Abstract
Previous work has established that activation of Mos, Mek, and p42 mitogen-activated protein (MAP) kinase can trigger release from G2-phase arrest in Xenopus oocytes and oocyte extracts and can cause Xenopus embryos and extracts to arrest in mitosis. Herein we have found that activation of the MAP kinase cascade can also bring about an interphase arrest in cycling extracts. Activation of the cascade early in the cycle was found to bring about the interphase arrest, which was characterized by an intact nuclear envelope, partially condensed chromatin, and interphase levels of H1 kinase activity, whereas activation of the cascade just before mitosis brought about the mitotic arrest, with a dissolved nuclear envelope, condensed chromatin, and high levels of H1 kinase activity. Early MAP kinase activation did not interfere significantly with DNA replication, cyclin synthesis, or association of cyclins with Cdc2, but it did prevent hyperphosphorylation of Cdc25 and Wee1 and activation of Cdc2/cyclin complexes. Thus, the extracts were arrested in a G2-like state, unable to activate Cdc2/cyclin complexes. The MAP kinase-induced G2 arrest appeared not to be related to the DNA replication checkpoint and not to be mediated through inhibition of Cdk2/cyclin E; evidently a novel mechanism underlies this arrest. Finally, we found that by delaying the inactivation of MAP kinase during release of a cytostatic factor-arrested extract from its arrest state, we could delay the subsequent entry into mitosis. This finding suggests that it is the persistence of activated MAP kinase after fertilization that allows the occurrence of a G2-phase during the first mitotic cell cycle.
INTRODUCTION
Immature, stage VI Xenopus oocytes are naturally arrested in a G2-like state. They contain sufficient levels of Cdc2 and B-type cyclins to bring about mitosis, but the Cdc2/cyclin complexes remain inactive due to inhibitory phosphorylations on Cdc2. Exposure to progesterone brings about the dephosphorylation and activation of the Cdc2/cyclin B complexes and releases the oocyte from the G2-like arrest into M-phase.
The mitogen-activated protein (MAP) kinase cascade, best known for its involvement in mitogenesis and cell fate induction (Blumer and Johnson, 1994; Cobb et al., 1994; Marshall, 1994; Waskiewicz and Cooper, 1995; Ferrell, 1996), is also involved in oocyte maturation (reviewed by Sagata, 1997). Maturation depends upon the synthesis of the Mos protein (a MAP kinase kinase kinase) and the consequent activation of Mek1 (a MAP kinase kinase) and p42 MAP kinase. Interfering with MAP kinase activation by injection of antisense Mos oligonucleotides (Sagata et al., 1988), Mek antibodies (Kosako et al., 1994, 1996), or MAP kinase phosphatase (Gotoh et al., 1995) delays Cdc2 activation and maturation, indicating that activation of the MAP kinase cascade is necessary for release of the oocyte from its G2-like arrest. In addition, microinjection of Mos protein (Yew et al., 1992), constitutively active Mek1 (Huang et al., 1995), or active thiophosphorylated MAP kinase (Haccard et al., 1995) can bring about Cdc2 activation and maturation, showing that activation of the cascade is sufficient to release the oocyte from G2 arrest. Studies in Xenopus extracts have yielded similar conclusions (Huang and Ferrell, 1996a).
The activity of MAP kinase remains high during interkinesis, the brief period between the inactivation of Cdc2 at the onset of metaphase I and its reactivation during anaphase I. Artificial inactivation of the MAP kinase cascade during interkinesis delays the reactivation of Cdc2 and permits DNA replication to occur (Furuno et al., 1994). Thus MAP kinase activation appears to promote Cdc2 activation before both of the meiotic divisions.
The MAP kinase cascade also plays an important role in maintaining the activity of Cdc2 in metaphase-arrested mature oocytes and unfertilized eggs. Mos and thiophosphorylated MAP kinase score as cytostatic factors (CSF; Masui and Markert, 1971; Masui, 1974) in the cleaving blastomere assay, indicating they have the potential to arrest cell cycles in M-phase (Sagata et al., 1989; Freeman et al., 1990; Yew et al., 1992; Haccard et al., 1993). Immunodepleting Mos from egg extracts removes the extracts’ cytostatic activity (Sagata et al., 1989), and adding MAP kinase phosphatase to CSF-arrested extracts causes the extracts to degrade cyclins and enter interphase (Minshull et al., 1994). Furthermore, the oocytes from Mos knock-out mice fail to arrest properly in meiosis II (Colledge et al., 1994; Hashimoto et al., 1994). MAP kinase activity is also required to maintain a mitotic arrest in cells with defective spindles; that is, MAP kinase is essential for the spindle assembly checkpoint (Minshull et al., 1994; Takenaka et al., 1997; Wang et al., 1997). M-phase arrest is not, however, the inevitable consequence of MAP kinase activation. Oocytes progress through meiosis I in the face of fully active MAP kinase (Ferrell et al., 1991; Gabrielli et al., 1993; Fukasawa et al., 1994; Furuno et al., 1994), and fertilization-induced destruction of cyclins B1 and B2 occurs before any detectable inactivation of p42 MAP kinase (Ferrell et al., 1991; Watanabe et al., 1991).
We were interested in exploring how MAP kinase exerts its M-phase arresting effects. We began these studies by examining the responses of cycling Xenopus egg extracts or calcium-treated CSF-arrested extracts to activators of the MAP kinase cascade. Extracts are a particularly attractive system for such studies for several reasons. The cell cycle stage of extracts can be assessed conveniently by monitoring the morphology of nuclei formed from demembranated sperm chromatin (Lohka and Maller, 1983; Murray and Kirschner, 1989; Murray, 1991). Moreover, extracts permit a greater range of experimental manipulation than do intact oocytes, eggs, and embryos.
We found that when recombinant Mos (Yew et al., 1992) or constitutively active Mek R4F (Mansour et al., 1994, 1996) was added to extracts just before M-phase, the extracts arrested in M-phase with high Cdc2 activity, as expected. However, we unexpectedly found that if Mos or Mek were added earlier, they brought about an interphase arrest rather than an M-phase arrest.
Herein we have characterized this arrest state and found it to be essentially a G2 arrest, with mitotic cyclins present and complexed to Cdc2 but with the Cdc2 inactive due to inactivating phosphorylation. These findings demonstrate that MAP kinase activation can cause the cell cycle to arrest at two distinct points, G2-phase and M-phase, depending upon the time at which the activation occurs. Moreover, MAP kinase activation can have opposite consequences in two fairly similar contexts—it can promote G2 arrest in cycling extracts and release from G2 arrest in oocytes and oocyte extracts.
We also artificially delayed MAP kinase inactivation in calcium-treated CSF-arrested extracts by supplementing the extracts with recombinant MAP kinase. We found that the delay in MAP kinase inactivation had no effect on the rate of calcium-induced Cdc2 inactivation but did delay the onset of the first mitotic M-phase. This finding suggests that it is the persistence of activated MAP kinase after fertilization that allows the occurrence of a G2-phase during the first mitotic cell cycle.
MATERIALS AND METHODS
Recombinant Protein Production and Purification
Plasmids containing cDNAs for active and inactive (K90R) Xenopus Mos as maltose binding protein (malE) fusions were provided by George Vande Woude (Frederick Cancer Research and Development Center, Frederick, MD). The proteins were expressed in bacteria and purified as described (Yew et al., 1992).
A plasmid containing the cDNA for a constitutively active version of human Mek1 (Mek R4F) was provided Natalie Ahn (University of Colorado, Boulder, CO) (Ser-218 is changed to Glu, Ser-222 is changed to Asp and amino acids 32–51 are deleted; Mansour et al., 1994, 1996). The hexahistidine-tagged protein was expressed in bacteria, immobilized on Ni+ resin, and purified as described (Wang et al., 1997).
A cDNA for a mutated form of Xenopus p42 MAP kinase containing the mutation found in Sevenmaker, an activated form of the Drosophila Rolled MAP kinase (Brunner et al., 1994), was constructed by Mike Sohaskey (Stanford University) by changing Asp-324 to Asn with overlap polymerase chain reaction techniques (Umbhauer et al., 1995). By analogy, we refer to this protein as “Froggymaker” (Fm). The primer used to change amino acid 324 was 5′-GACCCAAGTAATGAGCCTGTAGCTGACGC-3′ and the two flanking primers were 5′-ATAAGGTGCCATGGAACCGAC-3′ and 5′-TCTAGAGTCGACCTGCAGCCC-3′. The polymerase chain reaction product was digested with NcoI and PstI and subcloned into pT7–7-Xp42. After expression in bacteria, Fm MAP kinase was immobilized on Ni+ resin, eluted with imidizole, concentrated with a Centricon 30 column, and dialyzed against extract buffer [XB: 100 mM KCl, 10 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES), 1 mM MgCl2, 0.1 mM CaCl2, 50 mM sucrose, pH 7.7].
Fusions of glutathione S-transferase (GST) to Xenopus Cdk2 and Xenopus cyclin E were expressed in bacteria and purified as previously described (Guadagno and Newport, 1996).
Extract Preparation and Staining
Cycling Xenopus egg extracts and CSF-arrested egg extracts were prepared as described (Murray and Kirschner, 1989; Murray, 1991). Demembranated sperm chromatin was prepared essentially as described (Smythe and Newport, 1991). Unless noted otherwise, chromatin was added to extracts at a concentration of 500 sperm/μl to allow monitoring of cell cycle progression. To assess the morphology of nuclei reconstituted in extracts, samples were fixed with 11% formaldehyde, stained with 4,6-diamidino-2-phenylindole (DAPI; 1 μg/ml), and viewed by phase-contrast and epifluorescence microscopy with a Zeiss Axioscop.
Antibodies
Antisera DC3 and X15 were both raised against a C-terminal 12 amino acid peptide from Xenopus p42 MAP kinase (Hsiao et al., 1994). DC3 was used for p42 MAP kinase immunoblots and X15 was used for immune complex kinase assays. A polyclonal anti-phosphotyrosine antiserum was provided by G. Schieven and G. S. Martin (Ferrell et al., 1991). Anti-Wee1 and anti-Cdc25 antisera were obtained from S. Guadagno (Zymed Laboratories, South San Francisco, CA). The Wee1 antiserum was raised against a C-terminal 16-amino acid peptide from Xenopus Wee1 (VGAKNTRSLSFTCGGY) conjugated to keyhole limpet hemacyanin. The Cdc25 antiserum was raised against purified bacterially expressed hexahistidine-tagged Xenopus Cdc25C and was affinity-purified on a Cdc25C column. A Cdc2 monoclonal antibody (sc-54) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoblotting
Aliquots of egg extracts were lysed in SDS sample buffer and submitted to electrophoresis on 10.5% SDS polyacrylamide gels (acrylamide:bisacrylamide, 100:1). Proteins were transferred to an Immobilon P (Millipore, Bedford, MA) blotting membrane, which was then blocked with 3% milk (for analysis of Wee1, Cdc25, MAP kinase, and Cdc2) or 3% bovine serum albumin (for analysis of phosphotyrosine) in Tris(hydroxymethyl)aminomethane (Tris)-buffered saline (20 mM Tris, 150 mM NaCl, pH 7.6), and incubated with primary antibody (Wee1, 1:1000 dilution; Cdc25, 1 μg/ml; MAP kinase, 1:1000 dilution; Cdc2, 1 μg/ml; phosphotyrosine, 2 μg/ml) for 2 h. After washing, the blots were probed with either a secondary antibody for detection through chemiluminescence (MAP kinase) or with 125I-labeled protein A for radioactive detection (Wee1, Cdc25, Cdc2, and phosphotyrosine). Blots were stripped for reprobing by incubation with 2% SDS, 100 mM 2-mercaptoethanol, and 100 mM Tris-HCl, pH 7.4, at 70° for 40 min.
Kinase Assays
H1 kinase assays were performed essentially as described (Dunphy and Newport, 1989). Extract samples (2 μl) were frozen in 2 μl of maturation-promoting factor extraction buffer [EB: 80 mM β-glycerophosphate, 20 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 15 mM MgCl2, pH 7.3] on dry ice for subsequent histone H1 kinase assays. Frozen extracts were rapidly thawed in 96 μl EB, and 10 μl were removed, added to 10 μl of reaction mixture consisting of 0.4 mM ATP, 0.25 μCi/μl [γ-32P]ATP, 0.5 mg/ml histone H1, 20 μM cAMP-dependent protein kinase inhibitor, 25.4 mM HEPES-NaOH, pH 7.3, 6.4 mM EGTA, and 12.8 mM MgCl2, and incubated at 30°C for 10 min. The reactions were stopped by the addition of SDS sample buffer. Proteins were subjected to electrophoresis through 10% SDS polyacrylamide gels (acrylamide:bisacrylamide, 29:1), and transferred to an Immobilon P (Millipore) blotting membrane. Radiolabel in the histone H1 band was quantified on a Molecular Dynamics PhosphorImager and/or exposed to x-ray film.
Immunoprecipitations were performed for analysis of MAP kinase activity in various extracts. Extracts (2 μl) were added to 2 μl of EB and frozen in dry ice. Later, the extract was diluted 1:50, 1 μl of X15 (MAP kinase specific antibody) was added to 90% of the sample, and the mixture was incubated on ice for 1 h. The mixture was then added to 10 μl of washed protein A-agarose and incubated with rocking for 1 h at 4°C. After three washes in EB containing 0.1% Nonidet P-40 and one wash in detergent-free EB, 25 μl of reaction mixture were added containing 10 μM cAMP-dependent protein kinase inhibitor, 0.75 mM Na3VO4, 5 mM EGTA, 0.3 mg/ml myelin basic protein, 0.08 μC/μl [γ-32P]ATP, 0.2 mM ATP, 13 mM HEPES-NaOH, pH 7.3, 3.2 mM EGTA, and 6.4 mM MgCl2. The reaction was stopped, after incubation at 25°C for 11 min, with SDS sample buffer, and the proteins were separated on 12.5% SDS polyacrylamide gels (acrylamide:bisacrylamide, 29:1) and transferred to an Immobilon P (Millipore) blotting membrane. Quantitation was done using a Molecular Dynamics PhosphorImager.
DNA Replication Assays
Fifty microliters of cycling egg extract was supplemented with buffer, Mos (0.5–1.2 μM) or Mek1 (0.6–1 μM) and sperm chromatin. [α-32P]dCTP (10 μCi) was added and 9-μl aliquots were removed periodically. Samples were analyzed as previously described (Dasso and Newport, 1990) and quantitated on a Molecular Dynamics PhosphorImager.
Suc1 Precipitations
Cycling egg extracts were prepared and supplemented with Mos (0.5 μM), sperm chromatin, and 25 μCi of [35S]methionine. Aliquots (8 μl) were removed every 15 min and frozen in 8 μl of EB for later analysis. The extract was diluted 1:8.25, mixed with 8 μl of p13suc1 agarose, and incubated at 4°C for 1 h with rocking. After centrifugation and three washes with EB containing 0.1% Nonidet P-40, SDS sample buffer was added and the proteins were electrophoresed through 10% SDS polyacrylamide gels (acrylamide:bisacrylamide, 29:1), transferred to Immobilon P (Millipore) blotting membrane, and exposed to x-ray film.
RESULTS
Activation of MAP Kinase Can Bring about an M-Phase Arrest in Cycling Extracts
We set out to determine whether we could produce a mitotic arrest, such as is seen in unfertilized eggs or in early embryos injected with MAP kinase cascade activators, by artificially activating the cascade in cycling extracts. The first activator we employed was recombinant Mos, which is capable of rapidly activating Mek and p42 MAP kinase in extracts (Nebreda et al., 1993; Posada et al., 1993; Shibuya and Ruderman, 1993; VanRenterghem et al., 1994; Huang and Ferrell, 1996a,b; Jones and Smythe, 1996; Shibuya et al., 1996).
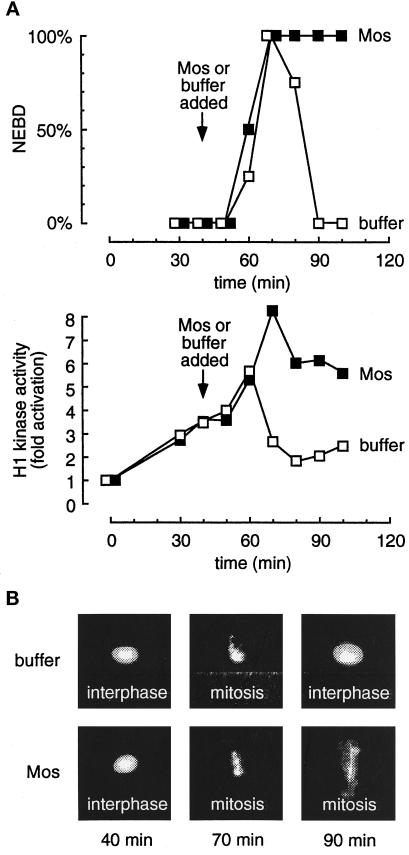
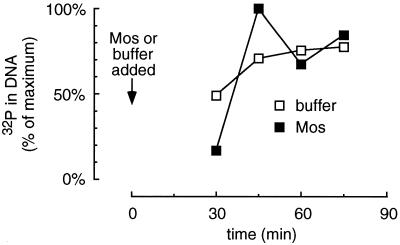
Figure 1 compares the behavior of a buffer-treated cycling extract with one treated with Mos just before M-phase. The extracts formed normal diffusely staining interphase nuclei by 40 min (Figure 1B). The buffer-treated extracts underwent mitosis at about 70 min; they underwent chromatin condensation (Figure 1B) and nuclear envelope breakdown (Figure 1, A, upper, and B). Mitosis was preceded by the transient activation of H1 kinase activity (Figure 1A, lower). Mos-treated (1 μM) extracts showed normal progression into mitosis (Figure 1B) with elevated H1 kinase activity (Figure 1A, lower) but, in contrast to the control extracts, remained arrested with condensed chromatin, dissolved nuclear envelopes, and high H1 kinase activity (Figure 1). The modest decrease in kinase activity seen at 80 min in the Mos-treated extracts may be due to destruction of A-type cyclins (Minshull et al., 1994). Addition of a constitutively active Mek (Mek R4F) just before M-phase also caused an M-phase arrest as judged by H1 kinase activity and nuclear morphology (our unpublished observations).
Figure 1.
Addition of Mos to a Xenopus cycling extract just before mitosis results in an M-phase arrest. Cycling extracts containing sperm chromatin were supplemented with Mos (▪) or buffer (□) 40 min after being placed at room temperature to initiate cycling. Aliquots were removed every 10 min for analysis. (A) The assembly and disassembly of the nuclear envelope was monitored by phase-contrast microscopy and expressed as percent nuclear envelope breakdown (NEBD; upper). In parallel, histone H1 kinase assays were performed and expressed as the fold increase over the activity at time 0 (lower). (B) Photographs of fixed nuclei stained with DAPI and observed by epifluorescence microscopy.
Thus, Mos caused the extracts to arrest in prometaphase or metaphase, as assessed by both morphological and biochemical criteria, indicating that the extracts recapitulate the behavior of embryos injected with Mos (Sagata et al., 1989; Yew et al., 1992) or activated p42 MAP kinase (Haccard et al., 1993). Similar findings have been reported by others (Abrieu et al., 1996).
Early Activation of MAP Kinase Can Result in an Interphase Arrest
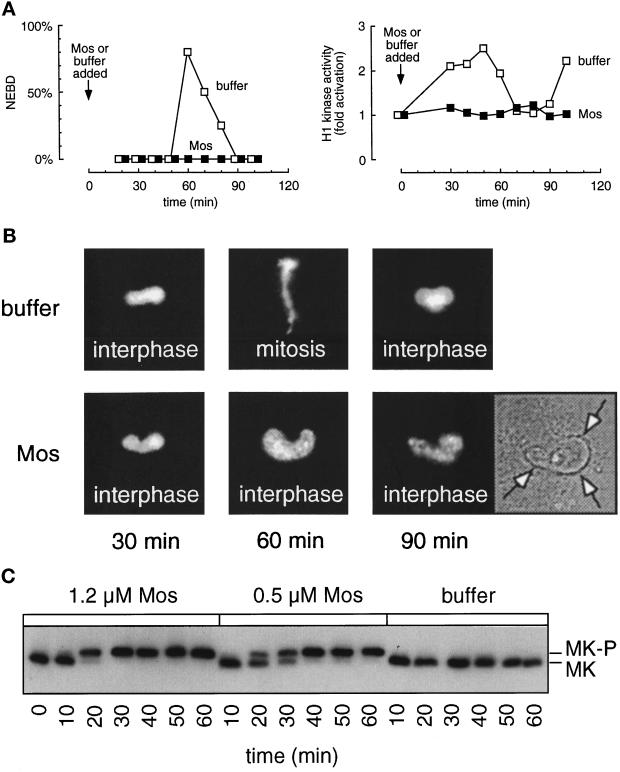
When Mos was added to the extracts earlier in the cell cycle, we observed an interphase arrest rather than an M-phase arrest. Mos brought about quantitative activation of the endogenous p42 MAP kinase, whereas MAP kinase was inactive or very transiently activated in the buffer-treated extracts (Figure 2C). Both the buffer-treated and Mos-treated extracts formed normal diffusely staining interphase nuclei by 30 min (Figure 2B). However, whereas nuclei in the buffer-treated extracts then underwent chromatin condensation and nuclear envelope breakdown by 60 min, the nuclei in Mos-treated extracts underwent only partial chromatin condensation with no nuclear envelope breakdown (Figure 2, A, left, and B). The nuclei remained in this interphase or early prophase state throughout the course of the experiment. Biochemical analysis further revealed that while the control extract displayed a normal peak of histone H1 kinase activity at mitosis, the Cdc2 activity in the Mos-treated extracts never increased past interphase levels (Figure 2A, right).
Figure 2.
Addition of Mos to a cycling extract early in interphase prevents Cdc2 activation and entry into mitosis. Cycling extracts containing sperm chromatin were supplemented with Mos (▪) or buffer (□) as soon as they were brought to room temperature (time 0) and aliquots were removed every 10 min for analysis. (A) Left, percent nuclear envelope breakdown as assessed by phase contrast microscopy. Right, histone H1 kinase activities from parallel samples. (B) Photographs of fixed nuclei stained with DAPI and observed by epifluorescence microscopy and phase-contrast microscopy. The arrows in the phase-contrast micrograph indicate the intact nuclear envelope. (C) MAP kinase immunoblots. MK, p42 MAP kinase; MK-P, phosphorylated p42 MAP kinase, which is detected by its higher apparent molecular mass.
To determine whether the block was due to the enzymatic activity of the added Mos protein, we performed the same experiment with an inactive version of Mos (Mos K90R). Mos K90R had no effect on the phosphorylation state of p42 MAP kinase (our unpublished observations). Mos K90R-treated extracts entered and exited mitosis, as judged by nuclear morphology, and Cdc2 was activated and inactivated normally (our unpublished observations). Therefore, the kinase activity of Mos is necessary for both the activation of MAP kinase and the resulting interphase arrest.
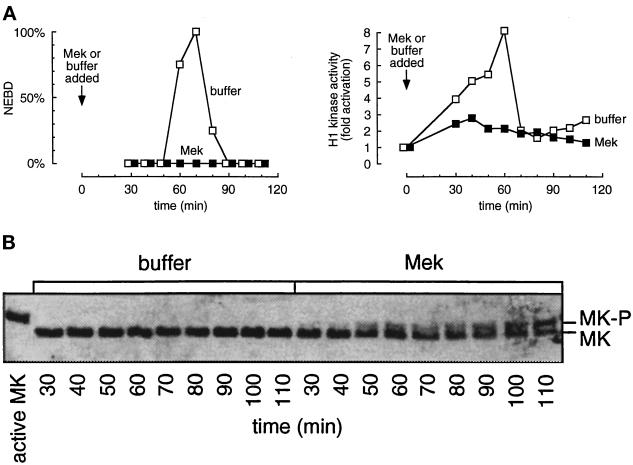
Similar results were obtained with extracts treated early with Mek R4F (Figure 3). Nuclear envelope breakdown did not occur (Figure 3A, left) and Cdc2 activity remained at interphase levels (Figure 3A, right). At the concentrations of Mek R4F that could be achieved (about 1 μM, compared with an endogenous Mek concentration of about 1.2 μM; Ferrell and Bhatt, 1997), we observed shifting of only about half of the p42 MAP kinase to the phosphorylated form as seen on the immunoblot in Figure 3B. However, this was sufficient to bring about an interphase arrest or to markedly delay entry into mitosis.
Figure 3.
Addition of constitutively active Mek to cycling extracts in early interphase activates MAP kinase and prevents Cdc2 activation. Cycling extracts containing sperm chromatin were supplemented with Mek (▪) or buffer (□) at time 0, and aliquots were removed every 10 min for analysis. (A) Left, percent nuclear envelope breakdown, monitored by phase-contrast microscopy. Right, H1 kinase activity. (B) MAP kinase immunoblots. Phosphorylated (shifted) MAP kinase is visible in the Mek-treated extracts but not in the buffer-treated extracts. The active MK lane shows fully active MAP kinase from a CSF-arrested extract. MK, p42 MAP kinase.
Thus, upstream activators of MAP kinase are able to prevent Cdc2 activation in the cycling extract system. As neither of these components have known targets other than members of the MAP kinase pathway, this inhibition was most likely due to the subsequent activation of MAP kinase.
The Timing of MAP Kinase Inactivation Correlates with the Timing of Cdc2 Activation
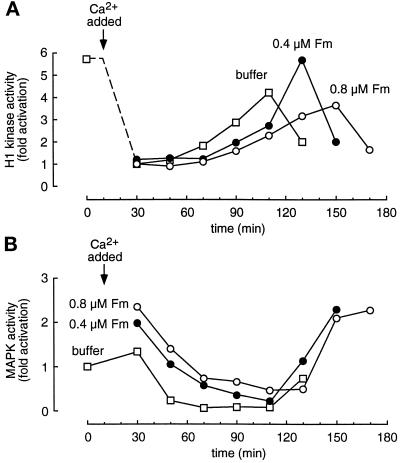
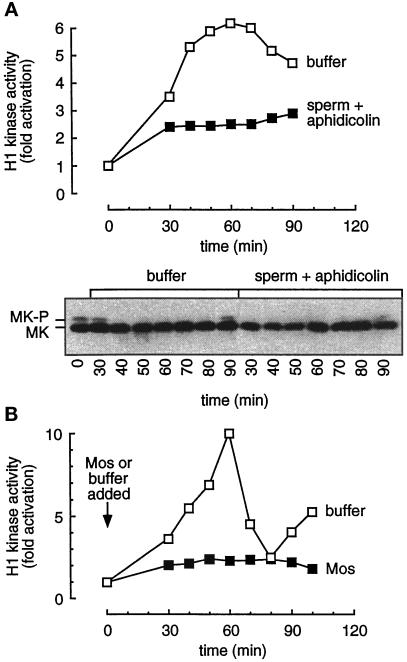
To further test the connection between MAP kinase activation and interphase arrest, we added excess recombinant MAP kinase protein to extracts to delay the inactivation of MAP kinase and assessed the consequences. We used a mutant Froggymaker (Fm) form of MAP kinase in which Asp-324 was changed to Arg. This mutation, analogous to that found in the gain of function Sevenmaker allele of the Drosophila Rolled MAP kinase, has been shown to render mammalian and Xenopus MAP kinases less susceptible to deactivating dephosphorylation (Bott et al., 1994; Umbhauer et al., 1995). We incubated recombinant Fm (0.4 and 0.8 μM, compared with an endogenous p42 MAP kinase concentration of about 0.3 μM; Ferrell and Bhatt, 1997) with CSF-arrested extracts, which have high Mos and Mek activities, for 10 min to allow phosphorylation and activation of the Fm. Calcium was then added to release the extracts into interphase. As shown in Figure 4A, calcium caused rapid inactivation of Cdc2, and the added Fm protein had no measurable effect on the rate of this inactivation.
Figure 4.
Addition of Fm MAP kinase can delay the onset of mitosis in activated CSF-arrested extracts. Extracts were supplemented with buffer (□), 0.4 μM Froggymaker MAP kinase (Fm, •), or 0.8 μM Fm (○) and incubated for 10 min before activation with 0.4 mM CaCl2. Aliquots were removed every 20 min for analysis. (A) Histone H1 kinase activity. (B) MAP kinase activity, assessed by an immune complex kinase assay with antiserum X15.
In the extract with no added Fm, the endogenous p42 MAP activity was essentially completely inactivated by the 50 min time point (Figure 4B). The extracts with added Fm showed higher initial levels of MAP kinase activity, and the inactivation of MAP kinase was slower and less complete (Figure 4B).
The delay in MAP kinase inactivation was accompanied by a delay in Cdc2 reactivation (Figure 4A). Cdc2 activity peaked at 110 min in the buffer-treated extracts, 130 min in the extracts treated with 0.4 μM Fm, and 150 min in the extracts treated with 0.8 μM Fm (Figure 4A). The delays observed in the inital inactivation of MAP kinase (Figure 4B) correlated with the delays in reactivation of Cdc2. These findings suggest that active MAP kinase is sufficient to cause a G2 arrest and that inactivation of the MAP kinase allows progression into M-phase.
Mos-treated Extracts Arrest in G2 Phase
We performed DNA replication assays on cycling extracts treated with Mos to determine whether the extracts were becoming arrested in S-phase or G2-phase. We added [α-32P]dCTP to Mos- or buffer-treated cycling extracts and assessed the incorporation of radiolabel into DNA after proteinase K treatment and agarose gel electrophoresis. As shown in Figure 5, there was little overall difference in DNA synthesis between Mos- and buffer-treated extracts. Similar results were obtained with Mek R4F-treated extracts (our unpublished observations). Thus Mos and Mek induce what is essentially a G2 arrest.
Figure 5.
DNA replication in Mos-treated extracts. Cycling extracts containing buffer (□) or Mos (▪) were supplemented with sperm chromatin and [α32P]dCTP to allow assessment of DNA replication. Samples were analyzed by agarose gel electrophoresis and autoradiography. Mos had no significant effect on the extent of DNA synthesis.
A- and B-Type Cyclins Accumulate Normally in Mek-treated Extracts
We set out to determine why Cdc2 is held inactive in the G2-arrested extracts. First we examined whether cyclin proteins were accumulating and associating with Cdc2 normally. We monitored the status of the cyclins in Mos-treated extracts by adding [35S]methionine, which became incorporated into newly synthesized proteins. Samples were taken periodically throughout the course of the experiment and subjected to precipitation with p13suc1 beads, which bind Cdc2, Cdk2, and their associated cyclins (Ducommun et al., 1991).
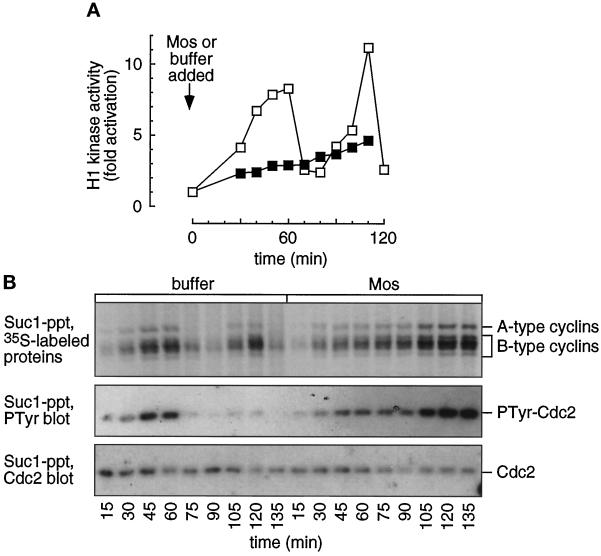
In the buffer-treated extracts, Cdk-associated 35S-labeled A- and B-type cyclin proteins underwent two cycles of accumulation and destruction (Figure 6B), accompanied by cycles of H1 kinase activation and inactivation (Figure 6A). The 35S-labeled cyclin A and B bands in the Mos-treated extracts progressively increased to levels almost twofold higher than the highest levels seen in buffer-treated extracts (Figure 6B, upper), but H1 kinase activity never reached mitotic levels (Figure 6A). The inability of Cdc2 to become activated is not, therefore, due to a scarcity of the cyclin subunit. Furthermore, this experiment demonstrates that the newly formed cyclins were able to form complexes with Cdc2, because p13suc1 beads precipitate only the Cdk-associated cyclins.
Figure 6.
Cyclins accumulate and associate with Cdc2 in MAP kinase-activated extracts. Cycling extracts were supplemented with sperm chromatin, [35S]methionine, and buffer (□) or Mos (▪). (A) Histone H1 kinase assays were performed on samples removed every 10 min. (B) Cyclin accumulation and Cdc2 tyrosine phosphorylation. Parallel samples were removed every 15 min and subjected to p13suc1 agarose precipitation and SDS-polyacrylamide gel electrophoresis. Suc1-precipitated 35S-labeled proteins were detected by autoradiography (upper). The cyclins exhibit an abrupt decrease in signal at mitosis (75 min) in the buffer-treated extracts while continuing to accumulate in the Mos-treated extracts. The lower half of the blot was probed with a phosphotyrosine antibody (middle), stripped, and reprobed with a Cdc2 antibody (lower). The level of phosphotyrosine increases over time in the Mos-treated extracts, and cycles in the buffer-treated extracts. Levels of cyclin, Cdc2, and Cdc2-PTyr were quantified by PhosphorImager scanning.
Wee1 and Cdc25 Remain Unphosphorylated in Mos-treated Extracts
The activity of Cdc2/cyclin complexes is regulated in part through phosphorylation of the Cdc2 moiety (reviewed by Morgan, 1995). Phosphorylation of either Thr-14 or Tyr-15 results in inactivation of the protein kinase; phosphorylation of Thr-161 is necessary for activation. In Xenopus egg extracts, Wee1 and Myt1 appear to be the kinases responsible for maintaining Cdc2 in an inactive form, and Cdc25 is the phosphatase that activates the kinase by removing those phosphate groups (Dunphy and Kumagai, 1991; Gautier et al., 1991; Kumagai and Dunphy, 1991; Mueller et al., 1995a,b).
Before mitosis, the activity of Wee1 is turned off and the activity of Cdc25 is turned on. This is believed to be accomplished in both cases through phosphorylation (Izumi et al., 1992; Kumagai and Dunphy, 1992; Mueller et al., 1995a). These modifications can be easily detected through changes in the proteins’ electrophoretic mobilities on immunoblots. We therefore used immunoblotting to examine whether Cdc25 and Wee1 were being properly regulated themselves in Mos- and Mek-treated extracts.
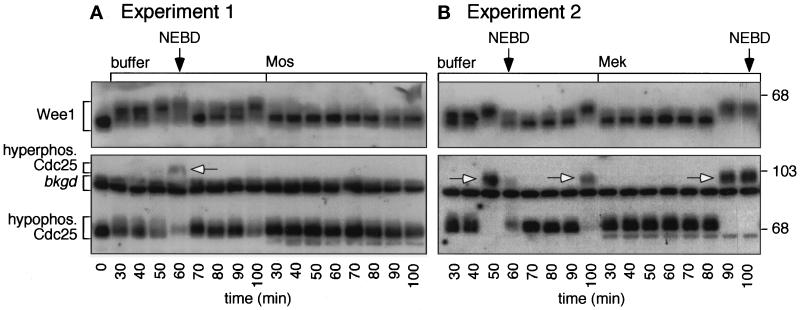
Figure 7 shows the results of two such experiments. In the first, extracts were treated with buffer or Mos, and the Mos-treated extracts exhibited a G2 arrest as assessed by nuclear morphology (Figure 7A). In the second, extracts were treated with buffer or Mek, and the Mek-treated extracts exhibited a mitotic delay rather than a G2 arrest (Figure 7B).
Figure 7.
Cdc25 and Wee1 phosphorylation in MAP kinase-activated extracts. Cycling extracts were prepared and supplemented with sperm chromatin and Mos, Mek, or buffer. Samples were removed every 10 min for analysis. (A and B) Immunoblots of Cdc25 and Wee1. Top, autoradiograms show the periodic accumulation of hyperphosphorylated Wee1 in the buffer-treated samples (left), with the most highly shifted forms peaking at nuclear envelope breakdown (NEBD). In addition, at mitosis the intensity of the hypophosphorylated Cdc25 band is reduced (lower), and some hyperphosphorylated Cdc25 is detectable (arrows). Some hyperphosphorylated Cdc25 may be obscured by the indicated background band. There is no change in electrophoretic mobility of either Wee1 or Cdc25 in the Mos- or Mek-treated extracts (right); both proteins remain in their hypophosphorylated interphase forms.
In both experiments, the buffer-treated cycling extracts exhibited cycles of Wee1 and Cdc25 hyperphosphorylation and dephosphorylation. Wee1 and Cdc25 became hyperphosphorylated before mitosis, dephosphorylated during mitosis, and hyperphosphorylated again during the next cycle (Figure 7, left sides of A and B). In contrast, in the Mos-treated extracts Wee1 and Cdc25 showed no differences in phosphorylation state throughout the time course (Figure 7A, right side). In the Mek-treated extracts, Wee1 and Cdc25 exhibited a delay in hyperphosphorylation commensurate with the observed delay in mitotic entry (Figure 7B, right side). Thus, Mos and Mek prevented or delayed the hyperphosphorylation (and, presumably, the activation) of Cdc25 and prevented or delayed the hyperphosphorylation (and, presumably, the inactivation) of Wee1. We infer that Cdc2 was kept in an inactive state due to the combination of low Cdc25 activity and high Wee1 activity.
To confirm and extend this result, we examined the state of Cdc2’s tyrosine phosphorylation in p13suc1 precipitations. As shown in Figure 6B, middle, the level of Cdc2 tyrosine phosphorylation in the Mos-treated extracts steadily increased to about 2.5 times the maximal levels seen in premitotic buffer-treated extracts. The variation in phosphotyrosine signal is not due to differences in protein levels, as evidenced by reprobing the same blot with Cdc2 antibody (Figure 6B, lower). Therefore, Mos prevents Cdc2 activation, at least in part, through the maintenance of Cdc2 tyrosine phosphorylation.
Relationship of the MAP Kinase Interphase Arrest to the DNA Replication Checkpoint
Dasso and Newport (1990) demonstrated that unreplicated DNA can produce a premitotic block in cycling egg extracts. They showed that the addition of aphidicolin to extracts containing sperm chromatin at concentrations of greater than 250 sperm/μl prevented DNA replication and resulted in an arrest. These S-phase arrested extracts were similar to the Mos-treated G2-arrested extracts seen here, in that both types of extracts possessed mitotic levels of cyclin proteins and tyrosine-phosphorylated, inactive Cdc2 (Figures 6 and 7; Dasso and Newport, 1990). We hypothesized that the Mos-induced G2 arrest we observed might be related to this DNA replication checkpoint. For example, unreplicated DNA could bring about MAP kinase activation, which then prevents entry into M-phase, or MAP kinase activation could subtly perturb replication in a way that triggers the DNA replication checkpoint.
We first determined whether p42 MAP kinase was phosphorylated in response to the treatment of extracts with aphidicolin and sperm nuclei. Such treatment did prevent Cdc2 activation (Figure 8A, upper), in agreement with Dasso and Newport (1990), but did not bring about MAP kinase phosphorylation as assessed by immunoblotting (Figure 8A, lower). Thus it appears that p42 MAP kinase does not mediate the DNA replication checkpoint.
Figure 8.
Mos-induced interphase arrest appears unrelated to the DNA replication checkpoint. (A) Upper, H1 kinase activity in extracts supplemented with buffer (□) or aphidicolin (0.4 mg/ml) and 2000 sperm/μl (▪). Lower, immunoblot of parallel samples probed with a MAP kinase-specific antibody (DC3). Note the absence of shifted MAP kinase in the aphidicolin-treated samples. (B) The presence of DNA is not required for the Mos-induced arrest. Cdc2 kinase activity in cycling extracts supplemented with buffer (□) or Mos (▪) and no added sperm chromatin.
Secondly, we determined whether the Mos-induced arrest required the presence of added sperm chromatin. We added either buffer or Mos to cycling extracts and assayed the resulting H1 activity. Figure 8B shows that Mos can prevent Cdc2 activation in the absence of added sperm; the arrest is therefore not DNA-dependent. Thus there appears to be no obvious connection between the Mos-induced arrest and the DNA replication checkpoint.
Added Cdk2/Cyclin E Does Not Block the Arrest
It has been previously demonstrated that Cdk2 activity is essential for progression into M-phase in cycling extracts (Guadagno and Newport, 1996). Extracts where Cdk2 has been inactivated by addition of the stoichiometric inhibitor p21 or by immunodepletion of Cdk2 arrest in G2-phase with mitotic levels of cyclins, tyrosine-phosphorylated Cdc2, and hypophosphorylated Cdc25 (Guadagno and Newport, 1996), just as do Mos-treated extracts. Therefore, we set out to determine whether Cdk2 was inhibited in the MAP kinase-activated extracts and thus responsible for the arrest.
Cdk2 was immunoprecipitated from extracts treated with buffer or Mos, and histone H1 kinase assays were performed to compare relative kinase activities. We consistently found a slight reduction in detectable kinase activity in the Mos-treated arrested extracts (Figure 9, left). Although the amount of decrease was quite small (and in the experiment shown, was within experimental error), it was nevertheless possible that this degree of inhibition was sufficient to effect a G2 arrest.
Figure 9.
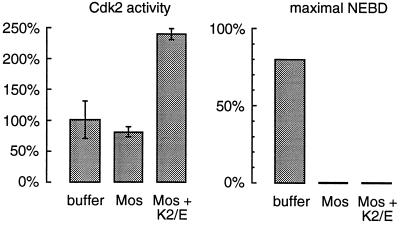
Mos-induced interphase arrest is not due to inhibiton of Cdk2/cyclin E. Cycling extracts containing sperm chromatin were supplemented with buffer, Mos, or Mos plus recombinant Cdk2/cyclin E proteins. Left, Cdk2 kinase activity in the treated extracts at 90 min. Cdk2 complexes were immunoprecipitated with a Cdk2-specific antibody. The extracts with Mos alone showed at most a small reduction in activity while the extracts with additional Cdk2/cyclin E display an approximately 2.5-fold increase in measureable activity. Right, maximal percent nuclear envelope breakdown (NEBD) was scored in the same experiment through phase-contrast microscopy. The addition of excess Cdk2/cyclin E activity did not reverse the MAP kinase-induced G2-phase arrest as assessed by phase-contrast microscopy.
To address this possibility, we determined whether addition of exogenous Cdk2/cyclin E proteins to an extract would prevent Mos from bringing about a G2 arrest. We added buffer, Mos, or Mos plus bacterially expressed GST-Cdk2 and GST-cyclin E to cycling extracts and monitored the resulting Cdk2 activity and cell cycle progression. The added Cdk2/cyclin E increased the levels of Cdk2 activity to well above normal interphase or M-phase levels (Figure 9, left) but did not restore the ability to enter mitosis as determined by monitoring nuclear envelope breakdown (Figure 9, right). These data indicate that the modest degree of Cdk2 inhibition seen in Mos-treated extracts cannot be the sole cause of the Mos-induced G2-phase block.
DISCUSSION
We have shown that activation of the MAP kinase cascade by treatment of cycling extracts with Mos or Mek just before M-phase can bring about a mitotic arrest (Figure 1), as expected from studies of microinjected embryos. However, when extracts are treated with Mos or Mek earlier in the cycle, they arrest in a state that morphologically and biochemically resembles interphase or early prophase (Figures 2 and 3).
Extracts that arrest in interphase after Mos/Mek treatment have completed DNA replication (Figure 5) and possess mitotic levels of A- and B-type cyclins complexed with Cdc2 (Figure 6B), but the Cdc2/cyclin complexes are inactive due to tyrosine phosphorylation of Cdc2 (Figure 6B). In the arrest state, the Cdc2 activator Cdc25 is present in its hypophosphorylated (and presumably inactive) form, and the Cdc2 inhibitor Wee1 is present in its hypophosphorylated (and presumably active) form (Figure 7). It seems plausible that active MAP kinase is exerting an effect on Wee1, Cdc25, and/or the upstream regulators of these proteins.
The MAP kinase-induced interphase arrest is not due to interference with DNA replication; replication is only minimally affected (Figure 5), and the arrest occurs in extracts not supplemented with nuclei (Figure 8B). The arrest is not a recapitulation of the DNA replication checkpoint, because activating the checkpoint by addition of sperm and aphidicolin did not trigger MAP kinase activation (Figure 8A). Finally, the arrest is not mediated through inhibition of Cdk2/cyclin E, because supplementing extracts with Cdk2/cyclin E did not prevent the G2 arrest (Figure 9). It appears that activation of the MAP kinase cascade induces G2 arrest by a novel mechanism.
G2 Arrest versus Mitotic Arrest
On the basis of the experiments described herein, there appears to be a point in interphase that is critical for determining the downstream effects of MAP kinase activation. Should MAP kinase be activated before this time, Cdc2 will not become activated and the cycle will arrest in interphase. If this point is passed, the activation of MAP kinase will bring about an M-phase arrest instead. Note that MAP kinase activation can prevent Cdc2 activation but does not reverse Cdc2 activation once it has occurred. Evidently MAP kinase can stop the cell cycle at two distinct points but cannot make the cycle run backwards.
MAP kinase function is required to halt M-phase progression in cells with defective mitotic spindles, a conclusion initially inferred from studies of cycling extracts (Minshull et al., 1994; Takenaka et al., 1997) and recently extended to somatic cells (Wang et al., 1997). This spindle assembly checkpoint is probably analogous to the M-phase arrest seen here in cycling extracts supplemented with Mos or Mek just before mitosis. The G2 arrest seen here in extracts supplemented early with Mos or Mek may also have a counterpart in somatic cells. Vande Woude and colleagues (Fukasawa et al., 1994) found that when Swiss 3T3 cells are infected with virus containing the v-mos oncogene, two distinct phenotypes are obtained depending upon the amount of v-Mos protein that was expressed. Cells that contained low levels of v-Mos were often transformed, whereas cells that contained 10-fold higher amounts of v-Mos rounded up, detached from the monolayer, and resembled growth-arrested cells. Interestingly, these growth-arrested cells had interphase levels of histone H1 activity and also possessed partially condensed chromosomes—both characteristics of the Mos-induced interphase arrest we describe here—as well as high levels of MAP kinase activity (Fukasawa et al., 1994). Raf-1, like Mos, is a MAP kinase kinase kinase, and Lloyd et al. (1997) have recently reported that induction of Raf-1 causes primary Schwann cells to arrest in G1-phase. Likewise, sustained activation of the MAP kinase pathway causes differentiation of PC12 cells, a process that presumably includes some sort of interphase arrest (Cowley et al., 1994; Dikic et al., 1994; Traverse et al., 1994; Fukuda et al., 1995). Thus, activation of MAP kinase may promote an interphase arrest in both cycling egg extracts and somatic cells.
G2 Arrest versus Release from G2 Arrest
Our findings dramatically underscore the fact that activation of the MAP kinase cascade can have very different consequences in different contexts. Here we have found that activation of the cascade can prevent the tyrosine dephosphorylation and activation of Cdc2 in Xenopus egg extracts. However, in Xenopus oocytes and oocyte extracts, activation of the MAP kinase cascade has the opposite effect. Immature oocytes are naturally arrested in a G2-like state with Cdc2 inactivated by tyrosine phosphorylation, and activation of the MAP kinase cascade causes the tyrosine dephosphorylation and activation of Cdc2 and the subsequent release of these G2-arrested oocytes into meiotic M-phase (Yew et al., 1992; Kosako, et al., 1994; Gotoh et al., 1995; Haccard et al., 1995; Huang and Ferrell, 1996a). Evidently there is some difference between immature oocytes and eggs that causes opposite biochemical events to be triggered by the same initial stimulus. For example, perhaps MAP kinase can only arrest early G2-phase cells in interphase, and oocytes have already passed beyond that cell cycle point.
MAP Kinase Activity and the Establishment of a G2-Phase
In Xenopus embryos, the 2nd through 12th mitotic cycles are very rapid (25–30 min). The embryo does not need to grow between divisions, and the cycles consist of S- and M-phases without discernible G1- or G2-phases. Little, if any, tyrosine-phosphorylated Cdc2 can be detected during these cycles, consistent with the absence of a G2-phase, and little MAP kinase activation can be detected (Ferrell et al., 1991; Hartley et al., 1996).
The first mitotic cycle differs from the 2nd through 12th cycles in a number of important ways. The cycle is made up of not only an S-phase and an M-phase but also an interposed about 25-min G2-phase. The extra time provided by this G2-phase allows the egg’s pronucleus and the sperm’s pronucleus to migrate toward each other and fuse (Gerhart, 1980; Hausen and Riebesell, 1991). During this G2-phase, Cdc2 is complexed to cyclins but held inactive by tyrosine phosphorylation (Ferrell et al., 1991; Watanabe et al., 1991; Hartley et al., 1996). The first cycle also differs from the subsequent cycles in that it is preceded by an extended period where p42 MAP kinase is fully active—MAP kinase is active in the metaphase-arrested egg and remains active in the fertilized egg during the about 30 min while meoisis is completed and the first mitotic cycle begins (Ferrell et al., 1991; Watanabe et al., 1991).
The present studies suggest a connection between the activation of p42 MAP kinase before the first mitotic cell cycle and the presence of a G2-phase in that cycle. Here we have shown that MAP kinase activation can bring about a G2 arrest (Figures 2 and 3) and that delaying the timing of MAP inactivation causes a corresponding delay in the subsequent activation of Cdc2 (Figure 4). If it is assumed that it takes an egg or extract about 60–70 min to undo the effects of MAP kinase activation., then it would follow that the G2-phase present in the first mitotic cell cycle is simply the period after S-phase during which the effects of MAP kinase are being undone. Subsequent to the first M-phase, there is no need for a G2-phase, and so only low levels of MAP kinase activity and Cdc2 tyrosine phosphorylation are seen. It will be of interest to test these speculations further through various manipulations of MAP kinase activity in intact embryos.
Note added in proof. We have recently found that Cdk2 activity must be inhibited by more than 95% to achieve an interphase arrest (Guadagno and Ferrell, unpublished observations). This finding corroborates our conclusion that Cdk2 inhibition does not mediate the G2 arrest described here.
ACKNOWLEDGMENTS
We thank N. Ahn, M. Cobb, J. Cooper, T. Geppart, J. Posada, and G. Vande Woude for providing MAPK, Mek, and Mos encoding plasmids; Sarah Guadagno and our collaborators at Zymed for providing the Cdc25 and Wee1 antisera; G. Schieven and G.S. Martin for the anti-phosphotyrosine antisera; R.R. Bhatt for expressing and purifying the Mek R4F; Chi-Ying Huang for expressing and purifying malE-Mos; and M.L. Sohaskey for cloning Froggymaker and for helpful comments on this manuscript. This work was supported by a grant from the National Institutes of Health to J.E.F. (GM-46383) and a Bank of America Giannini Foundation Fellowship to T.M.G. J.E.F. is a Howard Hughes Junior Faculty Scholar.
REFERENCES
- Abrieu A, Lorca T, Labbe JC, Morin N, Keyse S, Doree M. MAP kinase does not inactivate, but rather prevents the cyclin degradation pathway from being turned on in Xenopus egg extracts. J Cell Sci. 1996;109:239–246. doi: 10.1242/jcs.109.1.239. [DOI] [PubMed] [Google Scholar]
- Blumer KJ, Johnson GL. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Bott CM, Thorneycroft SG, Marshall CJ. The sevenmaker gain-of-function mutation in p42 MAP kinase leads to enhanced signalling and reduced sensitivity to dual specificity phosphatase action. FEBS Lett. 1994;352:201–205. doi: 10.1016/0014-5793(94)00958-9. [DOI] [PubMed] [Google Scholar]
- Brunner D, Oellers N, Szabad J, Biggs WH, III, Zipursky SL, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Hepler JE, Cheng M, Robbins D. The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer Biol. 1994;5:261–268. [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994;370:65–68. doi: 10.1038/370065a0. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Dikic I, Schlessinger J, Lax I. PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr Biol. 1994;4:702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Ducommun B, Brambilla P, Draetta G. Mutations at sites involved in Suc1 binding inactivate Cdc2. Mol Cell Biol. 1991;11:6177–6184. doi: 10.1128/mcb.11.12.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Newport JW. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989;58:181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr MAP kinases in mitogenesis and development. Curr Top Dev Biol. 1996;33:1–60. doi: 10.1016/s0070-2153(08)60336-1. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Bhatt RR. Mechanistic studies of the dual phosphorylation of mitogen-activated protein kinase. J Biol Chem. 1997;272:19008–19016. doi: 10.1074/jbc.272.30.19008. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RS, Kanki JP, Ballantyne SM, Pickham KM, Donoghue DJ. Effects of the v-mos oncogene on Xenopus development: meiotic induction in oocytes and mitotic arrest in cleaving embryos. J Cell Biol. 1990;111:533–541. doi: 10.1083/jcb.111.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K, Murakami MS, Blair DG, Kuriyama R, Hunt T, Fischinger P, Vande Woude GF. Similarities between somatic cells overexpressing the mos oncogene and oocytes during meiotic interphase. Cell Growth Differ. 1994;5:1093–1103. [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Tachibana T, Dell K, Hattori S, Yoneda Y, Nishida E. Induction of neurite outgrowth by MAP kinase in PC12 cells. Oncogene. 1995;11:239–244. [PubMed] [Google Scholar]
- Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, Ogawa Y, Sagata N. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli BG, Roy LM, Maller JL. Requirement for Cdk2 in cytostatic factor-mediated metaphase II arrest. Science. 1993;259:1766–1769. doi: 10.1126/science.8456304. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gerhart JC. Biological Regulation and Development. Vol. 2. R.F. Goldberger, New York: Plenum Press; 1980. Mechanisms regulating pattern formation in the amphibian egg and early embryo; pp. 133–316. [Google Scholar]
- Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Guadagno TM, Newport JW. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Rempel RE, Maller JL. In vivo regulation of the early embryonic cell cycle in Xenopus. Dev Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice [published erratum appears in Nature, Aug 4, 1994;370(6488), 391] Nature. 1994;370:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- Hausen P, Riebesell M. The early development of Xenopus laevis: an atlas of the histology. Berlin: Springer-Verlag; 1991. [Google Scholar]
- Hsiao K-M, Chou S-y, Shih S-J, Ferrell JE., Jr Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc Natl Acad Sci USA. 1994;91:5480–5484. doi: 10.1073/pnas.91.12.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-YF, Ferrell JE., Jr Dependence of Mos-induced Cdc2 activation on MAP kinase function in a cell-free system. EMBO J. 1996a;15:2169–2173. [PMC free article] [PubMed] [Google Scholar]
- Huang C-YF, Ferrell JE., Jr Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1996b;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Smythe C. Activation of the Xenopus cyclin degradation machinery by full-length cyclin A. J Cell Sci. 1996;109:1071–1079. doi: 10.1242/jcs.109.5.1071. [DOI] [PubMed] [Google Scholar]
- Kosako H, Akamatsu Y, Tsurushita N, Lee KK, Gotoh Y, Nishida E. Isolation and characterization of neutralizing single-chain antibodies against Xenopus mitogen-activated protein kinase kinase from phage display libraries. Biochemistry. 1996;35:13212–13221. doi: 10.1021/bi960956f. [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J. 1994;13:2131–2138. doi: 10.1002/j.1460-2075.1994.tb06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Lloyd AC, Obermuller F, Staddon S, Barth CF, McMahon M, Land H. Cooperating oncogenes converge to regulate cyclin/cdk complexes. Genes Dev. 1997;11:663–677. doi: 10.1101/gad.11.5.663. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Maller JL. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1983;101:518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour SJ, Candia JM, Matsuura JE, Manning MC, Ahn NG. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochemistry. 1996;35:15529–15536. doi: 10.1021/bi961854s. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Masui Y. A cytostatic factor in amphibian oocytes: its extraction and partial characterization. J Exp Zool. 1974;187:141–147. doi: 10.1002/jez.1401870116. [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995a;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995b;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Hill C, Gomez N, Cohen P, Hunt T. The protein kinase mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett. 1993;333:183–187. doi: 10.1016/0014-5793(93)80401-f. [DOI] [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn NG, Vande Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N. What does Mos do in oocytes and somatic cells? BioEssays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Morris J, Rapp UR, Ruderman JV. Activation of the Xenopus oocyte mitogen-activated protein kinase pathway by Mos is independent of Raf. Cell Growth Differ. 1996;7:235–241. [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe C, Newport JW. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Gotoh Y, Nishida E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J Cell Biol. 1997;136:1091–1097. doi: 10.1083/jcb.136.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature. 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- VanRenterghem B, Browning MD, Maller JL. Regulation of mitogen-activated protein kinase activation by protein kinases A and C in a cell-free system. J Biol Chem. 1994;269:24666–24672. [PubMed] [Google Scholar]
- Wang XM, Zhai Y, Ferrell JE., Jr A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J Cell Biol. 1997;137:433–443. doi: 10.1083/jcb.137.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Hunt T, Ikawa Y, Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991;352:247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- Yew N, Mellini ML, Vande Woude GF. Meiotic initiation by the mos protein in Xenopus. Nature. 1992;355:649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]