Abstract
PURPOSE
The human conjunctiva is a natural target for herpes simplex virus (HSV)-1 infection. The goals of this study were to investigate the cellular and molecular mechanisms of HSV-1 entry into human conjunctival epithelial (HCjE) cells. Specific features of entry studied included the method of initial viral binding to cells, pH dependency, and expression and usage of specific HSV-1 entry receptors.
METHODS
To observe HSV-1 initial binding, live cell imaging was performed on HSV-1–infected HCjE cells. Reporter HSV-1 virions expressing β-galactosidase were used to determine entry of wild-type HSV-1(KOS) and a mutant, HSV-1(KOS)Rid1, into HCjE cells. HSV-1 replication in HCjE cells was determined by plaque assays. Lysosomotropic agents were used to determine whether viral entry was pH dependent. Reverse transcription (RT)-PCR, flow cytometry, and immunohistochemistry were used to determine the expression of receptors. Receptor-specific siRNAs were used to define the role of individual entry receptors.
RESULTS
HSV-1 virions attach to filopodia present on HCjE cells and use them to reach the cell body for entry. Cultured HCjE cells demonstrate susceptibility to HSV-1 entry and form plaques confirming viral replication. Blocking vesicular acidification significantly reduces entry, implicating a pH-dependent mode of entry. Multiple assays confirm the expression of entry receptors nectin-1, HVEM, and 3-O-sulfated heparan sulfate (3-OS HS) on the HCjE cell membrane. Knocking down of gD receptors by siRNAs interference implicates nectin-1 and HVEM as the major mediators of entry.
CONCLUSIONS
HSV-1 entry into HCjE cells is a pH-dependent process that is aided by targeted virus travel on filopodia. HCjE cells express all three major entry receptors, with nectin-1 and HVEM playing the predominant role in mediating entry.
Herpes simplex virus (HSV)-1, a member of the alphaherpesvirus subfamily, can infect a variety of ocular cell types and is the most common cause of infectious blindness in the developed world.1,2 HSV-1 infection of the eye results in various diseases such as stromal keratitis, epithelial keratitis, and conjunctivitis.2–4 HSV-1 is the most frequently isolated virus in ocular infections, resulting in 20,000 primary and 28,000 reactivated ocular infections per year in the United States. In patients with conjunctivitis, HSV-1 is the second most common laboratory isolated virus and is responsible for approximately 21% of cases of acute viral conjunctivitis.1,2 The conjunctiva is responsible for protecting the eye and maintaining the moisture within it, and its infection can lead to serious complications.3,4 Conjunctival epithelium is also directly continuous with corneal epithelium, thus facilitating the spread of virus.5 This may ultimately lead to blindness through corneal scarring caused by recurrent infections from latent HSV-1.2,6 Detection and treatment of conjunctival HSV-1 infection is important because the frequency of recurrence has been shown to correlate highly with conjunctival severity compared with corneal severity. Reactivation of HSV-1 more often involves the conjunctiva, whereas reactivation infection of the cornea is rare.2
HSV-1 is an enveloped DNA virus that requires at least five envelope glycoproteins to enter host cells.7 Viral glycoproteins gB and gC first attach to heparan sulfate proteoglycans on the cell surface.8 After this activity, a conformational change brings glycoprotein D (gD) in the proximity of a gD receptor on the host cell.7 A collaborative effort among gD, the gD receptor, gB, gH, gL, and possibly an additional interaction of gH with other cell surface molecules, then leads to fusion of a cell membrane and viral envelope.7,9 Fusion with the plasma membrane is thought to be pH independent. However, depending on the cell type, HSV-1 can enter in a pH-dependent fashion and can fuse with the membrane of an intracellular vesicle.10
There are three distinct classes of known HSV-1 entry receptors.7 Among these, herpesvirus entry mediator (HVEM) is a member of the tumor necrosis factor receptor family.11 Nectin-1 (HveC) and nectin-2 (HveB) belong to the immunoglobulin superfamily. Nectin-2 is unique because it mediates the entry of some laboratory generated mutants but not wild-type strains of HSV-1.7 Lastly, specific sites on heparan sulfate, when modified by certain isoforms of 3-O-sulfotransferase (3-OST) such as 3-OST-3, 3-OST-5, and 3-OST-6, also work as entry receptors for HSV-1.12–14 The use of entry receptors varies with cell type. HVEM is the main entry receptor for human T lymphocytes and trabecular meshwork cells.11,15 Nectin-1 is thought to be important for the infection of cells of epithelial and neuronal origin.16,17 3-O–sulfated heparan sulfate (3-OS HS) is the major mediator of entry into corneal fibroblasts.18
Although conjunctiva is a target of HSV-1 infection, the identity of functional HSV-1 entry receptors in the cells of the conjunctiva remains unknown. Similarly, the pH dependence of entry remains undetermined. In the present study, we sought to examine the molecular and cellular aspects of initial interaction of HSV-1 virions with human conjunctival epithelial (HCjE) cells leading to HSV-1 entry and replication. This study demonstrates the pH-dependent nature of HSV-1 entry, implicating endocytosis in viral uptake. It also demonstrates that the expression of nectin-1 and HVEM predominantly determines HSV-1 entry into HCjE cells. This is the first report of a cell type in which both nectin-1 and HVEM are found critical for entry.
MATERIALS AND METHODS
Cells, Viruses, and Antibodies
HCjE cells were provided by Ilene K. Gipson (Harvard University, Boston, MA) and were originally obtained from Steven Foster (Massachusetts Eye and Ear Infirmary, Boston, MA). The cells were grown in keratinocyte serum-free media (SFM; Gibco-Invitrogen Corp., Rockville, MD) supplemented with 25 μg/mL bovine pituitary extract (BPE), 0.2 ng/mL epidermal growth factor (EGF), penicillin, streptomycin, and 0.4 mM calcium chloride and were cultured as described elsewhere.19
Patricia Spear (Northwestern University, Chicago, IL) kindly supplied wild-type Chinese hamster ovary (CHO-K1) cells, CHO cells stably expressing nectin-1 (HveC), and African green monkey kidney (Vero) cells. These cells were cultured as previously described.11 HSV-1(KOS)gL86 virus, HSV-1(KOS)Rid1tk12 virus, HSV-1(KOS), anti-Myc, and anti-HVEM antibodies were also supplied by Patricia Spear.11 HSV-1(K26GFP) was provided by Prashant Desai (Johns Hopkins University, Baltimore, MD).20 HSV-1(KOS)gL86 was grown in Vero cells that stably express gL.11 Virus stocks were at low multiplicity of infection (MOI) and propagated in complementing cell lines. Their titers were determined on Vero cells, and they were kept at —80°C for proper maintenance. HS4C3 antibody, which targets 3-OS HS, was obtained from Toin H. van Kuppevelt (Radboud University, Nijmegen, Netherlands).21,22 All experiments performed with HS4C3 antibody were performed in a 0.5 M NaCl solution to increase the specificity of antibody to 3-OS heparan sulfate. For such experiments, a control treated with only a 0.5 M NaCl solution was used to assess cytotoxic effects.21 Poliovirus receptor related 1 (PRR1) antibody, specific for nectin-1, was obtained from Beckman Coulter, and a mouse anti– nectin-1 antibody was purchase from Zymed Laboratories (catalog no. 37-5900). FITC-conjugated anti–rabbit IgG and FITC-conjugated anti–mouse IgG antibodies were obtained from Sigma-Aldrich (St. Louis, MO).
Live Cell Imaging
HCjE cells were plated on 35-mm glass-bottom dishes (MatTek Corp., Ashland, MA) coated with collagen (BD Biosciences, Franklin Lakes, NJ). Cells were washed with PBS and were placed in DMEM/10% FBS. HSV-1(K26GFP) was added to cells at an MOI of 200. After observing initial viral binding, images of HCjE cells were obtained every 15 seconds using an inverted fluorescence microscope (Eclipse TE2000; Nikon, Tokyo, Japan). All images and videos were processed by digital programs (Metamorph [Molecular Devices, Eugene, OR] and Photoshop [Adobe, San Jose, CA]).
Viral Entry Assays
HCjE cells and HSV-1 entry receptor lacking CHO-K1 cells were plated in a 96-well tissue culture dish with 2 × 104 cells per well. Cells were infected with serial dilutions of recombinant HSV-1(KOS)gL86 virus, which expresses β-galactosidase on entering cells. After 6 hours, cells were washed and incubated with o-nitrophenyl-β-D-galactopyranoside (ImmunoPure ONPG; Pierce, Rockford, IL), allowing quantitation of β-galactosidase activity to measure viral entry. Measurements were obtained by spectrophotometer (Molecular Devices) at an optical density of 410 nm. Similar experiments were repeated with HSV-1(KOS)Rid1tk12, a β-galactosidase– expressing virus that does not recognize HVEM.11
To provide qualitative data, 5-bromo-4-choro-3-indolyl-β-D-galactopyranoside (X-gal) assays were performed in six-well tissue culture dishes, as previously described.12 Briefly, after 6 hours of infection, cells were washed, fixed, permeabilized, and incubated in X-gal, which yields a blue color when reacting with β-galactosidase. Images were obtained with a 20× objective lens of an inverted microscope (Axiovert 100 M; Zeiss, Oberkochen, Germany).
Plaque Assay
Viral replication was assessed by plaque assay. A monolayer of HCjE cells was plated in a 24-well tissue culture dish.19 Vero cells and CHO-K1 cells were used as positive and negative controls, respectively. Cells were infected (0.01 MOI) with HSV-1(KOS) or mock infected with only PBS for 2 hours at 37°C. Virus was replaced with proper medium for each cell type: SFM for HCjE cells, Ham F-12 medium supplemented with 10% fetal bovine serum (FBS) for CHO-K1 cells, and DMEM with 2.5% heat-inactivated calf serum for Vero cells. Cells were incubated at 37°C and harvested at appropriate intervals (24, 48, 72, and 96 hours). At the time of each harvest, cells were washed, fixed, and stained with the Giemsa stain. Infectivity was measured by the number of plaque-forming units (PFUs). Images of plaques were obtained with a 20× objective lens of an inverted microscope (Axiovert 100 M; Zeiss). Viral titers were also determined by obtaining virus from infected HCjE and Vero cells and adding the inoculum to Vero cells.
pH Dependency of Entry
HCjE cells and HeLa cells were plated onto 96-well tissue culture dishes and were treated with BFLA-1 (1 μM) and chloroquine (1 mM), which were serially diluted. Cells were incubated with BFLA-1 and chloroquine for 1 hour, and then HSV-1(KOS)gL86 was added in equal amounts to each well. Concentrations for bafilomycin and chloroquine were within values indicated in previous reports.10,24 Viral entry assay was performed and β-galactosidase readings were obtained through the same protocol used throughout this study.
Reverse Transcription–Polymerase Chain Reaction
With the use of a Qiagen kit (RNeasy; Qiagen Co., Valencia, CA), total RNA from HCjE cells, HeLa cells, and CHO-K1 cells were isolated. HeLa and CHO-K1 were the positive and negative controls, respectively, for all four receptor mRNA. Superscript II reverse transcriptase was used to perform RT, and PCR amplification was completed with appropriate primers: 5′-TCTCTGCTGCCAGACA-3′ and 5′-GCCACAGCAGAACAGA-3′ for HVEM; 5′-TCCTTCACCGATGGCACTATCC-3′ and 5′-TCAACACCAGCAGGATGCTC-3′ for nectin-1; 5′-AGAAGCAGCAGCACCAGCAG-3′ and 5′-GTCACGTTCAGCCAGGA-3′ for nectin-2; and 5′-CAGGCCATCATCATCGG-3′ and 5′-CCGGTCATCTGGTAGAA-3′ for 3-OST-3. RT-PCR was performed as described elsewhere.18 Expected PCR product sizes were 1270 bp for HVEM, 738 bp for nectin-1, 616 bp for nectin-2, and 736 bp for 3-OST-3.
Immunocytochemistry
Thirty-five–millimeter glass-bottom culture dishes (Mat Tek Corp.) were plated with a monolayer of HCjE cells to image entry receptors on the cell membrane. Cells were blocked with 3% BSA in PBS-ABC for 40 minutes and were incubated with primary and FITC-conjugated secondary antibodies, respectively, for 1 hour each. Cells were fixed between incubations with primary and secondary antibodies with fixation buffer.
Each glass-bottom culture dish was treated with a different primary antibody, as follows: anti-HVEM (1:20 dilution), anti-PRR1 (1:100 dilution), and anti-HS4C3 (1:10 dilution). The secondary antibody used for the anti-HVEM sample was FITC-conjugated anti–rabbit IgG (Sigma), whereas FITC-conjugated anti–mouse IgG (Sigma) was used for the remaining samples. Secondary antibodies were administered at a 1:500 dilution. Control glass-bottom culture dishes with HCjE were prepared as controls that were treated with secondary antibody only. Laser-scanning spectrum confocal microscopy (TCS SP2; Leica) was used for imaging.
Flow Cytometry
Cell surface expression of receptors was investigated through the use of flow cytometry. Fluorescence-activated cell sorter (FACS) analysis was performed on HCjE cells using the same primary and secondary antibodies used with immunocytochemistry experiments. The cells were treated with the antibodies, as described previously.9 Controls were used with HCjE treated with secondary antibody alone. Antibodies were administered at appropriate dilutions: 1:20 for anti–HVEM antibody, 1:100 for anti–nectin-1 antibody (Zymed), and 1:10 for HS4C3 antibody.
Animal Treatment
BALB/c (wild-type) mice were obtained from Harlan. All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Immunohistochemistry
Primary antibodies used in this study included polyclonal antibody against nectin-1 (R166, courtesy of Roselyn J. Eisenberg, Gary H. Cohen, and Claude Krummenacher, University of Pennsylvania, Philadelphia, PA) and a rabbit polyclonal antibody against HVEM (courtesy of Patricia Spear) at 1:50 and 1:200 dilutions, respectively. Immunohistochemistry was performed as described previously.25 Briefly, tissue sections for immunohistochemistry were deparaffinized with xylene and rehydrated through a series of graded ethanols. Tissue sections were hydrated with distilled water, and antigen retrieval was performed using solution concentrate (Target Retrieval Solution 10× Concentrate; Dako, Carpinteria, CA). Nonspecific staining was blocked using an H2O2solution for 10 minutes, followed by a protein block for 10 minutes. Sections were incubated with nectin-1–specific or HVEM-specific antiserum at room temperature for 1 hour. Nectin-1 and HVEM staining were detected using the streptavidin-biotin kit (Envision P; Dako).
Antibody Blocking of Major Receptors
Antibodies against nectin-1, HVEM, and 3-OS HS were used to block receptors on HCjE cells. HCjE cells were first plated on a 96-well tissue culture dish, and the respective antibodies were added for 2 hours. Viral entry assays were performed as previously described with serial dilutions of HSV-1(KOS)gL86, and a spectrophotometer (Molecular Devices) at an optical density of 410 nm was used to measure β-galactosidase activity.12
SiRNA Interference of Major Receptors
SiRNAs that downregulate HVEM, nectin-1, and 2-OST RNA expression (Invitrogen) were used in HCjE cells to interfere with receptor formation. Interfering with 2-OST RNA is significant because 3-OS heparan sulfate isoforms, such as 3-OST-3, depend on 2-O-sulfation of heparan sulfate.12 Therefore, the downregulation of 2-OST RNA indirectly downregulates 3-OST-3 receptor expression. HCjE cells were plated onto a six-well tissue culture dish and were transfected with appropriate RNA duplexes that interfere with their corresponding receptors. SiRNA-downregulating nectin-1, HVEM, and 2-OST RNA were individually transfected to assess the synergistic effect of interfering with all receptors. Cells were also transfected simultaneously with nectin-1, HVEM siRNA, and all three RNA duplex types. After 24 hours, cells were loosened with cell dissociation buffer (Invitrogen) and replated onto 96-well tissue culture dishes. Viral entry assays were performed as previously described with serial dilutions of HSV-1(KOS)gL86.12 As stated earlier, a spectrophotometer (Molecular Devices) at an optical density of 410 nm was used to measure β-galactosidase activity.
Cell ELISA Measurement of Entry Receptor Surface Expression
Entry receptors that were downregulated by siRNA, as described, were tested for surface expression by cell ELISA, as described elsewhere.12 Receptor expression was identified by incubating cells with primary antibodies for 1 hour at specific dilutions: 1:20 for HVEM, 1:100 for nectin-1, and 1:10 for 3-OS HS. These dilution factors were also used for HCjE cells, for which combinations of more than one receptor were downregulated (nectin-1 and HVEM; nectin-1, HVEM, and 2-OST). After treatment with primary antibodies, cells were washed, and biotinylated anti–rabbit IgG (1:5000 in PBS with 3% BSA) for HVEM and biotinylated anti–mouse IgG (1:5000 in PBS with 3% BSA) for nectin-1 and 3-OS HS were added. To detect binding, streptavidin-conjugated horseradish peroxidase (AMDEX; Amersham, Arlington Heights, IL) was used at a 1:10,000 dilution. The substrate for the reaction is the slow kinetic form of 3,3′,5,5′-tetramethylbenzidine (Sigma). Optical density was read at 650 nm with a spectrophotometer (Molecular Devices).
RESULTS
HSV-1 Surfing to Cell Membrane of HCjE Cells
To study the initial interaction of HSV-1 virions with cells, live cell imaging was performed. Green fluorescence protein– tagged HSV-1 virions (K26GFP)20 were added to HCjE cells plated at a low population density. Our time-lapse images demonstrated that many virions directly reached the cell body and that many others first attached to filopodia on HCjE cells and traveled along the filopodia to reach the cell body for entry (Fig. 1; Movie S1, online at http://www.iovs.org/cgi/content/full/49/9/4026/DC1). Movements along filopodia were unidirectional toward the cell body. On average the virions covered a distance of 15 to 21 μm in 5 minutes, corresponding to an average speed of approximately 3.5 μm/min. This phenomenon seems to mimic viral surfing previously described with retroviruses.26 Surfing of other viruses has yet to be observed on conjunctival cells; however, HSV-1 surfing has been observed in many additional cell types (M-JO and DS, unpublished results, 2008).
FIGURE 1.
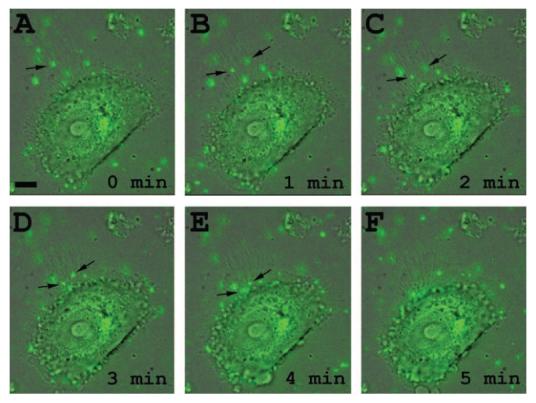
HSV-1 travels along filo-podia on HCjE cells. (A–F) Time course of green fluorescence protein expressing HSV-1 (K26GFP) interaction with filopodia. Virions (MOI, 200) were added to HCjE cells and monitored under a fluorescence microscope. Time 0 (A) is when virus (green) is first detected on filopodial surface (arrow). Scale bar, 10 μm.
HCjE Cell Susceptibility to HSV-1 Entry and Viral Replication
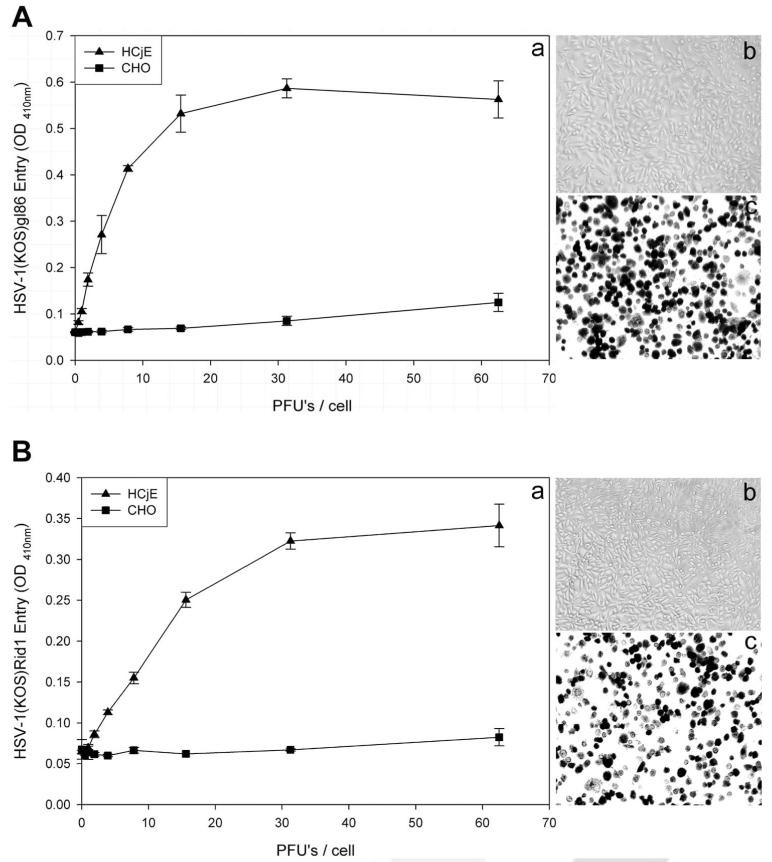
To determine HSV-1 entry, confluent monolayers of HCjE cells were infected with serial dilutions of recombinant HSV-1(KOS) gL86 representing wild-type virus and in parallel with a mutant form, HSV-1(KOS)Rid1tk12. Both recombinant viruses expressed β-galactosidase on entry into cells. CHO-K1 cells naturally resistant to HSV-1 entry were used as a negative control. The entry of HSV-1 was measured after 6 hours of viral infection. As shown in Figure 2, compared with CHO-K1 cells, both wild-type (Fig. 2Aa) and mutant (Fig. 2Ba) forms of HSV-1 entered HCjE cells in a dosedependent manner. An identical observation was made using an insoluble substrate, X-gal, for β-galactosidase and monitoring individual cells (Figs. 2Ab– c, 2Bb– c). The ability of HSV-1(KOS)Rid1tk12 to enter HCjE cells suggests that an entry receptor other than HVEM must be able to mediate the entry process because the mutant virus cannot use HVEM for entry.
FIGURE 2.
Wild-type and a mutant form of HSV-1(KOS) can enter into HCjE cells. (A) Analysis of wild-type HSV-1 entry into HCjE cells. HCjE or control CHO-K1 cells were plated in 96-well culture dishes and inoculated by serial dilutions of β-galactosidase– expressing recombinant HSV-1(KOS) gL86 virus at the plaque-forming unit (PFU)/cell indicated (a). The soluble substrate, ONPG, was added to cells at 6 hours after infection, and the enzymatic activity was measured with a spectrophotometer (a). Similar as-says were repeated with CHO-K1 (b) and HCjE (c) using an insoluble substrate, X-gal, to monitor individual cells turning blue because of virus entry (shown in black). (B) Analysis of HSV-1(KOS) rid1 mutant entry into HCjE cells. HCjE or the control CHO-K1 cells were plated in 96-well culture dishes and inoculated by serial dilutions of β-galactosidase–expressing recombinant HSV-1(KOS)Rid1tk12 virus at the PFU/cell indicated. ONPG (a) and X-gal (b, c) entry assays were repeated, as described.
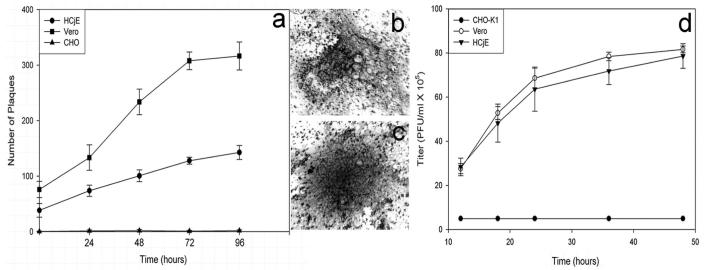
Given that HSV-1 was able to enter HCjE cells, we next evaluated whether the entry of HSV-1 into HCjE cells led to productive replication of the virus. Yields of infectious virus were determined by plaque assays in HCjE and Vero cells. As shown in Figure 3, HCjE and Vero cells exposed to HSV-1 (KOS) at 0.01 MOI produced higher numbers of plaques over time than the entry-resistant CHO-K1 cells infected with identical dosages of the same virus. Plaques containing 10 or more nuclei were counted. Typical plaques formed in Vero and HCjE cells are shown in Figure 3b-c. Vero and HCjE cells show similar levels of virus yield, as shown in Figure 3d. These results demonstrate that HSV-1 can enter and replicate in cultured HCjE cells.
FIGURE 3.
HSV-1 can productively replicate in HCjE cells. Confluent monolayers of HCjE, Vero, and wild-type CHO-K1 cells were infected with HSV-1(KOS) at 0.01 PFU/cell for 90 minutes at 37°C. Inoculums were harvested at 24 to 96 hours after infection. Infectious virus titer (PFU/mL) determined in triplicate in Vero cells by plaque assay indicates that the viral titer in cultured HCjE during that time. Data represent the mean ± SD of results in triplicate wells in a representative experiment (a). A typical plaque formed by Vero cells stained with Giemsa stain at 48 hours of infection with HSV-1(KOS) virus is shown (b). A typical Giemsa-stained plaque formed by HCjE cells at 48 hours of infection with HSV-1(KOS) is shown (c). Viral titers, to prove infectivity, were determined on Vero cells (d).
pH Dependency of Viral Entry
It has been demonstrated recently that based on cell type, HSV-1 entry can be pH dependent or independent.10,27 Therefore, we decided to examine the pH dependence of HSV-1 entry into HCjE cells. Effects of lysosomotropic agents capable of interfering with vesicular acidification were tested. These included bafilomycin (BFLA-1) and chloroquine.10 Monolayer cultures of HCjE cells were pretreated with BFLA-1 or chloroquine before infection with β-galactosidase expressing HSV-1(KOS)gL-86. As shown in Figure 4, there was a strong dosagedependent inhibition of HSV-1 entry by these drugs. Clearly, as BFLA-1 and chloroquine concentrations increased, signals for viral entry decreased correspondingly. This indicates that HSV-1 entry into HCjE cells requires acidification during infection; therefore, HSV-1 entry into HCjE cells is a pH-dependent process, and the virus uptake may involve an endocytotic uptake.
FIGURE 4.
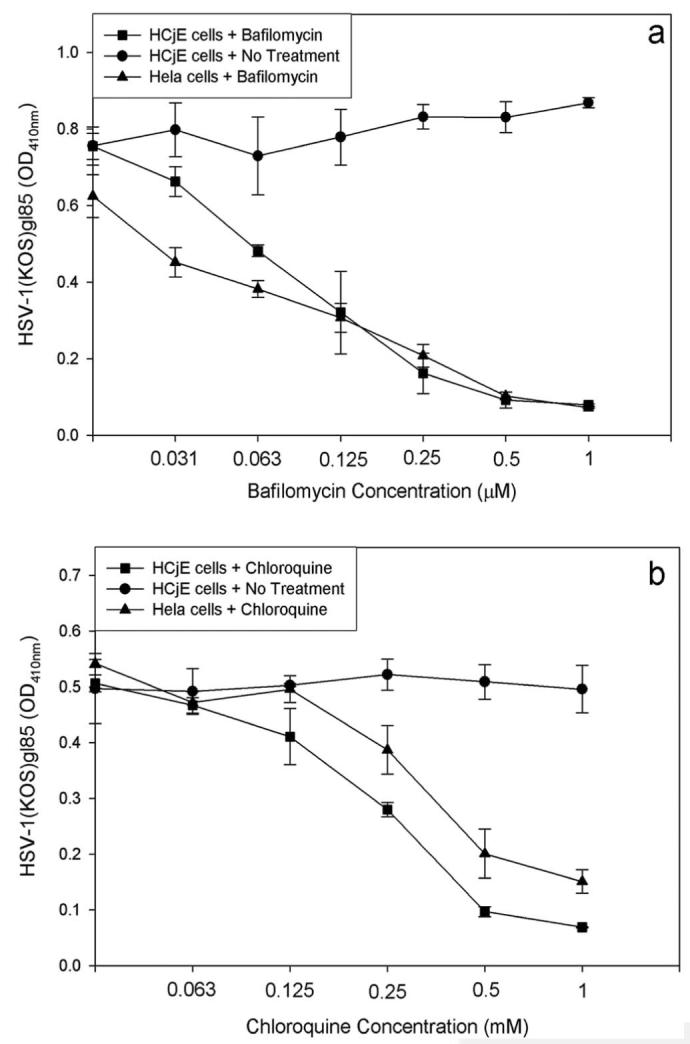
Lysosomotropic agents negatively affect HSV-1 entry. Monolayers of HCjE cells were either mock treated (No Treatment) or treated with indicated concentrations of bafilomycin (a) or chloroquine (b) and exposed to HSV-1 (50 PFU/cell). HeLa cells were also subjected to the same treatments and served as the positive control. Viral entry was measured using a spectrophotometer 6 hours after infection. Data shown are the means of triplicate measures and are representative of three independent experiments. The control (No Treatment) represents entry into corresponding mock-treated cells.
Identification of Entry Receptors
To determine which key entry receptors are expressed on HCjE cells, RT-PCR, immunofluorescence, and flow cytometry were performed. RT-PCR results demonstrated that mRNA specific for nectin-1, nectin-2, HVEM, and 3-OST-3 receptor genes exist in HCjE cells (Fig. 5). HeLa cells also expressed nectin-1, nectin-2, HVEM, and 3-OST-3 mRNA, whereas CHO-K1 cells failed to show any HSV-1 entry receptor mRNA, as expected (data not shown). Next, to examine protein expression, laser-scanning spectrum confocal microscopy was used for immunofluorescence assay. We excluded nectin-2 from subsequent studies because it mediates the entry of some laboratory-generated mutants but not wild-type strains of HSV-1.7 Receptor-specific primary antibodies combined with FITC-conjugated secondary antibodies were used. Immunofluorescence data shown in Figure 6 demonstrate that nectin-1, HVEM, and 3-OS HS are expressed along the cell membrane of HCjE cells (Fig. 6). The control cells, with only incubation of secondary FITC-conjugated antibody, did not show any fluorescence on the cell membrane. To verify and estimate the relative levels of receptor expression, flow cytometry was used. Although the expression of all the three receptors was evident again, it was also apparent that nectin-1 was likely the most abundant (Fig. 7a), followed by HVEM and 3-OS HS (Figs. 7b, 7c). 3-OS HS is a rare modification of HS, which may be responsible for the low expression of 3-OS HS detected by flow cytometry.12 To verify our receptor expression findings in vivo, immunohistochemistry was performed using sections of palpebral conjunctiva obtained from adult (8-month-old) female BALB/c mice. As shown (Fig. 8), strong nectin-1 expression was detected in the conjunctival epithelium and sebaceous glands with weak to modest staining in the lamina propria. Weak HVEM staining was detected in the conjunctival epithelium and sebaceous glands, and local HVEM staining was detected in the lamina propria. However, the weak staining may be attributable to poor reactivity of the rabbit HVEM antibody raised against human HVEM to the murine tissue. No clear signals were reported for 3-OS HS (data not shown).
FIGURE 5.
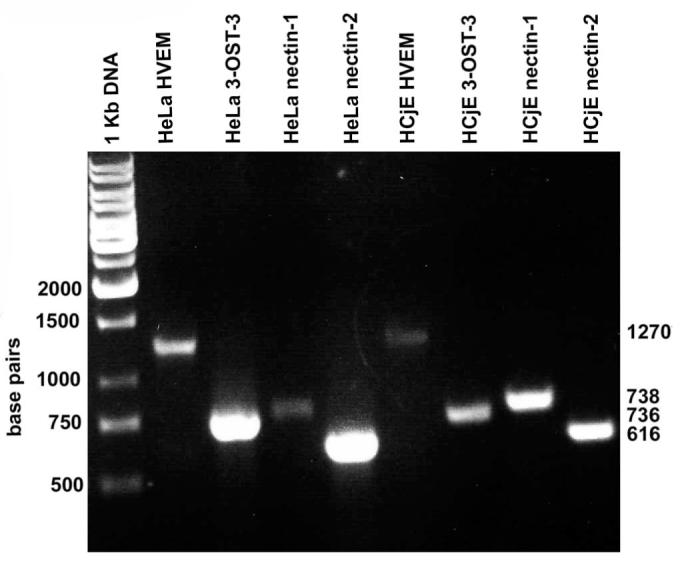
Detection of entry receptor mRNA. RT-PCR analysis of the expression of nectin-1, HVEM, and 3-OST-3 (as a surrogate marker for 3-OS HS)–specific messages in HCjE and HeLa cells (served as a control). cDNAs were produced from total RNA isolated from the cells. PCR products were separated by electrophoresis on an agarose gel and stained with ethidium bromide. Molecular weight markers are indicated.
FIGURE 6.
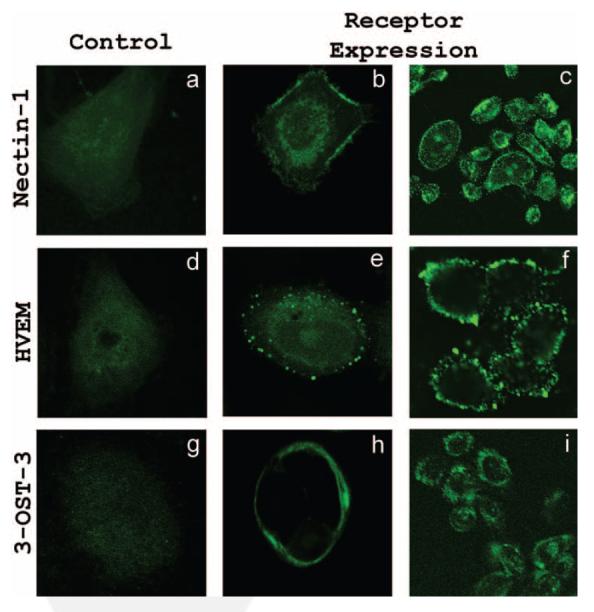
Immunofluorescence imaging of receptors on HCjE cell membrane. Images shown were taken using the FITC channel of confocal microscope. Cells were blocked for 40 minutes, washed, and either mock treated with buffer alone (a, d, g) or treated with primary antibodies for nectin-1 (b, c), HVEM (e, f), and 3-OS HS(h, i). Images were taken after the incubation of HCjE cells with FITC-conjugated secondary antibodies. Staining of cells with green demonstrated receptor expression.
FIGURE 7.
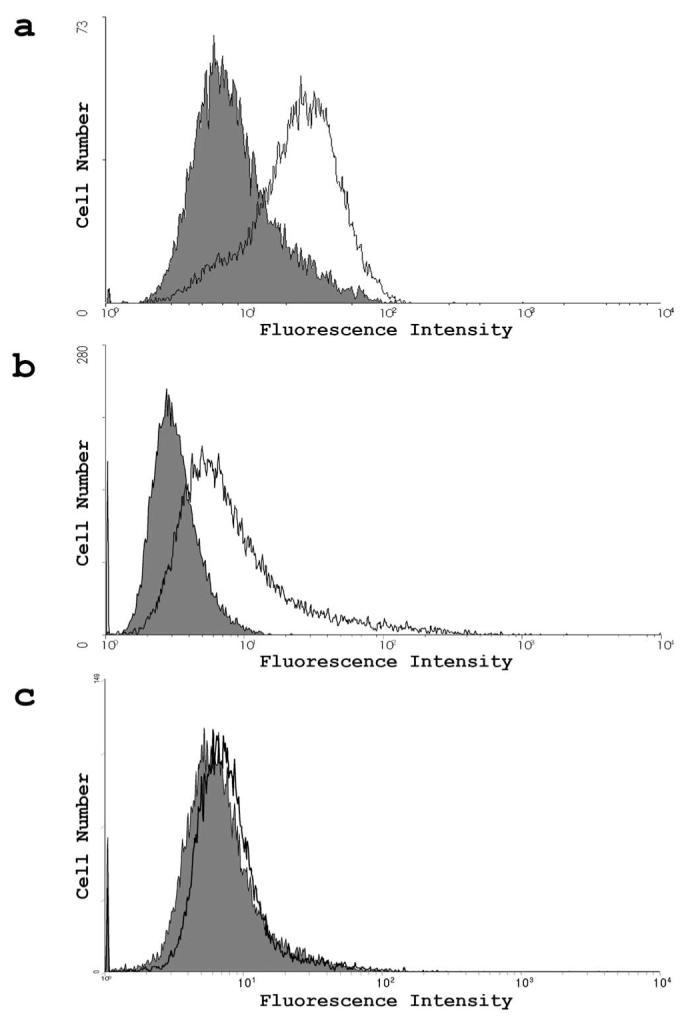
Flow cytometry measurement of receptor expression. (a-c) Expression was detected by FACS analysis. Cells were treated with primary antibodies for nectin-1 (a), HVEM (b), and 3-OS HS(c). Controls were treated with secondary antibodies only and are shown in gray on the graph.
FIGURE 8.
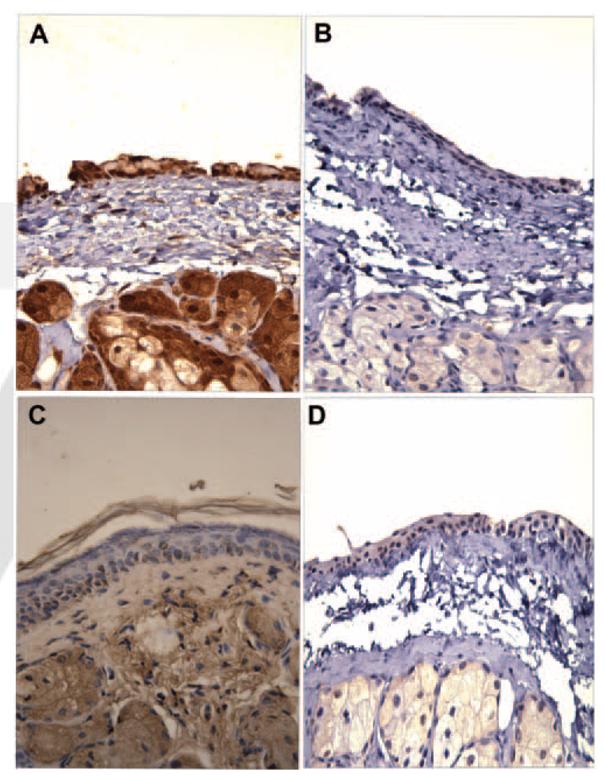
Confirmation of receptor expression by immunohisto-chemistry. Sections of palpebral conjunctiva from adult female BALB/c mice were reacted with anti–nectin-1 (A) or anti–HVEM (C) antibody, and immunohistochemistry was performed as described. Specific staining of the tissues is shown in brown. Note that staining for nectin-1 (A) was relatively stronger than for HVEM (C). Sections stained without primary antibodies were used as controls (B, D).
Blocking and Downregulation of the Entry Receptors
To prove that the observed entry receptors are responsible for viral entry, receptor expression in cells was blocked by receptor-specific antibodies. Less entry was observed for cells when antibodies against nectin-1 and HVEM (Figs. 9a, 9b) were used; however, anti-HS4C3 showed no decrease in entry (Fig. 9c), suggesting that nectin-1 and HVEM have more significant roles in the entry process than 3-OS HS.
FIGURE 9.
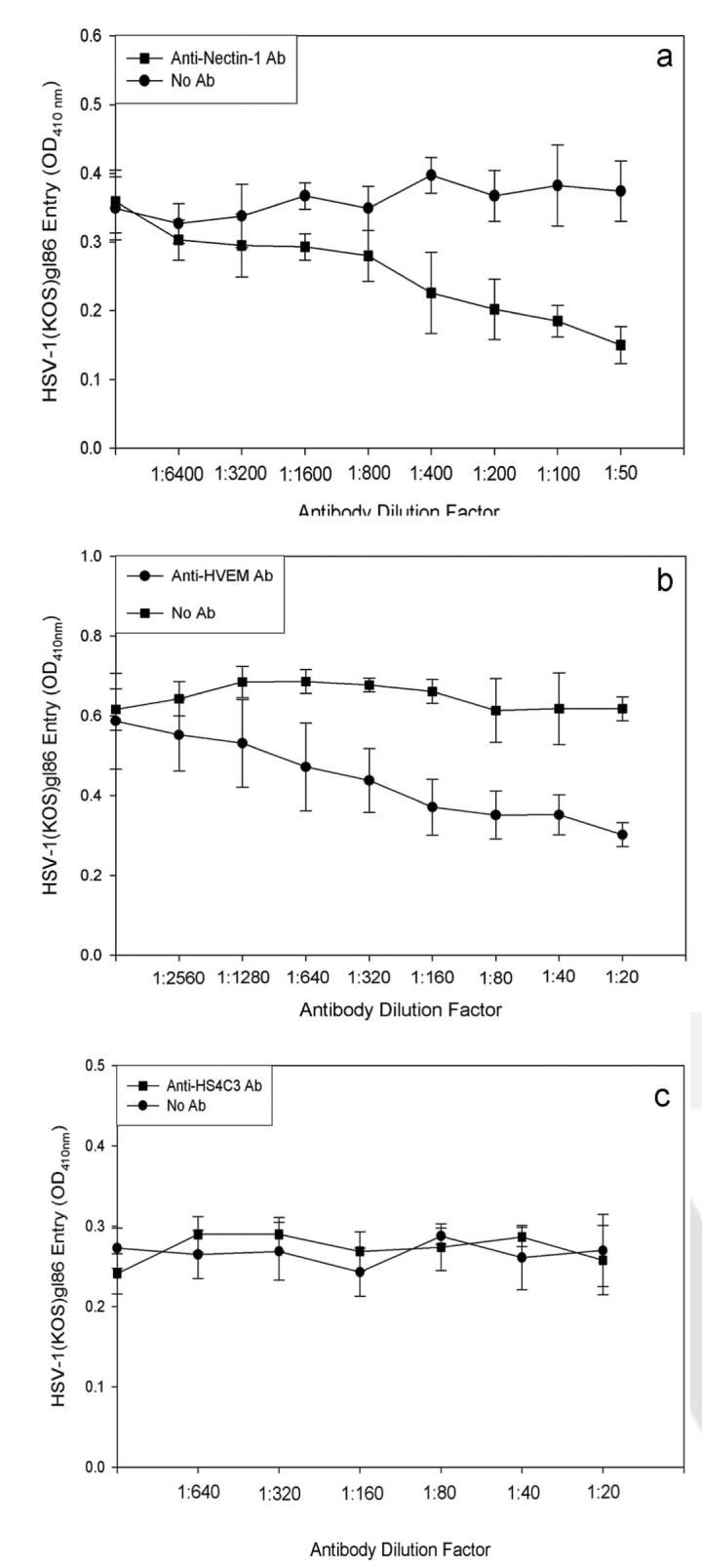
Antibody blocking of receptors. Monolayers of HCjE cells were blocked with anti-nectin-1 antibody (a), anti–HVEM antibody (b), and anti–HS4C3 antibody for 2 hours and then were exposed to HSV-1 (50 PFU/cell). Viral entry was measured using a spectrophotometer 6 hours after infection. Data shown are the means of triplicate measures and are representative of three independent experiments. Control (No Ab) represents entry into corresponding mock-treated cells.
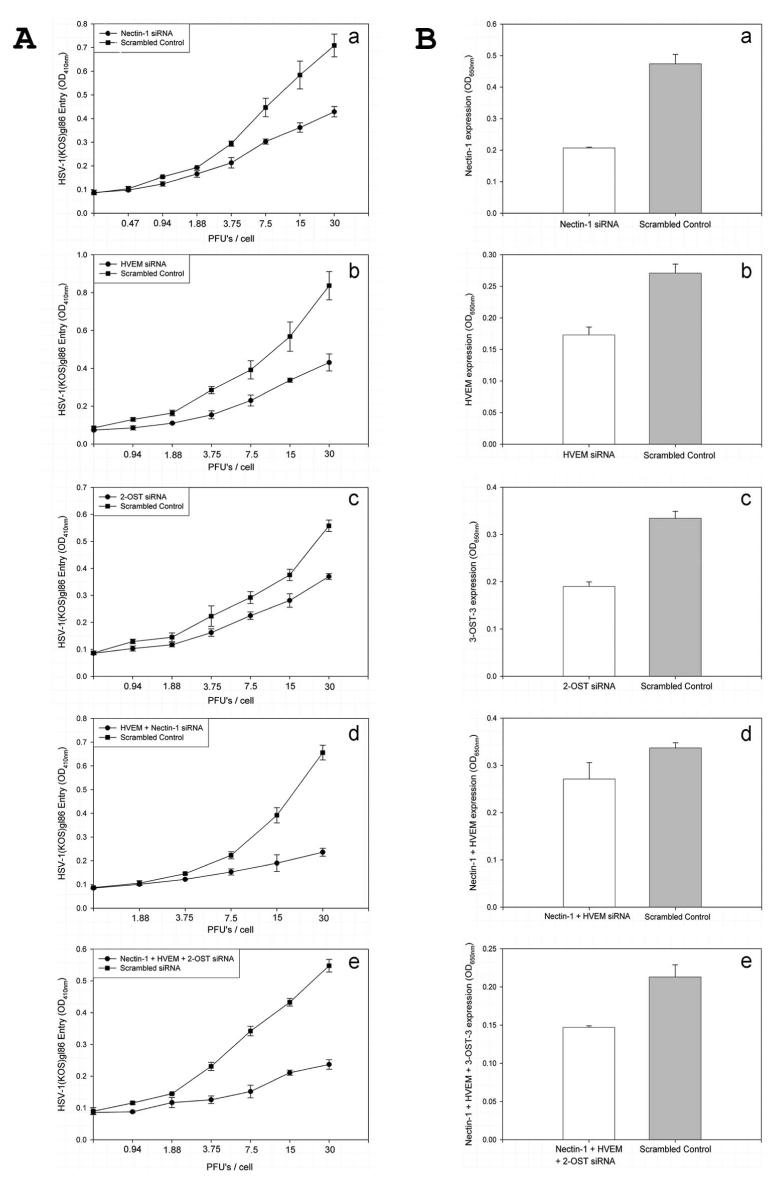
Receptors were also downregulated by receptor-specific siRNAs. Significantly less entry was observed when nectin-1, HVEM, or 3-OST was downregulated by its respective siRNA (Fig. 10Aa– c). Further reduction in entry was observed when nectin-1– and HVEM-specific siRNAs were cotransfected into HCjE cells (Fig. 10Ad), suggesting that nectin-1 and HVEM may be the major mediators of HSV-1 entry into HCjE cells. The downregulation of receptors from cell surfaces was verified in parallel by cell-ELISA using receptor-specific antibodies (Fig. 10B). When all three entry receptors (nectin-1, HVEM, and 3-OS HS) were simultaneously downregulated, the combined inhibition was similar or only slightly pronounced (Fig. 10Ad) than when only two receptors (nectin-1 and HVEM) were simultaneously downregulated (Fig. 10Ae). This suggests that 3-OS HS participation in entry may be minimal.
FIGURE 10.
SiRNA downregulation of receptor expression. (A) Viral entry was determined in cells transfected with siRNA against entry receptors: nectin-1 (a), HVEM (b), 2-OST (c), nectin-1 and HVEM (d), and nectin-1, HVEM, and 2-OST (e). Cells transfected with an equal amount of scrambled siRNA were used as control. (B) Determination of surface expression of the receptors by cell-ELISA. Cell-ELISA was performed with cells transfected with siRNA against entry receptors nectin-1 (a), HVEM (b), 2-OST (c), nectin-1 and HVEM (d), and nectin-1, HVEM, and 2-OST. Primary antibodies specific to each receptor and FITC-conjugated secondary antibodies were used for the ELISA. Data shown are the means of triplicate measures.
DISCUSSION
Viral conjunctivitis is common among persons of all ages. A number of viruses can cause conjunctival infection.1 Although adenovirus is by far the most common cause, herpes simplex virus (HSV) appears to be the most problematic.1,2 HSV-related symptoms tend to last longer (2–4 weeks) and to involve a greater risk of viral spread to the cornea. Viral infection of the conjunctiva is usually characterized by an acute follicular conjunctival reaction and preauricular adenopathy.3,4 Primary ocular herpes simplex infection is more common among children than adults and is usually associated with follicular conjunctivitis. Although HSV type 2 may be a cause, especially in neonates, HSV-1 infection is usually the predominant cause. Recurrent infection, typically seen in adults, is commonly associated with corneal involvement.
Knowledge of the molecular mediators of primary infection, which is common among children, is crucial for preventing the disease. As a first step toward understanding the molecular and cellular basis of the virus’s ability to invade and infect the cells of the conjunctiva, our study provides novel information on HSV-1 entry into HCjE cells. It demonstrates, for the first time, that HCjE cells grown in vitro can be productively infected with HSV-1, and the initial infection process bears unique characteristics. Results from this study raise a strong possibility that filopodial surfing, which was recently demonstrated for retroviruses26 but not for herpesviruses, can play a role in HSV-1 entry into HCjE cells. Similar to retroviruses, it is likely that HSV-1 and perhaps many additional herpesviruses can exploit retrograde F-actin flow to facilitate their targeted delivery to cell body for entry.26 We also report for the first time that entry into the HCjE cell is a pH-dependent process that makes use of more than one major receptor. Many cells types use nectin-1, HVEM, or 3-OS HS as their major receptor, but HCjE cells are unique in that they express all three receptors, and HSV-1 likely involves, up to certain degree, all three receptors.15,28 Involvement of the three receptors is evident from the receptor downregulation by transfection of cells with individual or a combination of receptor-specific siRNAs. Although the siRNA data suggest that nectin-1 and HVEM are important receptors for entry, 3-OS HS appears to play a less significant role in the entry process (Fig. 9). Interestingly, the residual entry, even when combinations of siRNA are used, may imply an additional receptor(s) responsible for HSV-1 entry; however, it is also possible that the siRNA are downregulating only a portion of their respective receptor mRNAs. Use of multiple entry receptors is consistent with receptor knockout mice studies performed for genital herpes, which shares some but not all HSV-1 entry receptors.7 It was found that knocking out one receptor was not enough to prevent entry into the cells of the mouse vaginal epithelium.28 Most drastic effects were observed when nectin-1 and HVEM were knocked out. Nectin-1 and HVEM are also expressed in the murine conjunctiva (Fig. 8).
Finer points of the study also show that distribution of the various entry receptors studied may differ. Figure 6 shows distinct differences in receptor distribution. Nectin-1 and 3-OS HS have a smooth, diffuse distribution along the cell membrane, whereas HVEM has a punctuate distribution. This may imply that HVEM tends to cluster in particular locations in HCjE cells. Whether this is relevant to HSV-1 entry into HCjE cells is something that may have to be studied in future studies. Another interesting finding that requires further scrutiny is the pH dependency of HSV-1 entry into HCjE cells. Previous studies have shown that pH dependency of HSV-1 entry is associated with an endocytotic mode of entry.10,27 It is unclear whether gD receptors have any role in deciding which mode, endocytosis or fusion with the plasma membrane, will be preferred.
These are important discoveries that have major implications for future therapy for conjunctival HSV-1 infection. Pharmaceutical drugs to treat HSV-1 infection of the conjunctiva must target both major receptors because targeting one receptor will not significantly decrease overall viral infectivity. Targeting the endocytotic mechanism is another aspect that should be studied further for the creation of HSV-1 treatments. Additional studies must be performed to assess the in vivo participation of HVEM, nectin-1, and 3-OS HS entry receptors in HSV-1 infection.
Acknowledgments
Supported by National Institute of Allergy and Infectious Diseases Grant RO1 A1057860 (DS), National Eye Institute Core Grant P30 EY01792, and a Research to Prevent Blindness career award (DS). JA was supported in part by a Fight for Sight summer fellowship. VT was supported by American Heart Association Fellowship AHA0525768Z and by a grant from the Illinois Society for the Prevention of Blindness.
Footnotes
Disclosure: J. Akhtar, None; V. Tiwari, None; M.-J. Oh, None; M. Kovacs, None; A. Jani, None; S.K. Kovacs, None; T. Valyi-Nagy, None; D. Shukla, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Marangon FB, Miller D, Alfonso E. Laboratory results in ocular viral diseases: implications in clinical-laboratory correlation. Arq Bras Oftalmol. 2007;79:189–194. doi: 10.1590/s0004-27492007000200002. [DOI] [PubMed] [Google Scholar]
- 2.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Uchio E, Takeuchi S, Itoh N, Matsuura N, Ohno S, Aoki K. Clinical and epidemiological features of acute follicular conjunctivitis with special reference to that caused by herpes simplex virus type 1. Br J Ophthalmol. 2000;84:968–972. doi: 10.1136/bjo.84.9.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritterband DC, Friedberg DN. Virus infections of the eye. Rev Med Virol. 1998;8:187–201. doi: 10.1002/(sici)1099-1654(1998100)8:4<187::aid-rmv221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995;36:1818–1827. [PubMed] [Google Scholar]
- 6.Hill JM, Gebhardt BM, Wen R, et al. Quantitation of herpes simplex virus type 1 DNA and latency-associated transcripts in rabbit trigeminal ganglia demonstrates a stable reservoir of viral nucleic acids during latency. J Virol. 1996;70:3137–3141. doi: 10.1128/jvi.70.5.3137-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 8.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scanlan PM, Tiwari V, Bommireddy S, Shukla D. Cellular expression of gH confers resistance to herpes simplex virus type-1 entry. Virology. 2003;312:14–24. doi: 10.1016/s0042-6822(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 10.Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174:1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/ NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 12.Shukla D, Liu J, Blaiklock P, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 13.Xia G, Chen J, Tiwari V, et al. Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus, type 1. J Biol Chem. 2002;277:37912–37919. doi: 10.1074/jbc.M204209200. [DOI] [PubMed] [Google Scholar]
- 14.Xu D, Tiwari V, Xia G, Clement C, Shukla D, Liu J. Characterization of heparan sulphate 3-O-sulphotransferase isoform 6 and its role in assisting the entry of herpes simplex virus type 1. Biochem J. 2005;385:451–459. doi: 10.1042/BJ20040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari V, Clement C, Scanlan PM, Tue BYJT, Shukla D. A role for HVEM as the receptor for herpes simplex virus-1 entry into primary human trabecular meshwork cells. J Virol. 2005;79:13173–13179. doi: 10.1128/JVI.79.20.13173-13179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haarr L, Shukla D, Rodahl E, Canto MC Dal, Spear PG. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology. 2001;287:301–309. doi: 10.1006/viro.2001.1041. [DOI] [PubMed] [Google Scholar]
- 17.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari V, Clement C, Xu D, et al. Role for 3-O-sulfated heparan sulfate as the receptor for herpes simplex virus type 1 entry into primary human corneal fibroblasts. J Virol. 2006;80:8970–8980. doi: 10.1128/JVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SH, Lee JH, Yoon JH, Lee HK, Seo KY. Multi-layered culture of primary human conjunctival epithelial cells producing MUC5AC. Exp Eye Res. 2007;85:226–233. doi: 10.1016/j.exer.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Desai P, Pearson S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72:7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dam GB Ten, Kurup S, van de Westerlo EM, et al. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J Biol Chem. 2006;281:4654–4662. doi: 10.1074/jbc.M506357200. [DOI] [PubMed] [Google Scholar]
- 22.Tiwari V, Dam GB ten, Yue BY, van Kuppevelt TH, Shukla D. Role of 3-O-sulfated heparan sulfate in virus-induced polykaryocyte formation. FEBS Lett. 2007;581:4468–4472. doi: 10.1016/j.febslet.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiwari V, O’Donnell C, Copeland RJ, Scarlett T, Liu J, Shukla D. Soluble 3-O-sulfated heparan sulfate can trigger herpes simplex virus type 1 entry into resistant Chinese hamster ovary (CHO-K1) cells. J Gen Virol. 2007;88:1075–1079. doi: 10.1099/vir.0.82476-0. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H([ H11001])-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 25.Valyi-Nagy T, Sheth V, Clement C, et al. Herpes simplex virus entry receptor nectin-1 is widely expressed in the murine eye. Curr Eye Res. 2004;29:303–309. doi: 10.1080/02713680490516756. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actinand myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytotic pathway. J Virol. 2005;79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor JM, Lin E, Susmarski N, et al. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]