Abstract
Significant decreases in the protein levels of potassium-chloride co-transporter 2 (KCC2) were detected in the ipsilateral spinal dorsal horn 4h following loose ligation of the sciatic nerve. These decreases were associated with a change in hindlimb weight distribution suggestive of pain behavior. In contrast, no changes in GABA-A receptor subunit alpha-1 levels were detected. The decreases in KCC2 coincided with a significant ipsilateral increase in BDNF protein levels. Both the decreases in KCC2 levels and the early pain behavior were prevented by intrathecal pre-treatment with the BDNF-sequestering TrkB/Fc chimera protein or the tyrosine kinase blocker K252a. The ligation-associated decreases in KCC2 levels were transient. In the ipsilateral spinal dorsal horn of ligated animals exhibiting weight-bearing pain behavior 7 days after the ligation the KCC2 levels were identical to those in control or sham-operated animals. These data suggested that TrkB-dependent reduction in KCC2 protein levels in the spinal dorsal horn was an early consequence of peripheral nerve injury. This decrease in KCC2 may have elicited an early increase in overall dorsal horn neuronal excitability perhaps through a loss of GABA inhibition which is critically dependent on KCC2 activity. The increased neuronal excitability may in turn have caused enhanced and exaggerated communication between primary afferents and dorsal horn neurons to contribute to the early behavioral signs of pain.
Keywords: Chronic constriction injury, Neuropathic pain, GABA, Neurotrophins, Potassium-chloride co-transporters, Synaptic plasticity
1. Introduction
The loss of GABA inhibition may at least partly contribute to the development of pain following peripheral nerve injury (reviews by Hammond, 2001; Eaton, 2002; also recent reports by Malan et al., 2002; Baba et al., 2003; Miletic et al., 2003). GABA-mediated inhibition appears critically dependent on the activity of the potassium-chloride co-transporter 2 (KCC2), one of four currently known members of the family of potassium-chloride co-transporters (Payne et al., 2003).
During early brain development, activation of GABA-A receptors typically elicits excitation because of the action of another co-transporter, the sodium-potassium-chloride co-transporter NKCC (Ben-Ari, 2002). However, during maturation, the expression of KCC2 progressively increases, and KCC2 activity replaces that of NKCC. This then shifts the early GABA-A receptor-mediated excitatory responses into inhibitory in adults. Nevertheless, GABA-A receptor-mediated excitations have also been observed in adults following tetanic stimulation, axotomy, neuronal trauma, or epileptiform activity (Kapur and Coulter, 1995; van den Pol et al., 1996; Kaila et al, 1997; Nabekura et al., 2002).
A reduction in KCC2 mRNA and protein levels in the spinal dorsal horn may similarly shift the normally inhibitory synaptic currents to excitatory thereby increasing lamina I neuronal excitability following partial nerve injury induced by a sciatic cuff. The injury-associated disruption of anion homeostasis appears at least partly dependent on the release of brain-derived neurotrophic factor (BDNF) from microglia (Coull et al., 2003; 2005; also recent review by Price et al., 2005).
Much present evidence suggests an important contribution of BDNF to nociceptive information signaling in the spinal dorsal horn (e.g., recent review by Merighi et al., 2004; also recent reports by Groth and Aanonsen, 2002; Pezet et al., 2002; Yajima et al., 2005). In the adult brain enhanced expression of both BDNF and its high-affinity TrkB receptors appear to predispose certain cortical areas to seizures (Binder et al., 2001). This BDNF-induced hyper-excitability may be at least in part due to the loss of GABA inhibition mediated by the TrkB-dependent down-regulation of KCC2 (Wardle and Poo, 2003; Rivera et al., 2002; 2004).
Little is presently known about the potential interaction between BDNF and KCC2 in the spinal dorsal horn following peripheral nerve injury. In this study we examined whether loose ligation of the sciatic nerve would be associated with changes in KCC2 protein in the spinal dorsal horn as well as early behavioral signs of pain. We also sought to determine if both the pain and the protein reduction would be alleviated by intrathecal pre-treatment with either the TrkB/Fc chimera protein, which sequesters endogenously released BDNF and in effect ‘blocks’ TrkB receptors, or with K252a, a nonspecific tyrosine kinase receptor blocker.
2. Materials and Methods
2.1 Animals and anesthesia
Male Sprague-Dawley rats (Harlan, ~350 g) were used. Water and food were provided ad libitum. Experiments were conducted in accordance with guidelines accepted by the International Association for the Study of Pain (Zimmermann, 1983). The animal protocol was approved by the Animal Care Committee of the School of Veterinary Medicine at the University of Wisconsin-Madison.
Animals were randomly assigned to control, sham-operated or ligated groups, and anesthetized with isoflurane using an anesthesia machine. Body temperature was kept at 37°C with a homeothermic blanket system. Anesthesia was sufficiently deep to prevent arousal, but light enough to permit spontaneous respiration. Adequate anesthesia was assessed by (1) monitoring blink or ear reflexes, (2) withdrawal to toe pinches, (3) respiratory rate, and (4) absence of spontaneous movements.
2.2 Sciatic ligation and tissue collection
Loose ligation of the sciatic nerve was performed as originally described by Bennett and Xie (1988). The sciatic nerve was exposed by blunt dissection, freed of adherent tissue, and four ligatures (4-0) were placed about 1mm apart. The ligatures were tied so that the nerve trunk was just barely constricted when viewed with a dissecting microscope at 40x. This degree of constriction retarded, but did not arrest, circulation through the superficial epineural vasculature. In sham-operated animals the sciatic was exposed in an identical manner except that no ligatures were placed around the nerve. Control animals were anesthetized but were not subject to surgery.
The animals recovered from the anesthesia and then 4h or 7 days after sciatic exposure or ligation, they were again anesthetized with isoflurane and euthanized with an intracardiac injection of saturated potassium chloride. A laminectomy rapidly (<2 min) exposed the lumbar spinal cord at L5 and about 1cm of the cord was excised and cut first into dorsal and ventral halves and then the dorsal half was further divided into ipsilateral and contralateral quadrants. All tissues were immediately placed into dry-ice cooled collecting tubes, and stored at −80°C until use.
2.3 Intrathecal drug application
The nonspecific Trk blocker K252a (2µg in 10µl PBS with 0.01% DMSO; Sigma-Aldrich), the TrkB/Fc chimera protein (5µg in 10µl PBS; Sigma-Aldrich), or vehicle (10µl) were injected intrathecally 15 min before the sciatic exposure/ligation using the procedure described by Mestre et al. (1994). Briefly, the animals were anesthetized with isoflurane and securely held in the sternal position. The tissues between the dorsal aspects of vertebrae L5 and L6 were punctured with a 25G needle connected to a 25µl Hamilton syringe. The needle was advanced until a slight but noticeable movement of the tail or the leg was observed which indicated that the needle entered into the intrathecal space. The animals recovered from the anesthesia and then four hours or seven days after exposure or ligation, the animals were re-anesthetized with isoflurane, and euthanized with an intracardiac injection of saturated potassium chloride. The spinal cords were then harvested, divided into quadrants and stored as detailed above. In a pilot study we confirmed that 10µl injections of 2% lidocaine using the same procedure produced the expected transient paralysis of the animals' hind quarters.
2.4 Weight distribution test
This test uses a dual channel scale (Incapacitance Meter™, Stoelting) that separately measures the force exerted by each hind limb (measured in grams). While normal rats distribute weight about equally, animals with a unilateral injury will shift the ratio of weight distribution between an injured and non-injured limb. This shift in weight distribution is taken as a measure of the level of discomfort in the injured limb. In other words, the less weight placed on an injured limb, the greater the pain. A 1s weighing period was used to average 20 measurements and obtain the weight borne by each hindlimb separately both before (baseline) and after sciatic exposure or ligation. A weight distribution ratio for each animal was then obtained by dividing the left (injured) leg weight by that of the right (noninjured) leg. We have reported recently that this test is equivalent to the thermal withdrawal latency test in detecting ligation-elicited neuropathic pain behavior (Miletic et al., 2005).
Baseline weight-distribution ratios were obtained for all animals before they were randomly assigned to sham-operated or ligated groups. The animals were then anesthetized and their sciatic nerves exposed or ligated as described above. Four hours or seven days after the sciatic exposure or ligation the weight distribution ratios of all animals were obtained again before they were anesthetized, euthanized and their tissues collected as described above.
2.5 Western Immunoblots
The collected spinal dorsal horn quadrants were homogenized and centrifuged at 7000g for 15 min. The 50mM Tris-HCl (pH 7.4) homogenizing buffer contained 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 1% NP-40, 0.25% sodium deoxycholate, and 5 µg/ml of a mixture of protease inhibitors [4-(2-aminoethyl) benzene-sulfonyl fluoride, pepstatin A, trans-epoxysuccinyl-L-leucyl-amido(4-guanidino) butane, bestatin, aprotinin, leupeptin]. Total protein content in the homogenates was determined with a commercially available kit (Pierce, #23236).
The homogenates were boiled and the proteins separated by SDS-PAGE electrophoresis (20 µg of total protein per well), and transferred onto PVDF membranes. The membranes were placed in a blocking solution (Tris-buffered saline containing 0.02% Tween and 5% non-fat dry milk) for 1 hr, and incubated overnight in primary antibodies to KCC2 (1:600; Chemicon), GABA-A (1:600; Upstate), BDNF (1:600; Promega) or CREB (1:2000; Upstate). After washing, incubation in appropriate peroxidase-conjugated secondary antibodies (1:10000) for 1 hr, and washing again, the proteins of interest were detected by chemiluminescence.
CREB was used as a protein loading control. In previous studies we reported that there were no detectable changes in the amount of the non activated form of CREB in the spinal dorsal horn either hours or days after the loose ligation (Miletic et al., 2002; Miyabe et al., 2005).
Protein levels were estimated from optical density measurements of scanned images of the respective bands using Image J (NIH, Bethesda, MD), a public domain Java image processing program based on NIH Image for the Macintosh. The protein levels in sham-operated or ligated animals were normalized to those in control, uninjured animals, i.e., control values served as baseline reference values. The average density of the protein bands in the control animals was denoted as 100% and the average density of the bands in the other animals was expressed as a percent change from this control.
2.6 Statistical Analysis
ANOVA was used for the statistical data analysis. The main emphasis was on detecting changes in the dependent measures both within and between groups (e.g., changes in protein levels or weight distribution due to the ligation, K252a or TrkB/Fc treatment). Significant effects were further analyzed with Scheffe's post-hoc test. Significance was inferred at the p ≤ 0.05 level.
3. Results
3.1 Early decreases in KCC2 protein levels were associated with loose ligation of the sciatic nerve
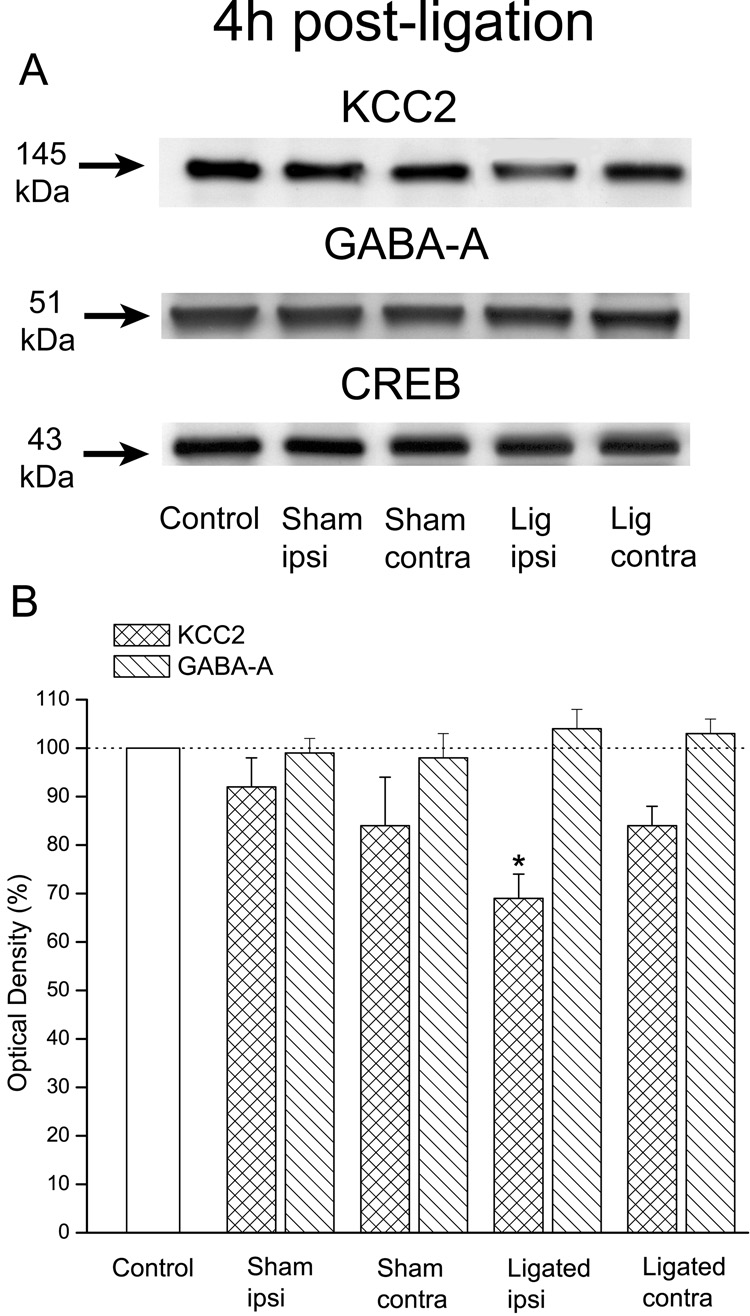
KCC2 protein levels in the injured, ipsilateral spinal dorsal horn of ligated animals (n=6) 4h after the ligation were substantially lower (69±5%) than those in control animals (100%; n=4). On the other hand, KCC2 levels in the contralateral dorsal horn of ligated animals (84±4%) or in either the ipsilateral (92±6%) or contralateral (84±10%) dorsal horn of sham-operated animals (n=4) were similar to those of controls.
Overall ANOVA indicated a significant difference in KCC2 protein levels among the five groups, F(4,19)=4.5, p=0.01. Scheffe's post-hoc analysis established that this difference was due to the significantly reduced KCC2 levels in the ipsilateral dorsal horn of ligated animals (p=0.016) rather than in the contralateral dorsal horn of these animals (p=0.4) or the ipsilateral (p=0.9) or contralateral (p=0.5) dorsal horn of sham-operated animals (Fig. 1).
Figure 1. Early decreases in the protein levels of KCC2 were associated with loose ligation of the sciatic nerve.
A: Immunoblots of KCC2, GABA-A (subunit α1) and CREB in control, sham-operated or ligated animals. B: Plots of the estimated protein content. Note that the levels of KCC2 in ligated animals (n=6) were significantly lower in the injured, ipsilateral but not contralateral dorsal horn 4h after the sciatic ligation. Note also that there were no significant differences in ipsilateral or contralateral dorsal horn levels between sham-operated (n=4) and control animals (n=4). Note further that there were no significant changes in the GABA-A receptor levels in the dorsal horn of any of the animals, and that CREB immunoblots confirmed equal protein loading. Error bars represent the SEM. *p=0.016.
Given the importance of GABA inhibition to normal sensory processing in the dorsal horn, we also assayed for protein levels of the GABA-A receptor subunit α1. Our data indicated that neither the ligation nor the sham surgery elicited significant changes in these levels at 4h post-ligation or exposure when compared to controls, i.e., 104±4%, 103±3%, 99±3% and 98±5%; F(4,19)=0.6, p=0.7 (Fig. 1).
3.2 Ligation-associated decreases in KCC2 protein levels were prevented by pretreatment with either K252a or TrkB/Fc
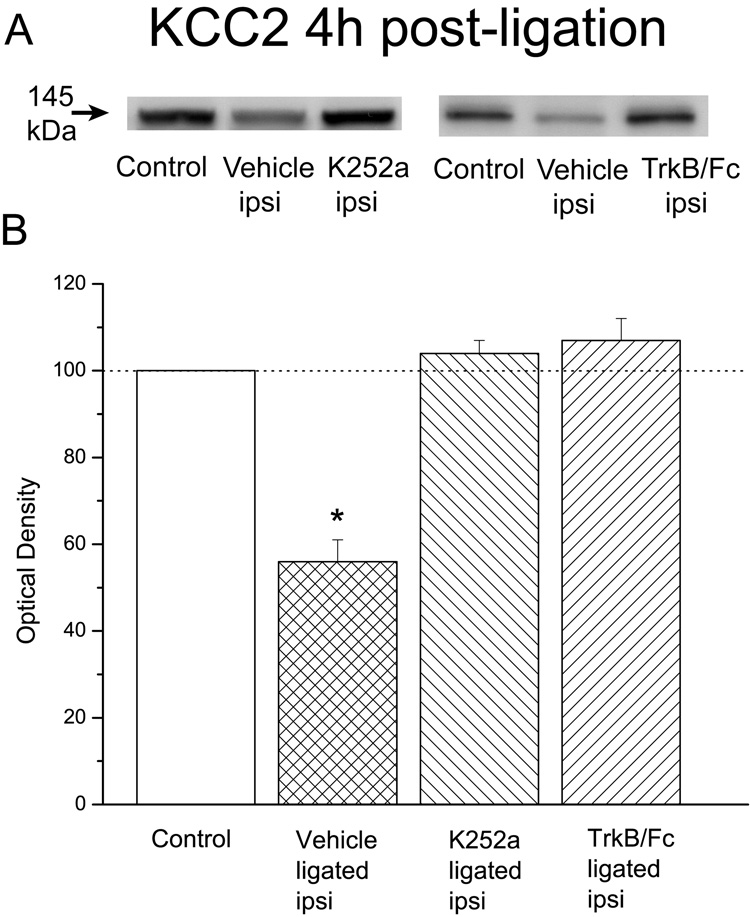
In our next experiment we sought to establish whether the tyrosine kinase receptor blocker K252a would prevent the ligation-associated decline in KCC2 protein levels. Our results established that the injury-associated reduction in these levels in the ipsilateral spinal dorsal horn of vehicle-treated animals (56±5%; n=6) was entirely prevented by K252a pre-treatment (104±3%, n=6; Fig. 2).
Figure 2. Ligation-associated decreases in KCC2 protein levels were prevented by intrathecal pretreatment with either K252a or TrkB/Fc.
A: KCC2 immunoblots in the ipsilateral dorsal horn of control or ligated animals treated with vehicle, K252a or TrkB/Fc. B: Plot of KCC2 levels. Note that the ligation-associated significant decrease in KCC2 protein levels in the ipsilateral spinal dorsal horn (n=6; different animals than illustrated in Fig.1) was entirely prevented by pre-treatment with either K252a (n=6) or TrkB/Fc (n=4) 15 min before the sciatic ligation. Error bars represent the SEM. *p<0.001.
Given that K252a is not a specific blocker of TrkB receptors we also pre-treated another four animals with the TrkB/Fc chimera protein which sequesters any endogenously released BDNF and in effect specifically ‘blocks’ TrkB receptors. Our results confirmed a TrkB-KCC2 connection because in these animals there was no evidence of an injury-associated reduction in KCC2 levels in the ipsilateral spinal dorsal horn following the sciatic ligation (107±5%, n=4). Statistical analysis confirmed a significant difference between groups, F(3,18)=47.7, p<0.001, which was due to the significantly reduced levels of KCC2 in vehicle-treated animals (p<0.001).
3.3 Increases in BDNF protein levels in the spinal dorsal horn coincided with the ligation-associated decreases in KCC2
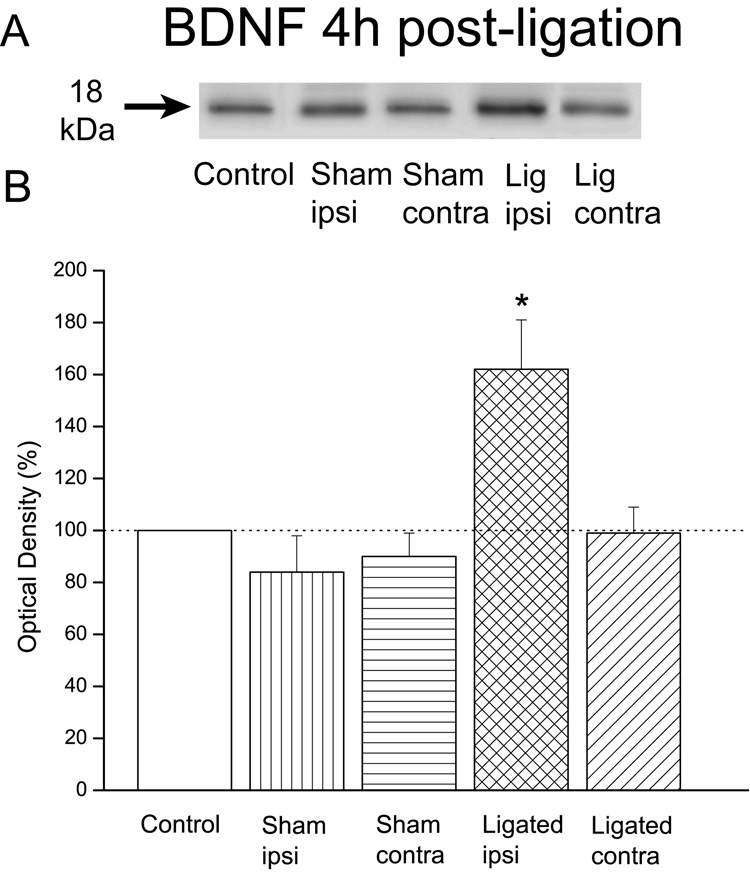
Previous studies in the hippocampus suggested that BDNF mediated KCC2 down-regulation (Rivera et al., 2002;2004). Consequently, in our study we sought to examine whether increases in BDNF coincided with the decreases in KCC2 levels post-ligation. Our results demonstrated that the levels of BDNF in ligated animals (n=6) were substantially higher in the injured, ipsilateral dorsal horn 4h after the sciatic ligation (162±19%) when compared to control animals (100%; n=4). In contrast, in the contralateral dorsal horn of ligated animals (99±10%) or the ipsilateral (84±14%) or contralateral (90±9%) dorsal horn in sham-operated animals (n=4) the levels of BDNF were similar to those in control animals (Fig. 3).
Figure 3. Early increases in the protein levels of BDNF were associated with loose ligation of the sciatic nerve.
A: Immunoblots of BDNF in control, sham-operated or ligated animals. B: Plot of the estimated protein content. Note that the levels of BDNF in ligated animals (n=6) were significantly higher in the injured, ipsilateral dorsal horn 4h after the sciatic ligation. Note also that the BDNF levels in the contralateral dorsal horn of these ligated animals, or the ipsilateral or contralateral dorsal horn in sham-operated animals (n=4) were not significantly different from controls (n=4). Error bars represent the SEM. *p=0.015.
ANOVA indicated a significant difference among groups, F(4,19)=6.9, p=0.004 which was due to the significant increases in BDNF levels in the ipsilateral spinal dorsal horn of ligated animals (p=0.015).
3.4 Early behavioral signs of neuropathic pain were attenuated by intrathecal pre-treatment with either K252a or TrkB/Fc
In these experiments we sought to determine whether intrathecal pre-treatment with K252a or TrkB/Fc would modify the early pain behavior given that this treatment successfully prevented the ligation-associated loss of KCC2 protein in the ipsilateral spinal dorsal horn (Fig. 4).
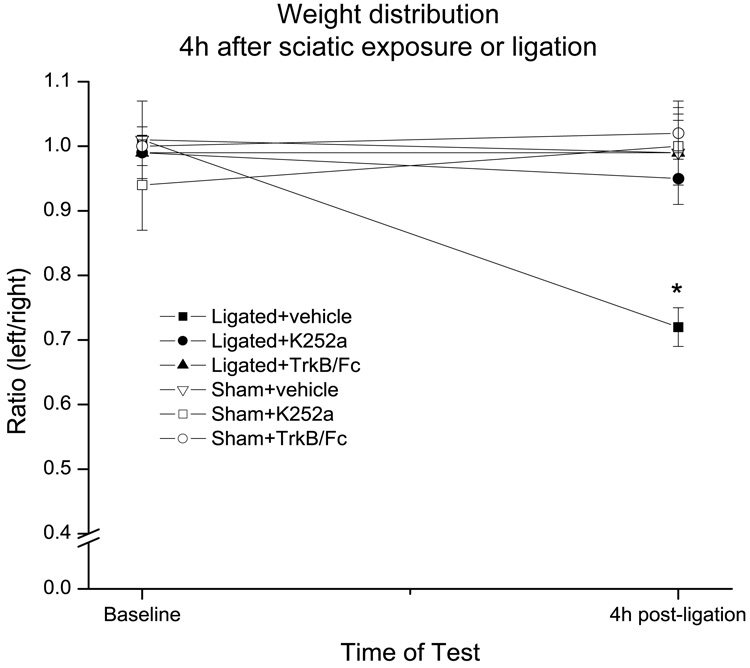
Figure 4. Early behavioral signs of pain were attenuated by intrathecal pre-treatment with either K252a or TrkB/Fc.
Baseline weight distribution ratios were obtained for all animals before sciatic exposure (sham) or ligation, and then again 4h later. Note that vehicle-treated ligated animals (n=6) exhibited a decrease in their weight distribution ratio suggestive of pain behavior. On the other hand, pre-treatment with either K252a (n=6) or TrkB/Fc (n=4) 15 min before the ligation prevented this decrease suggesting complete relief from the pain behavior. Note further that injection of vehicle, K252a or TrkB/Fc did not modify the weight distribution ratios in sham-operated animals (n=4 for each treatment). Error bars represent the SEM. *p=0.001.
As expected, 4h after the ligation vehicle-treated ligated animals (n=6) exhibited a shift in weight distribution from their ipsilateral to their contralateral leg. This resulted in a reduction in the post-injury weight-bearing ratio when compared to the pre-injury baseline value (0.72±0.03 vs. 1.01±0.02; n=6). In contrast, vehicle-treated sham-operated animals (n=4) did not show a change in their weight distribution ratio 4h after sciatic nerve exposure (0.99± 0.08 vs. 1.01± 0.06).
Intrathecal pre-treatment with either K252a (n=6) or TrkB/Fc (n=4) successfully prevented the injury-associated reduction in the weight-bearing ratio (0.95±0.04 vs. 0.99±0.04 and 0.99±0.05 vs. 0.99±0.04 respectively) suggesting complete relief from the early behavioral signs of pain (Fig. 4). Neither K252a nor TrkB/Fc pre-treatment modified the weight distribution ratio in sham-operated animals (1.00±0.05 vs. 0.94±0.07 and 1.02±0.04 vs. 1.00±0.03 respectively; n=4 in each group).
Between group ANOVA indicated no significant difference in weight-bearing ratio at baseline, F(5,22)=0.4, p=0.8, but a significant difference at 4h, F(5,22)=6.1, p=0.001. As expected, this difference was due to the reduced ratio in vehicle-treated ligated animals (p=0.02). Within group ANOVA confirmed a significant difference between the baseline and 4h post-ligation ratios for vehicle-treated ligated animals, F(1,5)=49, p=0.001.
On the other hand, the 4h weight-bearing ratio in K252a-treated ligated animals was indistinguishable from that of sham-operated animals treated with vehicle (p=0.99), K252a (p=0.99) or TrkB/Fc (p=0.96). Similarly, the 4h ratio in TrkB/Fc-treated ligated animals was statistically identical to that of sham-operated animals treated with vehicle, K252a or TrkB/Fc (p=1.0 for each).
3.5 The levels of KCC2 protein in the dorsal horn recovered 7 days after sciatic ligation in animals exhibiting differential weight-bearing behavior
In these experiments we sought to determine whether the injury-associated loss of KCC2 protein levels persisted 7 days after the sciatic ligation, typically the time of maximal hyperalgesia in our use of the loose ligation model (Miletic et al., 2003).
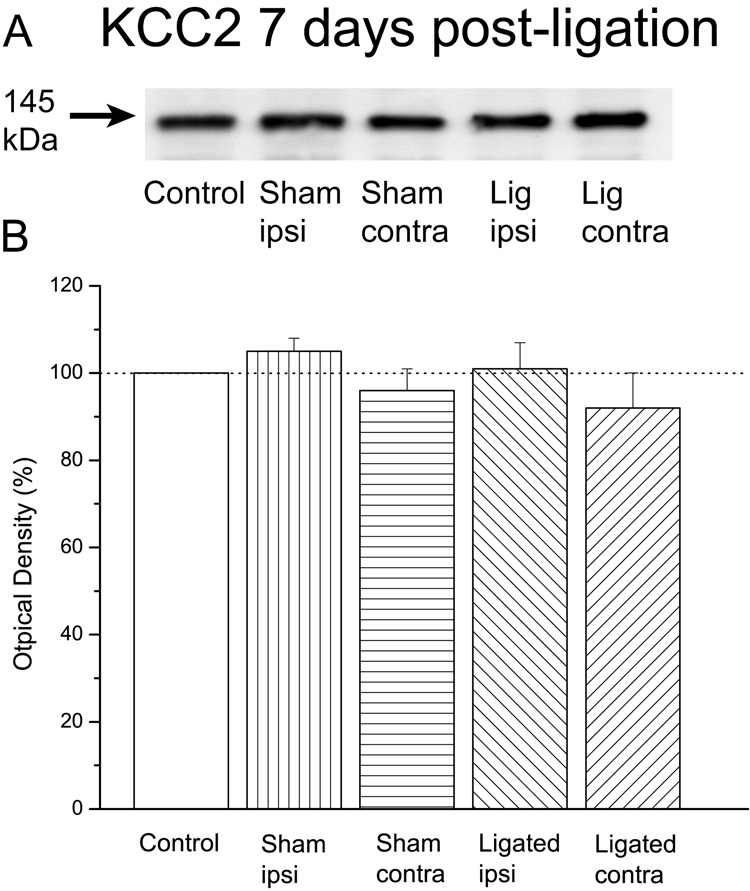
Immunoblot analysis revealed that there were no significant differences between ligated (n=4) and sham-operated (n=4) animals. In other words, KCC2 levels in the ligated ipsilateral (101±6%), ligated contralateral (92±8%), sham ipsilateral (105±3%), and sham contralateral (96±6%) spinal dorsal horn were essentially identical to those in control animals, F(4,15)=0.8, p=0.5 (Fig. 5). These data suggested that the early sciatic ligation-elicited down-regulation of KCC2 protein levels was transient, and that there may be significant differences in the cellular processes contributing to the early vs. late phases of nerve injury-associated pain.
Figure 5. The levels of KCC2 protein 7 days after loose ligation of the sciatic nerve were the same in all animal groups.
A: Immunoblots of KCC2 in control, sham-operated or ligated animals 7 days after surgery/ligation. B: Plot of the estimated protein content. Note that the levels of KCC2 were essentially the same across all animal groups (n=4 in each group).
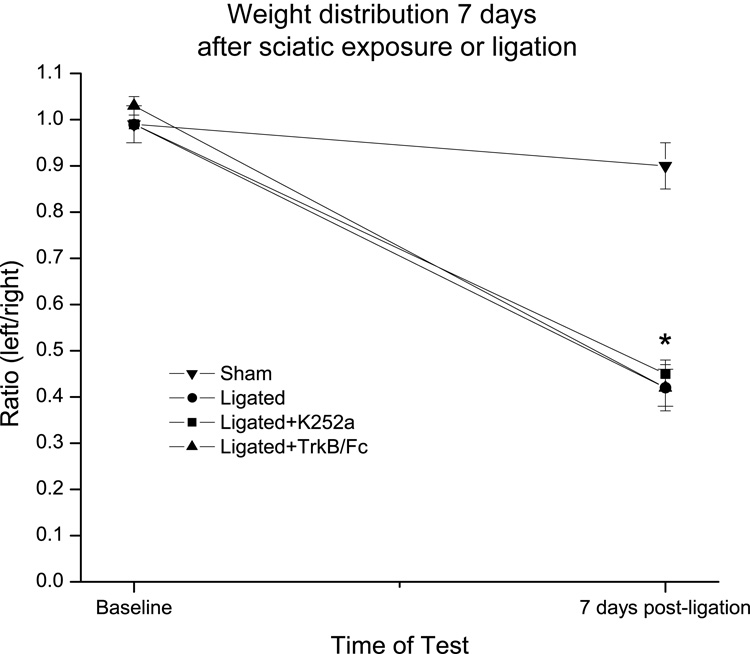
As expected, there was no difference in the baseline weight-bearing ratios between sham-operated (0.99±0.04, n=4) and ligated (0.99±0.04, n=4) animals (Fig. 6). However, 7 days post-ligation, the weight bearing ratio in ligated animals was substantially decreased (0.42±0.04) while that of sham-operated animals remained essentially unchanged (0.90±0.05). A single intrathecal injection of either K252a or TrkB/Fc 15 min before the ligation (n=4 for each treatment) was not effective in preventing the reduction in the weight-bearing ratio of these ligated animals 7 days after the ligation (0.99±0.01 vs. 0.45±0.03 for K252a, and 1.03±0.02 vs. 0.42±0.05 for TrkB/Fc).
Figure 6. Sciatic ligation animals exhibited a reduction in their weight distribution ratio 7 days post-ligation and this was not prevented by a single pre-treatment with either TrkB/Fc or K252a.
Baseline weight distribution ratios were obtained before sciatic exposure or ligation and then again 7 days after surgery. Note that ligated (n=4), but not sham-operated (n=4), animals exhibited a significant reduction in their weight distribution ration suggestive of pain behavior. Note also that a single injection of either K252a or TrkB/Fc 15 min before the ligation (n=4 for each treatment) did not prevent the development of the pain behavior. Error bars represent the SEM. * p<0.001.
Between group ANOVA indicated no significant difference in weight-bearing ratio at baseline, F(3,12)=0.3, p=0.8, but a significant difference at 7 days, F(3,12)=28, p<0.001. As expected, this difference was due to the significant reduction in the weight distribution ratio in all three ligated groups (p<0.001 for each). Within group ANOVA confirmed a significant difference between the baseline and 7 days post-ligation ratios for each of the three ligated groups, F(1,3)=169, p<0.001, F(1,3)=630, p<0.001 (K252a-treated), and F(1,3)=85, p=0.003 (TrkB/Fc-treated).
This finding on the failure of a single pre-emptive treatment was similar to our previous study in which we also found that only continual, rather than single, application of lidocaine was effective in abolishing pain behavior 7 days after loose ligation of the sciatic nerve (Smith et al., 2002).
4. Discussion
In this study we examined the early consequences of loose ligation of the sciatic nerve on the protein levels of potassium-chloride co-transporter 2 (KCC2) in the spinal dorsal horn, i.e., in the first few hours after the ligation. Our data established that: (1) KCC2 protein levels significantly decreased in the ipsilateral dorsal horn 4h after the sciatic ligation, (2) the decrease in KCC2 protein coincided with an ipsilateral increase in BDNF protein levels; (3) the decrease in KCC2 protein was associated with early behavioral signs of pain, and (4) both the decrease in protein and the early pain behavior were attenuated by a block of TrkB receptors. In addition, our data demonstrated that (5) the early ligation-associated decreases in KCC2 levels were transient and (6) they were not accompanied by a reduction in the levels of the α1 subunit of GABA-A receptors.
We surmise that the peripheral nerve injury elicited, among other mediators, the release of BDNF to activate TrkB receptors in the spinal dorsal horn. The activation of these receptors mediated some of the early consequences of injury-elicited plasticity, including the down-regulation of KCC2. GABA inhibition is critically dependent on KCC2 activity (Stein and Nicol, 2003), and the loss of this activity may have thus lead to a loss of GABA inhibition in the dorsal horn. We assume that at any one time a balance between excitatory and inhibitory processes determines the degree of overall neuronal excitability in the dorsal horn, and that the loss of GABA inhibition tipped the balance towards excitation. This increased excitability then sustained enhanced (and exaggerated) communication between primary afferents and dorsal horn neurons contributing to the early behavioral signs of pain. These early behavioral signs of pain appeared largely mediated by central processes because both the pain behavior and the decreases in KCC2 levels were alleviated by intrathecal treatment.
Our data provided further support for the notion that BDNF plays an important role in spinal nociception and injury-associated pain (e.g., recent review by Merighi et al., 2004; also recent reports by Groth and Aanonsen, 2002; Pezet et al., 2002; Yajima et al., 2005). Our study also established that the early behavioral signs of pain could be effectively attenuated by a single pre-treatment with the TrkB/Fc chimera protein or the nonspecific tyrosine kinase blocker K252a. A similar effective analgesic action of K252a has also been recently reported in a study of cyclophosphamide-elicited mechanical allodynia in the bladder (Guerios et al., 2006).
In the adult brain enhanced expression of both BDNF and TrkB receptors appears to predispose certain cortical areas to seizures (Binder et al., 2001). This BDNF-induced hyper-excitability may be at least in part due to the loss of GABA inhibition mediated by the TrkB-dependent down-regulation of KCC2 (Wardle and Poo, 2003). In two recent studies, Rivera and colleagues (2002; 2004) have provided substantial additional support for this notion. These authors have reported that BDNF application onto hippocampal neurons in culture or slices elicited a TrkB-mediated reduction in KCC2 mRNA and protein levels, and this resulted in the impairment of neuronal chloride extrusion capability. Moreover, the expression of KCC2 in vivo was similarly down-regulated following kindling-induced seizures. The down-regulation of KCC2 both in vitro and in vivo was evident within 2h, appeared maximal at 6h and disappeared by 24h. The decline in KCC2 protein levels was apparently due to the removal of the co-transporter from the cell membrane by rapid degradation of the protein. Rivera and colleagues concluded that the high turnover rate of KCC2 provides a time frame that is compatible with a role for this co-transporter in various manifestations of BDNF-mediated neuronal plasticity.
In the spinal dorsal horn a similar interaction between BDNF and KCC2 may be an important early step in the transition from normal to pathophysiological processing. The rapid down-regulation of KCC2 could quickly cause a loss of the all-important GABA inhibition before slower but more permanent changes in the overall circuitry can take place.
Our data provided further support for the studies of Coull and colleagues (2003; 2005) who have reported that partial nerve injury (induced by a sciatic cuff) was associated with a decrease in KCC2 mRNA and protein levels in lamina I neurons in the spinal dorsal horn. The injury also disrupted anion homeostasis in lamina I neurons and shifted the normally inhibitory synaptic currents to excitatory thereby increasing lamina I neuronal excitability in vitro. In addition, block of KCC2 activity in vivo reduced mechanical and thermal nociceptive thresholds in control, uninjured animals, and the anion shift in gradient appeared at least partly dependent on BDNF. Coull and colleagues concluded that injury-associated down-regulation of KCC2 may represent a novel mechanism of disinhibition in the spinal dorsal horn. Our data extended these observations by demonstrating that the loss of KCC2 appeared widespread throughout the spinal dorsal horn and was sufficient to elicit early behavioral signs of pain which were alleviated by a ‘block’ of TrkB receptors. A recent study similarly reported that a decrease in expression of KCC2, but not NKCC1, in the spinal dorsal horn accompanied the initial stage of formalin-evoked hyperalgesia (Nomura et al., 2006).
A sizable literature implicates the loss of GABA inhibition in the development of nerve injury-associated pain (e.g., reviews by Hammond, 2001; Eaton, 2002; also recent reports by Malan et al., 2002; Baba et al., 2003; Miletic et al., 2003). This is not surprising given the postulated critical role of GABA inhibitory action to the normal function of numerous neuronal circuits (Paulsen and Moser, 1998). The spinal cord is no exception, and any injury-induced modification of the GABA-inhibitory action has the potential to significantly modify the processing of nociceptive information in the spinal dorsal horn and in this way at least partly contribute to the development of pain.
Our observation that the injury-associated reduction in KCC2 protein levels was transient paralleled the results in the hippocampus (Rivera et al., 2004) and provided further support for the notion that, like activity-dependent plasticity, nerve injury-associated pain exhibits early, intermediate and late phases of development which may be mediated by different receptors, signaling pathways, transcription factors and genes (Ji et al., 2003; Malenka and Bear, 2004).
In conclusion, our data suggested that TrkB-dependent reduction in KCC2 protein levels in the spinal dorsal horn was an early consequence of peripheral nerve injury. The decrease in KCC2 may elicit an early increase in overall dorsal horn neuronal excitability perhaps through a loss of GABA inhibition which is critically dependent on KCC2 activity. The increased neuronal excitability may then sustain enhanced (and exaggerated) communication between primary afferents and dorsal horn neurons to contribute to the early behavioral signs of pain.
Acknowledgments
We are grateful to Tami A. Mueller for expert help with surgeries, behavioral testing and tissue collection. Supported in part by NIH grants NS055042 and NS034870.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Oakmoto M, Woolf CW. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission of the superficial spinal dorsal horn. Mol Cel Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, deKoninck P, deKoninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Coull JAM, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, deKoninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Eaton MJ. Emerging cell and molecular strategies for the study and treatment of painful peripheral neuropathies. J Peripher Nerv Syst. 2002;5:59–74. doi: 10.1046/j.1529-8027.2000.00006.x. [DOI] [PubMed] [Google Scholar]
- Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or TrkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- Guerios SD, Wang Z-Y, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett. 2006;392:193–197. doi: 10.1016/j.neulet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Hammond DL. J.J. Bonica Lecture--2001: role of spinal GABA in acute and persistent nociception. Reg Anesth Pain Med. 2001;26:551–557. doi: 10.1053/rapm.2001.27835. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABAmediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network driven, bicarbonate-dependent K-transient. J Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Coulter DA. Experimental status epilepticus alters gamma aminobutyric acid type A receptor function in CA1 pyramidal neurons. Ann Neurol. 1995;38:893–900. doi: 10.1002/ana.410380609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Merighi A, Carmignoto G, Gobbo S, Lossi L, Salio C, Vergnano AM, Zonta M. Neurotrophins in spinal cord nociceptive pathways. Prog Brain Res. 2004;146:291–321. doi: 10.1016/s0079-6123(03)46019-6. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier AJ. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Miletic G, Pankratz MT, Miletic V. Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany neuropathic pain following chronic constriction injury in rats. Pain. 2002;99:493–500. doi: 10.1016/S0304-3959(02)00242-7. [DOI] [PubMed] [Google Scholar]
- Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003;105:347–353. doi: 10.1016/s0304-3959(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Miletic G, Miyabe T, Gebhardt KJ, Miletic V. Increased levels of Homer1b/c and Shank1a in the post-synaptic density of spinal dorsal horn neurons are associated with neuropathic pain in rats. Neurosci Lett. 2005;386:189–193. doi: 10.1016/j.neulet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Miyabe T, Miletic G, Miletic V. Early activation of cyclic AMP response element binding protein (CREB) following loose ligation of the sciatic nerve in rats. Thal Rel Sys. 2005;3:19–23. [Google Scholar]
- Nabekura J, Ueno T, Okabe A, Furuta A, Iwaki T, Shimizu-Okabe C, Fukuda A, Akaike N. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J Neurosci. 2002;22:4412–4417. doi: 10.1523/JNEUROSCI.22-11-04412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Sakai A, Nagano M, Umino M, Suzuki H. Expression changes of cation chloride cotransporters in the rat spinal cord following intraplantar formalin. Neurosci Res. 2006;56:435–440. doi: 10.1016/j.neures.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval - GABAergic control of synaptic plasticity. TINS. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SWN, Williams RJ, McMahon SB. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvilli A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl contransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, Shih A, Miletic G, Miletic V. Continual systemic infusion of lidocaine provides analgesia in an animal model of neuropathic pain. Pain. 2002;97:267–273. doi: 10.1016/S0304-3959(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Stein V, Nicol RA. GABA generates excitement. Neuron. 2003;37:375–378. doi: 10.1016/s0896-6273(03)00056-4. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Obrietan K, Chen G. Excitatory actions of GABA after neuronal trauma. J Neurosci. 1996;16:4283–4292. doi: 10.1523/JNEUROSCI.16-13-04283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Hiroshi Wachi H, Yoshiyuki Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93:584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]