Abstract
Pituitary adenylyl cyclase activating polypeptide, 38 amino acids (PACAP38) is a brain-gut peptide with diverse physiological functions and is neuroprotective in several models of neurological disease. In this study, we show that systemic administration of PACAP38, which is transported across the blood-brain barrier, greatly reduces the neurotoxicity of methamphetamine (METH). Mice treated with PACAP38 exhibited an attenuation of striatal dopamine loss after METH exposure as well as greatly reduced markers of oxidative stress. PACAP38 treatment also prevented striatal neuroinflammation after METH administration as measured by overexpression of glial fibrillary acidic protein (GFAP), an indicator of astrogliosis, and glucose transporter 5 (GLUT5), a marker of microgliosis. In PACAP38 treated mice, the observed protective effects were not due to an altered thermal response to METH. Since the mice were not challenged with METH until 28 days after PACAP38 treatment, this suggests the neuroprotective effects are mediated by regulation of gene expression. At the time of METH administration, PACAP38 treated animals exhibited a preferential increase in the expression and function of the vesicular monoamine transporter (VMAT2). Genetic reduction of VMAT2 has been shown to increase the neurotoxicity of METH, thus we propose that the increased expression of VMAT2 may underlie the protective actions of PACAP38 against METH. The ability of PACAP38 to increase VMAT2 expression suggests that PACAP38 signaling pathways may constitute a novel therapeutic approach to treat and prevent disorders of dopamine storage.
Introduction
Pituitary adenylyl cyclase activating polypeptide, 38 amino acids (PACAP38) is a brain-gut peptide member of the vasoactive intestinal peptide (VIP) / secretin / glucagon family first isolated from sheep hypothalamus (Miyata, et al. 1989, Arimura 2007). PACAP38 and a related peptide, PACAP27, along with VIP have been shown to activate three G protein coupled receptors: PAC1, which has much higher affinity for PACAP variants, and VPAC1/VPAC2 which bind PACAP variants and VIP (Vaudry, et al. 2000, Fahrenkrug 2006). PACAP38 has been shown to have several roles in vertebrates such as a neurotransmitter, neuroprotectant, enzyme activity regulator and activator of neural stem cells (Arimura 1998, Hamelink, et al. 2002, Mercer, et al. 2004, Somogyvari-Vigh and Reglodi 2004, Dejda, et al. 2005, Shioda, et al. 2006, Yang, et al. 2006, Bobrovskaya, et al. 2007).
Several recent studies have demonstrated the ability of PACAP38 to protect neurons in culture. PACAP38 can attenuate hydrogen peroxide toxicity, while cells from mutant animals that do not express PACAP38 are more sensitive to oxidative damage (Vaudry, et al. 2002, Vaudry, et al. 2005). PACAP38 has also been shown to protect cells from a diverse array of toxic insults of clinical significance, such as β-amyloid fragements (Alzheimer’s), prion protein (Creuztfeldt-Jakob), glycoprotein-120 (HIV), and excitotoxicity (Morio, et al. 1996, Brenneman, et al. 2002, Onoue, et al. 2002a, Onoue, et al. 2002b, Atlasz, et al. 2006). Furthermore, PACAP38 has in vivo neuroprotective activity as well in models of traumatic brain injury, and focal as well as global ischemia (Uchida, et al. 1996, Reglodi, et al. 2002, Farkas, et al. 2004, Reglodi, et al. 2004a, Chen, et al. 2006). These properties make PACAP38 and its signaling pathways interesting therapeutic targets for the treatment and prevention of neurological disease. Most intriguing from the standpoint of neurological therapeutic development is that systemic administration of PACAP38 can have central nervous system effects by crossing the blood-brain barrier via a saturable transporter (Banks, et al. 1993, Uchida, et al. 1996, Dogrukol-Ak, et al. 2004).
The receptor with the highest affinity for PACAP38, PAC1, is present in both the substantia nigra and striatum, the cell bodies and terminal fields of the nigrostriatal dopamine system, which degenerate in Parkinson’s disease (Hornykiewicz 1975, Vaudry, et al. 2000). Additionally, PACAP38 can attenuate neuronal damage from 6-hydroxydopamine (6-OHDA), a Parkinson’s disease model toxicant, when administered to primary mesencephalic cultures or co-infused into the substantia nigra of adult rats (Takei, et al. 1998, Reglodi, et al. 2004b). Since PACAP38 has been shown to be neuroprotective against 6-OHDA, we decided to examine the effects of PACAP38 on the neurotoxicity of systemically-administered methamphetamine (METH).
METH neurotoxicity depends on the transporter proteins that package and recycle dopamine and PACAP38 has been suggested to alter this transport (Fumagalli, et al. 1998, Takei, et al. 1998, Fumagalli, et al. 1999, Vergo, et al. 2007). Dysfunction of the dopamine transporter (DAT) and the vesicular monoamine transporter 2 (VMAT2) caused by METH leads to a loss of dopamine from the neuron and oxidative stress (LaVoie and Hastings 1999, Miyazaki, et al. 2006). Furthermore, METH’s damaging effects on the neurons promote neuroinflammation (O'Callaghan and Miller 1994, Thomas, et al. 2004b, Kuhn, et al. 2006). Prolonged neuroinflammation, measured by indicator proteins expressed on activated astroglia and microglia, can lead to neurodegeneration (Aschner 1998, LaVoie, et al. 2004, Whitton 2007). Thus, we tested the ability of PACAP38 to prevent the possible neurochemical, oxidative, and neuroinflammatory damage induced by METH exposure.
Methods
Animals
7 month old retired breeder male C57BL6 mice from Charles River Laboratories were used in these studies in accordance with the Institutional Animal Care and Use Committee policies of Emory University. Mice received chow and water ad lib and were on a 12:12 light cycle (lights on at 7:00 AM).
Drug administration
PACAP38 lyophilized powder (Bachem; King of Prussia, PA) was dissolved in PBS with 0.1% mouse serum albumin (MSA; Sigma, St. Louis, MO). Subcutaneous osmotic minipumps (Alzet model 1007D; Cupertino, CA) were filled with PACAP38 solution and implanted mid-scapularly into mice. Seven days later, after a cumulative dose of 1 mg/kg PACAP38, the minipumps were removed. Mice were then administered methamphetamine or saline four weeks later in order for PACAP38 to exert a long term regulatory effect. Methamphetamine (Sigma; St. Louis, MO) was dissolved in 0.9% saline and administered subcutaneously in a volume of 100 µL. Mice received a total four doses of 15 mg/kg (free base) methamphetamine given two hours apart.
Core temperature measurement
Core body temperature was monitored rectally by use of a digital thermometer (VWR International, Westchester, PA) lubricated with AstroGlide (BioFilm, Inc., Vista, CA).
Western blotting
Western blots were used to quantify the amount of dopamine transporter (DAT), tyrosine hydroxylase (TH), glial fibrillary acidic protein (GFAP), glucose transporter 5 (GLUT5), vesicular monoamine transporter 2 (VMAT2), and α-tubulin present in samples of striatal tissue and were performed as previously described (Caudle, et al. 2007, Hatcher, et al. 2007). Briefly, unilateral striata were homogenized and subjected to polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes (PVDF). Blots were incubated in 7.5% nonfat dry milk in Tris-buffered saline for 1 hour room temperature. Membranes were then incubated overnight with an antibody to DAT (1:5,000; Chemicon, Temecula, CA). Primary antibody binding was detected using a goat anti-rat horseradish peroxidase secondary antibody (1:10,000; Jackson Immuno Research) and enhanced chemiluminescence (SuperSignal; Pierce, Rockford, IL). Luminescence was captured with a Fluorochem 8800 (Alpha Innotech, San Leandro, CA) imaging system. Densitometric analysis was performed and calibrated to co-blotted dilutional standards of pooled striata from all control samples. Membranes were stripped at for 15 minutes at room temperature with stripping buffer (Restore; Pierce, Rockford, IL) and reprobed with antibodies against TH (1:1,000; Chemicon, Temecula, CA), GFAP (1:5000; Sigma, St. Louis, MO), GLUT5 (1:5000; Chemicon, Temecula, CA), and VMAT2 (1:1,000; Chemicon, Temecula, CA) antibody. Alpha-tubulin (1:5000; Sigma, St. Louis, MO) blots were used to ensure equal protein loading across samples.
Vesicular dopamine uptake
Bilateral striata were prepared as previously described (Teng, et al. 1997, Caudle, et al. 2007). Striata were homogenized in buffer (HEPES 4 mM, sucrose 0.32 M, pH 7.4) Homogenates were centrifuged at 1000 × g for 10 minutes and the resulting supernatant was centrifuged at 20,000 × g for 20 minutes. The resulting pellet was resuspended in 1.6 mL 0.32 M sucrose before being transferred to a glass/Teflon homogenizer containing 6.4 mL water and subjected to 10 up-and-down strokes by hand. All contents of the homogenizer were then poured into a tube containing 1 mL each of 250 mM HEPES and 1M potassium tartrate and inverted to mix. The mixture was then centrifuged at 20,000 × g for 20 minutes and the resulting supernatant was placed in an ultracentrifuge tube and spun at 120,000 × g for 2 hours. Vesicles were resuspended in 1.8 mL buffer (potassium tartrate 100 mM, HEPES 25 mM, EDTA 0.1 mM, EGTA 0.05 mM, ascorbate 1.7 mM, pH 7.4) Uptake assays utilized 300 µL vesicle solution for each dopamine concentration, with 2% [3H]-dopamine as a tracer and 10 µM tetrabenazine to define specific uptake. Samples were incubated for 10 minutes at 30°C followed by the addition of [3H] dopamine and further incubation for 5 minutes at 30°C. The assay was terminated by addition of 5 mL ice-cold assay buffer before filtration through 0.5% PEI-soaked Whatman GF/F filters (Brandel, Inc., Gaithersburg, MD). Filters were then placed in scintillation fluid and counted using a Beckman LS6500 (Location). Velocity was expressed as pmol dopamine/mg protein/min and the kinetic parameters, KM and VMAX, were calculated by nonlinear regression using Prism 4.0 software (GraphPad, San Diego, CA).
Immunoautoradiography
Whole brains from control and PACAP38 treated animals were prepared and analyzed as previously decribed (Staley, et al. 1997). Briefly, animals were perfused transcardially with 4% paraformaldehyde. Brains were then removed, frozen, and cut to a thickness of 40 µm on a freezing microtome. Sections were incubated with a rabbit polyclonal antibody against VMAT2 (1:1000; Pel-Freez, Rogers, AK), for 48 hours at 4°C in 10% horse serum while adjacent non-primary control sections were incubated only in horse serum. Sections were then rinsed in TBS and incubated with an [125I] donkey anti-rabbit IgG (GE Healthcare, Fairfield, CT) for 2 hours at room temperature. Sections were rinsed in TBS, mounted on slides and allowed to air dry overnight. Slides and microscale standards (GE Healthcare, Fairfield, CT) were apposed to BioMax MS film (Kodak, Rochester, NY) for 24 hours and developed using an XOMAT (Kodak, Rochester, NY). Films were scanned (Expression 1680; Epson, Longbeach, CA), stored as digital files, and analyzed using Analytical Imaging Station 3.0 rev 1.7 (Imaging Research, Ontario, Canada). Images were analyzed for anatomical location and pixel intensity.
Neurotransmitter and metabolite detection
HPLC-EC analysis of dopamine and its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) was performed as previously described (Caudle, et al. 2006, Hatcher, et al. 2007). Dissected left striata were sonicated in 0.1 M perchloric acid containing 347 µM sodium bisulfite and 134 µM EDTA. Homogenates were centrifuged at 15,000 × g for 10 minutes at 4°C, the supernatant was removed, and filtered through a 0.22 µm filter by centrifugation at 15,000 × g for 10 minutes at 4°C. The supernatants were then analyzed for levels of DA, DOPAC, HVA using HPLC with a coulometric electrode detector (Waters, Inc., Milford, MA). Quantification was made by reference to calibration curves made with individual standards.
Immunohistochemistry
Tissue staining was performed as previously described (Caudle, et al. 2007). Briefly, animals were perfused transcardially with 4% paraformaldehyde. Brains were then removed, frozen, and cut to a thickness of 40 µm on a freezing microtome. Sections were incubated with antibodies against DAT (1:750; Chemicon, Temecula, CA), TH (1:2,000; Chemicon, Temecula, CA), or GFAP (1:1000; Sigma, St. Louis, MO) overnight at 4°C and then incubated in a biotinylated goat ant-rat secondary antibody for 1 hour at room temperature. Sections were then incubated 1 hour room temperature in avidin-biotin-HRP conjugate solution from Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Visualization was performed using 0.03% 3, 3’-diaminobenzidine (DAB) for 3 minutes at room temperature. Sections were incubated with biotinylated isolectin B4 (1:1000; Invitrogen, Carlsbad, CA) overnight at 4°C and then incubated 1 hour room temperature in avidin-biotin-HRP conjugate solution from Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Visualization was performed using DAB for 25 minutes at room temperature.
Protein carbonyl detection
Protein carbonyl levels in striatal tissue were determined using oxyblots as previously described (Caudle, et al. 2007, Hatcher, et al. 2007). Briefly, protein carbonyl levels were determined using the Oxyblot Protein Detection Kit (Chemicon, Temecula, CA) according to the manufacturer’s protocol. Protein carbonyls are derivatized to 2,4- dinitrophenylhydrazone (DNP) by reaction with concentrated 2,4-dinitrophenylhydrazine. DNP-derivatized protein samples were analyzed by dot blots, using a primary antibody against DNP (1:1500; Sigma, St Louis, MO) and an HRP conjugated goat anti-rabbit secondary (1:10000; Jackson Immuno Research, West Grove, PA).
Statistics
All statistical analysis was performed on raw data for each treatment group by oneway ANOVA or Student’s t-test using Prism 4.0 (GraphPad, San Diego, CA). Post-hoc analysis was performed using Student-Newman Keuls (SNK) test. Statistical significance is reported at the p < 0.05 level.
Results
PACAP38 does not alter dopamine levels and increases VMAT2
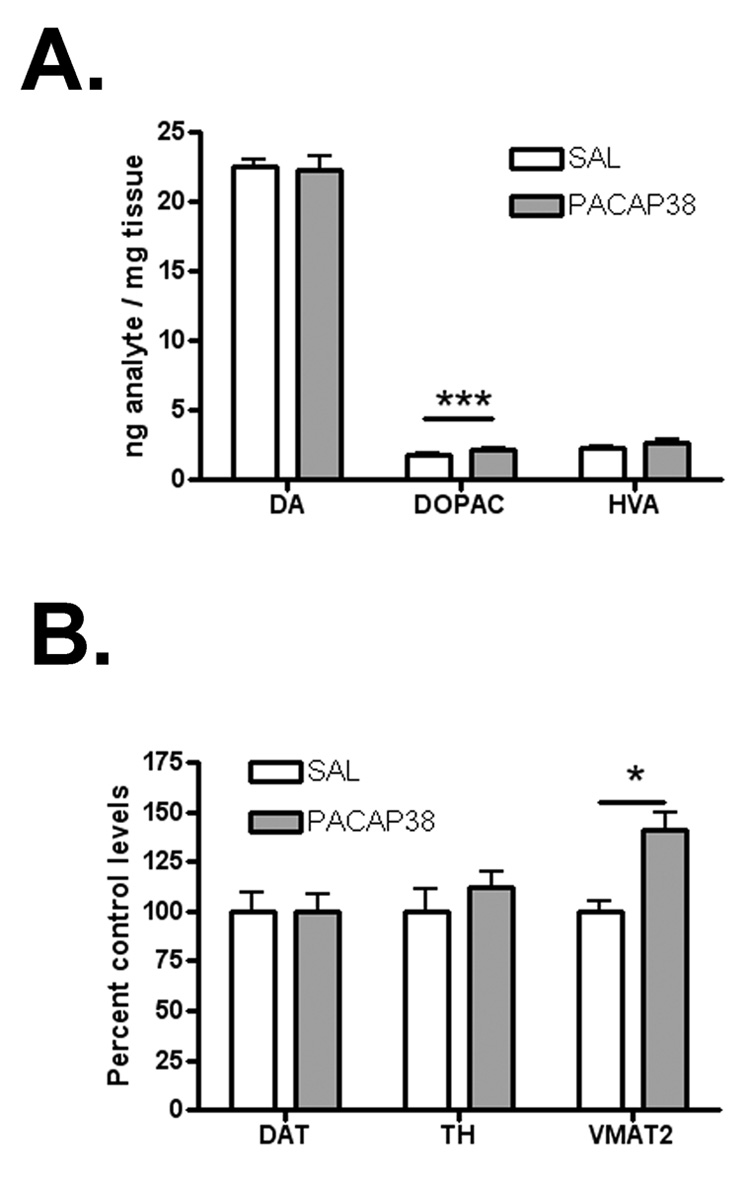
Four weeks after PACAP38 treatment, dopamine levels in the striatum were unchanged, from 22.56 ± 0.39 to 22.22 ± 1.05 ng/mg tissue (n = 6–8; ns; Figure 1A). DOPAC was increased by 22%, from 1.79 ± 0.14 to 2.18 ± 0.11 ng/mg tissue (n=6–8; p < 0.001) and HVA was not significantly changed, from 2.33 ± 0.07 to 2.70 ± 0.16 ng/mg tissue (n=6–8; ns). VMAT2 was selectively increased by 41% without a significant change in two other DA terminal markers, DAT and TH (n = 6–8; p < 0.05; Figure 1B).
Figure 1.
(A) Dopamine and HVA are not changed by PACAP38 treatment, while DOPAC is increased by 22%. (B) VMAT2 in the striatum is increased by 41% as measured by immunoblot without a change in DAT or TH. * indicates p < 0.05, *** indicates p < 0.001
PACAP38 increases striatal VMAT2 expression and function
Immunoautoradiographic densitometry was performed four weeks after PACAP treatment and showed that only the striatum has a significant increase of VMAT2 of 33% over control sections. Other regions that express VMAT2 displayed a trend of increased density but this did not reach the level of significance (n = 3; p < 0.05; Figure 2A).
Figure 2.
(A) regional densitometric analysis of VMAT2 immunoautoradiography shows a 33% increase in the striatum (STR) without a significant change in the cortex (CTX), midbrain (MB), or hippocampus (HIPP). (B) VMAX of dopamine transport in isolated striatal vesicles is increased by 56% after PACAP38 treatment (18.40 ± 1.17 to 28.64 ± 2.35 pmol/mg/min, p < 0.05), without a change in KM (236.1 ± 54.1 versus 269.1 ± 38.6 nM, ns). * indicates p < 0.05
Similarly, vesicles from the striatum of PACAP38 treated animals exhibited a 56% greater VMAX (28.64 ± 2.35 pmol/mg/min) versus controls (18.40 ± 1.17 pmol/mg/min) for tetrabenazine-sensitive dopamine transport (n = 6; p < 0.001; Figure 2B). The KM of dopamine for control vesicles (236.1 ± 54.1 nM) was not significantly different from vesicles derived from PACAP38 treated animals (269.1 ± 38.6 nM).
PACAP38 treatment attenuates dopamine loss after methamphetamine
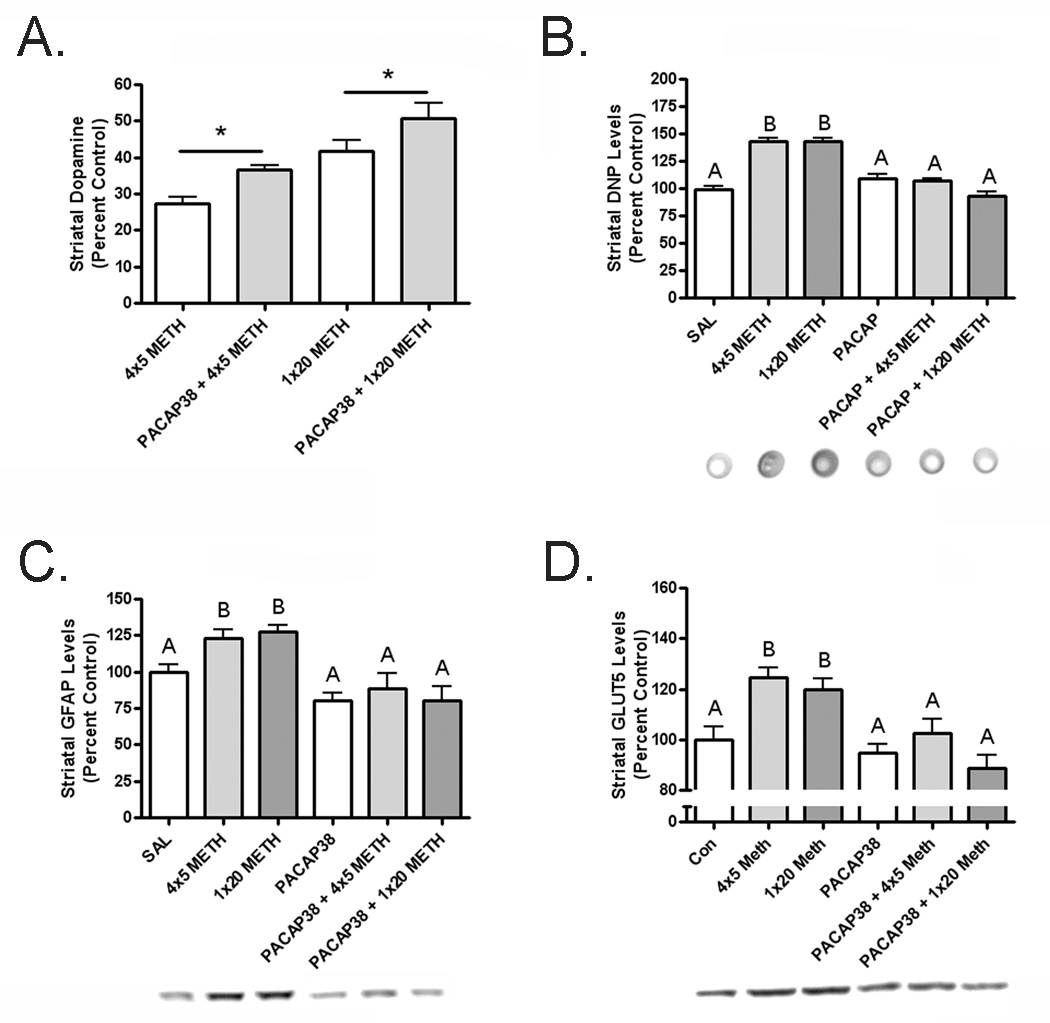
After treatment of PACAP38, animals were given a neurotoxic regimen of METH and assayed for neurochemistry two days later. Animals treated with four doses of 5 mg/kg METH exhibited a 72% loss of striatal DA, down to 6.18 ± 0.42, whereas those animals pretreated with PACAP38 only lost 62%, reduced to 8.27 ± 0.28 (n = 6–8; p < 0.05). Furthermore, animals administered one dose of 20 mg/kg METH lost 60% of striatal DA, decreased to 9.39 ± 0.74, but PACAP38 pretreated animals lose 50%, down to 11.42 ± 0.97 (n = 6–8; p < 0.05; Figure 3A).
Figure 3.
(A) Dopamine loss is attenuated after methamphetamine in PACAP38 treated mice; 4 × 5 mg/kg methamphetamine reduced dopamine 72% in control and 62% in PACAP38 treated mice, while 1 × 20 mg/kg methamphetamine caused a 60% loss of dopamine in controls but only 50% loss in PACAP38 treated mice. (B) Methamphetamine increased protein carbonyls in the control 4 × 5 mg/kg group by 42.8% and by 43.1% in the control 1 × 20 mg/kg group. PACAP38 treated mice are not different from saline control levels before or after methamphetamine. (C) GFAP is increased by methamphetamine by 23% in the control 4 × 5 mg/kg and 28% in control 1 × 20 mg/kg dose groups while PACAP38 treated mice have no change in GFAP. (D) Methamphetamine elevated GLUT5 levels by 24% in control 4 × 5 mg/kg and 20% in control 1 × 20 mg/kg dose groups but it did not change GLUT5 expression in PACAP38 treated mice. * indicates p < 0.05, different letters indicate a significant difference of at least p < 0.05.
Oxidative stress is reduced in PACAP38 treated mice after METH
Protein carbonyls, a measure of oxidative stress, have been shown to increase after METH exposure (Gluck, et al. 2001). Control mice given 4 × 5 mg/kg METH exhibited an increase of 42.8%, while control mice administered 1 × 20 mg/kg METH had a 43.1% elevation in protein carbonyls two days later. PACAP38 treated mice given saline did not differ from control mice given saline and both of these groups exhibited identical levels of protein carbonyls as PACAP38 treated mice given METH (Figure 3B). This abolishment of the formation of protein carbonyls is indicative of oxidative stress prevention.
PACAP38 treated mice do not exhibit neuroinflammation markers after METH
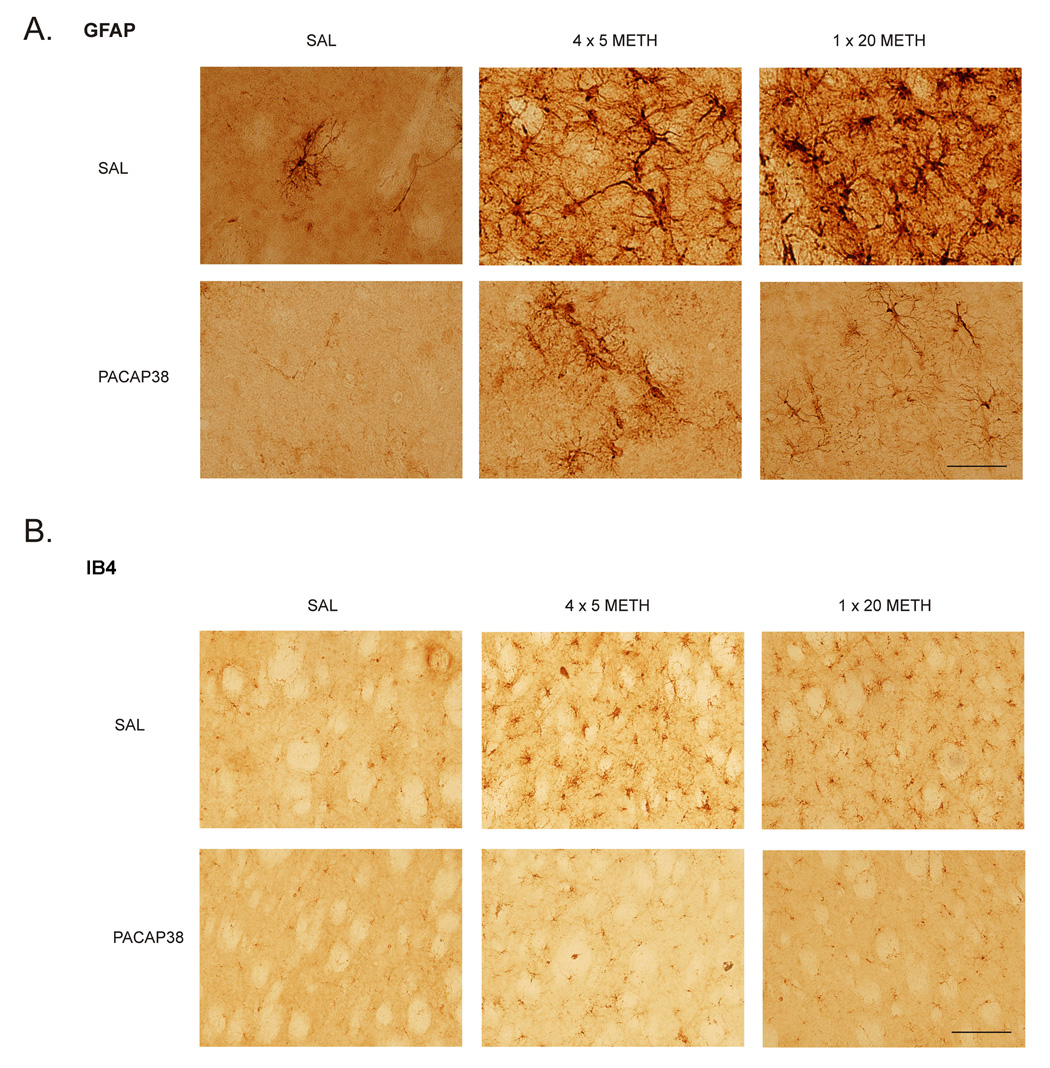
Activated astroglia, as measured by glial fibrillary acidic protein (GFAP) have been demonstrated to be present after neurotoxic insults, including METH administration (O'Callaghan and Miller 1994). Astroglia secrete chemokines that can be neuroprotective when present for a short time but become detrimental with a prolonged elevation. Control mice treated with 4 × 5 mg/kg METH had a 23% increase in striatal GFAP and 1 × 20 mg/kg METH treated mice showed a 28% elevation two days later. PACAP38 treated animals displayed nearly absent astroglial activation after METH. Furthermore, there was a non-significant trend towards an overall decrease in GFAP in PACAP38 treated mice (Figure 3C). The western blot data complemented that from the GFAP stain of the striatum in these animals (Figure 4A). The GFAP stained astrocytes in the PACAP38 treated mice appeared to have a more ramified morphology than the controls after METH, indicating less inflammation.
Figure 4.
(A) GFAP immunohistochemistry in the striatum. Robust astrogliosis after methamphetamine in the control groups is not present in PACAP38 striatum. The astroglia present in PACAP38 treated striatum are more ramified and less dense. Scale bar is 100 µm. (B) Isolectin IB4 staining in the striatum for microglia. Activated microglia are seen in methamphetamine treated control mice whereas their presence is diminished in the striatum of PACAP38 treated mice. Scale bar is 200 µm.
METH administration has also been shown to activate microglia in the striatum (Thomas, et al. 2004a). The sustained reactions of these neuroimmune cells are hypothesized to be a precursor to degeneration of the dopamine terminals (LaVoie, et al. 2004, Thomas, et al. 2004b). These activated microglia can be measured by observing the change in the glucose transporter 5 (GLUT5), a microglia-specific marker in the central nervous system (Payne, et al. 1997, Vannucci, et al. 1997, Richardson, et al. 2007). Control animals given METH at 4 × 5 mg/kg or 1 × 20 mg/kg had 24% and 20% increases in GLUT5 expression, respectively, two days after administration; PACAP38 pretreated animals did not show an increase at either dose of METH (Figure 3D). Stains for isolectin B4 (IB4) can also reveal activated microglia after METH administration (Thomas, et al. 2004a). Coronal sections from control mice treated with METH showed a robust increase in IB4 positive staining that is greatly attenuated in PACAP38 treated animals (Figure 4B).
PACAP38 does not alter thermal response to METH
Methamphetamine elevates core body temperature and some drugs that prevent methamphetamine neurotoxicity do so by decreasing the thermal response (Albers and Sonsalla 1995). One hour after subcutaneous injection of 5 mg/kg METH core body temperature in PACAP38 pretreated animals increased from 36.9 ± 0.2 °C to 37.8 ± 0.6 °C and control animals experienced an elevation from 36.7 ± 0.3 °C to 38.0 ± 0.5 °C. The METH induced increase in core temperature was not significant in control or PACAP38 animals until after the second s.c. injection of 5 mg/kg METH. Core temperature in control animals was elevated to 38.6 ± 0.5 °C and PACAP38 animals to 38.8 ± 0.6 °C. One hour after s.c. injection of 20 mg/kg METH, core temperature in control animals went up from 36.9 ± 0.2 °C to 38.7 ± 0.7 °C and PACAP38 treated animals increased from 36.7 ± 0.2 °C to 38.6 ± 0.6 °C (Figure 5).
Figure 5.

(A) Mice given 4 × 5 mg/kg methamphetamine did not show a temperature increase until after the second dose. Control and PACAP38 treated mice did not have different thermal responses to METH. (B) Mice treated with saline or PACAP38 then administered one dose of 20 mg/kg METH had identical temperature increases. * indicates p < 0.05
Discussion
PACAP38 has proved to be a highly versatile peptide in the central nervous system. Among its roles as a neurotransmitter and determinant of neuronal phenotype, it has several general neuroprotective actions: decreased oxidative stress, increased output of neurotrophic compounds by astrocytes, prevention of inflammation, and the attenuation of apoptosis (Delgado, et al. 2002, Vaudry, et al. 2002, Delgado, et al. 2003, Vaudry, et al. 2003, Reglodi, et al. 2004a, Ohtaki, et al. 2006, Yang, et al. 2006, Masmoudi-Kouki, et al. 2007). In reference to midbrain dopamine pathways, PACAP38 has demonstrated neuroprotective properties in the 6-hydroxydopamine model of Parkinson’s disease and PAC1, the receptor with the highest affinity for PACAP38, is present in high concentration in the nigrostriatal cell bodies and terminals (Vaudry, et al. 2000, Reglodi, et al. 2004b). This suggests that PACAP38 and PAC1 signaling pathways are positioned to directly confer neuroprotection to dopamine neurons against toxic insults.
Many studies investigating PACAP38 as a neuroprotective agent have utilized either a cell culture system or a direct infusion into the central nervous system immediately before or a short time after a challenge (Morio, et al. 1996, Reglodi, et al. 2000, Brenneman, et al. 2002, Dohi, et al. 2002, Onoue, et al. 2002a, Onoue, et al. 2002b, Reglodi, et al. 2004a). However, most intriguing from the standpoint of neurological therapeutic development is that systemic administration of PACAP38 can have central nervous system effects by crossing the blood-brain barrier (BBB) via a saturable transporter (Banks, et al. 1993, Dogrukol-Ak, et al. 2004). This transport efficiently allows systemic administration of PACAP to successfully prevent neuronal damage in models of stroke and ischemia (Uchida, et al. 1996, Reglodi, et al. 2002, Chen, et al. 2006, Ohtaki, et al. 2006).
PACAP38 can raise the activity of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis (Bobrovskaya, et al. 2007). However, dopamine content was not altered in the PACAP38 treated mice (Figure 1A). DOPAC was slightly increased, but this may be due to an effect of monoamine oxidase (MAO) regulation, as PACAP knockout mice have decreased 5-hydroxyindoleacetic acid (5-HIAA), from altered MAO metabolism of serotonin (Shintani, et al. 2006). Neither the dopamine transporter (DAT) nor TH, two markers of dopamine terminal integrity, was changed after PACAP38 treatment. However, the expression of vesicular monoamine transporter 2 (VMAT2), the protein responsible for packaging dopamine into secretory vesicles, was elevated by 41% (Figure 1B). Subsequent immunoautoradiography showed that this increase occurs preferentially in the striatum (Figure 2A). Furthermore, uptake experiments confirmed that the velocity (VMAX) of dopamine transport in vesicles is elevated by 60% without changing the KM, consistent with an increase in functional protein (Figure 2B).
Several investigations have shown that drugs such as lithium and cocaine can increase brain levels of VMAT2 in the short term, but no consequences of this elevation has been reported (Zucker, et al. 2001, Schwartz, et al. 2007). The effect PACAP38 on VMAT2 in the striatum four weeks after a systemic dose of the peptide is notable, especially considering a report suggesting that VMAT2 is not regulated by many drugs that act on the dopamine system (Kilbourn, et al. 1996). While the mechanism is not known, this is a demonstration that there are PACAP38-mediated pathways by which VMAT2 can be increased in the brain, possibly for therapeutic benefit.
VMAT2 is present on small synaptic and dense core neurotransmitter vesicles and transports dopamine into the vesicles by the exchange of protons (Henry, et al. 1994). Changing the amount or activity of VMAT2 in a dopamine neuron relative to the plasmalemmal DAT is hypothesized to determine the neuron’s ability to resist the damaging effect of toxicants that affect dopamine sequestration (Miller, et al. 1999). For example, genetically decreased VMAT2 in mice leads to the slow development of Parkinsonian pathology and increased methamphetamine (METH) neurotoxicity (Fumagalli, et al. 1999, Caudle, et al. 2007). Also, it has been shown that women who possess VMAT2 promoter variants which increase vesicular dopamine uptake are relatively protected from late-onset Parkinson’s disease (Glatt, et al. 2006). Furthermore, a recent study reported two intriguing findings: that reducing VMAT2 in midbrain cultures by shRNA leads to terminal loss and that increasing VMAT2 expression by the use of viral vectors in PC-12 cells attenuates METH cytotoxicity (Vergo, et al. 2007). However, no studies investigating the consequence of elevated VMAT2 on METH neurotoxicity in vivo have yet been reported.
Mice that have had VMAT2 expression elevated by PACAP38 lose less dopamine after METH administration and do not experience an increase in markers of oxidative damage (Figure 3A & B). This suggests that the increase in VMAT2 expression mediated by PACAP38 may lead to less dopamine release from, and/or increased reuptake into, the vesicles after METH administration. An increase in cytosolic dopamine can lead to the formation of dopamine quinones, which generate oxidative stress and lead to neurodegeneration (LaVoie and Hastings 1999, Sulzer and Zecca 2000, Miyazaki, et al. 2006). It is therefore possible that a decrease in cytosolic oxidized dopamine products, mediated by higher VMAT2 transport velocity, is responsible for the abolition of oxidation stress markers in PACAP38 treated mice after METH exposure.
Neuroinflammation accompanies many types of neurotoxic insults is hypothesized to promote and exacerbate neurodegenerative disease (van Muiswinkel, et al. 2004, Whitton 2007). Additionally, oxidized dopamine products and METH have been shown to cause neuroinflammation (O'Callaghan and Miller 1994, LaVoie, et al. 2004, Thomas, et al. 2004a). Activated astroglia and microglia secrete chemokines and activate enzymes that can immediately aid damaged neurons but are harmful if the neuroinflammation is persistent.
Control mice administered METH have robust responses in glial fibrillary acidic protein (GFAP), a marker of astrogliosis, and glucose transporter 5 (GLUT5), an indicator of microgliosis. When mice are treated with PACAP38 four weeks prior to METH exposure, there is a complete abolition of neuroinflammation (Figure 2C & D and Figure 3A & B). While PACAP38 has been shown to induce the immediate increase in secretion of neuroprotective factors from astrocytes, this abrogation of METH induced astrogliosis occurs four weeks after PACAP38 administration. This observation suggests that the prevention of neuroinflammation may be due a long term regulatory effect, such as the elevation of VMAT2 and the subsequent decrease in oxidative stress after METH administration.
PACAP38 did attenuate the loss of dopamine induced by METH, although the level of protection was small. This was not unanticipated, as mice with genetically reduced VMAT2 have severely impaired dopamine homeostasis and variable toxicity to METH as measured by dopamine depletion (Fumagalli, et al. 1999, Caudle, et al. 2007). However, the level of protection indicated by other measures of neurotoxicity was profound. A possible explanation could be that, while there is still transmitter loss after METH due to massive release, there is not a prolonged accumulation of cytosolic dopamine in PACAP38 treated mice. Sensitive time-course studies would be needed to examine the kinetics of DA build up in the cytosol against the opposing forces of VMAT2 drawing DA into the vesicle and DAT leading DA out of the cell in order to accurately test this hypothesis. Currently these types of experiments are limited to chromaffin cell models (Mosharov, et al. 2003). However, a recent study suggests that newly synthesized dopamine is responsible for the neurotoxic effects of METH, so it is logical that increased vesicular transport could mitigate this toxicity (Thomas, et al. 2008).
The attenuation of neurotoxicity after METH in PACAP38-treated animals, if primarily due to VMAT2 elevation, may pose a challenge to the weak base model of how METH interacts with the DA vesicle (Sulzer and Rayport 1990, Sulzer, et al. 1992). Specifically, if elevations in the amount of VMAT2 can reduce toxicity, presumably due to a decrease in cytosolic dopamine after METH, it suggests that the ability of METH to collapse the pH gradient in the vesicle may not be a critical determinant of in vivo toxicity. If the pH gradient were collapsed, it should make no difference how much VMAT2 is on the vesicular membrane, since it would not be able to exchange protons for neurotransmitter. However, if the gradient could still provide energy for DA sequestration, this could theoretically counteract the DA releasing action of METH.
Reports indicate that VMAT2 function in the dopamine neurons is depressed up to 75% for 24 hours after METH exposure and function is probably impaired for up to seven days (Brown, et al. 2002, Ugarte, et al. 2003). In addition, several studies have demonstrated that the function of VMAT2 is compromised by a promotion of oxidative and nitrative stress, as well as redistributed to non-vesicular compartments after METH administration (Brown, et al. 2002, Terland, et al. 2006, Eyerman and Yamamoto 2007, Fleckenstein, et al. 2007). Despite the effects on VMAT2, METH seems to leave the vesicular pH gradient at least partially intact as well as some functional vesicular transport, which likely provides a protective effect. Thus, the total amount of vesicular dopamine transport capacity, and therefore the capacity of the functional fraction after METH exposure, most likely mediates the differences in neurotoxicity observed in mice with increased VMAT2 expression.
It would also be predicted that decreased expression of VMAT2 may contribute to a feed-forward exacerbation of oxidative damage after METH: if the lower velocity of vesicular transport cannot take up vesicularly-released and newly synthesized cytosolic dopamine, this would allow a greater excess of dopamine oxidation and further damage the pool of VMAT2 protein. These converging lines of evidence suggest that elevating the expression of functional VMAT2 in dopamine neurons in vivo will defend against spikes in cytosolic dopamine after administration of amphetamine or METH.
Immunoautoradiographic experiments show that the elevation of VMAT2 is strongest in the striatum, but also hints at a trend of enhancement in other regions such the midbrain, hippocampus, and cortex. All of these regions have PAC1 expression, so it is possible that this receptor responsible for modulating VMAT2 increase. The PAC1 receptor, however, has eight different splice variants and their relative distributions have not been thoroughly mapped in the brain (Vaudry, et al. 2000). It may be that only one or a few subtypes of PAC1 can modify VMAT2 expression in neurons. It is also possible that this effect is not mediated by PAC1, but could be due to the interactions of PACAP at VPAC1, VPAC2, or some other, unknown, receptor.
PACAP38 knockout mice have a decreased ability to regulate core temperature due to reduced levels of dopamine and norepinephrine in brown adipose tissue and injection of PACAP38 is known to raise core temperature (Gray, et al. 2002, Pataki, et al. 2003). It has also been recognized that METH leads to an increase in core body temperature and that this is integral to its neurotoxicity (Miller and O'Callaghan 1994, Metzger, et al. 2000, Ugarte, et al. 2003). Several compounds that reduce METH neurotoxicity have also been shown oppose the increase in core body temperature, thereby confounding the results (Albers and Sonsalla 1995, Ali, et al. 1996). It was therefore necessary to determine the thermal effects of METH on PACAP38 treated mice to ensure this did not account for the observed neuroprotection. It was found that control mice and PACAP38 treated mice exhibited identical thermal responses to METH at each dose. Therefore, the normal core temperature was not affected by PACAP38 nor was the thermal response to METH responsible for the decreased neurotoxicity of METH in animals treated with PACAP38.
PACAP38 has demonstrated neuroprotective properties in several models of neuronal toxicity including ethanol, B-amyloid, prion protein, and cerebral artery occlusion. The short term effects of PACAP38 in these models were to decrease oxidative stress and thereby protect neurons. We have shown that PACAP38 administration, followed by a four week incubation time, leads to a prevention of METH toxicity, suggesting a long term regulatory mechanism. The promoter region of VMAT2 is responsive to the signaling cascade induced by the gut peptide gastrin, and results in increased VMAT2 protein expression in the gastric mucosa (Watson, et al. 2001). It is possible that PACAP38 activates an analogous pathway to regulate VMAT2 in the brain.
We have also shown that this PACAP38 administration increases the expression of VMAT2, a protein known to regulate the toxicity of METH, and propose that the elevation of VMAT2 is primarily responsible for the observed neuroprotection against METH. This would affect neurotoxicity and, potentially, the addictive properties of METH: without a sufficient increase in cytosolic DA, it is predicted there should be diminished dopamine release from DAT, with presumably less postsynaptic over-stimulation to promote the reinforcing properties of the METH.
In summary, PACAP38 treatment prevented METH-induced toxicity in mice. This included reductions in dopamine loss, protein carbonyl formation, astrogliosis, and microgliosis after METH expression. Our data suggest that an elevation in VMAT2 expression underlies the protection, but other mechanisms cannot be ruled out. Elucidation of how PACAP38 regulates VMAT2 expression could lead to novel approaches to treat neurological and neuropsychiatric disorders that involve altered packaging of dopamine and other monoamines.
Acknowledgements
We would like to thank Dr. Andrew Jenkins for his technical expertise.
This work was supported by the Emory Collaborative Center for Parkinson’s Disease Environmental Research U54ES012068 and American Parkinson’s Disease Association (G.W.M.), F32ES013457 and R21ES013828 (J.R.R.), and an Environmental Protection Agency Science to Achieve Results Fellowship #91643701-0 (T.S.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Ali SF, Newport GD, Slikker W., Jr Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann N Y Acad Sci. 1996;801:187–198. doi: 10.1111/j.1749-6632.1996.tb17441.x. [DOI] [PubMed] [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Arimura A. PACAP: the road to discovery. Peptides. 2007;28:1617–1619. doi: 10.1016/j.peptides.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Aschner M. Astrocytes as mediators of immune and inflammatory responses in the CNS. Neurotoxicology. 1998;19:269–281. [PubMed] [Google Scholar]
- Atlasz T, Koszegi Z, Babai N, Tamas A, Reglodi D, Kovacs P, et al. Microiontophoretically applied PACAP blocks excitatory effects of kainic acid in vivo. Ann N Y Acad Sci. 2006;1070:143–148. doi: 10.1196/annals.1317.002. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:690–696. [PubMed] [Google Scholar]
- Bobrovskaya L, Gelain DP, Gilligan C, Dickson PW, Dunkley PR. PACAP stimulates the sustained phosphorylation of tyrosine hydroxylase at serine 40. Cell Signal. 2007;19:1141–1149. doi: 10.1016/j.cellsig.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Hauser JM, Spong C, Phillips TM. Chemokine release is associated with the protective action of PACAP-38 against HIV envelope protein neurotoxicity. Neuropeptides. 2002;36:271–280. doi: 10.1016/s0143-4179(02)00045-8. [DOI] [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, et al. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J Pharmacol Exp Ther. 2002;302:497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, et al. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson's disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Samal B, Hamelink CR, Xiang CC, Chen Y, Chen M, et al. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul Pept. 2006;137:4–19. doi: 10.1016/j.regpep.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejda A, Sokolowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep. 2005;57:307–320. [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Juarranz MG, Leceta J, Ganea D, et al. PACAP in immunity and inflammation. Ann N Y Acad Sci. 2003;992:141–157. doi: 10.1111/j.1749-6632.2003.tb03145.x. [DOI] [PubMed] [Google Scholar]
- Delgado M, Jonakait GM, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit chemokine production in activated microglia. Glia. 2002;39:148–161. doi: 10.1002/glia.10098. [DOI] [PubMed] [Google Scholar]
- Dogrukol-Ak D, Tore F, Tuncel N. Passage of VIP/PACAP/secretin family across the blood-brain barrier: therapeutic effects. Curr Pharm Des. 2004;10:1325–1340. doi: 10.2174/1381612043384934. [DOI] [PubMed] [Google Scholar]
- Dohi K, Mizushima H, Nakajo S, Ohtaki H, Matsunaga S, Aruga T, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) prevents hippocampal neurons from apoptosis by inhibiting JNK/SAPK and p38 signal transduction pathways. Regul Pept. 2002;109:83–88. doi: 10.1016/s0167-0115(02)00190-8. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J. PACAP--a multifacetted neuropeptide. Chronobiol Int. 2006;23:53–61. doi: 10.1080/07420520500464569. [DOI] [PubMed] [Google Scholar]
- Farkas O, Tamas A, Zsombok A, Reglodi D, Pal J, Buki A, et al. Effects of pituitary adenylate cyclase activating polypeptide in a rat model of traumatic brain injury. Regul Pept. 2004;123:69–75. doi: 10.1016/j.regpep.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem. 2001;79:152–160. doi: 10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–3954. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, et al. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Richardson JR, Guillot TS, McCormack AL, Di Monte DA, Jones DP, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204:619–630. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP, Botton D, Sagne C, Isambert MF, Desnos C, Blanchard V, et al. Biochemistry and molecular biology of the vesicular monoamine transporter from chromaffin granules. J Exp Biol. 1994;196:251–262. doi: 10.1242/jeb.196.1.251. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Brain monoamines and parkinsonism. Natl Inst Drug Abuse Res Monogr Ser. 1975:13–21. doi: 10.1037/e472122004-001. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Frey KA, Vander Borght T, Sherman PS. Effects of dopaminergic drug treatments on in vivo radioligand binding to brain vesicular monoamine transporters. Nucl Med Biol. 1996;23:467–471. doi: 10.1016/0969-8051(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi-Kouki O, Gandolfo P, Castel H, Leprince J, Fournier A, Dejda A, et al. Role of PACAP and VIP in astroglial functions. Peptides. 2007 doi: 10.1016/j.peptides.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Mercer A, Ronnholm H, Holmberg J, Lundh H, Heidrich J, Zachrisson O, et al. PACAP promotes neural stem cell proliferation in adult mouse brain. J Neurosci Res. 2004;76:205–215. doi: 10.1002/jnr.20038. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Haughey HM, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid decrease in dopamine transporter function: role of dopamine and hyperthermia. J Pharmacol Exp Ther. 2000;295:1077–1085. [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Diaz-Corrales FJ, Fukuda M, Kitaichi K, Miyoshi K, et al. Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinine-formation-related molecules. Faseb J. 2006;20:571–573. doi: 10.1096/fj.05-4996fje. [DOI] [PubMed] [Google Scholar]
- Morio H, Tatsuno I, Hirai A, Tamura Y, Saito Y. Pituitary adenylate cyclase-activating polypeptide protects rat-cultured cortical neurons from glutamate-induced cytotoxicity. Brain Res. 1996;741:82–88. doi: 10.1016/s0006-8993(96)00920-1. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Gong LW, Khanna B, Sulzer D, Lindau M. Intracellular patch electrochemistry: regulation of cytosolic catecholamines in chromaffin cells. J Neurosci. 2003;23:5835–5845. doi: 10.1523/JNEUROSCI.23-13-05835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue S, Endo K, Ohshima K, Yajima T, Kashimoto K. The neuropeptide PACAP attenuates beta-amyloid (1-42)-induced toxicity in PC12 cells. Peptides. 2002a;23:1471–1478. doi: 10.1016/s0196-9781(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Onoue S, Ohshima K, Endo K, Yajima T, Kashimoto K. PACAP protects neuronal PC12 cells from the cytotoxicity of human prion protein fragment 106-126. FEBS Lett. 2002b;522:65–70. doi: 10.1016/s0014-5793(02)02886-7. [DOI] [PubMed] [Google Scholar]
- Pataki I, Adamik A, Jaszberenyi M, Macsai M, Telegdy G. Involvement of transmitters in pituitary adenylate cyclase-activating polypeptide-induced hyperthermia. Regul Pept. 2003;115:187–193. doi: 10.1016/s0167-0115(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Payne J, Maher F, Simpson I, Mattice L, Davies P. Glucose transporter Glut 5 expression in microglial cells. Glia. 1997;21:327–331. doi: 10.1002/(sici)1098-1136(199711)21:3<327::aid-glia7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Fabian Z, Tamas A, Lubics A, Szeberenyi J, Alexy T, et al. Effects of PACAP on in vitro and in vivo neuronal cell death, platelet aggregation, and production of reactive oxygen radicals. Regul Pept. 2004a;123:51–59. doi: 10.1016/j.regpep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004b;151:303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Vigh S, Maderdrut JL, Arimura A. Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann N Y Acad Sci. 2000;921:119–128. doi: 10.1111/j.1749-6632.2000.tb06958.x. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Somogyvari-Vigh A, Szanto Z, Kertes E, Lenard L, et al. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 2007;23:2227–2234. doi: 10.1016/s0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Guillot TS, Watson JL, Nakamaru-Ogiso E, Seo BB, et al. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicol Sci. 2007;95:196–204. doi: 10.1093/toxsci/kfl133. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Nachman R, Yossifoff M, Sapir R, Weizman A, Rehavi M. Cocaine, but not amphetamine, short term treatment elevates the density of rat brain vesicular monoamine transporter 2. J Neural Transm. 2007;114:427–430. doi: 10.1007/s00702-006-0549-8. [DOI] [PubMed] [Google Scholar]
- Shintani N, Hashimoto H, Tanaka K, Kawagishi N, Kawaguchi C, Hatanaka M, et al. Serotonergic inhibition of intense jumping behavior in mice lacking PACAP (Adcyap1−/−) Ann N Y Acad Sci. 2006;1070:545–549. doi: 10.1196/annals.1317.079. [DOI] [PubMed] [Google Scholar]
- Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, et al. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci. 2006;1070:550–560. doi: 10.1196/annals.1317.080. [DOI] [PubMed] [Google Scholar]
- Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des. 2004;10:2861–2889. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- Staley JK, Talbot JZ, Ciliax BJ, Miller GW, Levey AI, Kung MP, et al. Radioligand binding and immunoautoradiographic evidence for a lack of toxicity to dopaminergic nerve terminals in human cocaine overdose victims. Brain Res. 1997;747:219–229. doi: 10.1016/s0006-8993(96)01196-1. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Pothos E, Sung HM, Maidment NT, Hoebel BG, Rayport S. Weak base model of amphetamine action. Ann N Y Acad Sci. 1992;654:525–528. doi: 10.1111/j.1749-6632.1992.tb26020.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox Res. 2000;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- Takei N, Skoglosa Y, Lindholm D. Neurotrophic and neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. J Neurosci Res. 1998;54:698–706. doi: 10.1002/(SICI)1097-4547(19981201)54:5<698::AID-JNR15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. Lobeline and nicotine evoke [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. J Pharmacol Exp Ther. 1997;280:1432–1444. [PubMed] [Google Scholar]
- Terland O, Almas B, Flatmark T, Andersson KK, Sorlie M. One-electron oxidation of catecholamines generates free radicals with an in vitro toxicity correlating with their lifetime. Free Radic Biol Med. 2006;41:1266–1271. doi: 10.1016/j.freeradbiomed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004a;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Uchida D, Arimura A, Somogyvari-Vigh A, Shioda S, Banks WA. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996;736:280–286. doi: 10.1016/0006-8993(96)00716-0. [DOI] [PubMed] [Google Scholar]
- Ugarte YV, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases mouse vesicular dopamine uptake: role of hyperthermia and dopamine D2 receptors. Eur J Pharmacol. 2003;472:165–171. doi: 10.1016/s0014-2999(03)01911-3. [DOI] [PubMed] [Google Scholar]
- van Muiswinkel FL, de Vos RA, Bol JG, Andringa G, Jansen Steur EN, Ross D, et al. Expression of NAD(P)H:quinone oxidoreductase in the normal and Parkinsonian substantia nigra. Neurobiol Aging. 2004;25:1253–1262. doi: 10.1016/j.neurobiolaging.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Basille M, Pamantung TF, Fontaine M, Fournier A, et al. Pituitary adenylate cyclase-activating polypeptide prevents C2-ceramide-induced apoptosis of cerebellar granule cells. J Neurosci Res. 2003;72:303–316. doi: 10.1002/jnr.10530. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vaudry D, Hamelink C, Damadzic R, Eskay RL, Gonzalez B, Eiden LE. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides. 2005;26:2518–2524. doi: 10.1016/j.peptides.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Pamantung TF, Basille M, Rousselle C, Fournier A, Vaudry H, et al. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci. 2002;15:1451–1460. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Watson F, Kiernan RS, Deavall DG, Varro A, Dimaline R. Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J Biol Chem. 2001;276:7661–7671. doi: 10.1074/jbc.M006697200. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yang J, Yang Z, Chen P, Fraser A, Zhang W, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther. 2006;319:595–603. doi: 10.1124/jpet.106.102236. [DOI] [PubMed] [Google Scholar]
- Zucker M, Weizman A, Harel D, Rehavi M. Changes in vesicular monoamine transporter (VMAT2) and synaptophysin in rat Substantia nigra and prefrontal cortex induced by psychotropic drugs. Neuropsychobiology. 2001;44:187–191. doi: 10.1159/000054941. [DOI] [PubMed] [Google Scholar]