.06.099. doi: 10.1016/j.fertnstert.2007.06.099. [DOI] [PubMed] [Google Scholar]
- Guzick DS. Ovulation induction management of PCOS. Clin Obstet Gynecol. 2007;50:255–267. doi: 10.1097/GRF.0b013e31802f361e. [DOI] [PubMed] [Google Scholar]
- Healey S, Tan SL, Tulandi T, Biljan MM. Effects of letrozole on superovulation with gonadotropins in women undergoing intrauterine insemination. Fertil Steril. 2003;80:1325–1329. doi: 10.1016/j.fertnstert.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertil Steril. 2006;85:277–284. doi: 10.1016/j.fertnstert.2005.05.078. [DOI] [PubMed] [Google Scholar]
- Hu Y, Cortvrindt R, Smitz J. Effects of aromatase inhibition on in vitro follicle an oocyte development analyzed by early preantral mouse follicle culture. Mol Reprod Dev. 2002;61:549–559. doi: 10.1002/mrd.10107. [DOI] [PubMed] [Google Scholar]
- Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst Rev. 2000:CD000056. doi: 10.1002/14651858.CD000056. [DOI] [PubMed] [Google Scholar]
- Jee BC, Ku SY, Suh CS, Kim KC, Lee WD, Kim SH. Use of letrozole versus clomiphene citrate combined with gonadotropins in intrauterine insemination cycles: a pilot study. Fertil Steril. 2006;85:1774–1777. doi: 10.1016/j.fertnstert.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Kerin JF, Liu JH, Phillipou G, Yen SS. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61:265–268. doi: 10.1210/jcem-61-2-265. [DOI] [PubMed] [Google Scholar]
- Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update. 1997;3:359–365. doi: 10.1093/humupd/3.4.359. [DOI] [PubMed] [Google Scholar]
- Kurl RN, Morris ID. Differential depletion of cytoplasmic high affinity oestrogen receptors after the in vivo administration of the antioestrogens, clomiphene, MER-25 and tamoxifen. Br J Pharmacol. 1978;62:487–493. doi: 10.1111/j.1476-5381.1978.tb07752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology. Recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- Miller RM, Dixon Antiaromatase agents: preclinical data and neoadjuvant therapy. Clin Breast Cancer. 2000;1(Suppl 1):S9–S14. doi: 10.3816/cbc.2000.s.002. [DOI] [PubMed] [Google Scholar]
- Mitwally MFM, Casper RF. Aromatasa inhibition: a novel method of ovulation induction in women with polycystic ovarian syndrome. Reprod Technol. 2000;10:244–247. [Google Scholar]
- Mitwally MFM, Casper RF. Single dose administration of the aromatase inhibitor, letrozole: a simple and convenient effective method of ovulation induction. Fertil Steril. 2001;76(Suppl 1):S94–S95. [Google Scholar]
- Mitwally MF, Casper RF. Potential of aromatase inhibitors for ovulation and superovulation induction in infertile women. Drugs. 2006;66:2149–2160. doi: 10.2165/00003495-200666170-00001. [DOI] [PubMed] [Google Scholar]
- Mitwally MFM, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;22:61–78. doi: 10.1055/s-2004-823028. [DOI] [PubMed] [Google Scholar]
- Mitwally MF, Biljan MM, Casper RF. Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol. 2005;a 192:381–386. doi: 10.1016/j.ajog.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Mitwally MF, Casper RF, Diamond MP. The role of aromatase inhibitors in ameliorating deleterious effects of ovarian stimulation on outcome of infertility treatment. Reprod Biol Endocrinol. 2005;b 3:54. doi: 10.1186/1477-7827-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K. Options for preservation of fertility in women. N Engl J Med. 2006;29:1418–1420. [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;19:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, Bang H. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- Plourde PV, Dyroff M, Dukes M. Arimidex: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103–111. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- Plourde PV, Reiter EO, Jou HC, Desrochers PE, Rubin SD, Bercu BB, Diamond FB, Jr, Backeljauw PF. Safety and efficacy of anastrozole for the treatment of pubertal gynecomastia: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:4428–4433. doi: 10.1210/jc.2004-0082. [DOI] [PubMed] [Google Scholar]
- Schoolcraft W, Surrey E, Minjarez D, Gardner DK. Antagonist/letrozole protocol for patients failing microdose agonist flare stimulation. Fertil Steril. 2004;78(Suppl 1):S234. [Google Scholar]
- Schoolcraft WB, Surrey ES, Minjarez DA, Stevens JM, Gardner DK. Management of poor responders: can outcomes be improved with a novel gonadotropin-releasing hormone antagonist/letrozole protocol? Fertil Steril. 2008;89:152–156. doi: 10.1016/j.fertnstert.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Seli E, Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005;17:299–308. doi: 10.1097/01.gco.0000169108.15623.34. [DOI] [PubMed] [Google Scholar]
- Sereepapong W, Suwajanakorn S, Triratanachat S, Sampatanukul P, Pruksananonda K, Boonkasemsanti W, Reinprayoon D. Effects of clomiphene citrate on the endometrium of regularly cycling women. Fertil Steril. 2000;73:287–291. doi: 10.1016/s0015-0282(99)00509-9. [DOI] [PubMed] [Google Scholar]
- Sohrabvand F, Ansari SH, Bagheri M. Efficacy of combined metformin–letrozole in comparison with metformin–clomiphene citrate in clomiphene-resistant infertile women with polycystic ovarian disease. Hum Reprod. 2006;21:1432–1435. doi: 10.1093/humrep/del020. [DOI] [PubMed] [Google Scholar]
- The ESHRE Capri Workshop Group. Anovulatory infertility. Hum Reprod. 1995;10:1549–1553. [PubMed] [Google Scholar]
- Thiruppathi P, Shatavi S, Dias JA, Radwanska E, Luborsky JL. Gonadotrophin receptor expression on human granulosa cells of low and normal responders to FSH. Mol Hum Reprod. 2001;7:697–704. doi: 10.1093/molehr/7.8.697. [DOI] [PubMed] [Google Scholar]
- Tiboni GM. Aromatase inhibitors and teratogenesis (Letter) Fertil Steril. 2004;81:1158–1159. doi: 10.1016/j.fertnstert.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Tredway DR, Buraglio M, Hemsey G, Denton G. A phase I study of the pharmacokinetics, pharmacodynamics, and safety of single- and multiple-dose anastrozole in healthy, premenopausal female volunteers. Fertil Steril. 2004;82:1587–1593. doi: 10.1016/j.fertnstert.2004.04.059. [DOI] [PubMed] [Google Scholar]
- Tulandi T, Martin J, Al-Fadhli R, Kabli N, Forman R, Hitkari J, Librach C, Greenblatt E, Casper RF. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85:1761–1765. doi: 10.1016/j.fertnstert.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpoest WM, Kolibianakis E, Papanikolaou E, Smitz J, Van Steirteghem A, Devroey P. Aromatase inhibitors in ovarian stimulation for IVF/ICSI: a pilot study. Reprod Biomed Online. 2006;13:166–172. doi: 10.1016/s1472-6483(10)60611-6. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–2485. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- Wu HH, Wang NM, Cheng ML, Hsieh JN. A randomized comparison of ovulation induction and hormone profile between the aromatase inhibitor anastrozole and clomiphene citrate in women with infertility. Gynecol Endocrinol. 2007;23:76–81. doi: 10.1080/09513590601137509. [DOI] [PubMed] [Google Scholar]
- Young SL, Opsahl MS, Fritz MA. Serum concentrations of enclomiphene and zuclomiphene across consecutive cycles of clomiphene citrate therapy in anovulatory infertile women. Fertil Steril. 1999;71:639–644. doi: 10.1016/s0015-0282(98)00537-8. [DOI] [PubMed] [Google Scholar]