Abstract
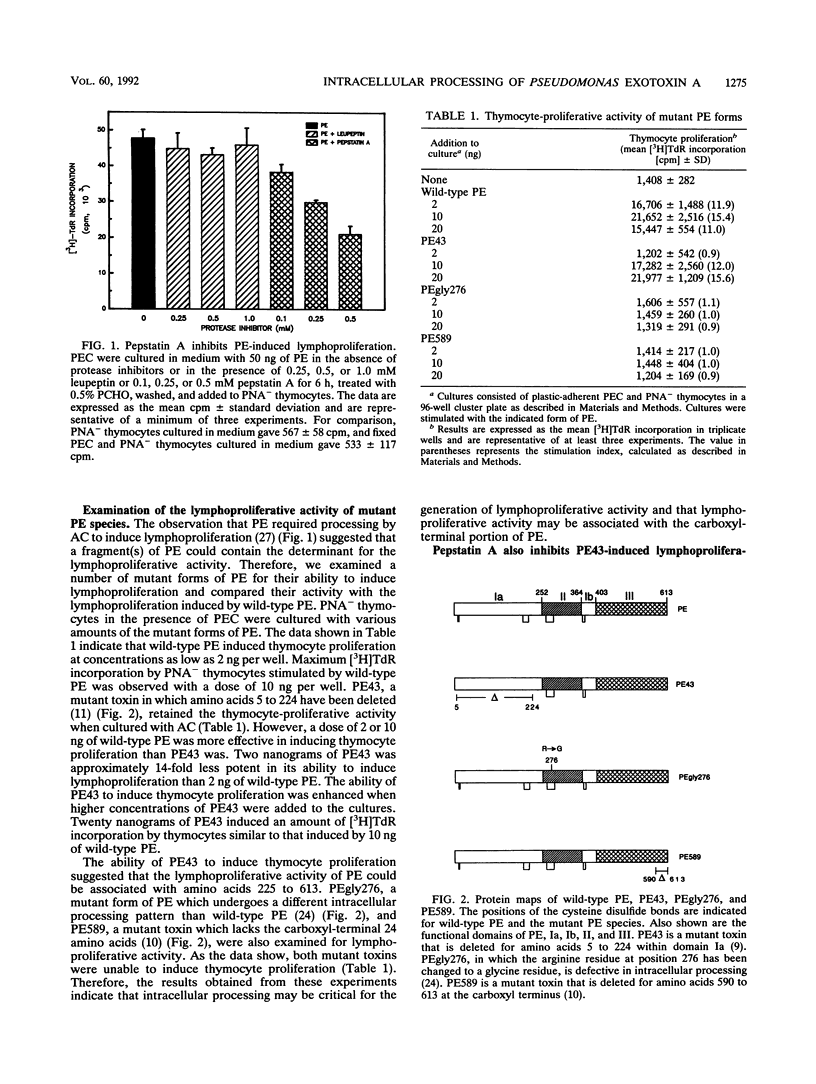
Pseudomonas aeruginosa exotoxin A (PE) represents a microbial superantigen that requires processing by accessory cells in order to induce the proliferation of V beta 8-bearing murine T lymphocytes. In this study, we have observed that PE requires intracellular processing by a protease in order to induce lymphoproliferation. Pepstatin A, an inhibitor of acid proteases, inhibited PE-induced lymphoproliferation, whereas leupeptin, an inhibitor of serine and thiol proteases, had no effect on PE-induced lymphoproliferation. A number of mutant forms of PE were examined for their ability to induce lymphoproliferation. The mutant form which lacks amino acids 5 to 224 of the receptor-binding domain, PE43, was capable of inducing murine thymocytes to proliferate in the presence of accessory cells. However, neither PEgly276, a mutant toxin which undergoes a different intracellular processing pattern than wild-type PE, nor PE589, a mutant toxin which lacks amino acids 590 to 613 at the carboxyl terminus, was able to induce thymocyte proliferation. In addition, the lymphoproliferation induced by the PE43 mutant form of PE could also be inhibited by pepstatin A. Therefore, our data indicate that intracellular processing by a proteolytic enzyme which is inhibited by pepstatin A is critical for PE-induced lymphoproliferation. Furthermore, the lymphoproliferative activity of PE is associated with the carboxyl-terminal portion of PE.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Dingle J. T. The inhibition of tissue acid proteinases by pepstatin. Biochem J. 1972 Apr;127(2):439–441. doi: 10.1042/bj1270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Rutenfranz I., Kirchner H. Processing requirements for T cell activation by Mycoplasma arthritidis-derived mitogen. Eur J Immunol. 1988 Dec;18(12):2109–2112. doi: 10.1002/eji.1830181239. [DOI] [PubMed] [Google Scholar]
- Buus S., Werdelin O. A group-specific inhibitor of lysosomal cysteine proteinases selectively inhibits both proteolytic degradation and presentation of the antigen dinitrophenyl-poly-L-lysine by guinea pig accessory cells to T cells. J Immunol. 1986 Jan;136(2):452–458. [PubMed] [Google Scholar]
- Callahan J. E., Herman A., Kappler J. W., Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990 Apr 1;144(7):2473–2479. [PubMed] [Google Scholar]
- Calvano S. E., Quimby F. W., Antonacci A. C., Reiser R. F., Bergdoll M. S., Dineen P. Analysis of the mitogenic effects of toxic shock toxin on human peripheral blood mononuclear cells in vitro. Clin Immunol Immunopathol. 1984 Oct;33(1):99–110. doi: 10.1016/0090-1229(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Chaudhary V. K., Jinno Y., FitzGerald D., Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Xu Y. H., FitzGerald D., Adhya S., Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli. Proc Natl Acad Sci U S A. 1988 May;85(9):2939–2943. doi: 10.1073/pnas.85.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diment S. Different roles for thiol and aspartyl proteases in antigen presentation of ovalbumin. J Immunol. 1990 Jul 15;145(2):417–422. [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Diment S., Martin K. J., Stahl P. D. Cleavage of parathyroid hormone in macrophage endosomes illustrates a novel pathway for intracellular processing of proteins. J Biol Chem. 1989 Aug 15;264(23):13403–13406. [PubMed] [Google Scholar]
- Diment S., Stahl P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J Biol Chem. 1985 Dec 5;260(28):15311–15317. [PubMed] [Google Scholar]
- FitzGerald D., Morris R. E., Saelinger C. B. Receptor-mediated internalization of Pseudomonas toxin by mouse fibroblasts. Cell. 1980 Oct;21(3):867–873. doi: 10.1016/0092-8674(80)90450-x. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: evidence that domain I functions in receptor binding. Mol Microbiol. 1987 Jul;1(1):67–72. doi: 10.1111/j.1365-2958.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Chaudhary V. K., Kondo T., Adhya S., FitzGerald D. J., Pastan I. Mutational analysis of domain I of Pseudomonas exotoxin. Mutations in domain I of Pseudomonas exotoxin which reduce cell binding and animal toxicity. J Biol Chem. 1988 Sep 15;263(26):13203–13207. [PubMed] [Google Scholar]
- Jinno Y., Ogata M., Chaudhary V. K., Willingham M. C., Adhya S., FitzGerald D., Pastan I. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989 Sep 25;264(27):15953–15959. [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- Legaard P. K., LeGrand R. D., Misfeldt M. L. The superantigen Pseudomonas exotoxin A requires additional functions from accessory cells for T lymphocyte proliferation. Cell Immunol. 1991 Jul;135(2):372–382. doi: 10.1016/0008-8749(91)90282-g. [DOI] [PubMed] [Google Scholar]
- Misfeldt M. L., Legaard P. K., Howell S. E., Fornella M. H., LeGrand R. D. Induction of interleukin-1 from murine peritoneal macrophages by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990 Apr;58(4):978–982. doi: 10.1128/iai.58.4.978-982.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Chaudhary V. K., Pastan I., FitzGerald D. J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990 Nov 25;265(33):20678–20685. [PubMed] [Google Scholar]
- Puri J., Factorovich Y. Selective inhibition of antigen presentation to cloned T cells by protease inhibitors. J Immunol. 1988 Nov 15;141(10):3313–3317. [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Roederer M., Bowser R., Murphy R. F. Kinetics and temperature dependence of exposure of endocytosed material to proteolytic enzymes and low pH: evidence for a maturation model for the formation of lysosomes. J Cell Physiol. 1987 May;131(2):200–209. doi: 10.1002/jcp.1041310209. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Cease K. B., Berzofsky J. A. Identification of proteases that process distinct epitopes on the same protein. J Immunol. 1989 Apr 1;142(7):2221–2229. [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Vroegop S. M., Buxser S. E. Cell surface molecules involved in early events in T-cell mitogenic stimulation by staphylococcal enterotoxins. Infect Immun. 1989 Jun;57(6):1816–1824. doi: 10.1128/iai.57.6.1816-1824.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Wileman T., Boshans R., Stahl P. Uptake and transport of mannosylated ligands by alveolar macrophages. Studies on ATP-dependent receptor-ligand dissociation. J Biol Chem. 1985 Jun 25;260(12):7387–7393. [PubMed] [Google Scholar]
- Yagi J., Baron J., Buxser S., Janeway C. A., Jr Bacterial proteins that mediate the association of a defined subset of T cell receptor:CD4 complexes with class II MHC. J Immunol. 1990 Feb 1;144(3):892–901. [PubMed] [Google Scholar]



