Abstract
The distal human intestine harbors trillions of microbes that allow us to extract calories from otherwise indigestible dietary polysaccharides. The products of polysaccharide fermentation include short-chain fatty acids that are ligands for Gpr41, a G protein-coupled receptor expressed by a subset of enteroendocrine cells in the gut epithelium. To examine the contribution of Gpr41 to energy balance, we compared Gpr41−/− and Gpr41+/+ mice that were either conventionally-raised with a complete gut microbiota or were reared germ-free and then cocolonized as young adults with two prominent members of the human distal gut microbial community: the saccharolytic bacterium, Bacteroides thetaiotaomicron and the methanogenic archaeon, Methanobrevibacter smithii. Both conventionally-raised and gnotobiotic Gpr41−/− mice colonized with the model fermentative community are significantly leaner and weigh less than their WT (+/+) littermates, despite similar levels of chow consumption. These differences are not evident when germ-free WT and germ-free Gpr41 knockout animals are compared. Functional genomic, biochemical, and physiologic studies of germ-free and cocolonized Gpr41−/− and +/+ littermates disclosed that Gpr41-deficiency is associated with reduced expression of PYY, an enteroendocrine cell-derived hormone that normally inhibits gut motility, increased intestinal transit rate, and reduced harvest of energy (short-chain fatty acids) from the diet. These results reveal that Gpr41 is a regulator of host energy balance through effects that are dependent upon the gut microbiota.
Keywords: host-microbial interactions, energy balance, enteroendocrine cells, nutrient sensing, polysaccharide fermentation
Our ability to effectively digest food reflects the combined activities of enzymes encoded in our primate genome and in the genomes of the trillions of microbes that reside in our distal guts. This microbial community, or microbiota, affects both sides of the energy-balance equation, influencing both the harvest of calories and the activity of host genes involved in the metabolism and storage of absorbed energy (e.g., ref. 1).
Our proteome has a very limited repertoire of glycoside hydrolases needed to digest complex dietary plant polysaccharides: the microbiota synthesizes a large arsenal of these enzymes (2), and allows us to process complex dietary carbohydrates to short-chain fatty acids (SCFAs), principally acetate, propionate, and butyrate. Host recovery of SCFAs is generally efficient and occurs by both passive diffusion and via mono-carboxylic acid transporters (e.g., MCT1 in the case of butyrate and lactate) (3). Butyrate is the preferred source of energy for colonic epithelial cells. Absorbed acetate and propionate are delivered to hepatocytes, which consume most of the propionate for gluconeogenesis. Although acetate can be used for lipogenesis in colonocytes, hepatocytes and adipocytes are the principal sites for de novo lipogenesis, at least in rodents.
Studies in gnotobiotic mice have emphasized the contributions of the gut microbiota and microbial fermentation of dietary polysaccharides to host energy balance. Adult germ-free (GF) mice are leaner than their age- and gender-matched conventionally raised (CONV-R) counterparts who have acquired a microbiota beginning at birth (1). Transplantation of an unfractionated gut microbiota from a CONV-R donor to an adult GF recipient results in an increase in adiposity. This increase is greater if the donors are obese because they are homozygous for a null allele in the leptin gene (ob/ob), or if they have diet-induced obesity (4, 5). Comparative metagenomic studies of distal gut (cecal) microbial community DNA prepared from mice with either form of obesity and from lean controls, have shown that the obesity-associated microbiomes have a greater capacity to ferment carbohydrates to SCFAs (4, 5). In addition, colonization of adult GF mice, fed a standard polysaccharide-rich chow diet, with two organisms—Bacteroides thetaiotaomicron, a prominent saccharolytic bacterium in the normal distal human gut microbiota and an adept adaptive forager of polysaccharides (6), plus Methanobrevibacter smithii, a dominant methanogenic archaeon in this community (7) that promotes polysaccharide fermentation by removing the H2 end product— results in higher levels of SCFAs in the colon, and significantly greater host adiposity than colonization of GF animals with either organism alone (8).
SCFAs also act as signaling molecules. Propionate, acetate, and to a lesser extent butyrate and pentanoate, are ligands for at least two G protein-coupled receptors (GPCRs), Gpr41 and Gpr43 (9–11). Both GPCRs are broadly expressed, including in the distal small intestine, colon, and adipocytes (9–11). SCFAs (C1-C6), which are ligands for Gpr41, stimulate expression of leptin, a polypeptide hormone with pleiotropic effects on appetite and energy metabolism, in mouse-cultured adipocytes (11). However, little is known about the regulation of these GPCRs, their mechanism of action, and whether they represent a class of molecules, strategically placed in certain gut epithelial cell lineages that sense the biochemical milieu of the gut and “transduce” information about key metabolic activities of the microbiota, such as polysaccharide fermentation, in ways that impact host physiology, including energy balance.
In this report, we compare GF wild-type and Gpr41-deficient mice with and without a model fermentative microbial community composed of B. thetaiotaiomicron (Bt) and M. smithii (Ms). The results reveal a pivotal role for Gpr41 in a microbiota-dependent metabolic circuit that regulates the flow of calories between the diet and the host. Our results suggest that inhibition of SCFA activation of Gpr41 is a potential therapeutic target for modulating the efficiency of caloric extraction from a polysaccharide-rich diet.
Results and Discussion
Gpr41 Is Expressed in Enteroendocrine Cells.
Analysis of the tissue distribution of Gpr41 mRNA in CONV-R adult mice indicated that highest levels are present in the distal small intestine (ileum) and colon [supporting information (SI) Fig. S1]. Enteroendocrine cells are strategically positioned to transduce information about the nutrient milieu of the gut and the metabolic activity of the microbiota to the host: they produce different sets of peptide hormones, depending upon their location along the length of the gastrointestinal tract (12). These neuroactive and endocrine factors are secreted basolaterally into the portal and systemic circulation where they influence a wide variety of extraintestinal physiological activities.
In situ hybridization studies indicated that Gpr41 is expressed in cells with the morphologic appearance of enteroendocrine cells (Fig. S2A). Cholecystokinin (CCK) is a known biomarker of this gut epithelial-cell lineage. Therefore, we used flow-assisted cell sorting (FACS) to purify CCK-positive cells from the small intestines of CONV-R transgenic mice engineered to express GFP in this enteroendocine subpopulation (Fig. S2 B and C). qRT-PCR assays of the expression of Gpr41 and seven other known enteroendocrine biomarkers in the crude starting material and in the FACS-purified population confirmed that Gpr41 is expressed in this enteroendocrine subset (Fig. S2 D and E). A similar approach was used in different pedigrees of transgenic mice engineered to express GFP in NeuroD- and Neurogenin3-producing enteroendocrine subpopulations to show that Gpr41 is also localized to these cells (data not shown). Finally, intraepithelial lymphocytes, which have some of the morphologic features of enteroendocrine cells when viewed by light microscopy, were purified using a T-cell antibody plus magnetic bead sorting (see SI Materials and Methods): qRT-PCR established that they do not express appreciable levels of this GPCR (Fig. S2F).
Microbial Suppression of Gpr41 Expression.
Ligand-induced down regulation is a hallmark of GPCR activation (13). Therefore, we generated Gpr41−/− mice (Fig. S3 A and B), rederived them as GF, and examined whether colonization of 8- to 10-week-old male GF knockout (Gpr41−/−) mice and their WT (+/+) littermates (mixed C57Bl6/J:129/Sv background) for 28 days with Bt and Ms (see Methods) affected ileal expression of Gpr41, or three other known GCPRs that bind fatty-acid ligands: Gpr43, which, as noted above, is also responsive to short-chain (C2-C6) fatty acids, plus Gpr40 and Gpr120, which recognize ligands with longer (>C6) acyl chains (14–16).
Quantitative PCR assays established that levels of colonization of the distal gut (cecum) with each microbial species were not significantly affected by the presence or absence of Gpr41 (mean 8.2 ± 4.3 × 1012 organisms per gram of luminal contents for B. thetaiotaomicron; 2.4 ± 1.5 × 106 for M. smithii; n, 7–8 mice per genotype). Therefore, any phenotypic differences observed between gnotobiotic WT and knockout animals could not be attributed to differences in their gut microbial ecology.
qRT-PCR assays of ileal RNAs revealed that compared with GF +/+ controls, cocolonization of WT mice produced statistically significant twofold reductions in the steady state levels of Gpr41, Gpr43, and Gpr120 mRNAs (P < 0.05; ANOVA) (Fig. S4). Expression of Gpr40 mRNA in +/+ mice was also reduced by colonization, although the observed change did not quite achieve statistical significance (see Fig. S4). Importantly, the magnitude of the reduction in Gpr40, Gpr43, and Gpr120 gene expression was not affected by the absence of Gpr41 (see Fig. S4).
Together, these findings indicate that Gpr41−/− mice have a specific deficiency affecting only one of these four fatty-acid binding GPCRs and therefore can, in principle, be used to assess the role of Gpr41 in mediating the effects of the microbiota on host energy homeostasis.
Gpr41 Is Needed for Microbiota-Induced Increases in Host Adiposity.
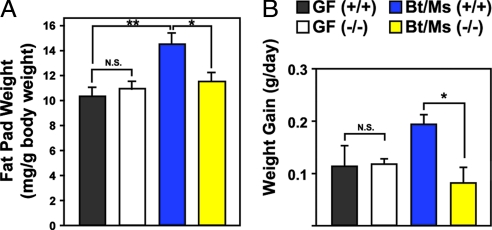
Eight to ten-week-old male GF Gpr41−/− mice, maintained on a standard polysaccharide-rich chow diet, exhibited no significant differences in their epididymal fat pad or total body weights compared with +/+ littermates (P > 0.05; n = 8–14 per group) (Fig. 1A and data not shown). In contrast, Gpr41−/− mice cocolonized with Bt/Ms had significantly lower epididymal fat pad weights than +/+ controls (11.4 ± 0.6 versus 14.4 ± 0.9 mg/g body weight, respectively; P < 0.05) (see Fig. 1A), gained significantly less body weight per day (0.08 ± 0.03 versus 0.19 ± 0.02 g/day, respectively; P < 0.05) (Fig. 1B), and weighed significantly less at the end of the 28-day colonization period (24 ± 0.4g versus 26 ± 0.4g; P < 0.05) (n, 13 to 14 animals per group, representing two independent experiments).
Fig. 1.
Microbiota-mediated increase in adiposity is blunted in Bt/Ms cocolonized gnotobiotic Gpr41−/− mice. (A) Weights of both epididymal fat pads in Gpr41−/− and +/+ littermates that are GF, or raised GF and then colonized at 4 to 6 weeks of age with Bt and Ms (n, 8–14 males per group; three independent experiments). (B) Weight gain (n, 4–9 mice per group; followed one to two times per week for up to 4 weeks, between 5 and 9 weeks of age in the case of GF animals, and for 4 weeks after gavage in the case of Bt/Ms cocolonized gnotobiotic animals). Mean values ± SEM are plotted. *, P < 0.05, **, P < 0.01. N.S., not significantly different.
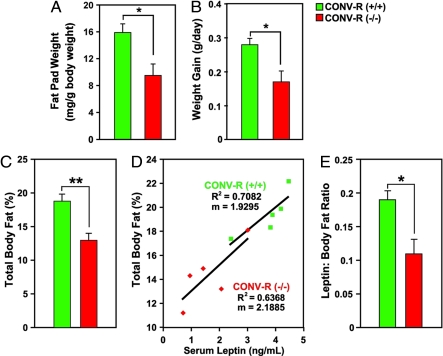
These gut microbiota-dependent differences were not a unique feature of the Bt/Ms gnotobiotic model. CONV-R Gpr41−/− animals, maintained on the same polysaccharide-rich, low-fat chow diet as their cocolonized gnotobiotic counterparts, also exhibited statistically significant decreases in weight gain, total body weight, and fat-pad weight compared with age- and gender-matched CONV-R +/+ littermates (P < 0.05) (Fig. 2 A and B plus data not shown). Dual energy X-ray absorptiometry (DEXA) confirmed their reduced adiposity (13 ± 1% versus 19 ± 1% of body weight in +/+ controls; P < 0.005; n, 9 to 13 per group) (Fig. 2C). The differences in body weight and adiposity observed in CONV-R Gpr41-deficient versus WT mice were not attributable to differences in their locomotor activity or body temperature (n, 4 animals per group; P > 0.05) (Fig. S5 A and B).
Fig. 2.
Microbiota-mediated increase in adiposity is blunted in CONV-R Gpr41−/− mice. (A) Epididymal fat-pad weights (values from both fat pads were combined for each animal; n, 12 males per group). (B) Weight gain (n, 6 per group; followed one to two times per week for up to 4 weeks, between the ages of 5 and 9 weeks). (C) Adiposity in CONV-R WT and knockout mice, defined by DEXA (n, 9–13 males per group; 8–10 weeks old). (D and E) Fasting (4 h) serum leptin levels plotted against percentage total body fat (n, 5 per group). *, P < 0.05, **, P < 0.01 (Student's t test).
Fasting serum levels of leptin were similar in GF Gpr41−/− and +/+ littermates, but significantly lower in Bt/Ms cocolonized and CONV-R Gpr41−/− animals (P < 0.05; n, 5–7 mice per genotype per treatment group) (Table S1). Moreover, serum leptin levels were significantly lower in CONV-R Gpr41−/− animals than would be expected based solely on the observed decrease in their adiposity (P = 0.02) (see Fig. 2 D and E). Together, these findings implicate Gpr41 in microbiota-dependent regulation of host adiposity and leptin production.
Loss of Gpr41 Is Associated with Increased Intestinal Transit Rate and Reduced Efficiency of Energy Harvest from the Diet.
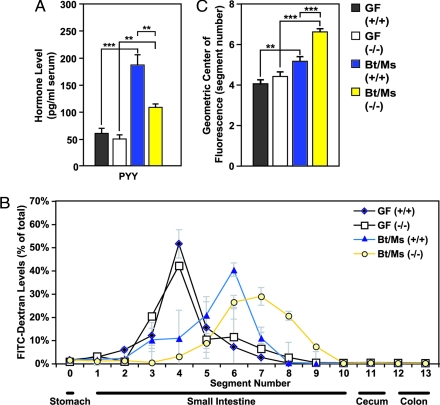
Signals communicated from the gut to the brain via enteroendocrine-cell derived hormones are important regulators of satiety and energy balance. Compared with GF +/+ controls, we found that Bt/Ms-colonization of +/+ mice led to significantly increased circulating levels of peptide YY (PYY). This increase was significantly blunted in their Gpr41−/− littermates (n, 4–8 per group; P < 0.05) (Fig. 3A).
Fig. 3.
Increased rate of intestinal transit in Bt/Ms colonized gnotobiotic Gpr41−/− mice. (A) Fasting serum PYY levels in GF and Bt/Ms cocolonized, Gpr41−/− and +/+ mice (n = four to eight per group; two independent experiments; all samples assayed in duplicate). (B and C) Gut transit time measured by oral gavage of a fluorescently labeled nonabsorbable substrate (fluorescine isothiocyanate-dextran; 70,000 MW) in GF and cocolonized WT and Gpr41-deficient animals. (B and C) Distribution of fluorescence signal intensity 60 min after gavage along the length of the gut; data are plotted as a fraction of total fluorescence in B. No signal was observed in the ceca or colons in any of the animals. Panel (C) presents the calculated geometric center of the fluorescence (n = 4–8 animals analyzed per group). *, P < 0.05, **, P < 0.01, ***, P < 0.005.
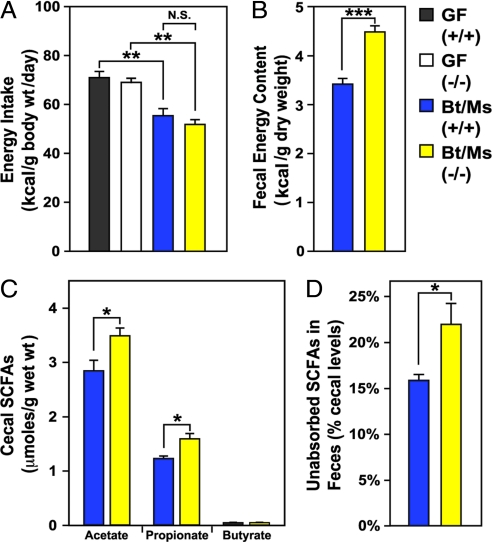
Despite decreases in levels of several anorexigenic hormones (leptin, PYY), Bt/Ms cocolonized and CONV-R Gpr41−/− animals consumed equivalent amounts of chow as their cocolonized and CONV-R +/+ counterparts (n, 11–16 animals per group; P > 0.3) (Fig. 4A and Fig. S5C). Although energy input was similar, bomb-calorimetric assays of feces demonstrated that the efficiency of caloric extraction from the diet was significantly reduced in cocolonized Gpr41-deficient versus +/+ animals (4.5 ± 0.1 versus 3.4 ± 0.2 kcal/g; n, 6–7 per group; P < 0.0001) (Fig. 4B).
Fig. 4.
Gnotobiotic Gpr41−/− mice extract fewer calories from their polysaccharide-rich chow diet and excrete more SCFAs. (A) Dietary energy intake in GF and Bt/Ms cocolonized +/+ and Gpr41−/− littermates (calories of chow consumed per day monitored concurrently with body weight, as in Fig. 1). (B) Bomb calorimetry-based assessment of remaining calories in the feces of colonized (Bt/Ms) Gpr41−/− and +/+ mice (n = 6–7 per group). (C) GC-MS assay of cecal SCFA levels (n = 5–7 per group; each sample assayed in duplicate). (D) GC-MS study of fecal SFCAs from the same mice assayed in (C). *,P < 0.05, **, P < 0.01, ***, P < 0.005. (Student's t test used for comparisons of two groups.)
PYY is also a regulator of gut motility. It produces a dose-related inhibition of transit rate along the length of the gut (17). To test whether Gpr41-deficiency and the associated decrease in PYY is accompanied by an increased transit rate, we gavaged GF and Bt/Ms-colonized Gpr41−/− and +/+ mice with a nonabsorbable fluorogenic marker (FITC-dextran; MW 70,000). Although no statistically significant differences in gastric emptying rates were observed (data not shown), intestinal transit rate was significantly faster in Bt/Ms-colonized Gpr41−/− versus +/+ littermates (P < 0.005; see Fig. 3 B and C). The effect of Gpr41-deficiency on intestinal transit rate was microbiota-dependent: No significant differences were noted among GF animals of either genotype (n, 4–8 per group) (see Fig. 3 B and C). The differences between Bt/Ms-colonized Gpr41−/− and +/+ littermates were not attributable to differences in the length of their small intestines, which were similar (1.8 ± 0.06 versus 1.9 ± 0.07 cm/g body weight, respectively; n, four to eight animals per group; P > 0.05).
Based on the observed increase in intestinal transit rate, we hypothesized that more undigested polysaccharides may reach the distal gut in Gpr41−/− versus +/+ mice. This was confirmed by microanalytic biochemical assays of glucans (glucose-containing polysaccharides) in cecal contents. Bt/Ms-colonized, Gpr41-deficient animals had significantly higher cecal glucan levels than their colonized +/+ littermates (19% increase; 2.2 ± 0.1 versus 1.8 ± 0.1 μmoles/g dry weight of cecal contents; P < 0.05; n, five to six per group; P < 0.05), and higher levels of monomeric glucose (28% increase; 2.7 ± 0.2 versus 2.1 ± 0.2 μmol/g dry weight of cecal contents; P < 0.05). In addition, GC-MS-based analyses of cecal SCFA levels revealed that the concentrations of propionate and acetate were significantly increased in Bt/Ms-colonized Gpr41−/− mice (n, 5–7 animals per group; P < 0.05) (Fig. 4C).
Follow-up whole-genome transcriptional profiling of Bt using (i) microbial RNA isolated from the cecal contents of Bt/Ms-colonized Gpr41-deficient and +/+ littermates, and (ii) custom Bt GeneChips containing probe sets specific for >98% of the organism's protein-coding genes, failed to reveal statistically significant differences in the expression of bacterial genes involved in fermentation of polysaccharides to SCFA between the two groups of animals (n, 5 per group; P < 0.05; FDR <1%). Follow-up qRT-PCR assays of these RNAs confirmed that several key genes in the pathway involved in SCFA production, including pyruvate formate lyase (BT4738; EC 2.3.1.54), and acetate kinase (BT3963; EC 2.7.2.1) exhibited no significant differences in their expression between Bt/Ms colonized Grp41−/− and +/+ mice (data not shown).
GC-MS analysis of feces indicated that Bt/Ms-colonized Gpr41−/− animals had a statistically significant (37 ± 9%) increase in total SCFAs compared with their +/+ littermates (n, 5–7 per group; P < 0.05) (Fig. 4D). In contrast, fecal levels of free C6-C18 fatty acids (FFA) and triglycerides were not significantly different between the two groups of mice [0.6 ± 0.1 versus 0.7 ± 0.1 mg/g weight of feces (FFA), and 1.9 ± 0.2 versus 1.9 ± 0.1 mg/g weight of feces (triglycerides); n = seven to eight animals assayed per group; P > 0.05]. qRT-PCR assays of distal small intestinal (ileal) RNA (SI Methods and Table S2) indicated that there were no significant differences in expression of key host genes involved in the active uptake or transepithelial transport of lipids [e.g., Mct1 (mono-carboxylate transporter), CD36 (lipid-binding glycoprotein) or ApoB (chylomicron-mediated transport); data not shown]. These findings are not surprising, given that the majority of long-chain fatty acids are absorbed in the proximal intestine.
We reasoned that the observed increase in fecal SCFAs reflects, at minimum, reduced host-passive absorption. SCFAs stimulate, and are substrates for, de novo lipogenesis in the liver. Bt/Ms-colonization of GF +/+ animals resulted in statistically significant increases in liver triglyceride levels (n, seven to eight per group; P < 0.05) (Fig. S6A). This effect of colonization was not seen in Gpr41−/− animals, nor were any differences observed between GF Gpr41−/− and +/+ mice (n, 7–8 per group; P > 0.05) (see Fig. S6A). qRT-PCR assays confirmed reduced expression of fatty acid synthase (Fas) in the livers of Bt/Ms-colonized Gpr41−/− mice compared to their +/+ littermates (77 ± 12% lower; n, 5–7 per group; P < 0.01) (Fig. S6B). In addition, fasting (4 h) serum triglyceride levels were decreased in Bt/Ms-colonized Gpr41-deficient versus +/+ animals (n, 7–8 per group) (see Table S1). These differences were not attributable to alterations in hepatic expression of genes involved in long-chain fatty-acid transport, trafficking, or fatty-acid reesterification (data not shown). Together, our results indicate that Gpr41-deficient mice have reduced hepatic lipogenesis, consistent with reduced intestinal absorption and delivery of SCFAs.
Prospectus.
A complete picture of how digestive physiology is regulated requires an understanding of how products generated by the microbiota interact with host nutrient sensors to modulate nutrient and energy flux. The data presented above indicate that Gpr41 mediates a key microbial-host communication circuit. Extrapolating from the phenotype of Gpr41-deficient mice, we posit that at least one feature of the interaction between SCFA products of microbial fermentation of dietary polysaccharides and Gpr41 is to increase circulating levels of enteroendocrine cell-derived hormones that reduce gut motility and thus increase absorption of SCFAs, which are used as substrates for lipogenesis in the liver. Analysis of these Gpr41-deficient mice suggests that an inhibitor of Gpr41 activation could result in decreased extraction of energy from the diet and leaner individuals.
Materials and Methods
Generation of Gpr41 Knockout Mice.
A 129/SV mouse BAC clone obtained from Children's Hospital Oakland Research Institute was used to construct the targeting vector shown in Fig. S3A. SM-1 ES cells (18), cultured on irradiated LIF-producing STO feeder layers, were electroporated with the linearized targeting vector and selected for resistance to G418 (18). Resistant ES cell clones were screened by Southern blotting using a flanking 3′ genomic fragment external to the targeting vector (see Fig. S3B). Two of these ES cell clones were microinjected into C57BL/6 blastocysts to produce germline transmitting chimeric mice. PCR genotyping used the primer set 5′-CACACTGCTCGATCCGGAACCCTT and 5′-GAGAACTGTCTGGAAAACGCTCAC to identify the mutant Gpr41 allele, and 5′-CGACGCCCAGTGGCTGTGGACTTA and 5′-GTACCACAGTGGATAGGCCACGC to detect the WT allele. This PCR genotyping protocol was validated by Southern blotting (see Fig. S3B).
Mice were provided with food and water ad libitum and maintained on a strict 12-h light–dark cycle. All procedures involving genetically engineered mice used in this study were approved by the Institutional Review Board for Animal Research of the University of Texas Southwestern Medical Center at Dallas.
Husbandry of Gnotobiotic Mice.
Gpr41−/− mice and their +/+ littermates (mixed C57BL/6J:129/Sv background) were rederived as GF and housed in flexible film plastic gnotobiotic isolators (19), where they were maintained on a strict 12-h light cycle (lights on at 0600 h) and fed a standard autoclaved polysaccharide-rich chow diet (B and K Universal) ad libitum. Four- to six-week-old male mice were inoculated with a single gavage of 108 CFU of the sequenced human gut-derived B. thetaiotaomicron strain VPI-5482 [harvested from overnight culture in TYG medium (6)], and M. smithii strain PS [cultured at 37°C for 5 d in 125-ml serum bottles containing 15 ml of MBC medium (8) supplemented with 3 g/L formate, 3 g/L acetate, and 0.3 ml of a freshly prepared oxygen-free solution of filter-sterilized 2.5% Na2S (note that the remaining volume or headspace in the bottle contained a 4:1 mixture of H2 and CO2, and the headspace was rejuvenated every 1 to 2 d) (8)]. All colonized mice were killed 28 d after gavage. The density of colonization was determined in cecal contents using qPCR assays that used species-specific primers (8). Age-matched, male CONV-R WT and Gpr41-deficient male mice were also fed the same autoclaved polysaccharide-rich chow diet ad libitum as the gnotobiotic animals. All experiments performed with gnotobiotic mice used protocols approved by the Washington University Animal Studies Committee.
Analysis of Host Adiposity and Energy Harvest.
All mice were fasted (4 h) before being killed. Epididymal fat pads, livers, and segments of the distal intestine (ileum) and colon were removed and flash-frozen in liquid nitrogen. Epididymal fat pad and liver weights were recorded before freezing.
Before killing, total body fat content was measured by DEXA (Lunar PIXImus Mouse, GE Medical Systems), 5 min after mice had been anesthesized with an intraperitoneal injection of ketamine (10 mg/kg body weight) and xylazine (10 mg/kg). Weight gain and chow consumption were monitored weekly in mice who were individually caged for the duration of the experiment. The energy content of fecal samples (freeze-dried immediately after collection) was defined using a bomb-calorimeter (Parr Instruments) and previously established methods (8).
Measurement of Physiological Parameters.
Locomotor activity and body temperature were assessed for 5 d using a telemetry device (minimitter PDT-4000; Mini Mitter) beginning 7 d after implantation (20). Locomotor activity data were processed using VitalView software (Mini Mitter).
Gastric emptying and gastrointestinal transit time was measured in GF and Bt/Ms-colonized Gpr41−/− and +/+ littermates using established methods, after an 18-h overnight fast (21, 22). FITC-labeled dextran (70,000 MW; Molecular Probes) was administered by gavage (100 μl of a 5-mg/ml solution prepared in PBS). Sixty minutes later, the entire GI tract from stomach to rectum was removed and placed in ice-cold PBS for 30 s to inhibit motility. The stomach, small intestine (divided into 10 equal length segments), cecum, and colon (subdivided into two equal-length segments) were each placed in a separate tube containing 1 ml of PBS (5 ml for stomach and cecum). The segments were coarsely chopped with scissors, and luminal contents suspended using a combination of vigorous washing and vortexing. A dilution series was completed for each sample (1:10 to 1:1000 in PBS) and the fluorescent signal quantified in a multiwell fluorescence plate reader (Stratagene Mx3000; excitation at 485 nm; emission at 530 nm). A histogram of the fluorescence signal distributed along the gastrointestinal tract was then plotted and the geometric center determined (SUM [% of total fluorescence per segment × segment number])/100) (23). Gastric emptying was calculated based on the amount of FITC-dextran left in the stomach compared with the total amount of fluorescence in the intestine.
The methods used for measurement of serum proteins, cecal, and fecal SCFAs, plus triglycerides are described in the SI Materials and Methods.
Statistical Analysis.
Unless otherwise noted, the significance of differences noted among different groups of mice was defined using ANOVA and Tukey's posthoc test.
Supplementary Material
Acknowledgments.
The authors thank Sabrina Wagoner, David O'Donnell, Maria Karlsson, Trey Coleman, Shelley Dixon, Randal Floyd, Amber Skach, and Marcus Thornton for technical assistance; Peter Turnbaugh, Peter Crawford, and Robert Heuckeroth for helpful advice; and Laura Kyro for graphics support. This work was supported in part by Graduate Research Fellowship DGE-0202737 from the National Science Foundation (to B.S.S.), by Grants DK70977 and DK30292 from the National Institutes of Health, and by grants from the W.M. Keck Foundation, Exploratory Research for Advanced Technology program of the Japan Science and Technology Agency, and The Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808567105/DCSupplemental.
References
- 1.Backhed F, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flint HJ, et al. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nature Rev Microb. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 3.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport L-lactate as well as butyrate. J Physiol. 1998;513:719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Turnbaugh P, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;17:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnenburg JL, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 7.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA. 2006;103:10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AJ, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 10.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth KA, Hertz JW, Gordon JI. Mapping enteroendocrine cell populations in transgenic mice reveals an unexpected degree of complexity in cellular differentiation within the gastrointestinal tract. J Cell Biol. 1990;110:1791–1801. doi: 10.1083/jcb.110.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunemann M, Hosey MM. G-protein coupled receptor kinases as modulators of G-protein signaling. J Physiol. 1999;517:5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briscoe CP, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa A, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 16.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 17.Lin HC, Neevel C, Chen JH. Slowing intestinal transit by PYY depends on serotonergic and opioid pathways. Am J Physiol. 2004;286:G558–G563. doi: 10.1152/ajpgi.00278.2003. [DOI] [PubMed] [Google Scholar]
- 18.Yanagisawa H, et al. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- 19.Hooper LV, et al. In: Mol Cell Microbiol. Sansonetti P, Zychlinsky A, editors. San Diego, CA: Academic; 2002. pp. 559–589. [Google Scholar]
- 20.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore BA, Otterbein LE, Turler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterol. 2003;124:377–391. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- 22.Wehner S, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981;6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.