Abstract
Advances in neuroscience have resulted in the development of new diagnostic and therapeutic agents for potential use in the central nervous system (CNS). However, the ability to deliver the majority of these agents to the brain is limited by the blood–brain barrier (BBB), a specialized structure of the blood vessel wall that hampers transport and diffusion from the blood to the brain. Many CNS disorders could be treated with drugs, enzymes, genes, or large-molecule biotechnological products such as recombinant proteins, if they could cross the BBB. This article reviews the problems of the BBB presence in treating the vast majority of CNS diseases and the efforts to circumvent the BBB through the design of new drugs and the development of more sophisticated delivery methods. Recent advances in the development of noninvasive, targeted drug delivery by MRI-guided ultrasound-induced BBB disruption are also summarized.
Introduction
Getting drugs and genes into the brain is a tall order [1]
Advances in neuroscience have resulted in the development of new diagnostic and therapeutic agents for potential use in the central nervous system (CNS). However, the ability to deliver the majority of these to the brain is limited by the blood–brain barrier (BBB), a specialized structure of the blood vessel wall that hampers transport and diffusion from the vasculature to the brain.
The BBB is formed by the brain capillary endothelium and excludes from the brain more than 98% of all small-molecule drugs and approximately 100% of large-molecule neurotherapeutics [2]. Only small- molecule drugs with high lipid solubility and a low molecular mass under a 400–500 Da can cross the BBB in pharmacologically significant amounts[3]; only a few diseases such as depression, affective disorders, chronic pain, and epilepsy, consistently respond to lipid-soluble small- molecule drugs [4,5]. There is no therapy for neurodegenerative diseases such as Alzheimer’s disease and Huntington’s disease, as well as for amyotrophic lateral sclerosis. Although the patients with multiple sclerosis (MS) are treated with cytokines that work on the peripheral immune system, cytokines do not stop the progression of MS within the central nervous system (CNS) [3]. There is no effective therapy for serious life-threatening CNS disorders such as brain cancer, stroke, brain and spinal cord trauma, HIV infection, either. Although L-Dopa therapy has been used for decades to treat Parkinson’s disease, there is no neuroprotective drug available to stop the neurodegeneration caused by this disorder. Nor is there effective treatment for the childhood disorders including autism, lysosomal storage disorders, fragile X syndrome, the ataxis, and blindness [2]. Many of these disorders could be treated with drugs, enzymes, genes, or large-molecule biotechnological products such as recombinant proteins. However, these drugs do not cross the BBB.
Blood Brain Barrier
Definition of the Blood-Brain Barrier (BBB)
The blood-brain barrier (BBB), the brain’s first line of defense from harmful substances in the blood stream, is composed of specialized capillaries. The capillary network in the brain is dense (it forms an area of about 20m2/1300-g human brain [6] and so intricate that no neuron or glial cell is more than 20 μm from neighboring capillary, so that every neuron is perfused by its own micro- vessel [7]. The brain capillary walls form a broad but thin barrier system, which is lined with single continuous layer of endothelial cells. The thickness of the endothelial cell is 200 nm; transport across the BBB involves movement across two membranes: the luminal and abluminal membranes of the capillary endothelium. The adjacent endothelial cells are cemented together by the tight junctions, the presence of which prevents a para-cellular pathway. Circulating molecules can only gain access to brain via a transcellular route through the brain capillary endothelial cells. The BBB structures also include a basal (basement) membrane supporting the ablumenal surface of the endothelium, pericytes, and astrocytes. Nearly 100% of the surface area of the capillary basement membrane is covered by end-feet of processes originating from brain astrocytes. In fact, the endothelium, the pericyte, and the astrocyte foot processes work in concert to tightly regulate the flux of molecules between blood and brain across the microvascular barrier. It is noteworthy that the BBB can even function under deleterious circumstances. For example, following stroke or brain trauma, though BBB function is initially disturbed, it can be restored within hours [8]. Likewise, the BBB is at least partially intact in tumors. While the BBB is often leaky in the malignant brain tumor center, the well-vascularized actively proliferating tumor edge and the normal brain tissue adjacent to the tumor have complex barrier integrity [9].
Transport across the BBB
Free Diffusion of Small Molecules: Certain small molecules can traverse the BBB via lipid-mediated transport. Lipid mediation of small molecules through biological membranes requires molecular movement through spaces within the lipid bilayer, and these spaces have a finite size. As a general rule, only lipid soluble (lipophilic) molecules with a molecular mass of less than 400 Da can cross from blood to brain. Otherwise, different molecules may gain access to the brain only via certain endogenous transport systems within the BBB. This occurs mostly through carrier-mediated transport (CMT) or receptor-mediated transport (RMT). Carrier- Mediated Transport (CMT): Small water-soluble nutrients and vitamins traverse the BBB via carrier-mediated transport (CMT). The CMT systems are also portals of entry for small-molecule drugs (for example, L-DOPA) that have a molecular structure similar to endogenous nutrients (transporter recognizes this structure). Receptor- Mediated Transport (RMT): Certain large molecule peptides or plasma proteins are selectively transported across the BBB via receptor- mediated transport systems. RMT is comprised of three sequential steps: (1) receptor-mediated endocytosis of the circulating peptide at the luminal membrane of the capillary endothelium; (2) movement through 200–300nm of endothelial cytoplasm; (3) exocytosis of the peptide into the brain interstitial fluid at the abluminal membrane of the capillary endothelium [10].
Trans- BBB Delivery Strategies
The traditional approaches for the drug delivery in the brain are small-molecule drugs, trans-cranial drug delivery via an invasive catheter, and BBB disruption. As we mentioned before, most small-molecule drugs do not cross the BBB, and only a few neurological diseases respond to small- molecule drugs [2]. Trans-cranial brain drug delivery requires an invasive catheter to be placed in the brain using one of three neurosurgical-based approaches: intracerebral implantation [11–13], intracerbroventricular infusion (ICV) [14,15], or convection enhanced diffusion (CED) [16–20]. While the intracerebral implantation and ICV infusion rely on passive diffusion of drugs into the brain, the CED forces fluid drugs through the brain. The limited penetration of drugs into the brain from the depot site and their rapid elimination by active transport from the brain tissue into the blood pose serious limitations with these methods [2,21,22]. So, to improve survival from gliomas (the most aggressive CNS malignant cancer) investigators have examined approaches ranging from direct intratumoral injections to implantable computer-driven constant infusion pumps and biocompatible/biodegradable devices bearing an anticancer drug such as a membranous sheet containing doxorubicin [23]. However, the prognosis of patients with malignant glioma is still very poor [24]. The mechanical delivery of drugs into the brain has also been hampered by serious drawbacks (for a review, see [25]). For example, CED has shown a preferential flow of the forced fluid along white matter tracts [18] leading to diffuse astrogliosis [16] and raised concerns regarding long-term effects of this delivery approach for humans [22].
Alternatively, to allow treatment with pharmacological agents, the BBB can be reversibly disrupted by intracarotid arterial infusion of noxious agents such as hyperosmolar solutions [26–29], vasoactive agents [30], solvents [31,32], akylating agents [33,34], immune adjuvants [35], cytokines [36,37], and other miscellaneous agents [38,39] (see review [2]). The major limitation is that BBB disruption causes a non-localized drug delivery and an increase in brain uptake of plasma albumin and other protein components of blood, which are toxic to brain cells [40]. The side effects include the potential risk of neuronal damage [41].
New drug delivery strategies have emerged from an understanding of the molecular and cellular biology of the brain microvasculature and BBB transport processes. Several approaches to overcome the BBB have been developed (for reviews [42–45]). Some methods have focused on modifying the agents to allow them to penetrate the BBB, while others have used drug carriers such as liposomes and nanoparticles [46,47], or conjugated the drugs with a protein [48], a peptide vector [49,50], or with an antibody and used BBB endogenous carrier system to ferry drug molecules across the BBB [51]. For example, drugs can be made transportable across the BBB by using “chimeric peptide” technology [52,53]. A chimeric peptide is formed when a small- or large-molecule drug that is normally not transported across the BBB is fused or conjugated to BBB transport vector that undergoes receptor-mediated transcytosis through the BBB, and which acts as a ”molecular Trojan horse” [1,41,54,55]. The discovery of “Trojan horse” peptides in the 90’s and the demonstration that proteins fused to the transport vector protein are capable of crossing the BBB raised hopes that it may have a huge impact on neurobiological therapy. However, it was only recently that delivery of proteins with therapeutic potential has been achieved in animal models of some human neurological disorders [41]. More studies must be undertaken which include genetically engineered fusion proteins or humanized antibodies for BBB drug targeting with reduced immunogenicity and minimized working burden in the chemical conjugation [56]. The limitation also is that only a finite amount of drug can be delivered due to a limited number of receptors and the fact that only a discrete quantity of molecules can be attached to a carrier. Furthermore, despite a high degree of target selectivity, the use of targeted therapies often has systemic toxicity [57].
Focused Ultrasound
Current advances in acoustic technology have made ultrasound a modality with high therapeutic and diagnostic applicability. Focused ultrasound techniques allow the concentration of acoustical energy in a focal spot deep in the body with minimal effects to the near-field tissue. It is a method of non-invasively inducing local biological effects deep inside the body without surgical intervention. Ultrasound-induced effects can be produced by two major mechanisms: thermal and non-thermal (such as radiation pressure and cavitation). Relatively low-level temperatures (~43°C) maintained for 30–60 min can be used for hyperthermia [58–61] to sensitize tumors to chemotherapy and radio-therapy. Using higher temperatures (>60°C), FUS is used as a thermal ablation method to treat tumors in many organs, including prostate, liver, kidney, breast, bone, uterus and pancreas [62–72] or as a technique of thermal coagulation of blood vessels [73–78]. One has great flexibility in designing ultrasonic applicators; spherical radiators, lenses, reflectors [58], or phased arrays [79] to tailor a focal zone to almost any application.
One major shortcoming of ultrasound is that it is strongly attenuated by bone; hence, for brain applications, an acoustic window must be made by a craniotomy. However, the experimental and theoretical studies [80–85] have shown that focal, trans-skull ultrasound exposure of brain tissue may be accomplished by using large surface area phased arrays and information derived from modern imaging methods to correct the ultrasound wave distortion produced by the skull These studies have also indicated that optimal trans-cranial focusing can be achieved at frequencies below 1 MHz[82]. By reducing the frequency to approximately 250kHz the need for patient specific correction is eliminated with the cost of a larger focal spot and reduced pressure gain [86]. Recently developed image-guided focused ultrasound clinical systems make it possible to deliver ultrasound to the targeted regions in the brain through the intact skull, and the animal experiments as well as the clinical trials showed encouraging results [62,85,87,88].
FUS-induced targeted BBB disruption may offer a solution to the problems of the delivery of either small or large molecule drugs to the brain, and FUS-based techniques could enter clinical drug development programs for most CNS disorders.
BBB disruption via ultrasound alone (a short history)
Several studies have shown that ultrasound-induced effects can result in localized BBB disruption, either accompanied by tissue necrosis or, in some cases, without any evident tissue damage at all. The very first study of BBB permeability following ultrasonic irradiation was provoked by the publication by Barnard, Fry, and Brennan [89] (University of Illinois) of results of histological studies of the lesions produced by FUS in the brain. They stated that blood vessels “appeared not to be altered morphologically by ultrasound”. In response, the rival team from MGH (Boston, MA) performed the study of the vasculature changes in the FUS-produced lesions and published their results the following year. By using a vital dye trypan blue and radioactive phosphate as tracers they demonstrated that despite “the lack of visible changes in structure of irradiated capillaries, there is evidence of a profound disturbance in capillary blood-brain barrier permeability, as evidenced by the rapid and massive deposition in the brain of trypan blue and radioactive phosphate” [90]. The BBB appeared to be altered, made easily permeable within the area damaged by FUS; this area was turned into “a non-barrier-protected region”, as occurs in such cases as cerebral injures (i.e. trauma, heat, and chemical, toxic, or allergic reactions) [90]. Since then, the Bakay’s technique of the use of the vital dye trypan blue has been widely used for detecting FUS-produced lesions [91–95] and verifying BBB disruption [91,96–101]. Later, Patrick et al. [102] noted that the BBB is also disrupted at the periphery of the ultrasound-produced lesions and suggested that this disruption could enhance the ultrasound treatment of brain tumors through combination of FUS-produced tumor ablation with an antineoplastic agents delivered via an ultrasonically modified BBB.
The ultrasound potential for BBB disruption without producing lesion was first reported by Ballantine, Bell, and Manlapaz [91]. By using a high-power defocused ultrasonic beam (frequency: 2.7 MHz, intensity: 4000 W/cm2, pulse width: 0.3s, pulse period: 1.0 s, pulse number: 15) they affected the BBB with a negligible damage to the surrounding parenchyma. Although the parenchyma appeared to be intensively stained with Trypan Blue and there were hemorrhagic areas in the sonicated regions, they found no evidence of discrete lesions. These results led them to the conclusion that it may be possible to select ultrasound parameters that allow for a BBB disruption without risk of producing lesions. The next step was taken by Vykhodtseva, who did not try to select US parameters to disrupt BBB, but vice versa, was intending to find exposure parameters for producing small, finely-controlled lesions in the deeply-located brain structure, that has been known to be responsible for Parkinson’s disease syndromes. She used a variety of short burst durations and pulse repetition frequencies at high acoustic intensity level as well as the Bakay’s technique to visualize FUS-produced effects in rabbit brains. However, in many cases, she observed areas that were diffusely stained with trypan blue and that sometimes contained tiny red blood cell extravasations without any discrete lesion. Attempts to produce lesions by increasing pulse duration resulted in hemorrhages and ill-controlled tissue disintegration. The disappointment was great and she published her findings only several years later [96]. That time she described these effects as BBB disruption, presumably caused by cavitation. Later, she returned to the study of the bio-effects produced by short pulses of FUS at intensity levels above the cavitation threshold in rabbit brain in vivo [103]. These effects included BBB disruption at the targeted locations without damage to the brain parenchyma (as detected by the histology evaluation). However, the parameters that would produce such disruption consistently have not been found. Continuing the attempts to disrupt BBB by FUS, in 1998–1999, Hynynen, Vykhodtseva, and McDannold performed extensive MRI/histological study on the brains of rabbits. They tested a wide range of FUS parameters at high acoustical power levels (up to 700 W) with a sharply focused transducer (f-number 0.8) and observed a variety of effects but failed to produce consistent BBB disruption (unpublished data) and accepted a limitation of this approach. More recently, Mesiwala et al. [104] reported similar findings in rat brain. In that work, they too found that it was sometimes possible to produce BBB disruption without visible damage to the brain parenchyma.
BBB disruption via ultrasound combined with an ultrasound contrast agent
A new approach to solving the problem of targeted BBB disruption has been developed by Hynynen et al [97]. They suggested to use focused ultrasound in combination with encapsulated gas-filled microbubbles (<8 μm diameter), commercially available as US contrast agents, instead to generate the bubbles in the brain blood vessels. Indeed, if the BBB disruption is related to an interaction between the ultrasound field and microbubbles, the acoustic energy needed to produce this interaction will be greatly reduced, since there is no need to generate the bubbles that requires high intensities [103]. This point makes the procedure more practical for application through the intact skull, since risks of overheating the skull would be greatly reduced. Finally, using these agents limits the interaction of the ultrasound to the endothelial cells reducing the chance of damage to other brain structures.
In the initial work, sonications at 1.63 MHz combined with the US contrast agent Optison (GE Healthcare, Milwaukee, WI) introduced by IV injection were tested using MRI (contrast enhanced imaging) and histology [97]. It was found that pulsed sonication: 100 ms pulses delivered at a repetition frequency of 1 Hz for 20s at pressure amplitudes of 0.7 and 1 MPa, repeatedly produced BBB disruption in the targeted locations of the rabbit brain. These pressure amplitudes are approximately two orders of magnitude lower than needed to produce thermal damage to brain under similar experimental conditions [105]. Lowering the pulse duration to 10 ms produced similar effects. The BBB was open at approximately 2h and closed at 48h after sonication as confirmed with the contrast enhanced MR imaging. In histology, the effects were found to be mostly related to tiny extravasations of red blood cells scattered around the sonicated areas indicating few affected capillaries. The evaluation for ischemia (Vanadium acid fuchsin, VAF staining) and apoptosis (ApopTag, CHEMICON International, Inc., CA), the effects one might expect if any even subtle damage to microcirculation occurred, detected only a few cells with evidence for apoptosis or ischemia indicating that the effects to the brain were negligible [106,107]. To test the method for safety, the long-term effects of these sonications have been investigated and a follow-up studies were performed using the parameters that did not produce neuronal damage [107]. No delayed effects were observed either by MRI or histology up to 4 wk after sonication, and no ischemic or apoptotic regions were detected that would indicate a compromised blood supply was induced by the sonications. Thus, the effects to the brain were found to be minimal – certainly less than what one would expect from any invasive intervention to deliver drugs.
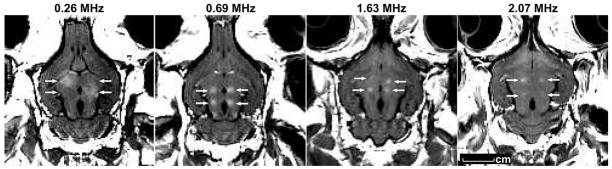
The frequency of 1.63 MHz tested in the initial studies, is not optimal for transcranial ultrasound application due to (i) small focal region volume; (ii) distorting effects of the skull bone on the ultrasound beam; and (iii) possible overheating due to high absorption of the ultrasound by bone. Thus, next studies investigated whether the BBB disruption could be produced using lower frequencies more suitable for transcranial sonication. First, a frequency of 690 kHz, which has been employed in a commercial focused ultrasound system designed for thermal ablation [100] was tested. Then, 260 kHz [101] a frequency at which the ultrasound can be applied through the skull without having to correct for focal distortion [80,86] investigated. At both frequencies, the BBB disruption has been produced, and again no ischemia, apoptosis, or any long-term damage has been detected in histology. The BBB disruption has been also produced through the intact rabbit skull. It is worth noting that the number of red blood cell extravasations was reduced at the lower frequencies and BBB disruption at 260 kHz was possible with no extravasations at all. Figure 1 shows examples of the focal BBB disruption in rabbit brain at four frequencies tested to date. A few hours after the sonications, the barrier is restored (Figure 2).
FIGURE 1.
Focal contrast enhancement in T1-weighted MR images demonstrating local BBB disruption in the rabbit brain produced by FUS at four ultrasound frequencies: 2.1 MHz, 1.63 MHz, 0.69 MHz, and 0.26 MHz. The images were acquired perpendicular to the direction of the ultrasound beam. The enhancing spots (arrows) show where the MRI contrast agent (Magnevist, Berlex Laboratories Inc, Wayne, NJ) passes through the FUS-induced BBB disruption. As the frequency decreases, the size of the BBB disrupted area increases. Lower ultrasound frequencies permit trans-cranial sonication with a little beam distortion. At 260 kHz, exposure will be possible without having to compensate for this distortion, making a relatively simple ultrasound device feasible.
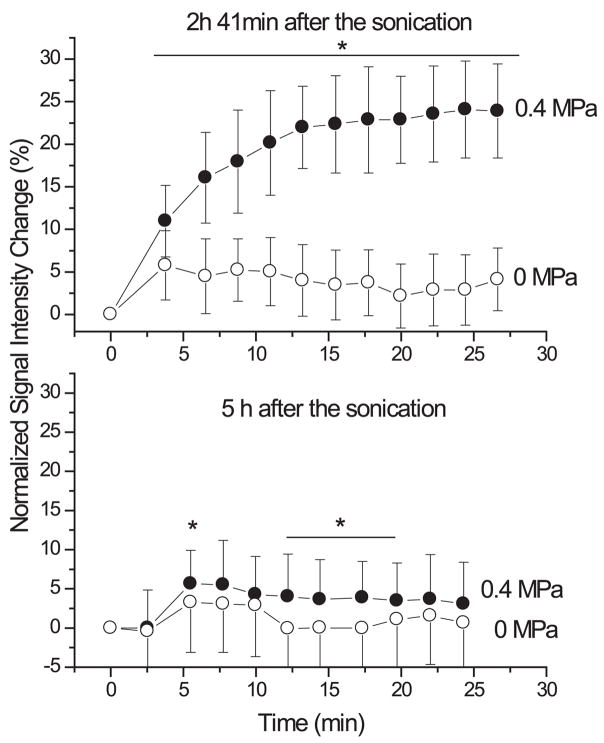
FIGURE 2.
The normalized signal intensity measured in contrast-enhanced T1-weighted MRI as a function of time after injection of contrast agent in the sonicated location (solid circle) and in a nonsonicated (control) location (open circle). Enhancement in the sonicated region was due to FUS-induced BBB disruption (ultrasound frequency: 0.26 MHz). At 2 h 41 min after sonication, the barrier was still open (upper), while at 5 hours it was almost closed (lower). Reproduced with permission from [101].
In a study of the effects of ultrasound frequency on the threshold for BBB disruption [108], the threshold at 2.1MHz for pulsed sonication (burst: 10ms, PRF: 1Hz, duration: 20s) was determined in rabbit brain and compared to the thresholds found previously at 0.26, 0.69, and 1.63MHz in rabbits and at 1.5MHz in rats under similar experimental conditions [97,100,101,107,109–111]. The BBBD thresholds (the pressure amplitude with 50% probability for BBBD) were 0.25, 0.35, 0.61, 0.60, and 0.69 MPa, for sonication at 0.26, 0.69, 1.5, 1.63, and 2.1 MHz, respectively. Converting the data to mechanical index (MI, which is defined as the pressure amplitude divided by the square root of frequency) and combining data from all of the frequencies yielded a MI threshold for BBB disruption of 0.47. This data suggests the use of MI as an index for ultrasound-induced bioeffects in the presence of microbubbles provided that other sonication parameters are kept constant.
Other work suggests that the pulse repetition frequency (PRF) and ultrasound contrast agent dose has no effect on the BBB disruption (BBBD), at least over a particular range (US frequency: 0.69 MHz, burst: 0.1, 1, 10 ms; PRF: 0.5, 1, 2, 5 Hz; duration: 20s peak; negative pressure amplitude: 0.1–1.5 MPa; Optison; 50, 100, 250 μl/kg), while both the BBB disruption threshold and magnitude depend on the burst length [112]. Reducing burst length from 10 ms to 0.1 ms resulted in significantly less contrast MRI enhancement. The BBB disruption thresholds were estimated to be 0.69, 0.47, and 0.36 MPa for 0.1, 1, and 10 ms bursts respectively.
A range of particle sizes has been tested for passage through the BBB disruption in the focal zone after sonication. It has been shown that MRI contrast agents such as Magnevist® (molecular weight: 938) and MION (monocrystalline iron oxide nanoparticles, molecular weight: 10000) [101,113], trypan blue (molecular weight: 961), horseradish peroxidase (molecular weight 40000) [100], and antibodies (molecular weight 150000) [114] can pass through the BBB disruption.
Thus, a noninvasive method for temporal disruption of the BBB in targeted regions in the brain using low-energy focused ultrasound pulses combined with an ultrasound contrast agent has been developed. It has been demonstrated that the method is reliable and that the associated effects in the brain tissue are negligible. It can also be applied using ultrasound frequencies suitable for transcranial sonication. The next step is to provide the data needed to move this technology to the clinic. For this purpose monoclonal antibodies have been identified.
Delivery of therapeutics
The use of monoclonal antibodies as targeted diagnostic and therapeutic agents has been intensively investigated since the 1970’s [115–119]. Monoclonal antibodies are a class of agents aimed at specific receptors on targeted cells, which bind to antigens present on cells (such as cancer cells). These anitibodies can be labeled with therapeutic or imaging agents. In addition to having direct cellular effects, antibodies can carry substances such as drugs, toxins, or radioactive isotopes to the targeted cells. Recently, a number of second-generation monoclonal antibody agents (such as Herceptin for treatment of metastatic HER-2-positive breast cancer; Rituximab and Ibritumomab Tiuxetan- for lymphomas, Gemtuzumab ozogamicin and Alemtuzumab for leukemia) have been approved by the FDA for use in malignancies, and many other agents are currently in clinical trials [117,120–123]. There is also evidence that treatment with antibodies to the amyloid beta protein (Abeta) can reduce cognitive dysfunction and reverse cognitive deficits in early Alzheimer’s Disease [124,125]. However, these large-molecule agents (150,000 Daltons) cannot pass the BBB; curative doses generally cannot be delivered without excessive toxicity to a normal brain [126,127]. Injecting agents directly to tumors or into the surgically created cavity -- as well as administrating drugs via the transient osmotic opening of the BBB -- has not been very efficient for patients with brain tumors, due to the limited diffusion of the drugs into brain, toxic side effects, and high complexity of the procedures [126,128–130].
The feasibility of the MRI-guided FUS technology to deliver antibodies to the brain has been demonstrated by Kinoshita et al. [114,131]. Using a mouse model, researchers delivered a polyclonal antibody against the extracellular domain of the dopamine D4 receptor to the targeted region of the brain. Intravenously administered dopamine D4 receptor-targeting antibodies crossed the BBB and recognized their antigens after the MRI-targeted FUS- induced BBB disruption occurred [131]. In brief, following the intravenous injection of the anti-dopamine D4 receptor antibodies, sonication was performed with the simultaneous injection of the microbubbles-based US agent (OPTSION, Amersham Health, Princeton, NJ). The exposure parameters were the following: US frequency: 0.69 MHz; burst length: 10 ms; PRF: 1 Hz; duration: 40s; peak pressure amplitude: 0.6–1.1MPa. The BBB disruption was confirmed by the contrast-enhanced MRI and Trypan Blue leakage in the targeted locations. The brain sections were stained with anti-rabbit IgG antibodies, and positive signals were detected at those locations where the Trypan Blue staining had been observed. This research confirms that the anti-dopamine D4 receptor antibody was delivered only in sonicated regions
More recently, Kinoshita et al. [114] used the same technique to deliver Herceptin (Trastuzumab, Genentech, San Francisco, CA) to the brain. Herceptin is a recombinant humanized IgG monoclonal antibody drug which works on the HER2/neu (erbB2) receptor. This receptor is amplified in approximately 25–30% of breast cancer patients [132] (about 50,000 patients in the US alone [133]). Herceptin targets the tumor cells, which overexpress the HER2/neu (erbB2) receptor. Herceptin was the first oncogene-targeted therapy to be developed for the treatment of metastatic breast cancer (for review see [134]). For patients with HER2/neu-positive tumors and for metastatic patients whose tumors overexpress this receptor, Herceptin has improved both survival and response rates [135]. Breast cancer is the second most common cause of brain metastases, and 10–15% of patients develop central nervous system disease [136]. However, for patients with metastases in the CNS, Herceptin success is significantly limited--due to a lack of drug penetration through the BBB [137,138,138]. The average survival time for these patients is only about one year [139].
Herceptin was successfully delivered into the brains of mice when the MRI-guided FUS-induced BBB disruption was performed [114]. Herceptin was detected in the sonicated locations (Figure 3), while in regions with an intact barrier its level was below detection limit. The amount of Herceptin delivered to the target tissue was correlated with the extent of BBB disruption monitored by MRI, enabling an indirect estimate of the amount of Herceptin delivered.
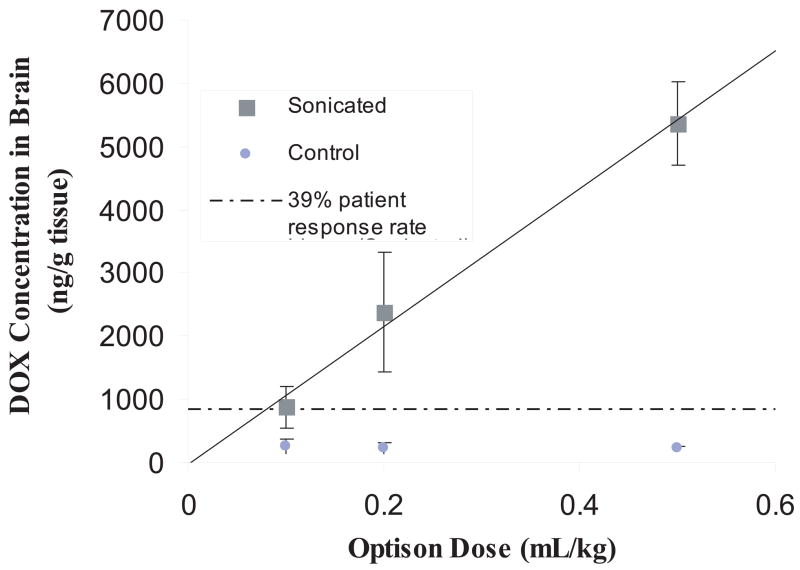
FIGURE 3.
Concentration of DOX delivered to the brain as a function of Optison dose. DOX concentration (mean ± SD) achieved in sonicated brain tissue met or exceeded the concentration shown to have a clinical response for human tumors (dotted line) [161]. Exposure parameters: frequency=1.7 MHz; Burst= 10 ms; PRF = 1 Hz; Duration: five 120-s sonications at 0.6 W followed by eight 60-s sonications at 0.3 W, sonications spaced 4 to 5 min apart. Reproduced with permission from [111].
Recently, the MRI-guided FUS technology to disrupt BBB has also been tested for targeted delivery of doxorubicin (DOX) [111], a chemotherapeutic drug with demonstrated anticancer efficacy and widespread clinical use. First isolated in the early 1960s, DOX remains among the most effective anticancer treatments ever developed (see reviews [140–143]). One of the most actively used agents for systemic chemotherapy, DOX functions either as a single agent or in combination with multidrug chemotherapy and/or radiotherapy to treat many types of malignancies including breast [144,145], ovarian [146,147], endometrial [148], gastric [149], lung [150], thyroid carcinomas [151], Hodgkin’s and AIDS-related non-Hodgkin’s lymphomas, [152,153], cutaneous lymphomas [154], osteosarcoma [155], and soft-tissue sarcomas [156]. DOX has also been suggested to be a strong candidate for chemotherapy of tumors in the CNS [157]. Although DOX showed its potential against glioma cells (the most invasive form of primary brain tumors) both in vitro [158] and in vivo [159,160], it has not been effective in patients with brain tumors due to the BBB, which prevents DOX cytotoxic concentrations in brain tumors from being achieved.
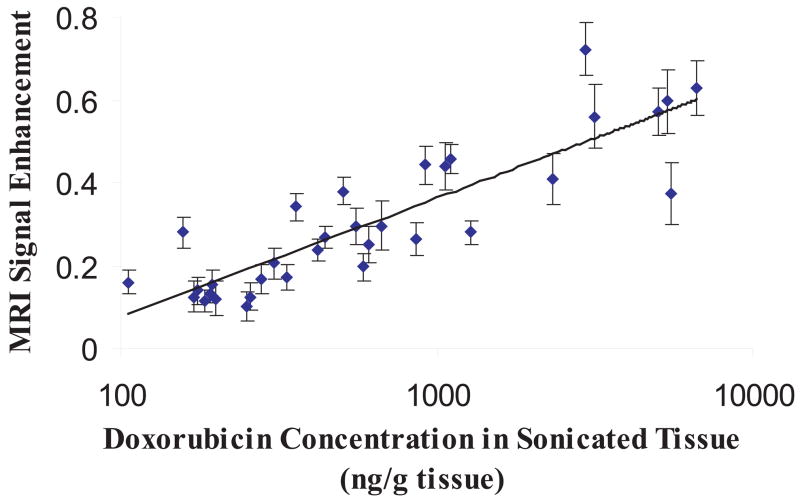
To test the feasibility of FUS+US contrast agent technology to delivery DOX to the brain, DOX hydrochloride encapsulated in long-circulating pegylated liposomes (Doxil; Ben Venue Laboratories, Bedford, OH; 5.67 mg/kg) was used [111]. In this form, the encapsulated DOX is released in the tissue once the liposomes pass the BBB. Concentrations within the therapeutic range [161] (886 ± 327 ng/g) have been achieved in the brain of rats following systemic administration of Doxil, when MRI-guided focused ultrasound with preformed microbubbles (Optison) was applied through the intact rodent skull (Figure 3). In contrast, the accumulation of DOX in untreated brain tissue remained low, reducing the risk of neurotoxic effects. Higher concentrations were possible with some associated brain tissue damage, which might be acceptable for cancer treatment. Importantly, MRI signal enhancement in the sonicated regions correlated with DOX concentrations, suggesting that contrast-enhanced MRI may provide a means for online treatment monitoring (Figure 4).
FIGURE 4.
Correlation of MRI signal enhancement and DOX delivered to the targeted brain following MRI-guided FUS-induced BBB disruption (logarithmic fit; r = 0.87). Reproduced with permission from [111].
Thus, FUS+US contrast agent method has potential to fundamentally change current practices in drug delivery and therapy for disorders of the central nervous system. In the future, the safety of repeatedly disrupting the BBB over the course of weeks and longer to mimic the schedule for patients receiving chemotherapy (such as Herceptin or doxorubicin) should be tested to prove that such repeated sessions do not cause brain damage and neuron loss. Then, it should be determined whether drugs can be delivered in a therapeutic dosage to the brain over the course of prolonged treatment. In the studies with antibodies and doxorubicin, a correlation was found between the magnitude of contrast-enhancement in MRI and the concentration of agents in the brain; this may provide a method to guide the procedure. It is worth noting that although doxorubicin is an efficient chemotherapeutic agent against many types of cancer, its use is limited due to severe cardiotoxic side-effects [162,163]. The FUS+US contrast agent technique might be useful for increased delivery of the drug into a tumor while leaving its concentration low outside of the tumor.
Physical Mechanisms of Blood-Brain Barrier Disruption
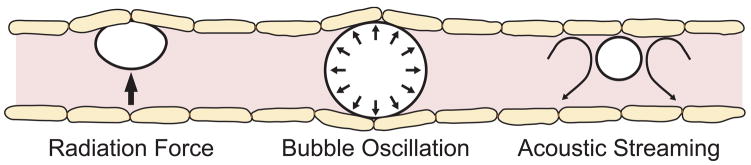
Despite recent successes in opening the blood-brain barrier with microbubble-enhanced ultrasound [97–101], the exact mechanisms through which the barrier is disrupted remain unknown. The effect may be attributed to a combination of cavitation activity and acoustic radiation forces. Cavitation defined as the acoustically induced activities of microscopic gas bubbles within the medium [164] is believed to be the most important of the variety of non-thermal bio-effects of ultrasound (see review [165]). These micro-bubbles can be seeded from pre-existing gas pockets in the media and act as nucleation sites. When microbubbles interact with an ultrasound beam, a range of biological effects can been observed [166]. At low acoustical power levels, the bubbles oscillate within the ultrasound field growing in size via rectified diffusion [167]. The bubble activity of this type called “stable” cavitation. Near an oscillating bubble, the medium undergoes a small-scale eddying “microstreaming” [166]. The biological effects of the stable cavitation are mostly attributed to shear stresses exerted on cells and large molecules by microstreaming [166,168,169]. Vascular endothelium is known to dynamically response to shear stress, and shear stress play a critical role in BBB homeostasis and vascular pathophysiology (for review see [170]). For endothelial cells to be affected, they must be in contact/near-contact with active bubbles. The acoustic radiation forces causing bubbles to move in the direction of the wave propagation [171] bring them in contact with vessel endothelium. Radiation pressure may be involved in FUS + microbubbles- based BBB opening through activating the stretch-sensitive or mechanosensitive (MS) ion channels in the vascular endothelium. To addition to mechanosensation, the MS channels are now known to be been implicated in many basic cell functions [172]. The channels can be activated by conformational changes during localized stretching of cell membranes [173,174]. Each of these effects can potentially affect the blood vessels and the blood flow within the microvasculature and be the source of the BBB disruption (Figure 5). At high enough acoustic pressures, the bubble can grow rapidly and collapse violently, a phenomenon known as inertial cavitation [164]. Bubble collapse can create extremely high local temperatures and pressures [175,176], high-velocity jets [177,178] and the generation of free radicals [179–181]. Both stable and inertial cavitation have been reported to produce bio-effects in vitro and in vivo (for review see [166,182,183]).
FIGURE 5.
Possible mechanisms for blood-brain barrier disruption via ultrasound + microbubbles. Assuming that the effect is not due to bubble collapse (inertial cavitation), possible effects include stimulation of the endothelial cells via radiation force on the bubbles, bubble oscillation or from microstreaming of the fluid around the bubbles.
The preformed microbubbles used in ultrasound contrast agents can produce all these effects, either with their shells intact or after the shells been cracked by the US exposure and their gaseous contents released. They can also act as cavitation nuclei when destabilized by the ultrasound wave [184]. The contrast agents may produce or greatly enhance effects at the lowest acoustic pressure levels at which the effects do not occur without contrast agent. Ultrasound in combination with US contrast agents produce pore formation [182] and increase permeability of cell membranes without affecting cell viability [185]. Cell deformations caused by oscillating microbubbles were suggested to be responsible for enhanced cell membrane permeability and such deformations were revealed under a microscope when a fast framing camera at 10 million frames per second was used [185].
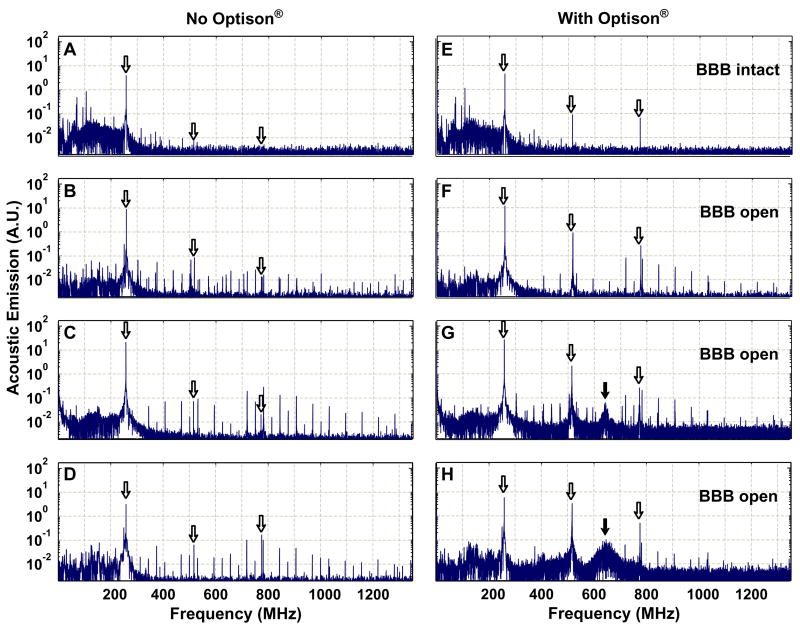
Inertial cavitation, if it occurs when the BBB disruption is produced, would likely be the most significant effect, due to the large energy concentrations in the vicinity of the collapsing bubbles. However, monitoring of acoustic emission during FUS exposures at the lowest therapeutic FUS frequency of 260 kHz (burst: 10 ms; PRF: 1 Hz; duration: 20 s) in conjunction with an US contrast agent (Optison), showed that the BBB disruption can be produced without wideband emission (Figure 6) [109], which has been used as a signature for inertial cavitation [186–188]. This finding, along with the lack of red blood cell extravasations, suggests that inertial cavitation is not necessary for the BBB disruption. Small extravasations found in histology were associated with the appearance of wideband emission at higher acoustical pressure levels (0.40 and 0.57 MPa). Interestingly, that significant increase in the emission was recorded at the second and third harmonics of the US driving frequency that indicates asymmetric oscillations. This increase correlated with BBB disruption and might be useful as an online method to indicate when the disruption occurs.
FIGURE 6.
Spectra acquired during 10 ms pulses at four different locations in the rabbit brain. The white arrows indicate the locations of the fundamental frequency of the ultrasound (260 kHz), as well as the second and third harmonics. (a)–(d): Spectra from sonications without Optison®. (e)–(h): Spectra from sonications at the same locations and exposure levels with Optison®. The sonication that produced the spectrum in (e) did not result in BBBD; in (f)–(h), BBB disruption was observed. In (g) and (h), the frequency spectrum was accompanied by wideband acoustic emission, which is indicated by a hump at approximately 650 kHz (black arrow), the central frequency of the receiver. The additional peaks (especially at lower frequencies) were artifacts in the signals presumably induced in the cables extending out of the MRI room. They were also observed in signals obtained with the ultrasound driving system disabled. (a), (e): 0.14 MPa; (b), (f): 0.29 MPa; (c), (g): 0.40 MPa; (d), (h): 0.57 MPa). Figure reproduced from [109] with permission.
Microbubbles in blood vessels
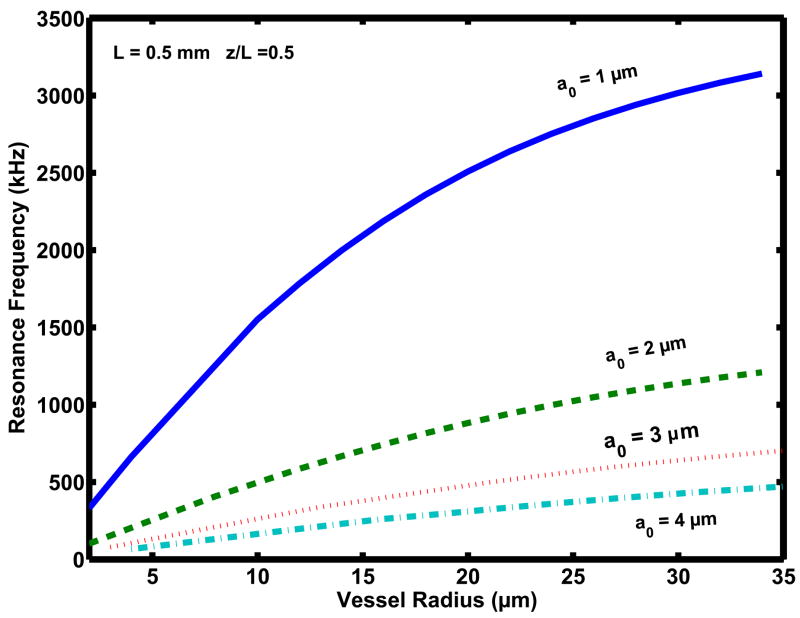
Most of our knowledge about bubble/ultrasound interaction derives from studies on bubbles contained within a vessel much larger than the bubble size resulting in negligible boundary effects. However, these conditions are not valid for bubbles in blood vessels such as capillaries, where the presence of boundaries may become significant. The investigation of bubbles acoustics in confined geometries such as blood vessels has only recently begun [189–195]. Interesting and unexpected results have emerged from these initial studies. The bubble resonant frequency has been found to be significantly affected by both the vessel diameter and length [190,191]. So, in the capillary of 5.0 μm radius and 0.5mm length, a 2 μm bubble resonates at about 250 kHz, while in the vessel of 30 μm radius and 0.7 mm length its resonance frequency is about 1.0 MHz (Figure 7). The upper limit for capillary diameter in rat brain has been found to be 10 μm [196] and typical ranges for arteriole- and venule diameter are 11μm −76 μm and 47μm −127 μm, respectively [197]. The transfer of energy from an ultrasonic field to a gas bubble occurs more effectively when the frequency of the ultrasonic field equals the resonance frequency of the bubble. These results suggest the possibility of using ultrasound in a range of frequencies that are, in general, lower than the ones used now for therapeutic applications of ultrasound [191]. Furthermore, the threshold for inertial cavitation is also affected by the vessel size, increasing in smaller vessels [195]. This can be understood by considering the effect of the vessel wall on the bubble inertia, which is known to greatly affect bubble collapse. The inertia is different for bubbles in a free field and bubbles in a tube [198,199]: while the effective mass of free-floating bubbles is relatively small and constant, the inertia of bubbles in a tube depends on tube size increasing in a tube of smaller diameter. As the bubble grows, its inertia increases and reaches the largest value in the smallest tube, such as a capillary. Increasing inertia slows down the bubble growth, and therefore more acoustical pressure needs to be applied for bubble expansion and subsequent collapse. In capillaries, where microbubbles can easily fill the vessel-cross section, their inertia is large and oscillations can be significantly hampered. As a consequence, the bubble collapse may be weakened. High-speed camera observations [189,193] have shown that in narrow tubes the bubble expansion is constrained leading to asymmetric elongation along the vessel wall. This intra-luminal expansion causes a significant dilation of the wall and eventually the tube rupture [189]. Obviously, the bubble’s expansion that stretches the capillary wall may produce significant bio-effects in the capillary and the inertial cavitation may not necessary for producing BBB disruption. As research on bubble acoustics in the blood vessels progresses, it is expected that useful insights for the control of microbubbles for BBB disruption would be provided.
FIGURE 7.
Resonance frequency as a function of the vessel radius, in the range between 2 and 35 μm for bubbles of different sizes (1–4 μm) located in the center of a vessel of length 0.5 mm. (Courtesy of E. Sassaroli).
Biological Mechanisms of BBB Disruption
Electron Microscopy Study
Electron microscopy of the brain following MRI-proven the BBB disruption in targeted locations, indicates that the sonication evokes transendothelial transport by both transcellular and paracellular pathways [200–202]. Several avenues of transcapillary passage following FUS exposure have been identified: 1. transcytosis; 2. endothelial cell cytoplasmic openings-fenestration and channel formation; 3. passage through opened tight junctions; 4. free passage through the injured endothelium (with the higher power sonications). An immunocytochemical study using endogenous immunoglobulinG (IgG) as a tracer, showed IgG passage from blood to brain parenchyma (IgG presence in the endothelial cell’s vesicles, in the cytoplasmic channels, and intercellular clefts as well as in neuropil around the blood vessels). Figure 8 shows examples of the electron microscopy findings. The rate of the transendothelial vesicular transport after FUS-induced modulation of the BBB estimated by using ultrastructural morphometry for horseradish peroxidase (HRP) as a tracer was found to be substantially increased, more pronounced in arterioles [201].
FIGURE 8.
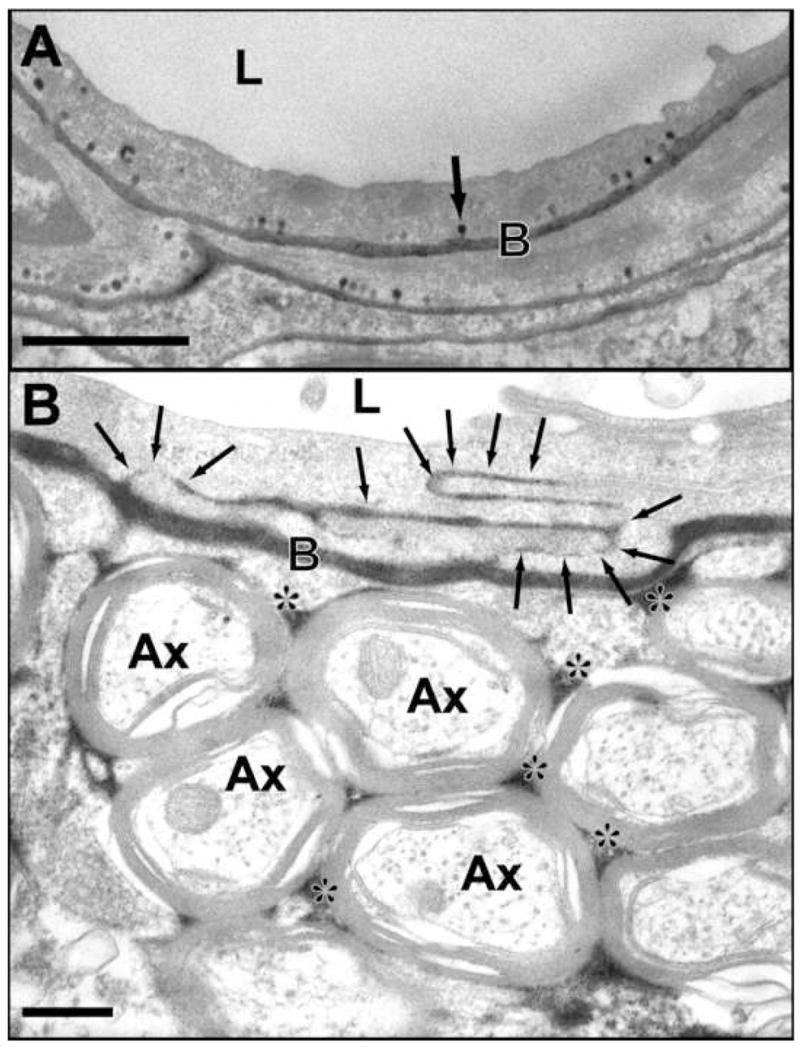
Electron micrographs showing transcellular and intracellular passage of horseradish peroxidase (HRP) after ultrasound-induced BBB disruption. This tracer appears black in the photomicrographs and has a molecular weight of 40 kDa. A: Intense vesicular transport is observed by numerous HRP-positive caveolae (example indicated by arrow) after sonication at 0.26 MHz in a rabbit brain. B: Passage of HRP through several interendothelial clefts (arrows) is observed after sonication at 1.5 MHz in a rat. The tracer has infiltrated the basement membrane and the interstitial space in the neuropil (*). (L: lumen; Ax: cross-sectioned axons; B: basement membrane). (Data from [201, 202]; Courtesy of N. Sheikov).
The paracellular passage of the tracer molecules was shown to be a result from junctional complexes disintegration [202]. That work was the first direct evidence that FUS causes disassembling of the tight junctional (TJs) molecular structure, leading to loss of the barrier functions in brain microvessels. The immunoelectron microscopy for the TJs-specific proteins showed a loss of immunosignals for occludin, claudin-5 and ZO-1 at 1 h, 2 h and, in a few vessels, at 4 h after sonication. BBB disruption was verified by the leakage of IV administered horseradish peroxidase (40,000 Da) and lanthanum chloride (La3+~139 Da). There was no tracer leakage observed at 6h and 24 h, and the barrier function of the TJs appeared completely restored at these time-intervals.
Multiphoton Microscopy Study In Vivo
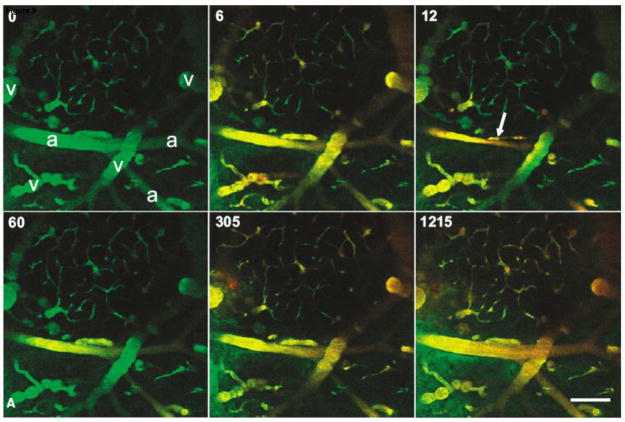
The temporal profile of BBB disruption has been studied in mice in vivo using simultaneous optical imaging and ultrasound exposure to the brain using a system developed by Raymond et al [203]. The ultrasound components were integrated into a multiphoton microscope and sonication was performed through the ventral surface, leaving the dorsal surface of the brain free for imaging through a cranial window (Figure 9). The fluorescent dye was used as a tracer to visualize the brain blood vessels during exposure to low-intensity US (0.2 W) with a coincident IV injection of Optison. This work indicates that vasoconstriction can occur during the ultrasound exposure, followed by leakage of the tracer shortly thereafter [204]. FUS produced arteriolar vasoconstriction that disrupted blood flow and lasted up to 5 min (Figure 9). The BBB disruption occurred via two distinct processes: a gradual increase (over minutes) in perivascular fluorescence along the length of the affected vessel without rupture of the vessel wall and a rapid increase (~seconds) in select, focal regions [204]. These data corroborated studies suggesting increased endothelial transcytosis and paracellular passage via tight junctions [200–202] and demonstrated vasoconstriction, which might alter BBB permeability by modifying cerebral blood flow. Similar vasoconstriction was observed earlier, when rabbit femoral artery was exposed to high-intensity pulsed FUS [77]. These results suggest that the temporary vasoconstriction (vessel spasm) might be related to the BBB disruption. This constriction might result in temporary ischemia, which can cause BBB disruption.
FIGURE 9.
In vivo multiphoton imaging of the mouse brain vasculature during FUS-induced BBB disruption. The animal received 0.1ml (2 mg/ml) 10 kDa, dextran-conjugated Alexa Fluor 488 intravenously ~5 min before imaging (green in images). Immediately after the t=0 frame was taken, a 45-s US exposure was initiated and a 0.1ml bolus (10 mg/ml) of 70 kDa, dextran-conjugated Texas Red was delivered intravenously (red in images). Almost total occlusion of the large vessel in the center of the field occurred 12 s after the initiation of ultrasound exposure (arrow). Beginning at 60 s and by 305 s, leakage in the green channel is apparent in the lower left of the field, and around the central vessel. (a=arterioles; v=veins; scale bar is 100 μm). Reproduced with permission from [204].
Spreading Depression
FUS has been shown to disrupt the BBB when used with an US contrast agent, allowing the delivery of both small- and large-molecule agents into the brain. However, the BBB disruption is sometimes accompanied by red blood cell extravasations, indicating a mild vascular injury. Such injury is permissible for tumor treatment, but would be unwanted for delivering neuroimaging agents to the targeted areas in the brain. A solution to disrupting the BBB without occasional injury may be FUS-induced spreading depression (SD). SD is an electrochemical wave that propagates through neural tissue, causing neuronal depolarization, transient loss of membrane ionic gradients, and cessation of neuronal bioelectrical activity without causing irreversible damage to the brain. A range of stimuli directly affecting the brain can trigger SD: high-frequency electrical pulses or direct current, mechanical stimuli such as pressure on or puncture of the cortex, alkaline pH, low osmolarity, and a variety of chemicals (see review [205]). First described by Leao in 1944 [206], SD remains a mysterious phenomenon and its exact functions remain unknown. SD is implicated in several neurological diseases including migraine and stroke. It may increase neuronal survivability against ischemia (infarct tolerance) [207], has potential to stimulate persistent neurogenesis [208] and alter BBB permeability by activating metalloproteinase (MMP-9), which is known to disrupt the BBB by cleaving extracellular matrix in the BBB basal membrane [209]. Previously the feasibility to initiate SD by FUS exposure through both the intact skull and exposed dura was established in animal experiments [210]. Recently, FUS’s potential to disrupt the BBB through initiating SD has been reported [211]. Cortical spreading depression (CSD) was induced by FUS (frequency: 4.89MHz) in the brain of rats and verified through electrocorticograms (ECG) recorded by using implanted EEG electrodes. Pulsed sonications (burst: 40–200ms, PRF: 2Hz, duration: 20–30s, total acoustic power in water: 2.3W) were delivered through a skull window or through the intact skull. Five to seven SD waves were initiated with an interval of 25–30 min in each animal at day “0” (electrodes implantation) and induced repeatedly over the next 1–7 days. In additional experiments, CSD waves were initiated by application of 4 μl of 2%KCl to the cortex. CSD initiated by both FUS and KCl applications produced BBB disruption, which was verified by leakage of Trypan Blue IV injected 18–20 hours after the last induced CSD. The brain parenchyma appeared to be intensively stained with the Trypan Blue in both areas sonicated with FUS and areas of KCl application. However, sonication through the skull revealed damage to the cerebral cortex presumably due to overheating the skull bone. These preliminary results indicate that FUS-induced spreading depression may be used for non-invasive BBB disruption in targeted locations although much more work is needed prior to any firm conclusions can be made.
Conclusion
A completely noninvasive method to selectively and temporarily disrupt the blood-brain barrier at desired locations in the brain using low-power focused ultrasound pulses combined with an injection of a commercially-available ultrasound contrast agent has been developed. By steering the focus, either by moving the transducer or electronically steering the focal region using a phased array transducer, one could disrupt the BBB within a desired region of the brain. Surrounding tissue will be protected by its intact BBB, thus limiting any potential dose-limiting neurotoxic side effects. Alternatively, it is possible to steer the ultrasound beam with the same way to deliver agents to the entire brain if needed. Since the procedure can be performed in an MRI scanner, it is possible to guide the procedure and evaluate tissue effects. The presence of the US contrast agent (pre-formed microbubbles in a lipid or albumin shell) enhances the local ultrasound effect and limits it to the brain vasculature, thus reducing the chance of damage to other structures. Use of these bubbles also greatly reduces the time average and peak acoustic energy needed to produce this interaction. This point makes the procedure practical for application through the intact skull, since the cost of the system and risks of overheating the skull would be greatly reduced. The effects of FUS-induced BBB disruption on the brain tissue appear to be negligible – certainly acceptable for cancer treatment.
Acknowledgments
Support: NIH (R01EB003268, R33EB000705, U41RR019703).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1(2):131–9. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105. 51. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 4.Ajay Bemis GW, Murcko MA. Designing libraries with CNS activity. J Med Chem. 1999;42(24):4942–51. doi: 10.1021/jm990017w. [DOI] [PubMed] [Google Scholar]
- 5.Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1(1):55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. Advances in cell biology of blood-brain barrier transport. Semin Cell Biol. 1991;2(6):419–26. [PubMed] [Google Scholar]
- 7.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36(4):555–8. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 8.Lo EH, Singhal AB, Torchilin VP, Abbott NJ. Drug delivery to damaged brain. Brain Res Brain Res Rev. 2001;38(1–2):140–8. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 9.Doolittle ND, Anderson CP, Bleyer WA, Cairncross JG, Cloughesy T, Eck SL, Guastadisegni P, Hall WA, Muldoon LL, Patel SJ, Peereboom D, Siegal T, Neuwelt EA. Importance of dose intensity in neuro-oncology clinical trials: summary report of the Sixth Annual Meeting of the Blood-Brain Barrier Disruption Consortium. Neuro -oncol. 2001;3(1):46–54. doi: 10.1093/neuonc/3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardridge WM. Brain drug targeting and gene technologies. Jpn J Pharmacol. 2001;87(2):97–103. doi: 10.1254/jjp.87.97. [DOI] [PubMed] [Google Scholar]
- 11.Krewson CE, Klarman ML, Saltzman WM. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain Res. 1995;680(1–2):196–206. doi: 10.1016/0006-8993(95)00261-n. [DOI] [PubMed] [Google Scholar]
- 12.Krewson CE, Dause R, Mak M, Saltzman WM. Stabilization of nerve growth factor in controlled release polymers and in tissue. J Biomater Sci Polym Ed. 1996;8(2):103–17. doi: 10.1163/156856296x00183. [DOI] [PubMed] [Google Scholar]
- 13.Guerin C, Olivi A, Weingart JD, Lawson HC, Brem H. Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest New Drugs. 2004;22(1):27–37. doi: 10.1023/b:drug.0000006172.65135.3e. [DOI] [PubMed] [Google Scholar]
- 14.Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp Neurol. 1994;127(1):23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- 15.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266(1 Pt 2):R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 16.Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, Gash DM. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461(2):250–61. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- 17.Reszka RC, Jacobs A, Voges J. Liposome-mediated suicide gene therapy in humans. Methods Enzymol. 2005;391:200–8. doi: 10.1016/S0076-6879(05)91012-4. [DOI] [PubMed] [Google Scholar]
- 18.Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, Kracht L, Coenen HH, Sturm V, Wienhard K, Heiss WD, Jacobs AH. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54(4):479–87. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 19.Grondin R, Zhang Z, Ai Y, Gash DM, Gerhardt GA. Intracranial delivery of proteins and peptides as a therapy for neurodegenerative diseases. Prog Drug Res. 2003;61:101–23. doi: 10.1007/978-3-0348-8049-7_4. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson S, Lesniak MS. Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr Drug Deliv. 2007;4(2):169–80. doi: 10.2174/156720107780362302. [DOI] [PubMed] [Google Scholar]
- 21.Fung LK, Shin M, Tyler B, Brem H, Saltzman WM. Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res. 1996;13(5):671–82. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- 22.Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, Gerhardt GA, Gash DM. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202(2):497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Manome Y, Kobayashi T, Mori M, Suzuki R, Funamizu N, Akiyama N, Inoue S, Tabata Y, Watanabe M. Local delivery of doxorubicin for malignant glioma by a biodegradable PLGA polymer sheet. Anticancer Res. 2006;26(5A):3317–26. [PubMed] [Google Scholar]
- 24.Yamanaka R, Itoh K. Peptide-based immunotherapeutic approaches to glioma: a review. Expert Opin Biol Ther. 2007;7(5):645–9. doi: 10.1517/14712598.7.5.645. [DOI] [PubMed] [Google Scholar]
- 25.Aebischer P, Ridet J. Recombinant proteins for neurodegenerative diseases: the delivery issue. Trends Neurosci. 2001;24(9):533–40. doi: 10.1016/s0166-2236(00)01899-3. [DOI] [PubMed] [Google Scholar]
- 26.Neuwelt EA, Barnett PA, McCormick CI, Frenkel EP, Minna JD. Osmotic blood-brain barrier modification: monoclonal antibody, albumin, and methotrexate delivery to cerebrospinal fluid and brain. Neurosurgery. 1985;17(3):419–23. doi: 10.1227/00006123-198509000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, McAllister LD, Bubalo JS, Kraemer DF, Fortin D, Nixon R, Muldoon LL, Neuwelt EA. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88(3):637–47. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Hall WA, Doolittle ND, Daman M, Bruns PK, Muldoon L, Fortin D, Neuwelt EA. Osmotic blood-brain barrier disruption chemotherapy for diffuse pontine gliomas. J Neurooncol. 2006;77(3):279–84. doi: 10.1007/s11060-005-9038-4. [DOI] [PubMed] [Google Scholar]
- 29.Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083–99. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- 30.Matsukado K, Sugita M, Black KL. Intracarotid low dose bradykinin infusion selectively increases tumor permeability through activation of bradykinin B2 receptors in malignant gliomas. Brain Res. 1998;792(1):10–5. doi: 10.1016/s0006-8993(97)01502-3. [DOI] [PubMed] [Google Scholar]
- 31.Broadwell RD, Salcman M, Kaplan RS. Morphologic effect of dimethyl sulfoxide on the blood-brain barrier. Science. 1982;217(4555):164–6. doi: 10.1126/science.7089551. [DOI] [PubMed] [Google Scholar]
- 32.Saija A, Princi P, Trombetta D, Lanza M, De Pasquale A. Changes in the permeability of the blood-brain barrier following sodium dodecyl sulphate administration in the rat. Exp Brain Res. 1997;115(3):546–51. doi: 10.1007/pl00005725. [DOI] [PubMed] [Google Scholar]
- 33.Gennuso R, Spigelman MK, Chinol M, Zappulla RA, Nieves J, Vallabhajosula S, Alberto PP, Goldsmith SJ, Holland JF. Effect of blood-brain barrier and blood-tumor barrier modification on central nervous system liposomal uptake. Cancer Invest. 1993;11(2):118–28. doi: 10.3109/07357909309024829. [DOI] [PubMed] [Google Scholar]
- 34.Cornford EM, Young D, Paxton JW, Finlay GJ, Wilson WR, Pardridge WM. Melphalan penetration of the blood-brain barrier via the neutral amino acid transporter in tumor-bearing brain. Cancer Res. 1992;52(1):138–43. [PubMed] [Google Scholar]
- 35.Rabchevsky AG, Degos JD, Dreyfus PA. Peripheral injections of Freund’s adjuvant in mice provoke leakage of serum proteins through the blood-brain barrier without inducing reactive gliosis. Brain Res. 1999;832(1–2):84–96. doi: 10.1016/s0006-8993(99)01479-1. [DOI] [PubMed] [Google Scholar]
- 36.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86(4):1245–57. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 37.Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8(16):923–6. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- 38.Oztas B, Kucuk M. Reversible blood-brain barrier dysfunction after intracarotid hyperthermic saline infusion. Int J Hyperthermia. 1998;14(4):395–401. doi: 10.3109/02656739809018241. [DOI] [PubMed] [Google Scholar]
- 39.Oztas B, Kucuk M. Intracarotid hypothermic saline infusion: a new method for reversible blood-brain barrier disruption in anesthetized rats. Neurosci Lett. 1995;190(3):203–6. doi: 10.1016/0304-3940(95)11542-5. [DOI] [PubMed] [Google Scholar]
- 40.Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc Natl Acad Sci U S A. 1995;92(5):1426–30. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27(2):85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47(1):65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 43.Pardridge WM. Intravenous, non-viral RNAi gene therapy of brain cancer. Expert Opin Biol Ther. 2004;4(7):1103–13. doi: 10.1517/14712598.4.7.1103. [DOI] [PubMed] [Google Scholar]
- 44.Pardridge WM. Tyrosine hydroxylase replacement in experimental Parkinson’s disease with transvascular gene therapy. NeuroRx. 2005;2(1):129–38. doi: 10.1602/neurorx.2.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koziara JM, Lockman PR, Allen DD, Mumper RJ. The blood-brain barrier and brain drug delivery. J Nanosci Nanotechnol. 2006;6(9–10):2712–35. doi: 10.1166/jnn.2006.441. [DOI] [PubMed] [Google Scholar]
- 46.Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA. 1996;93(24):14164–9. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16(10):1564–9. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 48.Singh M. Transferrin As A targeting ligand for liposomes and anticancer drugs. Curr Pharm Des. 1999;5(6):443–51. [PubMed] [Google Scholar]
- 49.Pardridge WM, Kang YS, Buciak JL. Transport of human recombinant brain-derived neurotrophic factor (BDNF) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. Pharm Res. 1994;11(5):738–46. doi: 10.1023/a:1018940732550. [DOI] [PubMed] [Google Scholar]
- 50.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 51.Pardridge WM. Non-invasive drug delivery to the human brain using endogenous blood-brain barrier transport systems. Pharm Sci Technolo Today. 1999;2(2):49–59. doi: 10.1016/s1461-5347(98)00117-5. [DOI] [PubMed] [Google Scholar]
- 52.Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev. 2001;46(1–3):247–79. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 53.Pardridge WM. Neurotrophins, neuroprotection and the blood-brain barrier. Curr Opin Investig Drugs. 2002;3(12):1753–7. [PubMed] [Google Scholar]
- 54.Schlachetzki F, Zhang Y, Boado RJ, Pardridge WM. Gene therapy of the brain: the trans-vascular approach. Neurology. 2004;62(8):1275–81. doi: 10.1212/01.wnl.0000120551.38463.d9. [DOI] [PubMed] [Google Scholar]
- 55.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6(5):494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Wu D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx. 2005;2(1):120–8. doi: 10.1602/neurorx.2.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Penas-Prado M, Gilbert MR. Molecularly targeted therapies for malignant gliomas: advances and challenges. Expert Rev Anticancer Ther. 2007;7(5):641–61. doi: 10.1586/14737140.7.5.641. [DOI] [PubMed] [Google Scholar]
- 58.Hynynen K. Biophysics and technology of ultrasound hyperthermia. In: Gautherie M, editor. Methods of External Hyperthermic Heating. New York: Springer-Verlag; 1990. pp. 61–115. [Google Scholar]
- 59.Diederich CHK. Ultrasound technology for hypethermia. 1999. [DOI] [PubMed] [Google Scholar]
- 60.Hunt JW. Principles of ultrasound used for generating localized hyperthermia. In: Field SB, Hand JW, editors. Principle Aspects of Clinical Hyperthermia. London: Taylor and Francis; 1990. pp. 371–422. [Google Scholar]
- 61.Lele PP. Local hyperthermia by ultrasound. In: Nussbaum GH, editor. Physical aspects of hyperthermia. New York: American Institute of Physics; 1982. pp. 393–440. [Google Scholar]
- 62.Hynynen K, Clement G, McDannold N, King R, White PJ, Jolesz F, Vitek S, Zadicario E. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain. Proceedings of the Eleventh Meeting of the International Society for Magnetic Resonance in Medicine; Toronto, ON. 2003. p. 77. [Google Scholar]
- 63.Chan AH, Fujimoto VY, Moore DE, Martin RW, Vaezy S. An image-guided high intensity focused ultrasound device for uterine fibroids treatment. Med Phys. 2002;29(11):2611–20. doi: 10.1118/1.1513990. [DOI] [PubMed] [Google Scholar]
- 64.Chapelon JY, Ribault M, Vernier F, Souchon R, Gelet A. Treatment of localised prostate cancer with transrectal high intensity focused ultrasound. Eur J Ultrasound. 1999;9(1):31–8. doi: 10.1016/s0929-8266(99)00005-1. [DOI] [PubMed] [Google Scholar]
- 65.Chen W, Wang Z, Wu F, Zhu H, Zou J, Bai J, Li K, Xie F. [High intensity focused ultrasound in the treatment of primary malignant bone tumor] Zhonghua Zhong Liu Za Zhi. 2002;24(6):612–5. [PubMed] [Google Scholar]
- 66.Gelet A, Chapelon JY, Poissonnier L, Bouvier R, Rouviere O, Curiel L, Janier M, Vallancien G. Local recurrence of prostate cancer after external beam radiotherapy: early experience of salvage therapy using high-intensity focused ultrasonography. Urology. 2004;63(4):625–9. doi: 10.1016/j.urology.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Wu F, Wang ZB, Cao YD, Chen WZ, Bai J, Zou JZ, Zhu H. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer. 2003;89(12):2227–33. doi: 10.1038/sj.bjc.6601411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res Treat. 2003;82(2):93–101. doi: 10.1023/B:BREA.0000003956.11376.5b. [DOI] [PubMed] [Google Scholar]
- 69.Kohrmann KU, Michel MS, Gaa J, Marlinghaus E, Alken P. High intensity focused ultrasound as noninvasive therapy for multilocal renal cell carcinoma: case study and review of the literature. J Urol. 2002;167(6):2397–403. [PubMed] [Google Scholar]
- 70.Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 71.ter Haar GR. High intensity focused ultrasound for the treatment of tumors. Echocardiography. 2001;18(4):317–22. doi: 10.1046/j.1540-8175.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 72.Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, Li KQ, Jin CB, Xie FL, Su HB. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004;11(12):1061–9. doi: 10.1245/ASO.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 73.Vaezy S, Noble ML, Keshavarzi A, Paun M, Prokop AF, Cornejo C, Sharar S, Chi EY, Crum LA, Martin RW. Liver hemostasis with high-intensity ultrasound: repair and healing. J Ultrasound Med. 2004;23(2):217–25. doi: 10.7863/jum.2004.23.2.217. [DOI] [PubMed] [Google Scholar]
- 74.Hwang JH, Vaezy S, Martin RW, Cho MY, Noble ML, Crum LA, Kimmey MB. High-intensity focused US: a potential new treatment for GI bleeding. Gastrointest Endosc. 2003;58(1):111–5. doi: 10.1067/mge.2003.322. [DOI] [PubMed] [Google Scholar]
- 75.Noble ML, Vaezy S, Keshavarzi A, Paun M, Prokop AF, Chi EY, Cornejo C, Sharar SR, Jurkovich GJ, Martin RW, Crum LA. Spleen hemostasis using high-intensity ultrasound: survival and healing. J Trauma. 2002;53(6):1115–20. doi: 10.1097/00005373-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 76.Vaezy S, Martin R, Crum L. High intensity focused ultrasound: a method of hemostasis. Echocardiography. 2001;18(4):309–15. doi: 10.1046/j.1540-8175.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 77.Hynynen K, Colucci V, Chung A, Jolesz F. Noninvasive arterial occlusion using MRI-guided focused ultrasound. Ultrasound Med Biol. 1996;22(8):1071–7. doi: 10.1016/s0301-5629(96)00143-3. [DOI] [PubMed] [Google Scholar]
- 78.Rivens IH, Rowland IJ, Denbow M, Fisk NM, ter Haar GR, Leach MO. Vascular occlusion using focused ultrasound surgery for use in fetal medicine. Eur J Ultrasound. 1999;9(1):89–97. doi: 10.1016/s0929-8266(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 79.Hynynen K, Sun J, Clement G, Thierman J, Daum D, Vykhodtseva NI, McDannold NJ. Phased array development for noninvasive focused ultrasound therapy of brain and liver. Proceedings of the First Joint BMES/EMBS Conference; 1999. p. 1270. [Google Scholar]
- 80.Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol. 1998;24(2):275–83. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 81.Sun J, Hynynen K. The potential of transskull ultrasound therapy and surgery using the maximum available skull surface area. J Acoust Soc Am. 1999;105(4):2519–27. doi: 10.1121/1.426863. [DOI] [PubMed] [Google Scholar]
- 82.Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47(8):1219–36. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- 83.Sun J, Hynynen K. Focusing of therapeutic ultrasound through a human skull: a numerical study. J Acoust Soc Am. 1998;104(3 Pt 1):1705–15. doi: 10.1121/1.424383. [DOI] [PubMed] [Google Scholar]
- 84.Pernot M, Aubry JF, Tanter M, Thomas JL, Fink M. High power transcranial beam steering for ultrasonic brain therapy. Phys Med Biol. 2003;48(16):2577–89. doi: 10.1109/TMI.2010.2076829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113(1):84–93. doi: 10.1121/1.1529663. [DOI] [PubMed] [Google Scholar]
- 86.Yin X, Hynynen K. A numerical study of transcranial focused ultrasound beam propagation at low frequency. Phys Med Biol. 2005;50(8):1821–36. doi: 10.1088/0031-9155/50/8/013. [DOI] [PubMed] [Google Scholar]
- 87.Hynynen K, Clement G, McDannold N, Vykhodtseva N. The feasibility of noninvasive image-guided treatments of brain disodereds by focused ultrasound. J Acoust Soc Am. 2004;115(5):2446. [Google Scholar]
- 88.Hynynen K, Clement GT, McDannold N, Vykhodtseva N, King R, White PJ, Vitek S, Jolesz FA. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: A preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52(1):100–7. doi: 10.1002/mrm.20118. [DOI] [PubMed] [Google Scholar]
- 89.BARNARD JW, Fry WJ, Fry FJ, KRUMINS RF. Effects of high intensity ultrasound on the central nervous system of the cat. J Comp Neurol. 1955;103(3):459–84. doi: 10.1002/cne.901030304. [DOI] [PubMed] [Google Scholar]
- 90.Bakay L, Hueter TF, BALLANTINE HT, Jr, Sosa D. Ultrasonically produced changes in the blood-brain barrier. Arch Neurol Psychat. 1956:457–67. doi: 10.1001/archneurpsyc.1956.02330290001001. [DOI] [PubMed] [Google Scholar]
- 91.Ballantine HT, Jr, Bell E, Manlapaz J. Progress and problems in the neurological applications of focused ultrasound. J Neurosurg. 1960;17:858–76. doi: 10.3171/jns.1960.17.5.0858. [DOI] [PubMed] [Google Scholar]
- 92.Astrom KE, Bell Ballantine HT, Jr, Heidensleben E. An experimental neuropathological study of the effects of high-frequency focused ultrasound on the brain of the cat. J Neuropathol Exp Neurol. 1961;20:484–520. doi: 10.1097/00005072-196120040-00002. [DOI] [PubMed] [Google Scholar]
- 93.Basauri L, Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: statistical evaluation of the effects of irradiation on the central nervous system of the cat. J Physiol. 1962;160(3):513–34. doi: 10.1113/jphysiol.1962.sp006863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Warwick R, Pond J. Trackless lesions in nervous tissues produced by high intensity focused ultrasound (high-frequency mechanical waves) J Anat. 1968;102(3):387–405. [PMC free article] [PubMed] [Google Scholar]
- 95.Vykhodtseva NI, Gavrilov LR, Mering TA, Iamshchikova NG. [Use of focused ultrasound for local destruction of different brain structures] Primenenie fokusirovannogo ul’trazvuka dlia lokal’nykh razrushenii razlichnykh struktur golovnogo mozga. Zh Nevropatol Psikhiatr. 1976;76(12):1810–6. [PubMed] [Google Scholar]
- 96.Vykhodtseva N. Effects of high intensity pulsed ultrasound on brain tissues. Proceedings of the 5th International Symposium on Ultrasound in Biol Med; Puschino, Russia. 1981. pp. 95–97. [Google Scholar]
- 97.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–6. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 98.Hynynen K, McDannold N, Martin H, Jolesz FA, Vykhodtseva N. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (Optison) Ultrasound Med Biol. 2003;29(3):473–81. doi: 10.1016/s0301-5629(02)00741-x. [DOI] [PubMed] [Google Scholar]
- 99.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Non-invasive opening of BBB by focused ultrasound. Acta Neurochir Suppl. 2003;86:555–8. doi: 10.1007/978-3-7091-0651-8_113. [DOI] [PubMed] [Google Scholar]
- 100.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 101.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105(3):445–54. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 102.Patrick JT, Nolting MN, Goss SA, Dines KA, Clendenon JL, Rea MA, Heimburger RF. Ultrasound and the blood-brain barrier. Adv Exp Med Biol. 1990;267:369–81. doi: 10.1007/978-1-4684-5766-7_36. [DOI] [PubMed] [Google Scholar]
- 103.Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol. 1995;21(7):969–79. doi: 10.1016/0301-5629(95)00038-s. [DOI] [PubMed] [Google Scholar]
- 104.Mesiwala AH, Farrell L, Wenzel HJ, Silbergeld DL, Crum LA, Winn HR, Mourad PD. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol. 2002;28(3):389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 105.Vykhodtseva NI, Sorrentino V, Jolesz FA, Bronson RT, Hynynen K. MRI detection of the thermal effects of focused ultrasound on the brain. Ultrasound Med Biol. 2000;26(5):871–80. doi: 10.1016/s0301-5629(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 106.Vykhodtseva N, McDannold N, Agabian S, Hynynen K. Histology findings after ultrasound exposure of the brain with ultrasound contrast agent- role of ischemia and apoptosis. in: <[11] Journal Name. 2003;(15):80. [Google Scholar]
- 107.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31(11):1527–37. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 108.McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. doi: 10.1016/j.ultrasmedbio.2007.10.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51(4):793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 110.McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound Med Biol. 2007;33(4):584–90. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121(4):901–7. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 112.McDannold N, Vykhodtseva N, Hynynen K. Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption. Ultrasound Med Biol. doi: 10.1016/j.ultrasmedbio.2007.11.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hynynen K, McDannold N, Josphson L, Vykhodtseva N, Weisleder R, Jolesz FA. Noninvasive MRI-guided focal opening of the blood brain barrier: Demonstration of large particle penetration. Proceedings of the Tenth Meeting of the International Society for Magnetic Resonance in Medicine; Honolulu, HI. 2002. p. 332. [Google Scholar]
- 114.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103(31):11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 116.Cersosimo RJ. Monoclonal antibodies in the treatment of cancer, Part 1. Am J Health Syst Pharm. 2003;60(15):1531–48. doi: 10.1093/ajhp/60.15.1531. [DOI] [PubMed] [Google Scholar]
- 117.Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004;5(5):292–302. doi: 10.1016/S1470-2045(04)01467-6. [DOI] [PubMed] [Google Scholar]
- 118.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. J Immunol. 1975;174(5):2453–5. 2005. [PubMed] [Google Scholar]
- 119.Gerber DE, Laterra J. Emerging monoclonal antibody therapies for malignant gliomas. Expert Opin Investig Drugs. 2007;16(4):477–94. doi: 10.1517/13543784.16.4.477. [DOI] [PubMed] [Google Scholar]
- 120.Segota E, Bukowski RM. The promise of targeted therapy: cancer drugs become more specific. Cleve Clin J Med. 2004;71(7):551–60. doi: 10.3949/ccjm.71.7.551. [DOI] [PubMed] [Google Scholar]
- 121.Ross JS, Schenkein DP, Pietrusko R, Rolfe M, Linette GP, Stec J, Stagliano NE, Ginsburg GS, Symmans WF, Pusztai L, Hortobagyi GN. Targeted therapies for cancer 2004. Am J Clin Pathol. 2004;122(4):598–609. doi: 10.1309/5CWP-U41A-FR1V-YM3F. [DOI] [PubMed] [Google Scholar]
- 122.Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(Suppl 1):115S–27S. [PubMed] [Google Scholar]
- 123.Zalutsky MR. Current status of therapy of solid tumors: brain tumor therapy. J Nucl Med. 2005;46(Suppl 1):151S–6S. [PubMed] [Google Scholar]
- 124.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408(6815):979–82. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 125.Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11(5):556–61. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 126.Kemper EM, Boogerd W, Thuis I, Beijnen JH, van Tellingen O. Modulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30(5):415–23. doi: 10.1016/j.ctrv.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Dietlein M, Pels H, Schulz H, Staak O, Borchmann P, Schomacker K, Fischer T, Eschner W, Pogge vS, Schicha H, Engert A, Schnell R. Imaging of central nervous system lymphomas with iodine-123 labeled rituximab. Eur J Haematol. 2005;74(4):348–52. doi: 10.1111/j.1600-0609.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 128.Neuwelt EA, Specht HD, Barnett PA, Dahlborg SA, Miley A, Larson SM, Brown P, Eckerman KF, Hellstrom KE, Hellstrom I. Increased delivery of tumor-specific monoclonal antibodies to brain after osmotic blood-brain barrier modification in patients with melanoma metastatic to the central nervous system. Neurosurgery. 1987;20(6):885–95. doi: 10.1227/00006123-198706000-00011. [DOI] [PubMed] [Google Scholar]
- 129.Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE, Cokgor I, McLendon RE, Pegram CN, Provenzale JM, Quinn JA, Rich JN, Regalado LV, Sampson JH, Shafman TD, Wikstrand CJ, Wong TZ, Zhao XG, Zalutsky MR, Bigner DD. Phase II trial of murine (131)I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J Clin Oncol. 2002;20(5):1389–97. doi: 10.1200/JCO.2002.20.5.1389. [DOI] [PubMed] [Google Scholar]
- 130.Haluska M, Anthony ML. Osmotic blood-brain barrier modification for the treatment of malignant brain tumors. Clin J Oncol Nurs. 2004;8(3):263–7. doi: 10.1188/04.CJON.263-267. [DOI] [PubMed] [Google Scholar]
- 131.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340(4):1085–90. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 132.Benz CC, O’Hagan RC, Richter B, Scott GK, Chang CH, Xiong X, Chew K, Ljung BM, Edgerton S, Thor A, Hassell JA. HER2/Neu and the Ets transcription activator PEA3 are coordinately upregulated in human breast cancer. Oncogene. 1997;15(13):1513–25. doi: 10.1038/sj.onc.1201331. [DOI] [PubMed] [Google Scholar]
- 133.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 134.Bell R. What can we learn from Herceptin trials in metastatic breast cancer? Oncology. 2002;63(Suppl 1):39–46. doi: 10.1159/000066200. [DOI] [PubMed] [Google Scholar]
- 135.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 136.Fenner MH, Possinger K. Chemotherapy for breast cancer brain metastases. Onkologie. 2002;25(5):474–9. doi: 10.1159/000067443. [DOI] [PubMed] [Google Scholar]
- 137.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–7. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 138.Burstein HJ, Lieberman G, Slamon DJ, Winer EP, Klein P. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol. 2005;16(11):1772–7. doi: 10.1093/annonc/mdi371. [DOI] [PubMed] [Google Scholar]
- 139.Lower EE, Drosick DR, Blau R, Brennan L, Danneman W, Hawley DK. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer. 2003;4(2):114–9. doi: 10.3816/cbc.2003.n.016. [DOI] [PubMed] [Google Scholar]
- 140.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–52. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 141.Scartozzi M, Galizia E, Verdecchia L, Berardi R, Antognoli S, Chiorrini S, Cascinu S. Chemotherapy for advanced gastric cancer: across the years for a standard of care. Expert Opin Pharmacother. 2007;8(6):797–808. doi: 10.1517/14656566.8.6.797. [DOI] [PubMed] [Google Scholar]
- 142.van Ditzhuijsen CI, van de WR, Haak HR. Adrenocortical carcinoma. Neth J Med. 2007;65(2):55–60. [PubMed] [Google Scholar]
- 143.von Minckwitz G. Docetaxel/anthracycline combinations for breast cancer treatment. Expert Opin Pharmacother. 2007;8(4):485–95. doi: 10.1517/14656566.8.4.485. [DOI] [PubMed] [Google Scholar]
- 144.Bristol IJ, Buchholz TA. Inflammatory breast cancer: current concepts in local management. Breast Dis. 2005;22:75–83. doi: 10.3233/bd-2006-22109. [DOI] [PubMed] [Google Scholar]
- 145.Ulrich-Pur H, Kornek GV, Haider K, Kwasny W, Payrits T, Dworan N, Vormittag L, Depisch D, Lang F, Scheithauer W. Phase II trial of pegylated liposomal doxorubicin (Caelyx) plus Gemcitabine in chemotherapeutically pretreated patients with advanced breast cancer. Acta Oncol. 2007;46(2):208–13. doi: 10.1080/02841860600897868. [DOI] [PubMed] [Google Scholar]
- 146.Thigpen JT, Aghajanian CA, Alberts DS, Campos SM, Gordon AN, Markman M, McMeekin DS, Monk BJ, Rose PG. Role of pegylated liposomal doxorubicin in ovarian cancer. Gynecol Oncol. 2005;96(1):10–8. doi: 10.1016/j.ygyno.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 147.Recchia F, Saggio G, Amiconi G, Di Blasio A, Cesta A, Candeloro G, Carta G, Necozione S, Mantovani G, Rea S. A multicenter phase II study of pegylated liposomal doxorubicin and oxaliplatin in recurrent ovarian cancer. Gynecol Oncol. 2007 doi: 10.1016/j.ygyno.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 148.Pignata S, Scambia G, Pisano C, Breda E, Di Maio M, Greggi S, Ferrandina G, Lorusso D, Zagonel V, Febbraro A, Riva N, De RV, Gallo C, Perrone F. A multicentre phase II study of carboplatin plus pegylated liposomal doxorubicin as first-line chemotherapy for patients with advanced or recurrent endometrial carcinoma: the END-1 study of the MITO (Multicentre Italian Trials in Ovarian Cancer and Gynecologic Malignancies) group. Br J Cancer. 2007 doi: 10.1038/sj.bjc.6603787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang X, Pang L, Feng J. A phase II study of etoposide, doxorubicin, and carboplatin in the treatment of advanced gastric cancer. Am J Clin Oncol. 2002;25(1):71–5. doi: 10.1097/00000421-200202000-00015. [DOI] [PubMed] [Google Scholar]
- 150.Ardizzoni A, Tjan-Heijnen VC, Postmus PE, Buchholz E, Biesma B, Karnicka-Mlodkowska H, Dziadziuszko R, Burghouts J, Van Meerbeeck JP, Gans S, Legrand C, Debruyne C, Giaccone G, Manegold C. Standard versus intensified chemotherapy with granulocyte colony-stimulating factor support in small-cell lung cancer: a prospective European Organization for Research and Treatment of Cancer-Lung Cancer Group Phase III Trial-08923. J Clin Oncol. 2002;20(19):3947–55. doi: 10.1200/JCO.2002.02.069. [DOI] [PubMed] [Google Scholar]
- 151.Gilliam LK, Kohn AD, Lalani T, Swanson PE, Vasko V, Patel A, Livingston RB, Pickett CA. Capecitabine therapy for refractory metastatic thyroid carcinoma: a case series. Thyroid. 2006;16(8):801–10. doi: 10.1089/thy.2006.16.801. [DOI] [PubMed] [Google Scholar]
- 152.Palmieri C, Treibel T, Large O, Bower M. AIDS-related non-Hodgkin’s lymphoma in the first decade of highly active antiretroviral therapy. QJM. 2006;99(12):811–26. doi: 10.1093/qjmed/hcl115. [DOI] [PubMed] [Google Scholar]
- 153.Laskar S, Gupta T, Vimal S, Muckaden MA, Saikia TK, Pai SK, Naresh KN, Dinshaw KA. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need? J Clin Oncol. 2004;22(1):62–8. doi: 10.1200/JCO.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 154.Pulini S, Rupoli S, Goteri G, Pimpinelli N, Alterini R, Tassetti A, Scortechini AR, Offidani M, Mulattieri S, Stronati A, Brandozzi G, Giacchetti A, Mozzicafreddo G, Ricotti G, Filosa G, Bettacchi A, Simonacci M, Novelli N, Leoni P. Pegylated liposomal doxorubicin in the treatment of primary cutaneous T-cell lymphomas. Haematologica. 2007;92(5):686–9. doi: 10.3324/haematol.10879. [DOI] [PubMed] [Google Scholar]
- 155.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98(11):2447–56. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 156.Grobmyer SR, Maki RG, Demetri GD, Mazumdar M, Riedel E, Brennan MF, Singer S. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15(11):1667–72. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 157.Leo E, Schlegel PG, Lindemann A. Chemotherapeutic induction of long-term remission in metastatic medulloblastoma. J Neurooncol. 1997;32(2):149–54. doi: 10.1023/a:1005721510659. [DOI] [PubMed] [Google Scholar]
- 158.Stan AC, Casares S, Radu D, Walter GF, Brumeanu TD. Doxorubicin-induced cell death in highly invasive human gliomas. Anticancer Res. 1999;19(2A):941–50. [PubMed] [Google Scholar]
- 159.Muldoon LL, Neuwelt EA. BR96-DOX immunoconjugate targeting of chemotherapy in brain tumor models. J Neurooncol. 2003;65(1):49–62. doi: 10.1023/a:1026234130830. [DOI] [PubMed] [Google Scholar]
- 160.Gupta B, Torchilin VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol Immunother. 2007;56(8):1215–23. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cummings J, McArdle CS. Studies on the in vivo disposition of adriamycin in human tumours which exhibit different responses to the drug. Br J Cancer. 1986;53(6):835–8. doi: 10.1038/bjc.1986.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol. 2006;41(3):389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 163.Ferreira AL, Salvadori DM, Nascimento MC, Rocha NS, Correa CR, Pereira EJ, Matsubara LS, Matsubara BB, Ladeira MS. Tomato-oleoresin supplement prevents doxorubicin-induced cardiac myocyte oxidative DNA damage in rats. Mutat Res. 2007 doi: 10.1016/j.mrgentox.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 164.Flynn HG. Physics of acoustic cavitation in liquids. In: Mason WP, editor. Physical Acoustics, Principles and Methods. New York: Academic Press; 1964. pp. 57–172. [Google Scholar]
- 165.Frizzell LA. Biologic Effects of Acoustic Cavitation. In: Suslick KS, editor. Ultrasound, Its Chemical, Physical, and biologig Effects. New York: VCH Publishers, Inc; 1998. pp. 287–303. [Google Scholar]
- 166.Nyborg WL. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound Med Biol. 2001;27(3):301–33. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 167.Hsieh DY, Plesset MS. Theory of rectified diffusion of mass into gas bubbles. J Acoust Soc Am. 1961;33:206–11. [Google Scholar]
- 168.Nyborg WL. Acoustic Streaming. In: Mason WP, editor. Physical Acoustics. New York: Academic Press; 1965. [Google Scholar]
- 169.Miller DL. A review of the ultrasonic bioeffects of microsonation, gas-body activation, and related cavitation-like phenomena. Ultrasound Med Biol. 1987;13(8):443–70. doi: 10.1016/0301-5629(87)90110-4. [DOI] [PubMed] [Google Scholar]
- 170.Krizanac-Bengez L, Mayberg MR, Janigro D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. Neurol Res. 2004;26(8):846–53. doi: 10.1179/016164104X3789. [DOI] [PubMed] [Google Scholar]
- 171.Leighton T. The Acoustic Bubble. Sand Diego: Academic Press; 1994. [Google Scholar]
- 172.Hamill OP. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006;453(3):333–51. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- 173.Mihran RT, Barnes FS, Wachtel H. Temporally-specific modification of myelinated axon excitability in vitro following a single ultrasound pulse. Ultrasound Med Biol. 1990;16(3):297–309. doi: 10.1016/0301-5629(90)90008-z. [DOI] [PubMed] [Google Scholar]
- 174.Mihran RT, Barnes FS, Wachtel H. Transient modification of nerve excitability in vitro by single ultrasound pulses. Biomed Sci Instrum. 1990;26:235–46. [PubMed] [Google Scholar]
- 175.Apfel RE. Acoustic cavitation: a possible consequence of biomedical uses of ultrasound. Br J Cancer Suppl. 1982;45(5):140–6. [PMC free article] [PubMed] [Google Scholar]
- 176.Flynn HG. Generation of transient cavities in liquids by microsecond pulses of ultrasound. J Acoust Soc Am. 1982;72:1926–32. [Google Scholar]
- 177.Brujan EA. The role of cavitation microjets in the therapeutic applications of ultrasound. Ultrasound Med Biol. 2004;30(3):381–7. doi: 10.1016/j.ultrasmedbio.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 178.Katz JI. Jets from collapsing bubbles. Proc R Soc London, Ser A. 1999;455:323–8. [Google Scholar]
- 179.Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med. 1992;13(3):247–70. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 180.Edmonds PD, Sancier KM. Evidence for free radical production by ultrasonic cavitation in biological media. Ultrasound Med Biol. 1983;9(6):635–9. doi: 10.1016/0301-5629(83)90009-1. [DOI] [PubMed] [Google Scholar]
- 181.Kondo T, Kodaira T, Kano E. Free radical formation induced by ultrasound and its effects on strand breaks in DNA of cultured FM3A cells. Free Radic Res Commun. 1993;19(Suppl 1):S193–S200. doi: 10.3109/10715769309056s193. [DOI] [PubMed] [Google Scholar]
- 182.Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol. 2004;30(4):519–26. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 183.Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol. 1996;22(9):1131–54. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- 184.Miller DL, Thomas RM. Ultrasound contrast agents nucleate inertial cavitation in vitro. Ultrasound Med Biol. 1995;21(8):1059–65. doi: 10.1016/0301-5629(95)93252-u. [DOI] [PubMed] [Google Scholar]
- 185.van Wamel A, Bouakaz A, Versluis M, de Jong N. Micromanipulation of endothelial cells: ultrasound-microbubble-cell interaction. Ultrasound Med Biol. 2004;30(9):1255–8. doi: 10.1016/j.ultrasmedbio.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 186.Coakley WT. Acoustic detection of single cavitation events in a focused field in water at 1 MHz. J Acoust Soc Am. 2006;49:792. [Google Scholar]
- 187.Neppiras EA. Acoustic Cavitation. Phys Rep. 1980;61:160. [Google Scholar]
- 188.Lele PP. MGAR. In: Effects of ultrasound on solid mammalian tissues and tumors in vivo. Repacholi MH, editor. New York: Plunum Publishing Corporation; 1987. pp. 275–306. [Google Scholar]
- 189.Zhong P, Zhou Y, Zhu S. Dynamics of bubble oscillation in constrained media and mechanisms of vessel rupture in SWL. Ultrasound Med Biol. 2001;27(1):119–34. doi: 10.1016/s0301-5629(00)00322-7. [DOI] [PubMed] [Google Scholar]
- 190.Sassaroli E, Hynynen K. Forced linear oscillations of microbubbles in blood capillaries. J Acoust Soc Am. 2004;115:3235–43. doi: 10.1121/1.1738456. [DOI] [PubMed] [Google Scholar]
- 191.Sassaroli E, Hynynen K. Resonance frequency of microbubbles in small blood vessels: a numerical study. Phys Med Biol. 2005;50(22):5293–305. doi: 10.1088/0031-9155/50/22/006. [DOI] [PubMed] [Google Scholar]
- 192.Cui J, Hamilton MF, Wilson PS, Zabolotskaya EA. Bubble pulsations between parallel plates. J Acoust Soc Am. 2006;119(4):2067–72. doi: 10.1121/1.2172545. [DOI] [PubMed] [Google Scholar]
- 193.Caskey CF, Kruse DE, Dayton P, Kitano TK, Ferrara KW. Microbubble oscillations in tubes with diameters of 12, 25, and 195 microns. Appl Phys Lett. 2006;88:033902. [Google Scholar]
- 194.Qin S, Ferrara KW. Acoustic response of compliable microvessels containing ultrasound contrast agents. Phys Med Biol. 2006;27:5063–88. doi: 10.1088/0031-9155/51/20/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sassaroli E, Hynynen K. Cavitation thresholds of microbubbles in gel tunnels by focused ultrasound. Ultrasound Med Biol. 2007;33(10):1651–60. doi: 10.1016/j.ultrasmedbio.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tata DA, Anderson BJ. A new method for the investigation of capillary structure. J Neurosci Methods. 2002;113(2):199–206. doi: 10.1016/s0165-0270(01)00494-0. [DOI] [PubMed] [Google Scholar]
- 197.Seylaz J, Charbonne R, Nanri K, Von Euw D, Borredon J, Kacem K, Meric P, Pinard E. Dynamic in vivo measurement of erythrocyte velocity and flow in capillaries and of microvessel diameter in the rat brain by confocal laser microscopy. J Cereb Blood Flow Metab. 1999;19(8):863–70. doi: 10.1097/00004647-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 198.Leighton TG, White PR, Marsden MA. The one-dimensional bubble: An unusual oscillator, with applications to human bioeffects of underwater sound. Eur J Phys. 1995;16:275–81. [Google Scholar]
- 199.Leighton TG, White PR, Marsden MA. Applications of one-dimensional bubbles to lithotripsy, and to diver response to low frequency sound. Acta Acoustica. 1995;3:517–29. [Google Scholar]
- 200.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30(7):979–89. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 201.Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32(9):1399–409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 202.Sheikov N, McDannold N, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. doi: 10.1016/j.ultrasmedbio.2007.12.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Raymond SB, Skoch J, Bacskai BJ, Hynynen K. Modular design for in vivo optical imaging and ultrasound treatment in the murine brain. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(2):431–4. doi: 10.1109/tuffc.2007.257. [DOI] [PubMed] [Google Scholar]
- 204.Raymond SB, Skoch J, Hynynen K, Bacskai BJ. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J Cereb Blood Flow Metab. 2007;27(2):393–403. doi: 10.1038/sj.jcbfm.9600336. [DOI] [PubMed] [Google Scholar]
- 205.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81(3):1065–96. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 206.Leao Pial circulation and spreading depression activity in cerebral cortex. J Neurophysiol. 1944;7:391–6. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 207.Yanamoto H, Xue JH, Miyamoto S, Nagata I, Nakano Y, Murao K, Kikuchi H. Spreading depression induces long-lasting brain protection against infarcted lesion development via BDNF gene-dependent mechanism. Brain Res. 2004;1019(1–2):178–88. doi: 10.1016/j.brainres.2004.05.105. [DOI] [PubMed] [Google Scholar]
- 208.Yanamoto H, Miyamoto S, Tohnai N, Nagata I, Xue JH, Nakano Y, Nakajo Y, Kikuchi H. Induced spreading depression activates persistent neurogenesis in the subventricular zone, generating cells with markers for divided and early committed neurons in the caudate putamen and cortex. Stroke. 2005;36(7):1544–50. doi: 10.1161/01.STR.0000169903.09253.c7. [DOI] [PubMed] [Google Scholar]
- 209.Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, Wang X, Rosenberg GA, Lo EH, Moskowitz MA. Cortical spreading depression activates and upregulates MMP-9. J Clin Invest. 2004;113(10):1447–55. doi: 10.1172/JCI21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vykhodtseva N, Koroleva VI. Steady Potential Changes and Spreading Depression in Rat Brains Produced by Focused Ultrasound. In: Clement G, et al., editors. Therapeutic Ultrasound; AIP Conference proceedings ISTU2005; Boston, MA. 2006. pp. 313–317. [Google Scholar]
- 211.Vykhodtseva N, Konopatskaya II, Koroleva VI. Focused Ultrasound Potential to Initiate Spreading Depression for Disruption of Blood-Brain Barrier. 2007 IEEE Ultrasonics Symposium; New York City, NY. 28–31 October 2007; pp. 428–31. [Google Scholar]