Abstract
BACKGROUND
Prosurvival signaling kinases inhibit glycogen synthase kinase-3β (GSK-3β) activity and stimulate apoptotic protein p53 degradation. Helium produces cardioprotection by activating prosurvival kinases, but whether GSK and p53 inhibition mediate this process is unknown. We tested the hypothesis that inhibition of GSK or p53 lowers the threshold of helium cardioprotection via a mitochondrial permeability transition pore (mPTP)-dependent mechanism.
METHODS
Rabbits (n = 85) instrumented for hemodynamic measurement and subjected to a 30 min left anterior descending coronary artery (LAD) occlusion and 3 h reperfusion received 0.9% saline (control), or 1, 3, or 5 cycles of 70% helium-30% oxygen administered for 5 min interspersed with 5 min of an air-oxygen mixture (fraction of inspired oxygen concentration = 0.30) before LAD occlusion. Other rabbits received the GSK inhibitor SB 216763 (SB21; 0.2 or 0.6 mg/kg), the p53 inhibitor pifithrin-α (PIF; 1.5 or 3.0 mg/kg), or SB21 (0.2 mg/kg) or PIF (1.5 mg/kg) plus helium (1 cycle) before LAD occlusion in the presence or absence of the mPTP opener atractyloside (5 mg/kg).
RESULTS
Helium reduced (P < 0.05) myocardial infarct size (35 ± 6 [n = 7], 25 ± 4 [n = 7], and 20 ± 3% [n = 6] of area at risk, 1, 3, and 5 cycles, respectively) compared with control (44 ± 6% [n = 7]). SB21 (0.6 [n = 7] but not 0.2 mg/kg [n = 6]) and PIF (3.0 [n = 6] but not 1.5 mg/kg [n = 7]) also reduced necrosis. SB21 (0.2 mg/kg) or 1.5 mg/kg PIF (1.5 mg/kg) plus helium (1 cycle; n = 6 per group) decreased infarct size to an equivalent degree as three cycles of helium alone, and this cardioprotection was blocked by atractyloside (n = 7 per group).
CONCLUSIONS
Inhibition of GSK or p53 lowers the threshold of helium-induced preconditioning via a mPTP-dependent mechanism in vivo.
Administration of noble gases before prolonged coronary artery occlusion and reperfusion protects myocardium against infarction.1,2 Our laboratory recently demonstrated that the cardioprotective effects of brief, repetitive exposure to the nonanesthetic noble gas helium in vivo are mediated by activation of prosurvival signaling kinases (including phosphotidylinositol-3-kinase [PI3K], extracellular signal-regulated kinases [Erk1/2], and mammalian target of rapamycin-70-kDa ribosomal protein s6 kinase [mTOR/p70s6K]) and inhibition of the mitochondrial permeability transition pore (mPTP).1 The β isoform of glycogen synthase kinase-3β (GSK-3β) is an important mediator of cellular function3 whose activation has been implicated in the pathogenesis of diabetes mellitus4 and Alzheimer’s disease.5 In contrast, GSK-3β inhibition has been shown to play a critical role in ischemic, pharmacologic, and anesthetic pre- and postconditioning.6–12 Several prosurvival kinases (e.g., PI3K, mTOR/p70s6K, protein kinase C) converge on and inhibit GSK-3β, thereby favorably modulating mPTP and producing cardioprotection.13 Activated GSK-3β binds to and promotes the actions of the apoptotic protein p53,14 and this latter enzyme also independently facilitates mPTP opening through its interactions with another apoptotic moiety, Bax.15 Thus, it is not surprising that a role for inhibition of p53 in myocardial and neural protection against cellular injury has also been described.16–18 Whether GSK or p53 inhibition mediates the protective effects of helium is unknown. We tested the hypothesis that inhibition of GSK or p53 lowers the threshold of helium cardioprotection via an mPTP-dependent mechanism in vivo.
METHODS
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Furthermore, all conformed to the Guiding Principles in the Care and Use of Animals of the American Physiologic Society and were in accordance with the Guide for the Care and Use of Laboratory Animals.
Experimental Preparation
Male New Zealand white rabbits weighing between 2.5 and 3.0 kg were anesthetized with IV sodium pentobarbital (30 mg/kg) as previously described.1 Additional doses of pentobarbital were titrated as required to assure that pedal and palpebral reflexes were absent throughout the experiment. Briefly, a tracheostomy was performed through a midline incision, and each rabbit was ventilated with positive pressure using an air-oxygen mixture (fractional inspired oxygen concentration = 0.30). Arterial blood gas tensions and acid-base status were maintained within a normal physiological range by adjusting the respiratory rate or tidal volume throughout the experiment. A pulse oximeter was placed on the right hindpaw of each rabbit for measurement of continuous arterial oxygen saturation. Heparin-filled catheters were positioned in the right carotid artery and the left jugular vein for measurement of arterial blood pressure and fluid or drug administration, respectively. Maintenance fluids (0.9% saline; 15 mL · kg−1 · min−1) were continued for the duration of each experiment. A thoracotomy was performed at the left fourth intercostal space, and the heart was suspended in a pericardial cradle. A prominent branch of the left anterior descending coronary artery (LAD) was identified, and a silk ligature was placed around this vessel approximately halfway between the base and the apex for the production of coronary artery occlusion and reperfusion. IV heparin (500 U) was administered immediately before LAD occlusion. Coronary artery occlusion was verified by the presence of epicardial cyanosis and regional dyskinesia in the ischemic zone, and reperfusion was confirmed by observing an epicardial hyperemic response. Systemic hemo-dynamics was continuously recorded on a polygraph throughout each experiment.
Experimental Protocol
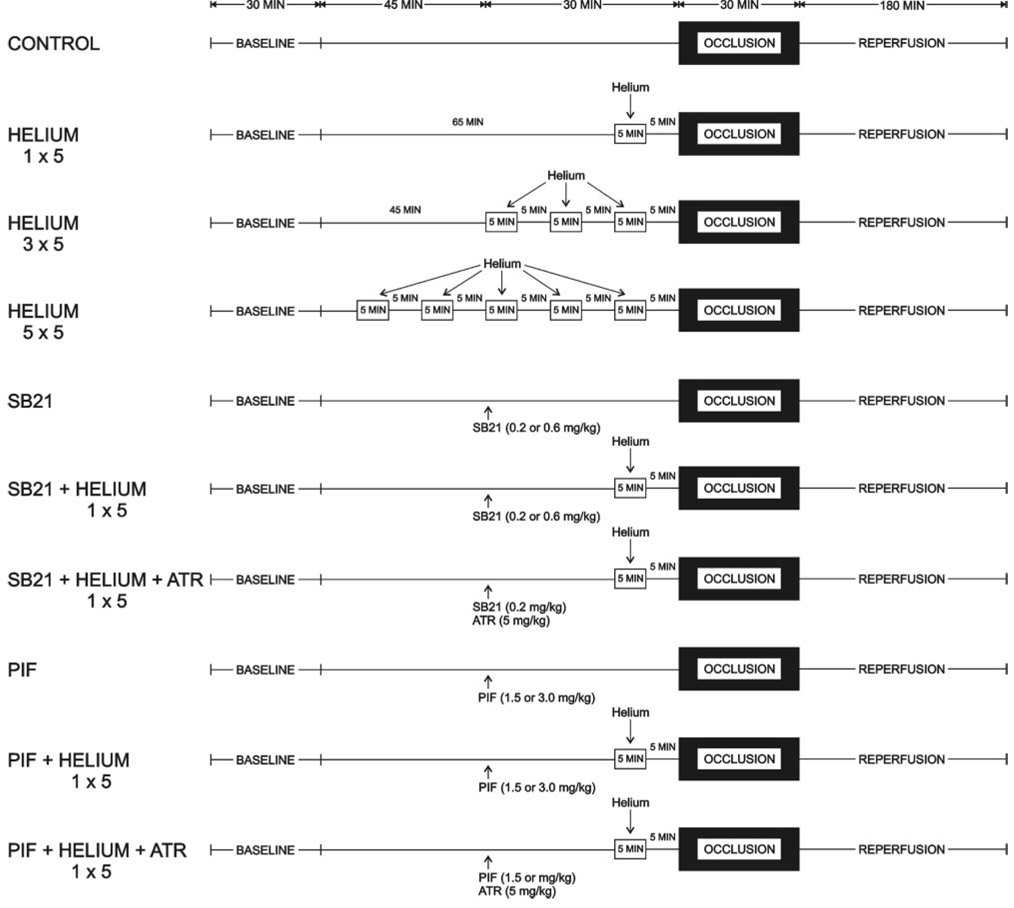
The experimental design is illustrated in Figure 1. Baseline hemodynamics and arterial blood gas tensions were recorded 30 min after instrumentation was completed. All rabbits underwent a 30 min LAD occlusion after 3 h of reperfusion. In 12 separate groups, rabbits (n = 85) were randomly assigned to receive 0.9% saline (control), 1, 3, or 5 cycles of 70% helium-30% oxygen administered for 5 min interspersed with 5 min of an air-oxygen mixture (fractional inspired oxygen concentration = 0.30), the selective GSK inhibitor SB 216763 (SB21; 0.2 or 0.6 mg/kg), the p53 inhibitor selective pifithrin-α (1.5 or 3.0 mg/kg), or the combination of SB21 (0.2 mg/kg) or pifithrin-α (1.5 mg/kg) and 1 cycle of 70% helium-30% oxygen before LAD occlusion in the absence and presence of the mPTP opener atractyloside (5 mg/kg). SB21 and atractyloside were dissolved in dimethyl-sulfoxide and administered by IV infusion 30 min before coronary occlusion. Pifithrin-α was dissolved in dimethylsulfoxide and administered by intraperitoneal injection 30 min before LAD occlusion. We have previously shown that the lower doses of SB21 and pifithrin-α do not produce hemodynamic effects nor affect infarct size in an identical rabbit model.12,18 We have also shown that the dose of atractyloside used in the current investigation is devoid of hemodynamic effects, does not alter the extent of myocardial necrosis compared with 0.9% saline when administered alone, and abolishes reductions in infarct size produced by the selective mPTP inhibitor cyclosporin A.19 Dimethylsulfoxide alone also did not affect infarct size in rabbits.20
Figure 1.
Schematic illustration depicting the experimental protocol used in the current investigation. SB21 = SB 216763; PIF = pifithrin-α; ATR = atractyloside.
Measurement of Myocardial Infarct Size
Myocardial infarct size was measured as previously described.1 Briefly, the LAD was reoccluded at the completion of each experiment and 3 mL of patent blue dye was injected IV. The left ventricular area at risk for infarction was separated from surrounding normal areas (stained blue), and the two regions were incubated at 37°C for 20 min in 1% 2,3,5-triphenyltetrazolium chloride in 0.1 M phosphate buffer adjusted to pH 7.4. Infarcted and noninfarcted myocardium within the area at risk were carefully separated and weighed after storage overnight in 10% formaldehyde. Myocardial infarct size was expressed as a percentage of the area at risk. Rabbits that developed intractable ventricular fibrillation and those with an area at risk <15% of total left ventricular mass were excluded from subsequent analysis.
Statistical Analysis
A power analysis indicated that a group size of n≥6 was required for a minimal difference in infarct size of 20% (α error <0.05; β error <20%) with a power of 95%. Statistical analysis of data within and between groups was performed with analysis of variance for repeated measures followed by Bonferroni’s modification of Student’s t-test. Changes were considered statistically significant when P < 0.05. All data are expressed as mean ± sd (sd).
RESULTS
Eighty-five rabbits were instrumented to obtain 78 successful infarct size experiments. Four rabbits were excluded because the left ventricular area at risk was <15% of the total left ventricular mass. Three rabbits were excluded because intractable ventricular fibrillation occurred during coronary artery occlusion. Arterial blood gas tensions and acid-base status were maintained within the physiologic range during administration of helium in all groups (data not shown). No differences among groups were observed. Arterial oxygen saturation remained at 100% during and after administration of helium with or without other drug interventions (data not shown). Baseline systemic hemodynamics were similar among groups (Table 1). No changes in hemodynamics were observed during administration of helium. SB21, pifithrin-α, and atractyloside did not affect hemodynamics in the absence or presence of helium. Significant (P < 0.05) decreases in rate–pressure product occurred during reperfusion in all experimental groups except for rabbits receiving 1 cycle of helium, 1.5 mg/kg pifithrin-α, and 5 mg/kg atractyloside.
Table 1.
Hemodynamics
| Reperfusion (min) | ||||||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Occlusion | 60 | 120 | 180 | |
| HR (min−1) | ||||||
| CON | 249 ± 40 | 241 ± 34 | 241 ± 34 | 237 ± 21 | 231 ± 25 | 220 ± 23 |
| He (1 Cycle) | 263 ± 29 | 254 ± 25 | 239 ± 18 | 230 ± 31* | 224 ± 31* | 220 ± 24* |
| He (3 Cycles) | 251 ± 33 | 243 ± 27 | 223 ± 18* | 216 ± 26* | 209 ± 22* | 199 ± 25* |
| He (5 Cycles) | 256 ± 28 | 245 ± 22 | 218 ± 20* | 217 ± 14* | 204 ± 9* | 194 ± 7* |
| SB21 (0.2 mg/kg) | 272 ± 24 | 265 ± 23 | 253 ± 23 | 243 ± 27* | 235 ± 24* | 229 ± 22* |
| SB21 (0.6 mg/kg) | 259 ± 31 | 252 ± 19 | 239 ± 17 | 227 ± 14* | 218 ± 22* | 209 ± 25* |
| He (1 Cycle) + SB21 (0.2 mg/kg) | 232 ± 10 | 224 ± 13 | 228 ± 23 | 222 ± 28 | 215 ± 29 | 207 ± 27 |
| He (1 Cycle) + SB21 (0.2 mg/kg) + ATR (5 mg/kg) | 273 ± 27 | 265 ± 21 | 257 ± 16 | 235 ± 31 | 234 ± 10* | 228 ± 8* |
| PIF (1.5 mg/kg) | 271 ± 29 | 257 ± 26 | 259 ± 26 | 251 ± 27* | 235 ± 26* | 215 ± 12* |
| PIF (3.0 mg/kg) | 275 ± 21 | 243 ± 22 | 243 ± 22 | 231 ± 30* | 224 ± 11* | 220 ± 12* |
| He (1 Cycle) + PIF (1.5 mg/kg) | 262 ± 19 | 238 ± 15 | 237 ± 22 | 238 ± 15 | 228 ± 17* | 215 ± 12* |
| He (1 Cycle) + PIF (1.5 mg/kg) + ATR (5 mg/kg) | 274 ± 24 | 267 ± 33 | 259 ± 36 | 249 ± 47 | 243 ± 47 | 246 ± 48 |
| MAP (mm Hg) | ||||||
| CON | 74 ± 7 | 68 ± 8 | 64 ± 8 | 63 ± 6 | 64 ± 6 | 64 ± 7 |
| He (1 Cycle) | 71 ± 7 | 75 ± 7 | 67 ± 9 | 67 ± 9 | 68 ± 12 | 68 ± 10 |
| He (3 Cycles) | 77 ± 8 | 82 ± 12 | 61 ± 11 | 60 ± 9* | 63 ± 9* | 60 ± 7* |
| He (5 Cycles) | 65 ± 11 | 65 ± 9 | 55 ± 9 | 57 ± 4 | 56 ± 6 | 58 ± 5 |
| SB21 (0.2 mg/kg) | 61 ± 7 | 63 ± 10 | 64 ± 5 | 57 ± 5 | 60 ± 7 | 61 ± 7 |
| SB21 (0.6 mg/kg) | 67 ± 11 | 70 ± 13 | 70 ± 12 | 64 ± 11 | 64 ± 10 | 63 ± 9 |
| He (1 Cycle) + SB21 (0.2 mg/kg) | 64 ± 8 | 68 ± 7 | 58 ± 8 | 58 ± 5 | 58 ± 7 | 58 ± 6 |
| He (1 Cycle) + SB21 (0.2 mg/kg) + ATR (5 mg/kg) | 69 ± 11 | 70 ± 13 | 60 ± 12 | 58 ± 10 | 59 ± 8 | 62 ± 12 |
| PIF (1.5 mg/kg) | 69 ± 7 | 74 ± 9 | 68 ± 11 | 60 ± 5 | 60 ± 8 | 63 ± 10 |
| PIF (3.0 mg/kg) | 64 ± 6 | 70 ± 12 | 54 ± 16 | 56 ± 9 | 58 ± 9 | 59 ± 11 |
| He (1 Cycle) + PIF (1.5 mg/kg) | 65 ± 9 | 73 ± 13 | 69 ± 12 | 59 ± 7 | 65 ± 10 | 60 ± 8 |
| He (1 Cycle) + PIF (1.5 mg/kg) + ATR (5 mg/kg) | 69 ± 12 | 71 ± 11 | 65 ± 8 | 64 ± 11 | 61 ± 9 | 61 ± 10 |
| RPP (min−1 · mm Hg · 10−3) | ||||||
| CON | 21.1 ± 4.3 | 19.2 ± 3.7 | 17.9 ± 3.2 | 18.0 ± 3.0 | 17.7 ± 2.5 | 16.4 ± 2.3* |
| He (1 Cycle) | 20.7 ± 1.4 | 21.2 ± 2.5 | 18.0 ± 2.8 | 17.6 ± 4.3 | 17.4 ± 4.6 | 16.7 ± 3.2* |
| He (3 Cycles) | 21.9 ± 4.2 | 22.7 ± 4.1 | 16.0 ± 3.8* | 15.3 ± 3.7* | 15.6 ± 2.6* | 14.1 ± 4.9* |
| He (5 Cycles) | 19.1 ± 3.3 | 18.1 ± 2.6 | 14.1 ± 2.4* | 14.4 ± 1.0* | 13.4 ± 1.3* | 13.1 ± 1.3* |
| SB21 (0.2 mg/kg) | 19.3 ± 2.7 | 19.5 ± 3.8 | 18.9 ± 2.7 | 16.2 ± 2.2 | 16.5 ± 2.5 | 16.2 ± 2.1* |
| SB21 (0.6 mg/kg) | 20.0 ± 3.8 | 22.7 ± 4.1 | 19.0 ± 3.5 | 16.7 ± 2.7 | 16.1 ± 3.3* | 15.3 ± 2.5* |
| He (1 Cycle) + SB21 (0.2 mg/kg) | 17.2 ± 2.2 | 17.6 ± 2.2 | 15.1 ± 2.4 | 15.1 ± 2.0 | 14.5 ± 2.6 | 13.9 ± 1.8* |
| He (1 Cycle) + SB21 (0.2 mg/kg) + ATR (5 mg/kg) | 22.5 ± 4.4 | 21.4 ± 5.1 | 17.9 ± 4.4 | 16.2 ± 3.7 | 16.2 ± 2.3* | 16.5 ± 3.1 |
| PIF (1.5 mg/kg) | 21.3 ± 2.8 | 21.4 ± 2.0 | 20.1 ± 3.2 | 17.8 ± 2.2* | 16.5 ± 1.5* | 17.0 ± 2.2* |
| PIF (3.0 mg/kg) | 20.6 ± 2.4 | 19.6 ± 3.8 | 15.3 ± 4.4* | 15.2 ± 3.5* | 15.4 ± 2.3* | 15.4 ± 2.6* |
| He (1 Cycle) + PIF (1.5 mg/kg) | 19.6 ± 2.8 | 19.7 ± 4.0 | 18.7 ± 3.0 | 16.6 ± 2.1 | 17.2 ± 3.1 | 15.3 ± 2.2* |
| He (1 Cycle) + PIF (1.5 mg/kg) + ATR (5 mg/kg) | 22.6 ± 4.4 | 22.7 ± 4.7 | 20.1 ± 4.0 | 19.2 ± 5.4 | 18.1 ± 5.0 | 17.6 ± 4.9 |
Data are mean ± sd.
HR = heart rate; MAP = mean arterial pressure; RPP = rate pressure product; CON = control; He = helium; SB21 = SB213763; PIF = pifithrin-α ; ATR = atractyloside.
Significantly (P ± 0.05) different from baseline.
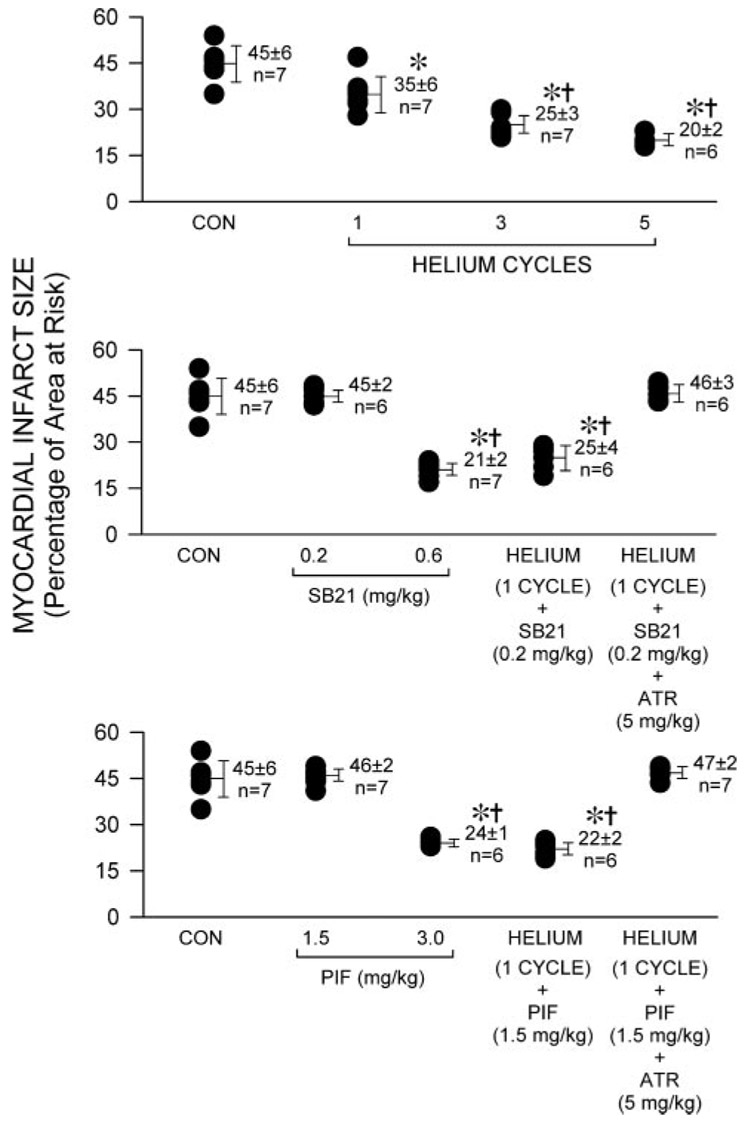
Body weight, left ventricular mass, area at risk weight, and the ratio of area at risk to left ventricular mass were similar among groups (Table 2). Helium significantly (P < 0.05) reduced infarct size (35 ± 6, 25 ± 4, and 20% ± 3%, 1, 3, and 5 cycles, respectively, of left ventricular area at risk; Fig. 2) compared with control rabbits (44% ± 6%). SB21 (0.6 but not 0.2 mg/kg) and pifithrin-α (3.0 but not 1.5 mg/kg) alone also reduced infarct size (21 ± 2, 45 ± 2, 24 ± 1, and 46% ± 2%, respectively). The combination of 0.2 mg/kg SB21 or 1.5 mg/kg pifithrin-α and 1 cycle of helium decreased infarct size (25 ± 4 and 22% ± 2%, respectively) to an equivalent degree as 3 cycles of helium exposure alone. The cardioprotective effects of combined administration of SB21 or pifithrin-α and helium were abolished by atractyloside pretreatment (46 ± 3 and 47% ± 2%, respectively).
Table 2.
Left Ventricular Area at Risk
| N | Body weight (g) | LV (g) | AAR (g) | AAR/LV (%) | |
|---|---|---|---|---|---|
| CON | 7 | 2769 ± 357 | 3.61 ± 0.28 | 1.37 ± 0.36 | 38 ± 8 |
| He (1 Cycle) | 7 | 2736 ± 196 | 3.45 ± 0.23 | 1.24 ± 0.11 | 36 ± 5 |
| He (3 Cycles) | 7 | 2526 ± 143 | 3.90 ± 0.26 | 1.30 ± 0.07 | 34 ± 2 |
| He (5 Cycles) | 6 | 2647 ± 462 | 3.55 ± 0.34 | 1.23 ± 0.14 | 35 ± 2 |
| SB21 (0.2 mg/kg) | 6 | 2590 ± 294 | 3.71 ± 0.22 | 1.61 ± 0.14 | 44 ± 4 |
| SB21 (0.6 mg/kg) | 7 | 2671 ± 415 | 3.56 ± 0.31 | 1.27 ± 0.08 | 36 ± 3 |
| He (1 Cycle) + SB21 (0.2 mg/kg) | 6 | 2503 ± 133 | 3.50 ± 0.34 | 1.30 ± 0.08 | 37 ± 3 |
| He (1 Cycle) + SB21 (0.2 mg/kg) + ATR (5 mg/kg) | 6 | 2702 ± 132 | 3.88 ± 0.40 | 1.44 ± 0.27 | 37 ± 6 |
| PIF (1.5 mg/kg) | 7 | 2563 ± 152 | 3.36 ± 0.49 | 1.25 ± 0.22 | 37 ± 4 |
| PIF (3.0 mg/kg) | 6 | 2438 ± 103 | 3.09 ± 0.25 | 1.22 ± 0.04 | 40 ± 2 |
| He (1 Cycle) + PIF (1.5 mg/kg) | 6 | 2428 ± 105 | 3.07 ± 0.19 | 1.15 ± 0.11 | 38 ± 3 |
| He (1 Cycle) + PIF (1.5 mg/kg) ± ATR (5 mg/kg) | 7 | 2720 ± 189 | 4.19 ± 0.27 | 1.66 ± 0.15 | 40 ± 4 |
Data are mean ± sd.
LV = left ventricle; AAR = area at risk; CON = control; He = helium; SB21 = SB213763; PIF = pifithrin-α; ATR = atractyloside.
Figure 2.
Myocardial infarct size depicted as a percentage of left ventricular area at risk in rabbits receiving 0.9% saline (control, CON) or 1, 3, or 5 cycles of 70% helium-30% oxygen administered for 5 min interspersed with 5 min of an air-oxygen mixture (fraction of inspired oxygen concentration = 0.30; panel A, top). Infarct sizes in rabbits receiving the selective glycogen synthase kinase inhibitor SB 216763 (SB21, 0.2 or 0.6 mg/kg) and the combination of one preconditioning cycle of helium and 0.2 mg/kg SB21 in the absence or presence of the mitochondrial permeability transition pore opener atractyloside (ATR; 5 mg/kg) are depicted in panel B (middle). Infarct sizes in rabbits receiving the selective p53 inhibitor pifithrin-α (PIF, 1.5 or 3.0 mg/kg) and the combination of one preconditioning cycle of helium and 1.5 mg/kg PIF in the absence of presence of ATR (5 mg/kg) are depicted in panel C (bottom). Each point represents a single experiment. All data are mean ± sd *Significantly (P < 0.05) different from CON; †Significantly (P < 0.05) different from one cycle of helium pretreatment.
DISCUSSION
The current results confirm previous observations1 demonstrating that 3 cycles of 5 min 70% helium-30% oxygen preconditioning interspersed with 5 min washout periods of an air-oxygen mixture reduce myocardial necrosis after prolonged coronary artery occlusion and reperfusion. Further, the results indicate for the first time that cardioprotection produced by the nonanesthetic noble gas is dependent on exposure frequency before ischemia and reperfusion. Although a single cycle of helium preconditioning produced only a small decrease in infarct size, progressively larger quantities of myocardium were salvaged from irreversible ischemic injury as the number of cycles of helium preconditioning increased. The 0.6 and 3.0 mg/kg doses of SB21 and pifithrin-α, respectively, also reduced infarct size in the current investigation, confirming that inhibition of GSK or p53 produces cardioprotection.9,12,16,18 Previous studies have shown that decreases in myocardial necrosis produced by SB21 or pifithrin-α were unaffected by administration of the selective PI3K antagonist wortmannin, but were abolished by the mPTP opener atractyloside,12,13,15,18 confirming that that GSK or p53 inhibition-mediated protection occurs downstream from PI3K and is dependent on the transition state of the mPTP. The current results also demonstrate that combined administration of sub-cardioprotective threshold doses of SB21 or pifithrin-α and helium reduce myocardial infarct size to a similar magnitude as three cycles of helium alone, and further, that these cardioprotective effects were abolished by atractyloside pretreatment. Thus, the results suggest that GSK or p53 inhibition lowers the threshold of helium preconditioning in barbiturate-anesthetized, acutely instrumented rabbits in a mPTP-dependent manner.
A role for GSK-3β inhibition in several forms of cardioprotection against irreversible ischemic injury has been established.6–11 Reductions in myocardial infarct size during ischemic preconditioning were associated with phosphorylation of GSK-3β through a PI3K-dependent mechanism.6 Similarly, GSK-3β inhibition mediated the protective effects of G protein-linked receptor ligands (e.g., δ1 opioid,7–9 adenosine subtype 3 [A3]10) and volatile anesthetics12,21,22 administered before ischemia or during early reperfusion. GSK-3β has been shown to function as a primary regulatory target of prosurvival kinases. Modulation of this enzyme’s activity appears to play a critical role in determining the transition state of the mPTP upon reperfusion and thus the extent of subsequent myocardial damage.13 Activation of PI3K and Erk1/2 and their target mTOR/p70s6K were shown to mediate helium preconditioning in rabbits.1 Thus, the current results suggest that the cardioprotection produced by the combination of a single 5 min helium exposure to a subthreshold dose of SB21 may be related to more pronounced inhibition of GSK-3β by helium-induced activation of these upstream signaling kinases. However, this conclusion requires qualification because the specific GSK isoform (α or β) was not identified, nor were GSK-3β phosphorylation or enzyme activity determined in the current investigation.
The precise mechanism(s) by which GSK-3β inhibition reduces mPTP opening during early reperfusion and preserves myocardial viability remains to be clearly defined. Activated GSK-3β was shown to bind and stimulate the actions of the tumor suppressor protein p5314 which, when activated, translocates to mitochondria, opens mPTP through its interactions with Bax, facilitates the release of cytochrome c, and causes rapid mitochondrial demise.15 Activation of p53 by hypoxia or large quantities of reactive oxygen species23 also produces cell death by enhancing signaling by24 and increasing transcription of apoptotic proteins.25 In contrast, ischemic preconditioning mitigated increases in p53 expression after hypoxia-reoxygenation in ventricular myocytes.26 Direct pifithrin-α-induced p53 inhibition or enhanced degradation of the protein by PI3K-mediated phosphorylation of murine double minute 2 (Mdm2) protein protected against ischemic injury.16 Pifithrin-α also enhanced isoflurane postconditioning.18 Furthermore, transgenic deletion of p53 prevented cardiac rupture after myocardial infarction.27 Taken together, these data suggest that inhibition of p53 activity or increases in p53 metabolism stimulated by PI3K activation mediates several forms of cardioprotection. The current results demonstrate that administration of a dose of pifithrin-α that did not affect infarct size alone lowered the threshold of helium preconditioning in vivo. This action was blocked by atractyloside, suggesting that the observed reductions in infarct size were mediated by the combined actions of the helium and selective p53 inhibition on mPTP. The PI3K downstream target Akt was shown to activate Mdm228 and, thus, activation of PI3K by helium may facilitate an interaction between phospho-Mdm2 and p53 that leads to the metabolism of the apoptotic protein29 and prevents its detrimental association with GSK-3β. Ischemic preconditioning was shown to activate Mdm2 and enhance phospho-Mdm2-p53 binding in a PI3K-dependent manner,16 but whether helium specifically causes phosphorylation of Mdm2 or produces a similar phospho-Mdm2-p53 interaction was not specifically examined in the current investigation.
In addition to previously described limitations, the current results must be interpreted within the constraints of several other potential shortcomings. SB21 and pifithrin-α have been shown to be selective inhibitors of GSK and p53,30,31 respectively, at the doses used in the current investigation. Similarly, atractyloside was shown to be a relatively selective mPTP opener.32 Nevertheless, the possibility that these drugs may have affected the activity of other protein kinases involved in helium preconditioning cannot be completely excluded. The route and duration of administration of SB21, pifithrin-α, and atractyloside, and were heterogeneous, and these pharmacokinetic factors may have influenced the results. Plasma concentrations of SB21, pifithrin-α, and atractyloside were also not determined. Myocardial infarct size is determined primarily by the size of the area at risk and the extent of coronary collateral perfusion. The area at risk expressed as a percentage of total left ventricular mass was similar among groups in the current investigation, and coronary collateral blood flow was shown to be minimal in rabbits. Thus, differences in collateral perfusion among groups probably did not account for the observed results, but coronary collateral blood flow was not specifically quantified. The reductions in myocardial necrosis produced by helium in the absence or presence of other drug interventions occurred independent of changes in major determinants of myocardial oxygen consumption. Nevertheless, coronary venous oxygen tension was not directly measured nor was myocardial oxygen consumption calculated. Notably, no significant differences in hemodynamics were observed among groups before and during coronary artery occlusion that may account for differences in infarct size observed among groups. Finally, the current results implicating a role for GSK, p53, and mPTP in helium-induced cardioprotection were obtained in barbiturate-anesthetized, acutely instrumented rabbits. It is unknown whether similar results occur in other animal species or humans.
In summary, the current results confirm that brief, intermittent administration of helium before prolonged coronary artery occlusion and reperfusion protects myocardium against infarction and demonstrate that this helium cardioprotection is dependent on exposure frequency before ischemia and reperfusion in rabbits. The findings further suggest that GSK or p53 inhibition lowers the threshold of helium cardio-protection via a mPTP-dependent mechanism in vivo.
Acknowledgments
Supported in part by National Institutes of Health grants HL 054820 and GM 066730 from the United States Public Health Service (Bethesda, MD) and by departmental funds. Dr. Amour is the recipient of research fellowship grants from the Société Française d’Anesthésie et de Réanimation (SFAR, Paris, France), Novo Nordisk® (Paris-LA Défense, France), and the Assistance Publique des Hôpitaux de Paris (APHP, Paris, France).
REFERENCES
- 1.Pagel PS, Krolikowski JG, Venkatapuram S, Shim YH, Kersten JR, Weihrauch D, Warltier DC, Pratt PF., Jr Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105:562–569. doi: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- 2.Preckel B, Weber NC, Sanders RD, Maze M, Schlack W. Molecular mechanisms transducing the anesthetic, analgesic, and organ-protective actions of xenon. Anesthesiology. 2006;105:187–197. doi: 10.1097/00000542-200607000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–6. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes. 1999;48:1662–1666. doi: 10.2337/diabetes.48.8.1662. [DOI] [PubMed] [Google Scholar]
- 5.Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion JP. The active form of glycogen synthase kinase-3β is associated with granulovacuolar degeneration in neurons in Alzheimer’ disease. Acta Neuropathol. 2002;103:91–99. doi: 10.1007/s004010100435. [DOI] [PubMed] [Google Scholar]
- 6.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3β during preconditioning through phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 7.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 8.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3beta. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 9.Gross ER, Hsu AK, Gross GJ. GSK3β inhibition and KATP channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol. 2007;102:341–349. doi: 10.1007/s00395-007-0651-6. [DOI] [PubMed] [Google Scholar]
- 10.Park SS, Zhao H, Jang Y, Mueller RA, Xu Z. N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide confers cardioprotection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3 beta. J Pharmacol Exp Ther. 2006;318:124–131. doi: 10.1124/jpet.106.101477. [DOI] [PubMed] [Google Scholar]
- 11.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol. 2007;292:R800–R809. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- 12.Pagel PS, Krolikowski JG, Neff DA, Weihrauch D, Bienengraeber M, Kersten JR, Warltier DC. Inhibition of glycogen synthase kinase potentiates isoflurane-induced protection against myocardial infarction during early reperfusion in vivo. Anesth Analg. 2006;102:1348–1354. doi: 10.1213/01.ane.0000202379.61338.37. [DOI] [PubMed] [Google Scholar]
- 13.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen sythase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watcharasti P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3β (GSK3β) binds to and promotes the actions of p53. J Biol Chem. 2003;278:48872–48879. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 16.Mocanu MM, Yellon DM. p53 down-regulation: a new molecular mechanism involved in ischaemic preconditioning. FEBS Lett. 2003;555:302–306. doi: 10.1016/s0014-5793(03)01260-2. [DOI] [PubMed] [Google Scholar]
- 17.Tomasevic G, Shamloo M, Israeli D, Wieloch T. Activation of p53 and its target genes p21WAF1/Cip1 and PAG608/Wig-1 in ischemic preconditioning. Brain Res Mol Brain Res. 1999;70:304–313. doi: 10.1016/s0169-328x(99)00146-1. [DOI] [PubMed] [Google Scholar]
- 18.Venkatapuram S, Wang C, Krolikowski JG, Weihrauch D, Kersten JR, Warltier DC, Pratt PF, Jr, Pagel PS. Inhibition of apoptotic protein p53 lowers the threshold of isoflurane-induced cardioprotection during early reperfusion in rabbits. Anesth Analg. 2006;103:1400–1405. doi: 10.1213/01.ane.0000240903.63832.d8e. [DOI] [PubMed] [Google Scholar]
- 19.Krolikowski JG, Bienengraeber M, Weihrauch D, Warltier DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: role of mitochondrial KATP channels. Anesth Analg. 2005;101:1590–1596. doi: 10.1213/01.ANE.0000181288.13549.28. [DOI] [PubMed] [Google Scholar]
- 20.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Waritier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Feng J, Fischer G, Lucchinetti E, Zhu M, Bestman L, Jegger D, Arras M, Pasch T, Perriard JC, Schaub MC, Zaugg M. Infarct-remodeled myocardium is receptive to protection by isoflurane postconditioning. Role of protein kinase B/Akt signaling. Anesthesiology. 2006;104:1004–1014. doi: 10.1097/00000542-200605000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase-3β. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Vousden KH. Activation of the p53 tumor suppressor protein. Biochem Biophys Acta. 2002;1602:47–59. doi: 10.1016/s0304-419x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 24.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 25.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 26.Maulik N, Sasaki N, Addya S, Das DK. Regulation of cardiomyocyte apoptosis by redox-sensitive transcription factors. FEBS Lett. 2000;485:7–12. doi: 10.1016/s0014-5793(00)02174-8. [DOI] [PubMed] [Google Scholar]
- 27.Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovasc Res. 2006;70:457–465. doi: 10.1016/j.cardiores.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Ashcroft M, Ludwig RL, Woods DB, Copeland TD, Weber HO, MacRae EJ, Vousden KH. Phosphorylation of HDM2 by Akt. Oncogene. 2002;21:1955–1962. doi: 10.1038/sj.onc.1205276. [DOI] [PubMed] [Google Scholar]
- 29.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 30.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudhov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 31.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecular inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 32.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]