Abstract
In the mammary gland, epithelial cells are embedded in a ‘soft' environment and become functionally differentiated in culture when exposed to a laminin-rich extracellular matrix gel. Here, we define the processes by which mammary epithelial cells integrate biochemical and mechanical extracellular cues to maintain their differentiated phenotype. We used single cells cultured on top of gels in conditions permissive for β-casein expression using atomic force microscopy to measure the elasticity of the cells and their underlying substrata. We found that maintenance of β-casein expression required both laminin signalling and a ‘soft' extracellular matrix, as is the case in normal tissues in vivo, and biomimetic intracellular elasticity, as is the case in primary mammary epithelial organoids. Conversely, two hallmarks of breast cancer development, stiffening of the extracellular matrix and loss of laminin signalling, led to the loss of β-casein expression and non-biomimetic intracellular elasticity. Our data indicate that tissue-specific gene expression is controlled by both the tissues' unique biochemical milieu and mechanical properties, processes involved in maintenance of tissue integrity and protection against tumorigenesis.
Keywords: atomic force microscopy, β1-integrin, laminin, microenvironment, tissue elasticity
Introduction
Signals from the microenvironment are essential to direct normal tissue development and, in the adult organism, to maintain tissue-specific functions (Nelson and Bissell, 2006). Studies in three-dimensional (3D) cultures have identified key biochemical microenvironmental cues underlying mammary-specific structure and function. The extracellular matrix (ECM) component, laminin-111 (LM1), is necessary to induce polarity and acinar morphogenesis (Gudjonsson et al, 2002) and, together with lactogenic hormones, is required for β-casein expression in mammary epithelial cells (MECs) (Streuli et al, 1991; Xu et al, 2007). In addition to a unique biochemical milieu, different tissue microenvironments exhibit distinct mechanical properties (Discher et al, 2005).
There is growing evidence that, rather than being a passive property of the tissue, the mechanical properties of the microenvironment have a direct impact on tissue-specific morphogenetic programmes in MECs and other cell types (Wozniak et al, 2003; Paszek et al, 2005; Engler et al, 2006). Furthermore, because abnormally high stiffness and loss of tissue function are hallmarks of solid tumours (Paszek et al, 2005), and increased mammographic density is a risk factor for breast cancer (Boyd et al, 1998), it has been suggested that aberrant tissue stiffness may facilitate the acquisition of a malignant phenotype (Wozniak et al, 2003; Paszek et al, 2005; Provenzano et al, 2006). Tissue elasticity is thought to be maintained by a mechanical homeostatic mechanism largely determined by the reciprocal mechanochemical interactions between cells and their surrounding ECM (Bissell et al, 1982; Discher et al, 2005; Paszek et al, 2005). Previous studies have examined the effects of either the biochemical composition or the elasticity of the substrata on functional differentiation or cell mechanics individually; however, a comprehensive approach aiming to dissect how these signals modulate each other and how these signals integrate to control tissue-specific gene expression have been lacking (Alcaraz et al, 2004).
To determine how the mechanochemistry of the cellular microenvironment affects tissue-specific gene expression, we used two mammary epithelial cell lines (SCp2 and EpH4) that in culture can be induced to functionally differentiate (defined here as expression of an abundant mouse milk protein, β-casein) in the presence of appropriate ECM. The fact that, in the presence of a LM1-containing gels, cell–cell contact is not required for β-casein expression (Streuli et al, 1991) (Supplementary Figure 1) allowed us to examine single cells cultured on top of gels under these conditions. This ‘single cell on top' assay allowed us to use atomic force microscopy (AFM) to assess the elasticity or stiffness (defined by the Young's elastic modulus E) of single cells and their underlying substrata independently. Using this quantitative and comprehensive approach, we unraveled the intimate interrelationship among LM1 binding, ECM stiffness, cell shape and cell stiffness, as well as the synergistic effects of these mechanochemical properties on tissue-specific gene expression. Furthermore, we show that variations of these properties from biomimetic values, that is, those comparable with normal physiological conditions, may be linked potentially to dedifferentiation that occurs in tumours.
Results
Single cells exhibit elastic moduli comparable with cells within primary organoids when exposed to LM1 and biomimetic extracellular elasticity
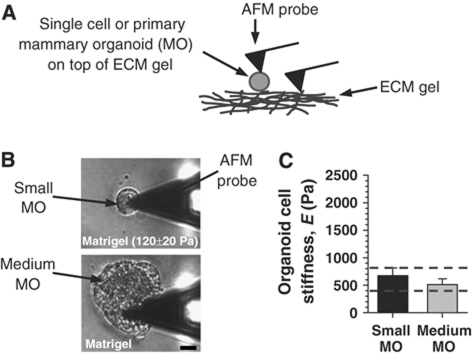
Previous studies have reported that normal mouse mammary tissue is very soft (E∼100 Pa) (Table I) (Paszek et al, 2005) and have suggested that this low stiffness is caused mainly by the ECM. However, the actual elastic modulus of epithelial cells within the mammary gland has not been measured. To estimate the elasticity of fully differentiated MECs in vivo, we isolated milk-synthesizing primary mammary organoids from early pregnant mice and cultured them on top of Matrigel in differentiation medium for 24 h. Matrigel is a suitable substratum because it is rich in LM1 and exhibits biomimetic elastic modulus, as measured by AFM and rheometry (Table I). A schematic representation of this experimental setup is shown in Figure 1A. Mammary organoids in culture are often heterogeneous in size. To account for this heterogeneity, we measured the elasticity of both small- and medium-sized organoids (Figure 1B), which typically contain about a dozen or several dozen cells, respectively. We found that irrespective of the size, cells within the organoids exhibited comparable average elastic moduli between 400 and 800 Pa (Figure 1C). We used this range as a reference for biomimetic cellular elasticity throughout this work.
Table 1.
Summary of mechanical parameters: comparison between rodent mammary tissue, different biological substrata and single MECs cultured on top of these substrata
| Sample | Tissue or substratum | Single cellsa | |
|---|---|---|---|
| EAFM (Pa)b | Ebulk (Pa)c | EAFM (Pa)b | |
| Normal mammary tissue | n.a. | 170±30d | |
| Average mammary tumour | n.a. | 4050±940d | |
| Mammary organoids on Matrigel | 120±20 | n.a. | 600±200 |
| Matrigel | 120±20 | 220±10 | 700±100 |
| Laminin-111 | 110±30 | n.a. | 730±110 |
| Laminin-111+collagen I (2 g/l) (40:60% v/v) | 72±8 | 150±40 | 600±90 |
| Collagen I (2 g/l) | 290±100 | 240±40 | 400±100 |
| PolyHEMA | n.a. | n.a. | 1700±300 |
| Borosilicate glass (2D) | n.a. | 63 × 109 e | 1300±200 |
| Data are mean±s.e.; n.a., not available. | |||
| aMean of all cell lines. | |||
| bYoung's modulus measured by AFM. | |||
| cYoung's modulus measured with a rheometer. | |||
| dFrom Paszek et al (2005). | |||
| eAccording to the manufacturer's instructions. | |||
Figure 1.
The ‘physiological' or ‘biomimetic' elasticity of MECs can be defined in culture. (A) Schematic representation of the experimental setup: either mammary organoids (MO) or single MECs (SCp2 or EpH4) were plated on top of an ECM gel. An AFM probe was used to quantify the elasticity of either the cell or the underlying ECM gel. (B) Morphology of primary mammary organoids isolated from mice in early pregnancy and cultured on top of a LM1-rich ECM gel (Matrigel) in differentiation medium for 24 h. The elastic modulus of the substrata as measured by AFM is indicated at the bottom of each image. Most mammary organoids had either small or medium sizes, corresponding to roughly a dozen or a few dozen cells, respectively. (C) Elastic moduli of cells within either small- or medium-sized primary mammary organoids cultured as in (B). Dashed horizontal lines correspond to the lower and upper values defined by the elasticity of cells within mammary organoids, used as a reference for biomimetic cell elastic moduli throughout this study. All scale bars correspond to 20 μm throughout the figures unless otherwise indicated.
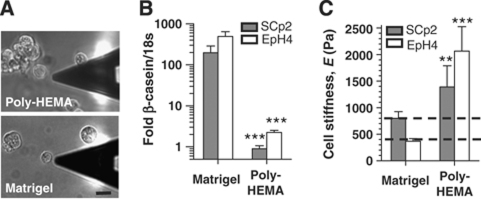
The two mammary cell lines, SCp2 and EpH4, have been used extensively to study functional differentiation of MECs in culture (Desprez et al, 1993; Pujuguet et al, 2001). The elastic moduli of either single SCp2 or EpH4 cells cultured on top of Matrigel for 24 h (Figure 2A, bottom panel) fell within the biomimetic range defined by cells in mammary organoids (parallel dashed lines) (Figure 2C). As MECs exhibit a round morphology both in vivo and when exposed to LM1 in culture (Roskelley et al, 1994), it is possible that their similar elastic moduli is due to the round shape per se (Le Beyec et al, 2007). To address this question, we measured the elasticity of the two cell lines rounded in the absence of LM1 signalling by culturing them on top of a substratum coated with the non-adhesive poly-(2-hydroxyethyl methacrylate) (poly-HEMA) (Figure 2A, top panel). Unlike cells on Matrigel, cells cultured on poly-HEMA were significantly stiffer (Figure 2C) and did not express β-casein, as assessed by quantitative RT–PCR (Figure 2B). The stiffening of MECs on poly-HEMA was not due to increased cell death (Muschler et al, 1999). These data confirm that LM1 signalling is necessary for functional differentiation of MECs and indicate that biomimetic cellular elasticity in MECs is cell line independent and downstream of cell–ECM rather than cell–cell adhesion or cell rounding per se.
Figure 2.
Laminin-111 signalling and biomimetic extracellular elasticity, but not cell shape per se, induce robust β-casein expression and a cellular elasticity comparable with cells within mammary organoids. Two MECs (SCp2 and EpH4) were cultured in the presence (Matrigel) or absence (poly-HEMA) of ECM signalling. (A–C) Both culture conditions produced a similarly round morphology in SCp2 (A) and EpH4 cells (data not shown), but differed dramatically in their effects on β-casein, as measured by quantitative RT–PCR (B) and cellular elasticity (C). Note that fold β-casein/18S rRNA data are plotted on a logarithmic scale throughout the figures. **P<0.05 and ***P<0.01 were determined by two-tailed Student's t-test with respect to (w.r.t.) Matrigel or the corresponding control throughout this work unless otherwise indicated.
Increasing extracellular elasticity beyond normal mammary tissue values promotes spreading and stiffening and inhibits -casein expression in MECs
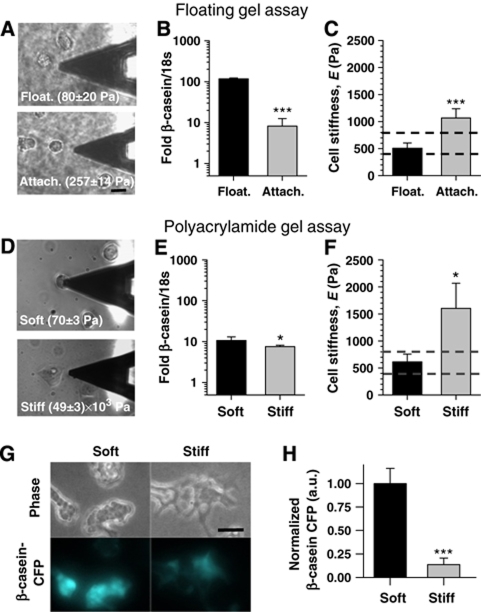
As normal mammary tissue is soft (Table I), we hypothesized that strong functional differentiation in culture would be achieved only by using substrata where the elasticity mimics normal tissue. Accordingly, we used two culture assays that allow increasing extracellular stiffness beyond biomimetic values, while maintaining biochemical signalling constant (Alcaraz et al, 2004). In the first assay (Figure 3A–C), EpH4 cells were cultured on top of gels containing LM1 mixed with collagen type I (COL-I) (3 g/l) (40:60% v/v). Four hours after plating, differentiation media was added and half of the gels were gently detached from their container along the gel's edges and rendered floating in the medium (Michalopoulos and Pitot, 1975). As AFM requires samples to be somewhat anchored, caution was taken to avoid complete gel detachment by leaving the bottom-center of the gel attached to the underlying glass surface. AFM measurements revealed that the average floating gel elasticity was close to that of bulk mammary tissue, whereas the attached gel was three-fold stiffer (Figure 3A, bottom image). Such gel stiffening was sufficient to dramatically downregulate β-casein (Figure 3B) and increase the elastic modulus of the cells (Figure 3C). In the second assay (Figure 3D–H), EpH4 cells were plated on top of polyacrylamide gels exhibiting elastic moduli either close to mammary tissue (referred to as ‘soft'), comparable with or even higher than mammary tumours (referred to as ‘stiff'). Only 24 h after plating on the stiffer substrata, cells displayed a spread morphology (Figure 3D) and non-biomimetic elasticity (Figure 3F). To induce β-casein, cells were overlaid with 2% Matrigel diluted in differentiation medium. In agreement with the first assay, stiffer substrata downregulated β-casein transcription, measured both by quantitative RT–PCR (Figure 3E) and by the fluorescence of cells transfected with a construct containing 16 concatenated copies of the mouse β-casein gene promoter driving cyan fluorescent protein (CFP) expression (Figure 3G and H). These findings support our hypothesis and indicate that LM1-dependent functional differentiation is modulated by the extracellular elasticity.
Figure 3.
Increasing extracellular elasticity beyond normal mammary tissue values inhibits β-casein expression and promotes spreading and stiffening in MECs. We used two independent culture assays to increase extracellular elasticity beyond normal mammary tissue values while maintaining biochemical composition constant. The elastic modulus of the substrata as measured by AFM is indicated at the bottom of each image. (A–C) Floating gel assay: EpH4 cells were cultured on top of LM1 mixed with COL-I (3 g/l) (40:60% v/v). Four hours after plating, differentiation media was added and half of the gels were gently detached from the container. The elastic modulus of the floating gel was comparable to normal tissue (A, top image), whereas that of the attached gel was three-fold stiffer (A, bottom image). Corresponding β-casein expression (B) and cell stiffness (C) in these culture conditions. (D–H) Polyacrylamide gel assay: EpH4 cells were cultured on top of gels coated with equal fibronectin concentration but exhibiting a stiffness comparable with normal tissue (referred to as ‘soft') or within the range of mammary tumours (referred to as ‘stiff'). Cell morphology (D) and elasticity (F) 24 h after plating on either soft or stiff polyacrylamide gels. (E) β-Casein expression 48 h after overlaying cells with 2% Matrigel diluted in differentiation media. (G) Phase contrast (top) and corresponding CFP fluorescence intensity images (bottom panels) of EpH4 cells stably transfected with 16 concatenated copies of the β-casein promoter fused to CFP cultured as in (E). (H) Corresponding quantification of CFP intensity of cells cultured as in (E). The two assays consistently reported a downregulation of β-casein expression as well as non-biomimetic cell shape and elasticity in gels with non-biomimetic elastic moduli (for more details see the Discussion). *P<0.1 and ***P<0.01 were determined by two-tailed Student's t-test.
Loss of LM1 signalling induces non-biomimetic cellular elasticity and/or morphology and downregulates -casein expression
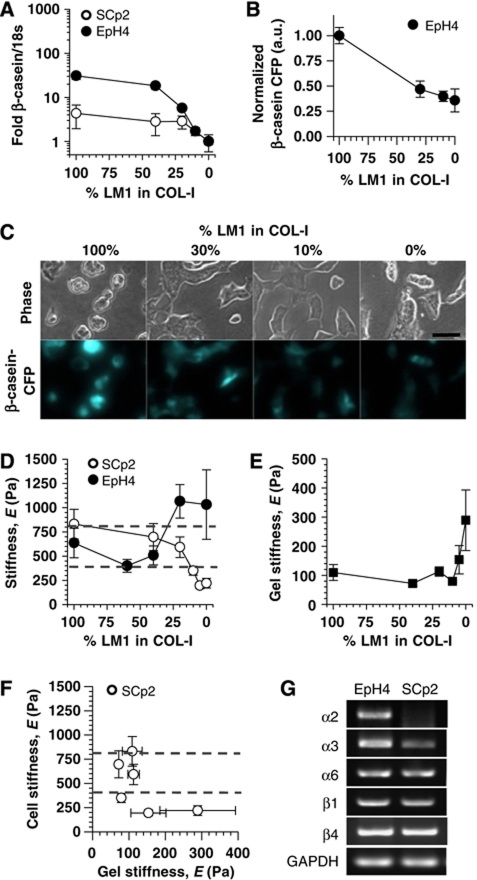
Mammary epithelial cells in vivo are in contact with a basement membrane, a specialized ECM rich in LM1 that physically separates mammary epithelium from the stroma; the latter is rich in COL-I (Provenzano et al, 2006) and contains much less LM1 (Klinowska et al, 1999). During tumour cell invasion, the integrity of the basement membrane is often compromised (Wetzels et al, 1989) and MECs can contact the stroma directly. To investigate how this loss of LM1 signalling affects functional differentiation and the mechanical properties of MECs, we examined SCp2 and EpH4 cells plated on top of LM1 gels mixed with increasing ratio of COL-I (2 g/l). We found that as little as 10% LM1 was sufficient to maintain β-casein expression and biomimetic extra- and intracellular elasticity (Figure 4A–E). Interestingly, reducing LM1 concentration below 10% led to a dramatic downregulation of β-casein expression (Figure 4A), a decrease in β-casein promoter activity (Figure 4B and C), non-biomimetic cellular elasticity (Figure 4D) and a sudden increase in the gel's elastic moduli (Figure 4E). Both SCp2 and EpH4 cells exhibited a similar relative downmodulation of β-casein as the COL-I/LM1 ratio increased. However, unlike SCp2 cells, loss of LM1 signalling in EpH4 cells induced stiffening (Figure 4D) and spreading comparable with that found on glass substrata (Supplementary Figure 2). The differences between SCp2 and EpH4 on these gels most probably arise from the distinct expression profiles of LM1 and COL-I integrin receptors (Figure 4G). SCp2 cells appeared stiffer on softer gels (Figure 4F), thereby revealing a strong non-linear relationship between extra- and intracellular elasticity in these cells, and confirming that our methodology to probe cell mechanics with AFM was not biased by the stiffness of the underlying substrata (further discussion on the lack of contribution by the underlying substrata elasticity on cell mechanical measurements is presented in Supplementary data). These experiments reveal that at a concentration of 10% or above, LM1 signalling is dominant over COL-I signalling, and that COL-I-dependent loss of β-casein expression is associated with non-biomimetic extra- and intercellular elastic moduli and changes in cell shape.
Figure 4.
Loss of LM1 signalling downregulates β-casein expression and induces non-biomimetic cellular elasticity and/or morphology in SCp2 and EpH4 MECs. (A) β-Casein expression of MECs cultured on top of COL-I (2 g/l) gels mixed with decreasing concentrations of LM1. (B and C) Visualization (C) and corresponding quantification (B) of the activity of the β-casein promoter in EpH4 cells cultured as in (A). (D) Elastic moduli of MECs cultured as in (A). Although both cell lines exhibited non-biomimetic elasticity for LM1 concentrations below 10%, the elastic moduli of SCp2 was lower than the physiological range (dashed lines), whereas that of EpH4 was higher. (E) Elasticity of LM1–COL-I mixed gels. (F) Elasticity of SCp2 cells as a function of gel elasticity. Note the non-linear relationship between these two properties. (G) Messenger RNA levels of LM1- and COL-I-specific integrin receptors in SCp2 and EpH4 MECs cultured in 2D assessed by RT–PCR.
Receptors involved in LM1-dependent biomimetic cellular elasticity
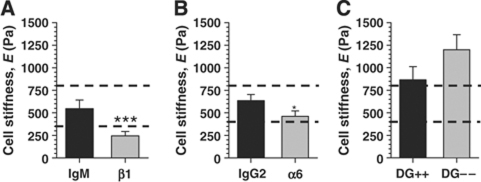
To start defining the molecular mechanisms underlying LM1-dependent biomimetic cellular elasticity, we examined the function of laminin-specific receptors, β1- and α6-integrins (Muschler et al, 1999) and dystroglycan (DG) (Weir et al, 2006), shown previously to be necessary for β-casein expression in culture and in vivo (Muschler et al, 1999; Naylor et al, 2005; Weir et al, 2006). To inhibit integrin receptors, we used function-blocking antibodies against either β1- or α6-integrins as described previously (Muschler et al, 1999). Blocking β1-integrin in SCp2 dramatically decreased their elastic moduli (Figure 5A), whereas blocking α6-integrin had only a weak effect (Figure 5B). Unlike β1-integrin blocking experiments, we did not observe a statistically significant difference between the elasticity of DG negative (DG−/−) and DG expressing (DG+/+) cells (kindly provided by Dr John Muschler at the California Pacific Medical Center) (P=0.14) (Figure 5C). These data suggest that a β1-integrin other than α6β1 mediates LM1-dependent biomimetic cellular elasticity.
Figure 5.
Receptors containing the β1-integrin subunit are major candidates for mediating LM1-induced cell biomimetic elasticity in MECs. (A and B) Elastic moduli of SCp2 cells cultured on top of Matrigel in the presence of function blocking antibodies against β1- (A) and α6-integrins (B) or corresponding isotype controls. (C) Elasticity of dystroglycan-expressing cells (DG+/+) or DG-negative (DG−/−). Both cell types remained fairly round under all conditions.
Biomimetic cellular elasticity is associated with low non-muscle myosin II activity and low actin polymerization
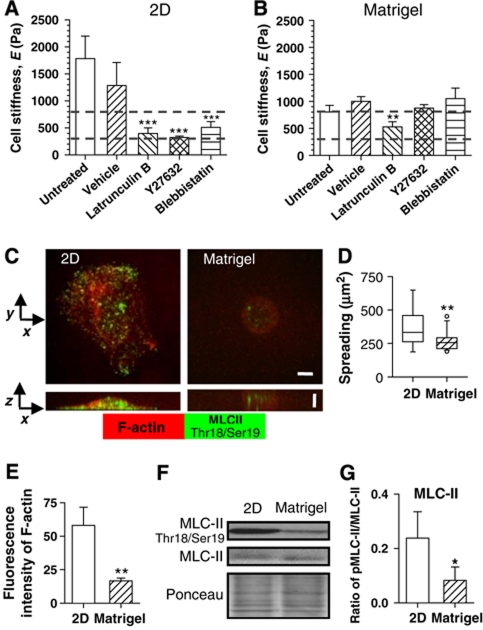
The actin–myosin cytoskeleton is the major contributor to cellular elasticity (Wakatsuki et al, 2003; Roca-Cusachs et al, 2008). When SCp2 cells cultured on two-dimensional (2D) glass substrata for 24 h were treated with specific inhibitors of actin polymerization (latrunculin B), myosin II ATPase activity (blebbistatin) and its upstream effector Rho kinase (ROCK) (Y27632), cellular elasticity markedly decreased towards the biomimetic range, although the difference was significantly smaller than cells cultured on top of Matrigel (Figure 6A). In contrast, two different inhibitors of microtubule polymerization essentially had no effect on cellular elasticity (Supplementary Figure 3). Treating SCp2 cells cultured on Matrigel with the same concentration of the inhibitors against the actin–myosin cytoskeleton revealed that actin polymerization still contributed to cellular elasticity. However, unlike 2D conditions, we did not observe any further reduction in cellular elasticity upon inhibition of ROCK or myosin II activities in Matrigel (Figure 6B). Confocal visualization in SCp2 cells of F-actin and phosphorylated myosin II light chain (MLC-II) fluorescence staining at Thr18 and Ser19, the latter being indicative of the specific activity of myosin II, revealed that cells on Matrigel were round, F-actin was mostly cortical and diphosphorylated myosin was scarce. In contrast, MECs on 2D spread and the corresponding F-actin and active myosin II were abundant both at the cell cortex and throughout the cytoplasm. (Figure 6C). Increased spreading and F-actin in 2D conditions were confirmed by quantitative analysis of the cell-projected area (Figure 6D) and of the average fluorescence intensity of phalloidin staining per cell (Figure 6E), respectively. Similar findings were obtained in EpH4 cells (data not shown). In agreement with the confocal images, immunoblot analysis of EpH4 cells showed that diphosphorylation of myosin II was significantly lower on Matrigel than on 2D tissue culture plastic (Figure 6F and G). These results indicate that LM1-dependent cellular biomimetic elastic modulus is mediated, at least in part, by targeting the actin–myosin cytoskeleton through downregulation of actin polymerization and myosin II activity.
Figure 6.
Laminin-111-induced cell biomimetic elasticity is partially mediated through downregulation of both actin polymerization and myosin II activity. (A, B) Comparison of the elasticity of SCp2 cells cultured for 24 h on 2D (A) or on top of LM1-rich gel (Matrigel) (B) after 30 min treatment with either vehicle (DMSO) or inhibitors of actin polymerization (latrunculin B), ROCK (Y26732) and myosin II ATPase (blebbistatin) activity using concentrations described in Materials and methods. *P<0.1, **P<0.05 and ***P<0.01 were determined by two-tailed Student's t-test w.r.t. untreated cells. (C) F-actin (red) and diphosphorylated MLC-II (green) organization in SCp2 cells spread on 2D substrata or rounded on top of Matrigel studied with confocal microscopy. Images correspond to the maximum intensity value projected on either the x–y plane (top images) or the z–x plane (bottom images). Both horizontal and vertical scale bars indicate 5 μm. (D) Box plot of cell spreading of SCp2 cells cultured as in (C). **P<0.05 was determined using Mann–Whitney rank sum test. (E) Quantification of the average fluorescence intensity of F-actin phalloidin staining per cell. (F) Immunoblot of total and diphosphorylated MLC-II in MECs cultured as in (C); (G) corresponding quantification by densitometry analysis of the ratio between diphosphorylated and total MLC-II. 2D substrata corresponds to either untreated borosilicate glass or tissue culture polystyrene dish.
Discussion
In vivo, signals from the microenvironment are essential for normal development and organ homoeostasis, and abnormalities in these signals contribute to various pathologies including tumorigenesis (Ingber, 2003; Nelson and Bissell, 2006). Nevertheless, the detailed mechanochemical signalling by which the microenvironment controls these processes are still ill-defined (Alcaraz et al, 2004). Previous studies using cultured cells have examined the effects of extracellular biochemical or biophysical cues on differentiation (Engler et al, 2004b; Le Beyec et al, 2007), morphology (Engler et al, 2004a) and mechanics (Solon et al, 2007), each in isolation. Here, we used a comprehensive approach to demonstrate quantitatively the intimate connection among LM1 binding, ECM elasticity, cell shape and cell elasticity in controlling tissue-specific gene expression in single epithelial cells. In addition, we found that variations of these properties from biomimetic values induce a loss of functional differentiation, which could potentially be linked to loss of differentiation during tumorigenesis.
Biochemical and mechanical extracellular cues synergize to maintain (or suppress) functional differentiation in culture
We used both biological and synthetic substrata to test the hypothesis that robust functional differentiation in culture requires substrata exhibiting elasticity within the range observed in normal mammary tissue, which we have defined here as biomimetic elasticity. The elastic modulus of the substrata was measured at the micro- and macroscopic scales by AFM and rheometry, respectively. In agreement with previous findings on synthetic gels (Engler et al, 2004a), the two techniques provided comparable values for biological gels (Table I), indicating that either method is suitable to probe the mechanics of compliant materials. In support of our hypothesis, we found that β-casein expression was maximal in MECs cultured on LM1-rich ECM gels containing ∼40% LM1 or more and exhibiting elastic moduli values within 75–120 Pa, a range comparable with normal mammary tissue. Conversely, dramatic reduction in β-casein expression (<3-fold) was observed in gels with less than 10% LM1 and a non-biomimetic elasticity of >250 Pa. Similarly, increasing the stiffness of synthetic polyacrylamide gels close to mammary tumour values was sufficient to downregulate functional differentiation induced by overlaying 2% Matrigel in the medium. However, the corresponding degree of β-casein expression on polyacrylamide gels was less than that observed on the biological gel assays. This lower β-casein induction is not surprising if one considers the important differences between the biological gels and the polyacrylamide gel assay in terms of how laminin is presented to the cell, that is, physically cross-linked within a gel versus in soluble form, and at what time point during the assay, that is, at plating time versus after 24 h. In support of the former explanation, we found that β-casein expression assessed by quantitative RT–PCR in MECs on top of Matrigel was an order of magnitude higher than on cells in 2D overlaid with 2% Matrigel (Supplementary Figure 4). Despite the different nature of these assays, it is remarkable that all consistently revealed that LM1-dependent functional differentiation is modulated by extracellular elasticity, and that, for a given LM1 concentration, β-casein expression is enhanced when the substrata exhibits biomimetic elasticity.
In addition to a requirement for biomimetic extracellular elasticity, we observed that strong β-casein expression (∼10–100-fold) was tightly associated with biomimetic cell elastic moduli (400–800 Pa), whereas β-casein expression was lost when values fell outside this range (data summarized in Supplementary Figure 5). Collectively, our findings reveal that functional differentiation of MECs is programmed to be highly responsive only in a narrow range of extra- and intercellular elasticity. These findings are qualitatively similar to recent findings on mechanically dependent stem cell commitment (Engler et al, 2006). Our results also suggest that the high compliance of the mammary tissue in vivo is essential for its functional differentiation (our data) and morphogenesis (Wozniak et al, 2003; Paszek et al, 2005). It is worth noting that all these observations underscore the importance of the strategy to potentially increase the efficacy of current scaffolds used in tissue engineering and regenerative medicine by mimicking the physiological elasticity of the target tissue (Engler et al, 2006).
Molecular mechanisms underlying LM1-dependent biomimetic cellular elasticity
The conditions of our ‘single cell on top' assay are not the traditional definition of 3D culture because cells are not completely surrounded by an ECM network. However, we and others have amply demonstrated that the ‘on top' conditions are much closer to 3D than commonly used 2D glass or plastic substrata: MECs form multicellular 3D colonies that mimic in vivo acinar structure and function when cultured on top of Matrigel (Barcellos-Hoff et al, 1989; Muschler et al, 1999; Lee et al, 2007). To our knowledge, our current results are the first mechanical measurements of single cells under conditions that mimic much of the 3D mechanochemical microenvironment in vivo. Our findings suggest that LM1-dependent mammary cell mechanics is primarily dominated by cell–ECM interactions rather than cell–cell interactions or changes in cell shape. Accordingly, we found that compromising signalling from the β1-integrin family of ECM receptors using function blocking antibodies in SCp2 was sufficient to inhibit LM1 effects on cellular elasticity. Furthermore, the elasticity of SCp2 on top of Matrigel treated with β1-integrin blocking antibodies was as low as that of the same cells cultured on COL-I gels, thereby indicating that a decrease in LM1 signalling induces non-biomimetic cellular elasticity. Our data support the finding that upregulation of β1-integrin clustering using stiff polyacrylamide substrata increases traction forces in fibroblasts and inhibits morphological differentiation in MECs, whereas using β1-integrin mutants with increased integrin self-association increases cell-generated forces and spreading on soft substrata in MECs (Paszek et al, 2005).
Among the different LM1 integrins, α3β1 is the major candidate in mediating LM1-dependent cell biomimetic elasticity in our experiments. Inhibiting α6-integrin in SCp2 cells had only a weak effect on cell mechanics; α2 is not expressed in these cells and α1-integrins are expressed only in myoepithelial cells in vivo (Taddei et al, 2003). We showed previously that inhibition of β1-integrin, and to a much lesser degree α6-integrin, could downregulate LM1-dependent β-casein expression in MECs, but did not prevent shape changes (Muschler et al, 1999). In contrast, inhibiting a non-integrin LM1 receptor that binds to the laminin LG4-5 domain was sufficient to inhibit both cell rounding and β-casein expression in MECs (Muschler et al, 1999). This non-integrin receptor has been recently identified as DG (Weir et al, 2006). We did not observe any effect of DG in the LM1-control of single cell mechanics, thereby indicating the specific function of β1-integrin in controlling elasticity of single cells under these conditions. These findings suggest that there is a further division of labour between different LM1 receptors in controlling cell shape, elasticity and β-casein expression.
Signals downstream of β1-integrin have been implicated in the control of the actin–myosin cytoskeleton in MECs (Wozniak et al, 2003; Paszek et al, 2005) and other cell types (Wang and Ingber, 1994; Galbraith et al, 2002; Wakatsuki et al, 2003). Actin–myosin contractility is the main mechanism by which non-muscle cells generate endogenous forces, and it is regulated in part by the Rho/ROCK pathway and its downstream effects on MLC-II phosphorylation (Wakatsuki et al, 2003; Wozniak et al, 2003). However, the function of these pathways on the mechanics of MECs had not been quantitatively examined in detail previously. We found that inhibiting ROCK, myosin II ATPase activity or actin polymerization in cells on 2D glass downmodulated cellular elastic moduli by ∼70%, which fell within the biomimetic range. However, none of these inhibitors fully mimicked the mechanical effects induced by LM1-rich ECM gels (Figure 6A and B). Furthermore, we observed that inhibiting actin polymerization, but not ROCK or myosin II, in cells on Matrigel further downmodulated cellular elasticity (∼30%), indicating that LM1-dependent cell biomimetic elasticity requires an intermediate level of actin polymerization and a low level of ROCK and myosin II activities achieved by moving cells from 2D conditions to 3D LM1-rich gels. In support of these findings, it was reported that treating primary MECs on Matrigel with an actin polymerization inhibitor downmodulated β-casein expression (Zoubiane et al, 2004), and that low Rho/ROCK activity was required for morphogenesis of human MECs (Wozniak et al, 2003; Paszek et al, 2005). These results illustrate that depending on the microenvironmental conditions, the response of the cells to the same drug may differ considerably, as we showed in human MECs treated with apoptotic agents (Weaver et al, 2002). Our data also reveal that downregulation of microtubule polymerization is unlikely to be involved in LM1-control of cell mechanics (Supplementary Figure 3). Nonetheless, we cannot rule out a function of microtubules in functional differentiation, as microtubule integrity (Zoubiane et al, 2004) and tubulin (Houdebine, 1990) have been implicated in β-casein expression.
On the basis of the strong correlation found between β-casein expression and biomimetic cell elastic moduli (Supplementary Figure 5), it is conceivable that the physiological range of cellular elasticity is necessary, although not sufficient, for functional differentiation of MECs. Defining the detailed molecular mechanisms by which cellular elasticity regulates β-casein expression would require further studies. However, we note that our observation of reduced myosin II activity brought about by a LM1-rich gel in functionally differentiated MECs (Figure 6C and G) is consistent with previous findings that Rho signalling needs to be reduced for acinar morphogenesis to occur (Paszek et al, 2005), and that integrin-linked kinase (ILK) activity and Rac1 signalling are involved in β-casein expression (Somasiri et al, 2001; Akhtar and Streuli, 2006). This previous evidence and our data suggest that biomimetic cellular elasticity is indicative of a unique level of signalling associated with β1 integrin as well as with Rac1/Rho, as these molecules are all important regulators of the actin–myosin cytoskeleton and are also necessary for β-casein expression. In addition, we can envision other contributions of cellular elasticity to β-casein expression, including a function in the localization and expression of the prolactin receptor as well as in the expression and assembly of endogenously synthesized laminin-containing basement membrane (Streuli and Bissell, 1990).
Does normal tissue mechanics have tumour suppressor-like functions?
As tissue mechanics is tissue specific (Discher et al, 2005), it is conceivable that there are homoeostatic mechanisms that maintain such specificity. In support of this hypothesis, we found a strong association between extra- and intracellular elasticity and functional differentiation in MECs. Specifically, we demonstrated that LM1 signalling and biomimetic extracellular elasticity are clearly necessary for functional differentiation of MECs; these cells in turn exhibit a strong correlation with biomimetic cellular elasticity, although the proof of a mechanistic relation between the latter and β-casein expression remains to be elucidated. As these signalling pathways go awry in tumorigenesis (Wetzels et al, 1989; Petersen et al, 1992; Gudjonsson et al, 2002; Paszek et al, 2005), it is tempting to speculate that, in addition to LM1 signalling, physiological tissue mechanics and/or its underlying homoeostatic mechanisms would have protective, that is, tumour suppressor, functions associated with maintenance of functional differentiation in vivo.
Materials and methods
Culture and transfection of cell lines
SCp2 (Desprez et al, 1993), EpH4 (Pujuguet et al, 2001) and MEpL DG−/− and DG+/+ cells (Weir et al, 2006) (kind gift from Dr J Muschler) were propagated in growth medium as described previously. Unless noted otherwise, cells were seeded at a density of ∼10 000 cells/cm2 on top of different substrata in growth medium supplemented with hydrocortisone (1 μg/ml) (Sigma). After 4 h, cells were treated with differentiation medium consisting of DMEM/F12, insulin, gentamicin, hydrocortisone and prolactin (3 μg/ml). Cell mechanics and β-casein expression were probed 24 and 48 h after adding differentiation medium, respectively. To pre-round cells in the absence of exogenous ECM signalling, cells were cultured on the nonadhesive substratum poly-HEMA (Sigma) (Muschler et al, 1999). EpH4 cells were transfected with a plasmid containing 16 concatenated copies of the mouse β-casein promoter fused to the CFP gene using Lipofectamine 2000 (Invitrogen). To screen for positive clones, 2% Matrigel (BD Biosciences) diluted in differentiation medium (∼15–200 μg/ml) was added to cells 24 h after plating on glass.
Mammary organoids
Primary organoids were isolated as described previously (Fata et al, 2007). Briefly, mammary glands were removed from 9-week-old pregnant (day 5) FVB mice and minced with two parallel razor blades (approved by the Animal Welfare and Research Committee (AWRC) at Lawrence Berkeley Labs; animal protocol no. 0510). Minced tissue (4–8 glands) was treated with collagenase/trypsin solution and DNase and washed, leaving a final pellet rich in epithelial pieces (referred to as mammary organoids) with virtually no stromal components or single cells. Mammary orgaoids was plated on top of Matrigel in basal media (Fata et al, 2007) supplemented with 5% fetal bovine serum. Differentiation medium was added 24 h after plating.
Preparation of substrata
For AFM experiments, 100 μl of ECM solutions were added to glass-bottomed culture dishes (MatTek) and incubated for 30 min at 37 °C to allow gelation unless otherwise indicated. Biological gels included Matrigel, LM1 (incubated overnight) (Trevigen) and neutralized COL-I (2 or 3 g/l) (ICN Biomedicals) (see Supplementary data for more details) mixed with increasing concentration of LM1. Traditional 2D substrata included uncoated borosilicate glass coverslips and polystyrene, referred to as glass and plastic, respectively. In the floating gel assay, gels containing 40% LM1 and 60% COL-I (3 g/l) were detached from the container edge with a spatula, whereas the gel centre remained attached to the underlying substratum for AFM measurements. The polyacrylamide gel assay was performed as described elsewhere (Pelham and Wang, 1997). Briefly, different volumes of 30% w/v acrylamide and 2% w/v bis-acrylamide solution (Bio-Rad) were mixed to form gels attached to a glass coverslip, with E comparable with normal rodent mammary tissue, average tumours or stiffer (Paszek et al, 2005). Gels were subsequenty coated with 40 μg/cm2 human fibronectin (BD) to facilitate cell adhesion.
Inhibitors
Actin polimerization was inhibited with latrunculin B (1 μM, Sigma). Microtubule polymerization was inhibited with nocodazole (10 μM, Sigma) and colcemid (23 μM, Sigma). ROCK and non-muscle MLC-II activity were inhibited using Y27632 (10 μM, Calbiochem) and blebbistatin (10 μM, Sigma), respectively. All drugs were added to the cells 30 min before AFM measurements. To inhibit the function of specific integrin subunits, cells were plated in the presence of 5 μg/ml azide- and endotoxin-free function-blocking antibodies against β1- (Ha2/5), α6-integrin (GoH3) or the corresponding isotype-matched control (IgM and IgG2, respectively) (BD Pharmingen) (Muschler et al, 1999).
RT–PCR analysis
Total cellular RNA was extracted using RNeasy kit (Qiagen). cDNA was synthesized with Superscript first-strand synthesis kit (Invitrogen) from 0.1–0.5 μg RNA samples. To assess the transcription profile of LM1 and COL-I-specific integrin receptors in SCp2 and EpH4, cDNA was used as a template for amplification of α2-, α3-, α6-, β1-, β4-integrin and GAPDH (used as an equal loading control), using an annealing temperature of 56 °C for 36 cycles. Quantitative real-time PCR analysis of β-casein and 18S rRNA (used as loading control) was performed with the Lightcycler System (Lightcycler FastStart DAN Master SYBR Green I kit, Roche). Description of the primers and protocol used to amplify β-casein cDNA and 18S rRNA can be found in the Supplementary data. All β-casein data were normalized to the corresponding 18S and averaged from three measurements for each independent experiment (n⩾2). To compare β-casein values from different experiments, we included cells on COL-I in each experiment and used their β-casein expression level as a common reference. Accordingly, all β-casein data (mean±s.e.) are given as fold with respect to COL-I.
Immunoblot analysis
Cells were cultured on uncoated tissue culture plastic or on top of Matrigel. Total cell lysates were obtained as described elsewhere (Lee et al, 2007) and treated with urea buffer (8 M Urea, 0.01 M Tris–HCl pH 8.0, 0.01 M NaH2PO4 and 150 mM NaCl) supplemented with phosphatase (sets 1 and 2, Sigma) and protease inhibitor cocktails (set I, Calbiochem). Equal protein amounts were separated on reducing SDS–PAGE gels (Invitrogen), transferred onto nitrocellulose membrane, blocked and incubated overnight with antibodies that recognize either total or phosphorylated (Thr18/Ser19) non-muscle MLC-II (Cell Signaling). Primary antibodies were detected with the Pierce SuperSignal detection kit and resulting bands were imaged with FluorChem 8900 analysis system (Alpha Innotech).
AFM elasticity measurements
We used a stand-alone AFM (Bioscope, Veeco) coupled to an inverted microscope as described in detail elsewhere (Alcaraz et al, 2003; Roca-Cusachs et al, 2008). In brief, force measurements were conducted using low spring constant cantilevers (k=0.03 N/m) (Microlever, Veeco). For cell measurements, the tip was positioned above the central part of the cell and approached to the cell surface using a stepping motor. Following the initial tip contact with the cell surface, three force versus piezo displacement recordings (F–z curves) were acquired at moderate loading force (∼1 nN) and low speed (∼5 μm/s). A similar procedure was used to probe the elasticity of the substrata. E and sample indentation δ were computed from F–z curves as described elsewhere (Alcaraz et al, 2003). Recordings from cells subjected to δ larger than 15% of the total height were discarded to rule out any artifactual contribution from the underlying substratum (Sridhar and Sivashanker, 2003). Data from cells or gels that yielded non-reproducible E values (coefficient of variation CV (CV=s.d./mean) higher than 15%) were also discarded. E data were screened for outliers using Chauvenet's criterion (Taylor, 1997). Application of these selection criteria led to ⩽15% discarded elastic moduli from either gels or cells. Examples of F–z curves on gels and on cells are shown in Supplementary Figure 6. Further details on F–z curve analysis are given as Supplementary data. All mechanical data are given as mean±s.e. and were calculated from at least nine measurements for each independent experiment (n⩾2).
Rheometry measurements
The bulk elasticity of biological gels prepared as in AFM experiments was assessed by measuring the complex modulus at low frequencies (∼1 Hz) using a parallel plate rheometer (Paar Physica MCR 300, kindly provided by Professor K Healy at UCB). Data are given as mean±s.e. and were calculated from at least six repeated measurements for each independent experiment (n⩾2).
Imaging
Bright-field images of cells and AFM cantilevers and CFP fluorescent images were acquired using an inverted microscope coupled to a CCD camera and a 10 × and 40 × objective, respectively.
To visualize F-actin and active myosin II in cells cultured on glass or on top of Matrigel, cells were fixed in 1% paraformaldehyde (Electron Microscopy Sciences) in the presence of phosphatase inhibitors, treated with 100 mM glycine (Sigma) in PBS, permeabilized with 0.5% Triton X-100 (Calbiochem) in PBS and blocked with 5% goat serum in 0.05% Tween-20 in PBS (Sigma) for 1 h at room temperature. Primary rabbit polyclonal antibody against phosphorylated MLC-II (Thr18/Ser19) (Cell Signaling) was incubated overnight in blocking buffer at 4 °C, followed by 3 × 15 min washes in 0.5% Triton X-100 in PBS. For detection of primary antibody and F-actin, samples were incubated with Alexa Fluor 488 secondary anti-rabbit antibody and Alexa Fluor 594 conjugated phalloidin (Invitrogen) for 1 h, respectively. Fluorescently labelled cells were examined on a spinning disc confocal system (Solamere Technology Group). Images were taken using a 63 × oil immersion objective (Zeiss). The average phalloidin fluorescence intensity per cell was calculated by adding the total fluorescence intensity of each confocal section after subtracting the background. Projections of the maximum intensity values in x–y and z–x planes were performed using Imaris 6.1 software (Bitplane).
Supplementary Material
Supplementary data
Acknowledgments
We thank D Fletcher and S Kumar from UC Berkeley (UCB) for critical reading of the manuscript, J Inman, C Ghajar and other members of the Bissell lab for helpful discussions and P Roca and D Navajas from the University of Barcelona, J Pollock (Healy lab, UCB), S Parekh, M Rosenbluth and Ailey Crow (Fletcher lab, UCB) and S Smith (Bustamante lab, UCB) for technical support. This work was supported by grants from the Department of Energy (DE-AC03-76SF00098 and a Distinguished Fellow Award to MJB, and DE-AC03-76DF00098 to CB), the National Institutes of Health (CA64786 and CA57621 to MJB, GM-071552 to CB), the Department of Defense (Innovator Award to MJB) and post-doctoral fellowships from the Catalonian Ministry of Universities, Research and Information Society (to JA), the Department of Defense (to RX, VAS and CMN), the Canadian Institute for Health Research (to VAS), the Susan G Komen Breast Cancer Foundation (to HM) and the American Cancer Society (to DCR).
References
- Akhtar N, Streuli CH (2006) Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J Cell Biol 173: 781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farre R, Navajas D (2003) Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys J 84: 2071–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J, Nelson CM, Bissell MJ (2004) Biomechanical approaches for studying integration of tissue structure and function in mammary epithelia. J Mammary Gland Biol Neoplasia 9: 361–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ (1989) Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105: 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G (1982) How does the extracellular matrix direct gene expression? J Theor Biol 99: 31–68 [DOI] [PubMed] [Google Scholar]
- Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ (1998) Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 7: 1133–1144 [PubMed] [Google Scholar]
- Desprez PY, Roskelley C, Campisi J, Bissell MJ (1993) Isolation of functional cell lines from a mouse mammary epithelial cell strain: the importance of basement membrane and cell–cell interaction. Mol Cell Differ 1: 99–110 [Google Scholar]
- Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143 [DOI] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D (2004a) Substrate compliance versus ligand density in cell on gel responses. Biophys J 86 (1 Part 1): 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE (2004b) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166: 877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689 [DOI] [PubMed] [Google Scholar]
- Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ (2007) The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev Biol 306: 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP (2002) The relationship between force and focal complex development. J Cell Biol 159: 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW (2002) Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115 (Part 1): 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdebine LM (1990) The possible involvement of tubulin in transduction of the prolactin signal. Reprod Nutr Dev 30: 431–438 [DOI] [PubMed] [Google Scholar]
- Ingber DE (2003) Mechanobiology and diseases of mechanotransduction. Ann Med 35: 564–577 [DOI] [PubMed] [Google Scholar]
- Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH (1999) Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol 215: 13–32 [DOI] [PubMed] [Google Scholar]
- Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ (2007) Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res 313: 3066–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GY, Kenny PA, Lee EH, Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4: 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G, Pitot HC (1975) Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res 94: 70–78 [DOI] [PubMed] [Google Scholar]
- Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ (1999) Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Mol Biol Cell 10: 2817–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH (2005) Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 171: 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22: 287–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254 [DOI] [PubMed] [Google Scholar]
- Pelham RJ Jr, Wang Y (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 89: 9064–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ (2006) Collagen reorganization at the tumor–stromal interface facilitates local invasion. BMC Med 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujuguet P, Radisky D, Levy D, Lacza C, Bissell MJ (2001) Trichostatin A inhibits beta-casein expression in mammary epithelial cells. J Cell Biochem 83: 660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Alcaraz J, Sunyer R, Samitier J, Farre R, Navajas D (2008) Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in G1 and proliferation. Biophys J 94: 4984–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ (1994) Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA 91: 12378–12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon J, Levental I, Sengupta K, Georges PC, Janmey PA (2007) Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93: 4453–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasiri A, Howarth A, Goswami D, Dedhar S, Roskelley CD (2001) Overexpression of the integrin-linked kinase mesenchymally transforms mammary epithelial cells. J Cell Sci 114 (Part 6): 1125–1136 [DOI] [PubMed] [Google Scholar]
- Sridhar I, Sivashanker S (2003) On the adhesion mechanics of multi-layer elastic systems. Surf Coat Tech 167: 181–187 [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ (1991) Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol 115: 1383–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ (1990) Expression of extracellular matrix components is regulated by substratum. J Cell Biol 110: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei I, Faraldo MM, Teuliere J, Deugnier MA, Thiery JP, Glukhova MA (2003) Integrins in mammary gland development and differentiation of mammary epithelium. J Mammary Gland Biol Neoplasia 8: 383–394 [DOI] [PubMed] [Google Scholar]
- Taylor JR (1997) An Introduction to Error Analysis. Sausalito: University Science Books [Google Scholar]
- Wakatsuki T, Wysolmerski RB, Elson EL (2003) Mechanics of cell spreading: role of myosin II. J Cell Sci 116 (Part 8): 1617–1625 [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE (1994) Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J 66: 2181–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ (2002) beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, Bissell MJ, Muschler JL (2006) Dystroglycan loss disrupts polarity and {beta}-casein induction in mammary epithelial cells by perturbing laminin anchoring. J Cell Sci 119 (Part 19): 4047–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels RH, Holland R, van Haelst UJ, Lane EB, Leigh IM, Ramaekers FC (1989) Detection of basement membrane components and basal cell keratin 14 in noninvasive and invasive carcinomas of the breast. Am J Pathol 134: 571–579 [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ (2003) ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol 163: 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Spencer VA, Bissell MJ (2007) Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors. J Biol Chem 282: 14992–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubiane GS, Valentijn A, Lowe ET, Akhtar N, Bagley S, Gilmore AP, Streuli CH (2004) A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. J Cell Sci 117 (Part 2): 271–280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data