Abstract
ING4 is a candidate tumor suppressor that plays a major role in gene regulation, cell cycle control, apoptosis, and angiogenesis. ING4 expression is downregulated in glioblastoma cells, and head and neck squamous cell carcinoma. Here, we identified liprin α1/PPFIA1, a cytoplasmic protein necessary for focal adhesion formation and axon guidance, as a novel interacting protein with ING4. ING4 and liprin α1 colocalized at lamellipodia in the vicinity of vinculin. Overexpressed ING4 suppressed cell spreading and cell migration. In contrast, overexpressed liprin α1 enhanced cell spreading and cell migration. Knockdown of endogenous ING4 with RNA interference induced cell motility, whereas knockdown of endogenous liprin α1 suppressed cell motility. ING4 also suppressed cell motility that was enhanced by liprin α1. However, ING4 did not further suppress cell motility when liprin α1 was suppressed with RNA interference, suggesting a functional and mechanistic interdependence between these proteins. In addition to its nuclear functions, cytoplasmic ING4 interacts with liprin α1 to regulate cell migration, and with its known anti-angiogenic function, may prevent invasion and metastasis.
Keywords: Adaptor Proteins, Signal Transducing, biosynthesis, genetics, metabolism, Cell Cycle Proteins, biosynthesis, genetics, metabolism, Cell Line, Tumor, Cell Membrane, metabolism, Cell Movement, physiology, Colonic Neoplasms, genetics, metabolism, pathology, Glioma, genetics, metabolism, pathology, Homeodomain Proteins, biosynthesis, genetics, metabolism, Humans, Protein Binding, RNA, Small Interfering, genetics, Transfection, Tumor Suppressor Proteins, biosynthesis, genetics, metabolism
Introduction
Inhibitor of growth (ING) family is comprised of six members in humans, including ING1–5 and INGx (1, 2). The first ING gene (ING1) was identified by using a strategy for tumor suppressor gene isolation (3). ING4 and other family members were subsequently identified by a sequence homology search (4–7). As a candidate tumor suppressor, ING4 is associated with a loss of heterozygocity on chromosomes in head and neck cancers (8). The ING genes negatively regulate cell proliferation (2, 9, 10). ING4 binds to p53 and acetyltransferase p300, and thus, facilitates p53 acetylation by p300, especially on Lys382 (7). The ING4 protein also associates with the HBO1 HAT (histone acetyltransferase) complex (11) and interacts with the methylated lysine 4 of histone H3 (12, 13), suggesting a role in chromatin remodeling and transcription regulation.
ING4 overexpression induces apoptosis in cells (7). In addition, ING4 plays a major role in cellular responses to hypoxia, repressing HIF (hypoxia inducible factor) activation by interacting with HIF prolyl hydroxylase (14). ING4 regulates angiogenic growth induced by human glioblastoma cells in SCID mice, by interfering with the NF-κB pathway (15). ING4 was also reported to suppress loss of contact inhibition (16). ING4 overexpression in breast cancer cells showed the attenuation of anchorage-independent cell growth in soft agar (16).
The conversion of a cancer cell into an invasive metastatic type is a hallmark of tumor progression, as well as the deregulation of cell proliferation (17). During this switch, cancer cells lose control of several morphological features such as cell polarization, filopodia and lamellipodia formation, cell spreading, and cell migration (18), which are key regulating steps between the static and metastatic transition of a cancer cell. The morphological transition towards a migrating cell involves multiple regulatory components at different steps (19). Elucidating the regulatory networks among the known tumor suppressors and identifying new candidate tumor suppressor genes may provide valuable therapeutic strategies for preventing tumor progression and metastasis.
To reveal the molecular basis of ING4 function, we examined ING4-interacting proteins by a protein pull-down assay. We identified several ING4-associated proteins. We show here that ING4 interacts and colocalizes in lamellipodia with liprin α1/PPFIA1 [protein tyrosine phosphatase, receptor type f polypeptide (PTPRF), interacting protein (liprin), alpha 1]. Liprin α1 was identified as a binding protein of leukocyte common antigen-related (LAR) family receptor tyrosine phosphatases and colocalized with LAR at focal adhesions (LAR-RPTP) (20). Liprin α1 is required for the trafficking of synaptic vesicles (21) and is involved in the development and maintenance of excitatory synapses (22). We further show that ING4 overexpression leads to the suppression of cell spreading and cell migration, and liprin α1 is required for these functions. We propose that ING4 regulates cell motility by interacting with liprin α1.
Materials and Methods
Cell lines and culture
RKO, HEK-293, and U-87 MG cells were purchased from American Type Culture Collection (ATCC; Rockville, MD) and cultured under recommended conditions. Transient transfections of plasmids were performed with Lipofectamine and Plus reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA).
Plasmid construction
pFLAG-CMV2-ING4, pFLAG-CMV6-ING2 and pFLAG-CMV2-ING5 were constructed previously (7, 24). The liprin α1 expression plasmid pMT2-myc-liprin α1 was a generous gift from Dr. Morgan Sheng (Massachusetts Institute of Technology, Cambridge, MA).
Antibodies and reagents
FLAG M2 mouse monoclonal antibody, rabbit anti-FLAG polyclonal antibody, and mouse anti-vinculin monoclonal antibody were purchased from Sigma-Aldrich (St. Louis, MO). Chicken anti-liprin α1 polyclonal antibody was purchased from Genway (San Diego, CA) and mouse anti-myc monoclonal antibody from Invitrogen. Fluorophor-conjugated secondary antibodies for immunofluorescence were purchased from Jackson ImmunoResearch Laboratory (West Grove, PA). Alexa Fluor 488 or rhodamine phalloidin was from Molecular Probes (Eugene, OR). Rabbit anti-ING4 polyclonal antibody was created as described previously (7), and mouse anti-ING4 monoclonal antibody was obtained from BD Biosciences (San Jose, CA).
Protein pull-down assay
RKO cells were transfected with FLAG-ING4 plasmid. After 48 h transfection, cells were harvested, resuspended in lysis buffer A (20 mM HEPES, pH 7.9, 20% glycerol, 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% NP-40, 1 mM DTT, and protease inhibitor cocktail, Calbiochem, La Jolla, CA), kept on ice for 10 min, and centrifuged at 500g for 5 min at 4°C. The supernatant, which contains mostly cytoplasmic extracts, was saved for future experiments. The precipitate that contains perinuclear and nuclear extracts was resuspended in lysis buffer B (same as lysis buffer A but with 500 mM NaCl), kept on ice for 30 min, and centrifuged at 13,000 × g for 15 min at 4°C. The supernatant was incubated with FLAG M2-conjugated resins (Sigma-Aldrich) for 4 h at 4°C (salt concentration is 250 mM), centrifuged, and washed three times with wash buffer 1 (20 mM HEPES, pH 7.9, 150 mM KCl, 0.2 mM EDTA, 0.2 mM EGTA, 0.1% NP-40, 10% glycerol, 1 mM DTT, and the protease inhibitor cocktail), followed by three times with wash buffer 2 (same as wash buffer 1 but with 300 mM KCl). The pull-down proteins were eluted by elution buffer (20 mM Tris-HCl, pH 8.0, 150 mM KCl, 1 mM DTT, and 5% glycerol) containing 0.2 mg/ml of FLAG peptide at 4°C for 4 h.
Coimmunoprecipitation
RKO cells transfected with FLAG-ING4 expression plasmids were harvested after 48 h transfection, resuspended in extraction buffer A (50 mM Tris-HCl, pH 8.0, 500 mM KCl, 20% glycerol, 0.1% NP-40, 1 mM DTT, and the protease inhibitor cocktail), subjected to three rounds of freeze-thawing (liquid N2, 37°C), and centrifuged at 13,000 × g for 10 min at 4°C. The cellular extracts (400 μg) were immunoprecipitated with FLAG M2 resin (salt concentration is 266 mM) for 4 h at 4°C, centrifuged and washed with extraction buffer A four times.
Subcellular fractionation
RKO cells transfected with FLAG-ING4 plasmid were grown for 48 h before being harvested. Cytoplasmic and nuclear fractions were separated by NE-PER kit (Pierce, Rockford, IL).
Immunofluorescence microscopy
Cells were plated on glass coverslips at 4 × 104/cm2 and cultured for designated incubation times with designated cell treatments. Cells were fixed in 4% paraformaldehyde, permeated by 0.1% Triton X-100, and blocked by 1% BSA in PBS. Cells were incubated with a primary antibody at room temperature for 1 h and then treated with fluorophor-conjugated secondary antibody for another hour at room temperature. The cells were examined by fluorescence microscopy (Axioplan 2 imaging: Carl Zeiss, Thornwood, NY).
siRNAs
Small interference RNA (siRNA) targeting ING4 and liprin α1 transcripts were designed and synthesized by QIAGEN (Valencia, CA). siRNA sequences for ING4 were as follows: ING4 siRNA-1 (5′–TCCCTTTGAATTACAGAGAAA-3′), ING4 siRNA-2 (5′-ATGCCTGTGGATCCCAACGAA-3′), ING4 siRNA-3 (5′-GAGGCTGATCTCAAGGAGAAA-3′), and ING4 siRNA-4 (5′-AAGGAGAAACAGATTGAGTCA-3′). siRNA sequences for liprin α1 were as follows: liprin α1 siRNA-1 (5′-CACGAGGTTGGTCATGAAAGA-3′) and liprin α1 siRNA-2 (5′-CTGGTGTTTCCGAGACGGATA-3′). Lipofectamine 2000 (Invitrogen) was used for transfection of the siRNAs.
Cell spreading assay
RKO cells transfected with ING4 expression plasmids were plated on glass coverslips in serum free medium and cultured overnight. The starved cells were stimulated by 5% fetal bovine serum (FBS) in DMEM without glutamine and antibiotics. At designated time points, cells were fixed and stained on F-actin by rhodamine-phalloidin. Membrane protrusion and spreading was observed by immunofluorescence microscopy based on the staining. The ratio of cells that had filopodia and/or lamellipodia (filo/lamellipodia) over cells with GFP expression was calculated by counting cells and is referred to as the percent of cell spreading. The average surface area per cell was measured by NIH ImageJ software. The statistical analysis was performed by Student’s t-test with data from more than three independent fields in the experiment.
Modified Boyden chamber cell migration assay
Modified Boyden chamber cell migration assay was performed using a migration chamber containing 8 mM pore-size polystyrene membranes coated with FluoroBlok materials (BD Biosciences). RKO cells were plated at 2 × 104 cells/cm2 in a 100 mm dish and cultured for 16 h. Cells were transfected with 1 μg of plasmid and incubated for 16 h. The transfected cells were harvested, washed once with serum-free DMEM, and resuspended in serum-free DMEM at a concentration of 1 × 105/ml. For the FluoroBlok transmembrane migration assay, 0.5 ml of the cell suspension was transferred to the upper chamber of the assay and 0.75 ml of complete DMEM was laid in the lower chamber. The 24-well plate of the migration assay was immediately incubated at 37°C for 22 h. Quantitative cell migration data were obtained by fluorescence spectrometry (Victor II, PerkinElmer Life and Analytical Sciences, Boston, MA) after the transverse cells in the lower chamber were stained with calcein AM (Molecular Probes) at 37°C for 1 h.
Results
ING4 interacts with liprin α1
We performed a pull-down assay using FLAG-tagged ING4 (ING4_v1) protein overexpressed in RKO cells to understand the network of ING4-interacting proteins. Several cytoplasmic proteins, Ubc4/5, Calcium binding protein 1 (CaBP1), Ras-GTPase activating protein, SH3 domain-binding protein 2a (G3BP2a), COP1-β’, liprin α1, and several ribosomal proteins, were identified as ING4 interacting proteins by mass spectrometry (Fig. 1A). Among these pull-down proteins, we focused on liprin α1 in this report. The mass spectrometry results were further confirmed by coimmunoprecipitation of FLAG-ING4 with endogenous liprin α1, which was detected by anti-liprin α1 antibody in a Western blot (Fig. 1B). This interaction was specific for ING4 among other ING family proteins; ING2 and ING5 did not coimmunoprecipitate with liprin α1 (Fig. 1B). We determined the ING4 motif responsible for the interaction using three C-terminal-truncated FLAG-ING4 constructs coexpressed with myc-tagged liprin α1. The peptide sequence between codons 135 and 180 was essential for ING4-liprin α1 interaction (Fig. 1C).
Figure 1.
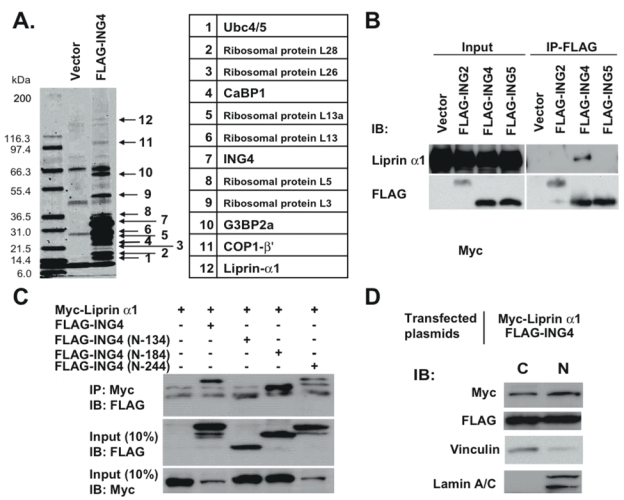
ING4 interacts with liprin α1. A, Cell extracts from RKO cells transfected with FLAG-ING4 were used for the pull-down assay. The binding proteins were separated by 12% SDS-PAGE, visualized by Coomassie blue staining, and extracted for mass spectrometry analysis. The identified proteins were listed. B, Whole cell lysates from RKO cells expressed FLAG-tagged ING2, 4 or 5 were used for immunoprecipitation (IP) using FLAG M2 resin. Liprin α1 was detected by immunoblot (IB) using chicken anti-liprin α1 antibody. C, Whole cell lysates from RKO cells expressed myc-tagged liprin α1 and three kinds of FLAG-tagged C-terminal-truncated ING4 (N-134, N-180 and N-244) were used for IP using anti-myc antibody. D, Subcellular fractionations of cell lysate from RKO cells expressed FLAG-tagged ING4 and myc-tagged liprin α1 were resolved in SDS-PAGE. C indicates cytoplasmic and N indicates nuclear.
Because liprin α1 is known to carry out its function mainly in the cytoplasm, and ING4 has been demonstrated to possess gene regulatory activities in the nucleus, we investigated whether ING4 was also present in the cytoplasm. By subcellular fractionation and Western blotting, we demonstrated that ING4 was present in both nuclear and cytoplasmic extracts (Fig. 1D). We also found that liprin α1 was present in both nuclear and cytoplasmic extracts, whereas the focal adhesion molecule, vinculin, was more strictly found in the cytoplasmic extract, and a nuclear marker, lamin A/C, was only detected in the nuclear extract (Fig. 1D).
ING4 colocalizes with liprin α1 in protruding membranes
The subcellular localization of ING4 and liprin α1 was examined by immunofluorescence. We have observed that overexpressed ING4 (ING4_v1) mainly localized in the nucleus when cells were in contact with each other (23). However, overexpressed ING4 had a tendency to be distributed in the cytoplasm under low cell density conditions in RKO cells (Fig. 2A). ING4 was widely spread out, but mostly in the cytoplasm, with elongated protruding membranes stimulated by serum in the cells (Fig. 2B). It is intriguing that ING4 proteins preferred to cluster around the perinuclear areas, and most remarkably, assembled into intensive foci along the protruding membrane. Looking closely at the protruding membrane, we found that ING4 localized densely at the forefront of the leading edge, suggesting a role for ING4 in mechanisms of membrane protrusion, such as focal adhesion (Fig. 2B). Similar to ING4, endogenous liprin α1 resided all around the cytoplasm and formed puncta in the polar lamellipodium. The colocalization of ING4 and liprin α1 in the protruding membrane or polar lamellipodium was significant (Fig. 2B). The colocalization was further confirmed by confocal microscopy analyses in RKO cells cotransfected with liprin α1 and ING4 (Supplementary Fig. S1A). We also observed similar colocalization of ING4 and liprin α1 in protruding membranes or polar lamellipodia in HEK-293 cells and U-87 MG glioblastoma cells (Supplementary Fig. S1B and C).
Figure 2.
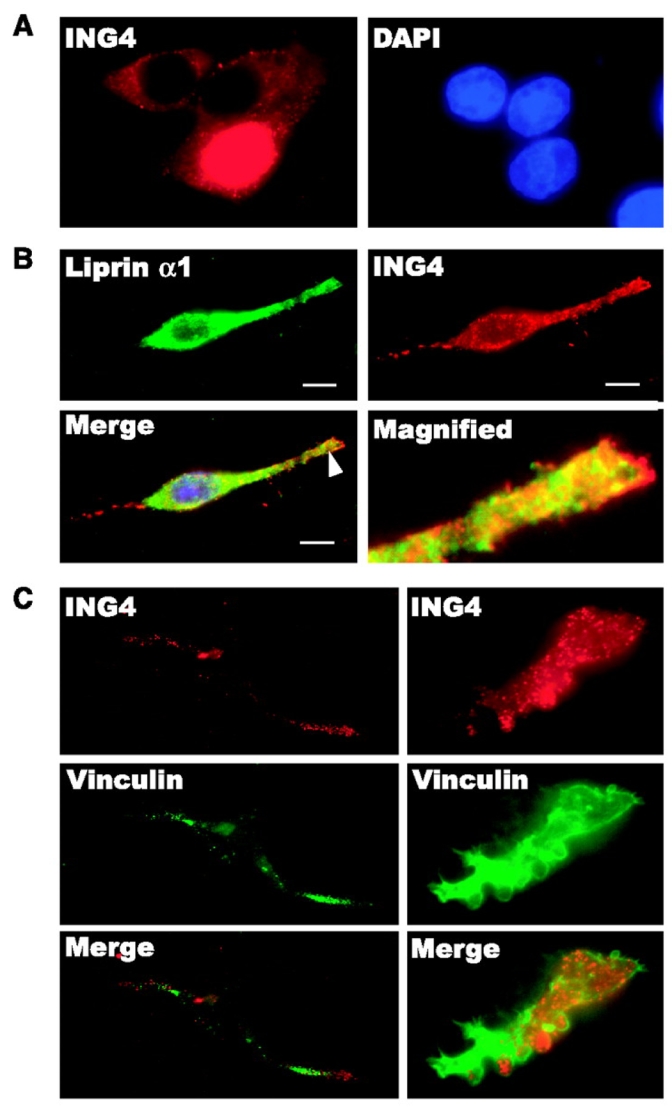
ING4 colocalizes with liprin α1 at lamellipodia in the vicinity of vinculin. A, RKO cells transfected with ENG4 plasmid were seeded on glass coverslips and cultured in completed medium for 24 h. ING4 was detected by rabbit anti-ING4 antibody (red). B, FLAG-ING4-transfected RKO cells were seeded on fibronectin-coated glass coverslips, starved overnight and stimulated by 5% FBS. Cells in growing status were observed by immunofluorescence microscopy. Liprin α1 (green) was detected by chicken anti-liprin α1 antibody and FLAG-ING4 (red) was detected by rabbit anti-FLAG antibody. Bar, 10 μm. C, RKO cells transfected with FLAG-ING4 were treated the same way as B. ING4 (red) was detected by rabbit anti-FLAG antibody and vinculin (green) was detected by mouse anti-vinculin antibody.
Because ING4 and liprin α1 colocalized mainly at the protruding membranes, we surmised that ING4 may be involved in a focal adhesion complex. We examined whether ING4 colocalizes with the focal adhesion molecule, vinculin, in the cells. ING4 clustered at the tip of polar lamellipodia in front of vinculin or at the front edge of membrane ruffles that was surrounded by vinculin (Fig. 2C).
Effect of overexpressed ING4 and liprin α1 on cell motility
Liprin α1 plays a major role in neuron cell growth, particularly in axon guidance and in terminal branches during dendrite extension (22). Because ING4 interacts with liprin α1, we were interested in understanding whether ING4 regulates cell spreading and cell motility. First, we performed a cell spreading assay. Cells were synchronized by serum starvation and stimulated by adding serum. The membrane morphology was observed by F-actin staining. The transfection efficiency, evaluated by a GFP expression vector, was high enough (80–90%) for the cells to be used for this experiment (Supplementary Fig. S2A). The surface area of ING4-overexpressed cells was significantly smaller than that of the control cells (Fig. 2A left and Supplementary Fig. S2B). The ratio of cells that had filopodia and/or lamellipodia (filo/lamellipodia) over cells with GFP expression was calculated and is referred to as the percent of cell spreading. The results showed that 1 h after serum stimulation, ING4 significantly delayed cell spreading by nearly 4-fold relative to the vector control (Fig. 3A right and Supplementary Fig. S2A). The mechanism by which ING4 suppresses cell spreading may also assist in cell migration regulation, particularly to the polarized protrusion membranes during migration. Thus, we next performed a modified Boyden chamber assay to measure transmembrane cell migration by fluorescence scoring. Overexpressed ING4 also significantly suppressed cell migration (Fig. 3B). This result was further confirmed by the in vitro scratch assay (Supplementary Fig. S3A, B, and Video 1). Time-lapsed motion pictures that monitored cell migration taken every 5 min over a 24-h period showed a marked suppression of cell migration when ING4 was overexpressed in RKO cells.
Figure 3.
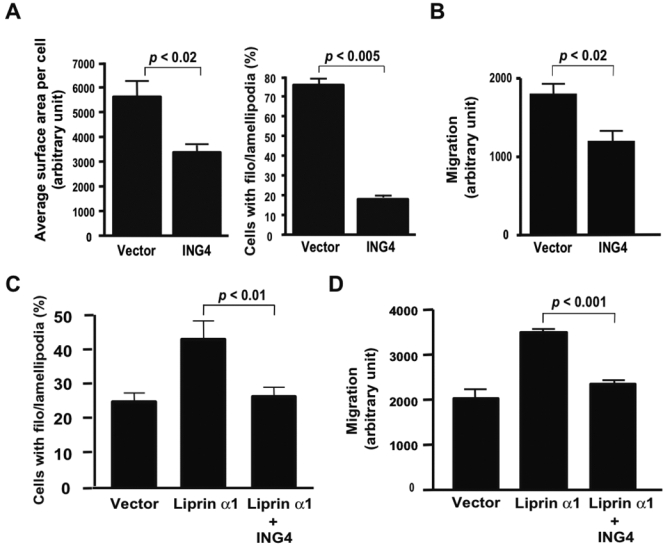
Effect of overexpressed ING4 and liprin α1 on cell spreading and migration. A, (left panel) RKO cells transfected with EGFP vector and EGFP-ING4 were starved overnight and stimulated by serum. After 1 h serum stimulation, average surface area per cell was measured (Supplementary Fig. S2B). (right panel) Starved RKO cells transfected with pFLAG vector or pFLAG-ING4 were subjected to the cell spreading assay as same as A. After 1 h serum stimulation, the ratio of cells with filo/lamellipodia formation versus cells transfected with EGFP or EGFP-ING4 was calculated (Supplementary Fig. S3A). B, RKO cells transfected with pcDNA vector or pcDNA-ING4 plasmid were used for the modified Boyden chamber assay. Cell migration was quantified by measuring the total fluorescence of cells that traveled to the lower chamber. C, Starved RKO cells transfected with pcDNA-ING4 and/or myc-liprin α1 expression plasmids were subjected to the cell spreading assay. After 45 min serum stimulation, the ratio of cells with filo/lamellipodia formation was calculated (Supplementary Fig. S4). D, RKO cells transfected with pcDNA-ING4 and/or myc-liprin α expression plasmids were used for a modified Boyden chamber assay as same as B.
Next, we examined the effect of overexpressed liprin α1 on cell motility. After 45 min serum stimulation, overexpressed liprin α1 enhanced cell spreading (Fig. 3C and Supplementary Fig. S4). Coexpression of ING4 with liprin α1 suppressed the cell spreading enhanced by liprin α1 (Fig. 3C). Overexpression of liprin α1 also induced cell migration, but this induction was suppressed by ING4 (Fig. 3D).
Effect of endogenous ING4 on cell motility
To understand the role of endogenous ING4, we reduced endogenous ING4 by four small interference RNAs (siRNAs). All four siRNAs reduced protein levels more than 50%, and siRNA-3 almost completely eliminated ING4 protein expression detected by anti-ING4 antibody (Fig. 4A). For the spreading assay, F-actin in the cells transfected with the siRNAs was stained after 30 min serum stimulation. Cells with control siRNA did not spread out much in 30 min, while the ING4 siRNA-transfected cells were swiftly extending their membranes in the form of filo/lamellipodia (Supplementary Fig. S5, Fig. 4B and C). siRNA-3 showed statistical significance in results determined either by scoring the percentage of cells with the formation of filo/lamellipodia (Fig. 4B) or by measuring the average cell surface area (Fig. 4C) consistent with ING4 protein levels (Fig. 4A). Next, we performed the modified Boyden chamber assay. The result showed that siRNA knockdown of ING4 also enhanced cell migration (Fig. 4D). Again, siRNA-3 enhanced cell migration most effectively consistent with the protein level (Fig. 4A).
Figure 4.
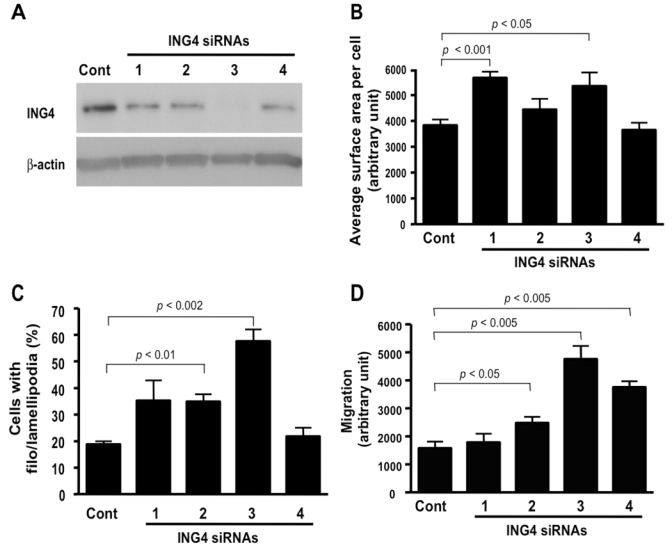
Downregulation of ING4 enhances cell motility. A, The downregulation of ING4 by four different siRNAs was detected by mouse monoclonal anti-ING4 antibody. B, RKO cells transfected with the four ING4 siRNAs for 72 h were starved and used for the cell spreading assay. After 30 min serum stimulation, average cell surface area was measured (Supplementary Fig. S5). C, RKO cells transfected with the four ING4 siRNAs for 72 h were used for the cell spreading assay as same as A. Quantitative data were presented as percent of cells with filo/lamellipodia (Supplementary Fig. S5). D, The modified Boyden chamber assay was carried out by using RKO cells transfected with ING4-siRNAs for 72 h.
Regulation of cell migration by ING4 and liprin α1
Two siRNAs against liprin α1 (Lip-KD1 and Lip-KD2) significantly decreased protein levels of liprin α1 in RKO cells and suppressed cell spreading after 1 h serum stimulation compared with control vector (Fig. 5A, Supplementary Fig. S6). But overexpressed ING4 in the cells with liprin α1 knockdown (Fig. 5A; lanes, Lip-KD1+ING4 and Lip-KD2+ING4) did not further suppress cell spreading. Next, we performed modified Boyden chamber cell migration assay. As shown in Fig. 5B, knockdown of liprin α1 by siRNAs suppressed cell migration, on the other hand, ING4 overexpression in liprin α1 knockdown cells (lanes, Lip-KD1+ ING4 and Lip-KD2+ING4) did not further confer migration suppression. We summarize these results as the following: 1) liprin α1 positively regulates cell spreading and migration; 2) ING4 counteracts liprin α1’s positive influence on spreading and migration; and 3) in the absence of liprin α1 (or dissociation of the ING4-liprin α1 interaction), ING4 loses the specificity to suppress cell spreading and migration.
Figure 5.
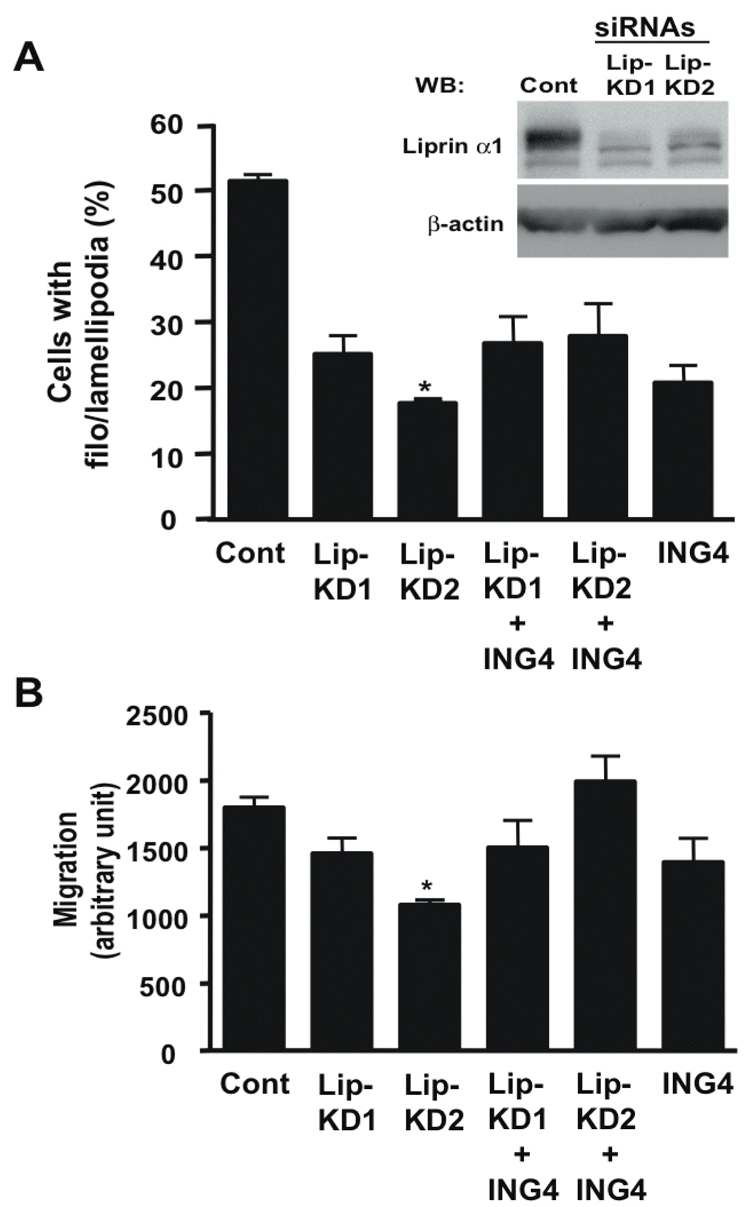
Interaction of liprin α1 and ING4 on regulating cell motility. A, Endogenous liprin α1 in RKO cells transfected with control vector (Cont) and two siRNAs (Lip-KDl and Lip-KD2) was detected by chicken liprin α1 antibody. RKO cells transfected with the control vector, pcDNA-ING4, the two liprin α1 siRNAs with/without ING4 for 72 h were used for the cell spreading assay. After 1 h serum stimulation, the ratio of cells with filo/lamellipodia was calculated. * Indicates p<0.0001 compare with the control (Student’s t-test). B, RKO cells transfected with the same vector and/or siRNAs used for A were subjected to a modified Boyden chamber assay. * Indicates p<0.002 compared with the control (Student’s t-test).
Discussion
In addition to the previously reported nucleus-based functions, we provided evidence of ING4 cytoplasmic functions. We show here that ING4 binds to a cytoplasmic protein, liprin α1, and translocated to lamellipodia where it is colocalized with liprin α1, suggesting a dynamic change of ING4 subcellular localization under some conditions. Liprin α1 was originally identified as a binding protein of the LAR family receptor protein tyrosine phosphatases (LAR-RPTP) and was shown to be colocalized with LAR at focal adhesions, where liprin α1’s location is proximal to the rear end of focal adhesion, which disassembles in migration during membrane protrusion (20). ING4 interaction with liprin α1 may explain the subcellular localization of ING4 being proximal to the focal adhesion molecule, vinculin. Although the exact function of liprin α1 in the formation and disassembly of focal adhesion is unknown, it has been suggested that liprin α1 may play a role in the regulation of focal adhesion disassembly. Recent studies in axon guidance indicated that liprin α1 is required for the trafficking of synaptic vesicles (21) and is involved in the development and maintenance of excitatory synapses (22). Thus, liprin α1 could serve as a scaffold protein that is able to assemble protein complexes in a transportation vehicle destined to the presynapse. Interestingly, in our study, ING4 tends to cluster at the perinuclear area or at lamellipodium in RKO, U-87 MG, and HEK-293 cells, suggesting a transportation pathway from the nucleus to lamellipodium. In addition, the physical interaction of ING4 with COP1-β′, another protein identified by the present experiment, may further support this hypothesis.
Cell migration is a highly coordinated process involving multiple components and steps that mirror the complexity of embryonic morphogenesis (18). A migration movement begins with cell polarization, which includes vesicle trafficking toward the leading edge, followed by membrane protrusion and adhesion. At the lagging end, adhesion disassembles to allow forward movement. Although liprin α1 has not been specifically studied for its role in the polarity of cell migration, its associated protein, GIT1, has been shown to play a major role in the regulation of membrane protrusive activity and cell migration (25). Here, we showed that ING4 suppressed membrane protrusion by delaying actin polymerization in the spreading cells. This negative regulation of actin polymerization may play a major role in suppressing cell migration. By liprin α1 knockdown and ING4 overexpression simultaneously, we found that liprin α1 was required for ING4’s capacity to suppress cell spreading and migration. These results suggest that appropriate subcellular localization and/or proper protein-protein interactions are critical for ING4 function in regulating the cell motility. Liprin α1 may play an essential role in the trafficking of ING4 to its destination and/or in assembling the required protein complex for ING4 activities.
We and others have previously shown that liprin α1 and ING4 are expressed in both normal tissue and multiple cancer types (23). Recently, ING4 was identified in a screening assay designed to search for genes that suppress loss of contact inhibition among cells. Ectopic expression of ING4 attenuated anchorage-independent cell growth and suppressed loss of contact inhibition (16). Garkavtsev et al (15) reported that an interaction between ING4 and NF-κB plays an important role during angiogenesis. The interaction between ING4 and liprin α1 may also participate in endothelial cell migration and angiogenesis. In addition, development and function of the mammary gland is impaired in homozygous LAR-deficient mice (26). Thus, the interaction of ING4 and liprin α1 may play a role during development. The role of ING4 interaction with liprin α1 in normal cell migration, e.g.) endothelial cells, and during development, warrants further investigation.
Previously, we reported that the endogenous ING4 expression level was induced by serum starvation and reduced by serum activation in normal fibroblast cells (23). This observation is consistent with the present observation using siRNAs against ING4. Therefore, endogenous ING4 seems to suppress cell migration in normal cells, and the decreased ING4 expression in cancer cells (8, 15) could lead to cancer cell migration and metastasis. In the same report (23), we described four splice variants of ING4 (ING4_v1, v2, v3 and v4). In this report, we focused on the initially described ING4, ING4_v1, because of its strong binding affinity to liprin α1. Other splice variants have a partially missing NLS (deletion is between 128aa to 132aa) that corresponds to a flanking region that is critical for binding with liprin α1 (135aa–180aa), identified in this report. One of the four splice variants, ING4_v4, showed dominant-negative effects on the functions of ING4_v1. Therefore, ING4 may regulate multiple cellular functions by changing its subcellular localization, binding partners, and associations with the variants.
In summary, we have identified an ING4-associated protein, liprin α1, that may function in directing ING4 to its cytoplasmic destination, where ING4 may function as a regulator of membrane dynamics, and consequently, cell motility. These findings shed a new light on our understanding of the biological role of the candidate tumor suppressor, ING4, in addition to its documented nuclear transcription regulation.
Supplementary Material
Note: Supplementary data for this article are available at Cancer Research Online, (http://cancerres.aacrjournals.org/).
Acknowledgments
This work was supported by the Intramural Research Program of Center for Cancer Research, National Cancer Institute, National Institute of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore, be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We are grateful to the IAB microscopy facility for the production of cell migration videos. We thank Drs. Paul Randazzo and Kensuke Kumamoto for scientific advice, Damien Nissou and Alexei Grichine for technique assistance, and Dr. Tom Holroyd and Dorothea Dudek-Creaven for editorial assistance.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online, (http://cancerres.aacrjournals.org/).
References
- 1.He GH, Helbing CC, Wagner MJ, Sensen CW, Riabowol K. Phylogenetic analysis of the ING family of PHD finger proteins. Mol Biol Evol. 2005;22:104–16. doi: 10.1093/molbev/msh256. [DOI] [PubMed] [Google Scholar]
- 2.Russell M, Berardi P, Gong W, Riabowol K. Grow-ING, Age-ING and Die-ING: ING proteins link cancer, senescence and apoptosis. Exp Cell Res. 2006;312:951–61. doi: 10.1016/j.yexcr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Garkavtsev I, Kazarov A, Gudkov A, Riabowol K. Suppression of the novel growth inhibitor p33ING1 promotes neoplastic transformation. Nat Genet. 1996;14:415–20. doi: 10.1038/ng1296-415. [DOI] [PubMed] [Google Scholar]
- 4.Nagashima M, Shiseki M, Miura K, et al. DNA damage-inducible gene p33ING2 negatively regulates cell proliferation through acetylation of p53. Proc Natl Acad Sci USA. 2001;98:9671–6. doi: 10.1073/pnas.161151798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagashima M, Shiseki M, Pedeux R, et al. A novel PHD-finger motif protein, p47ING3, modulates p53-mediated transcription, cell cycle control, and apoptosis. Oncogene. 2003;22:343–50. doi: 10.1038/sj.onc.1206115. [DOI] [PubMed] [Google Scholar]
- 6.Shimada Y, Saito A, Suzuki M, Takahashi E, Horie M. Cloning of a novel gene (ING1L) homologous to ING1, a candidate tumor suppressor. Cytogenet Cell Genet. 1998;83:232–5. doi: 10.1159/000015188. [DOI] [PubMed] [Google Scholar]
- 7.Shiseki M, Nagashima M, Pedeux RM, et al. p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity. Cancer Res. 1003;63:2373–8. [PubMed] [Google Scholar]
- 8.Gunduz M, Nagatsuka H, Demircan K, et al. Frequent deletion and down-regulation of ING4, a candidate tumor suppressor gene at 12p13, in head and neck squamous cell carcinomas. Gene. 2005;356:109–17. doi: 10.1016/j.gene.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Campos EI, Chin MY, Kuo WH, Li G. Biological functions of the ING family tumor suppressors. Cell Mol Life Sci. 2004;61:2597–613. doi: 10.1007/s00018-004-4199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi X, Gozani O. The fellowships of the INGs. J Cell Biochem. 2005;96:1127–36. doi: 10.1002/jcb.20625. [DOI] [PubMed] [Google Scholar]
- 11.Doyon Y, Cayrou C, Ullah M, et al. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Pena PV, Davrazou F, Shi X, et al. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature. 2006;442:100–3. doi: 10.1038/nature04814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi X, Hong T, Walter KL, et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature. 2006;442:96–9. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozer A, Wu LC, Bruick RK. The candidate tumor suppressor ING4 represses activation of the hypoxia inducible factor (HIF) Proc Natl Acad Sci U S A. 2005;102:7481–6. doi: 10.1073/pnas.0502716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garkavtse I, Kozin SV, Chernova O, et al. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428:328–32. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Chin K, Gray JW, Bishop JM. A screen for genes that suppress loss of contact inhibition: identification of ING4 as a candidate tumor suppressor gene in human cancer. Proc Natl Acad Sci U S A. 2004;101:16251–6. doi: 10.1073/pnas.0407158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 18.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 19.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–7. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 20.Serra-Pages C, Kedersha NL, Fazikas L, Medley Q, Debant A, Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 1995;14:2827–38. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KE, DeProto J, Kaufmann N, Patel BN, Duckworth A, Van VD. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr Biol. 2005;15:684–9. doi: 10.1016/j.cub.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Dunah AW, Hueske E, Wyszynski M, et al. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8:458–67. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- 23.Unoki M, Shen JC, Zheng ZM, Harris CC. Novel splice variants of ING4 and their possible roles in regulation of cell growth and motility. J Biol Chem. 2006;281(45):34677–86. doi: 10.1074/jbc.M606296200. [DOI] [PubMed] [Google Scholar]
- 24.Pedeux R, Sengupta S, Shen JC, et al. ENG2 regulates the onset of replicative senescence by induction of p300-dependent p53 acetylation. Mol Cell Biol. 2005;25:6639–48. doi: 10.1128/MCB.25.15.6639-6648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci. 2002;115:1497–510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- 26.Schaapveld RQ, Schepens JT, Robinson GW, et al. Impaired mammary gland development and function in mice lacking LAR receptor-like tyrosine phosphatase activity. Dev Biol. 1997;188:134–46. doi: 10.1006/dbio.1997.8630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


