Abstract
High-resolution solid-state NMR spectra of three full-length membrane proteins uniformly aligned in lipid bilayers between glass slides are observed at high magnetic field. The resolution of the specific amino acid labeled samples shows promise for large membrane protein structure determination utilizing aligned samples and shows resonance patterns known as PISA wheels. The tilt angles of the transmembrane helices are extracted from the resonance patterns in PISEMA spectra.
Progress has been made in membrane protein structure determination in lipid bilayer environments1. From the complete structure of gramicidin in 19931g to the structure of the M2 transmembrane domain with2 and without1e the antiviral drug amantadine, the structure of MerFt1a, etc., there are now ten structures in the Protein Data Bank characterized by aligned sample solid state NMR. Recent improvements in RF probe technology3 and in sample preparation have make it possible here to obtain spectra of uniformly aligned full-length membrane proteins displaying characteristic resonance patterns for their transmembrane (TM) α-helices4.
Sample preparation is the key to macromolecular structural characterization, whether it is crystallization for x-ray diffraction or cryo-EM, or homogeneous isotropic samples for solution NMR, or a uniformly aligned sample for solid state NMR. Here, from monomeric to octameric, from 3.5 to 82 kDa and from one to three TM helices per monomer we demonstrate uniform protein alignment in hydrated phospholipid bilayers on glass slides.
The helical structure prediction for three proteins is shown in Fig. 1. KdpF is a 30 residue protein (33 residues as expressed in E. coli) from the Mycobacterium tuberculosis genome. This protein appears to be a component of the Kdp K+ transporting complex8. It has a single putative TM helix and no predicted 2° structure for the terminal segments. Rv1861 is another putative membrane protein from the Mtb genome. It has a predicted ATP or GTP binding site in the N-terminal segment and three TM helices. Rv1861 is adjacent to the genes that code for molybdenum transport and may be involved in this transport function. Non-denaturing PAGE of this protein shows a narrow band at approximately 82 kDa suggesting an octameric state (see supplemental materials). Diacylglycerol kinase (DAGK) from E. coli is an extensively studied protein with an experimentally characterized secondary structure as illustrated in Fig. 112. In addition to the three TM helices there are two short amphipathic helices thought to be associated with the bilayer interface. Overall, this protein forms a native state trimer and therefore, a nine-helix bundle.
Figure 1.
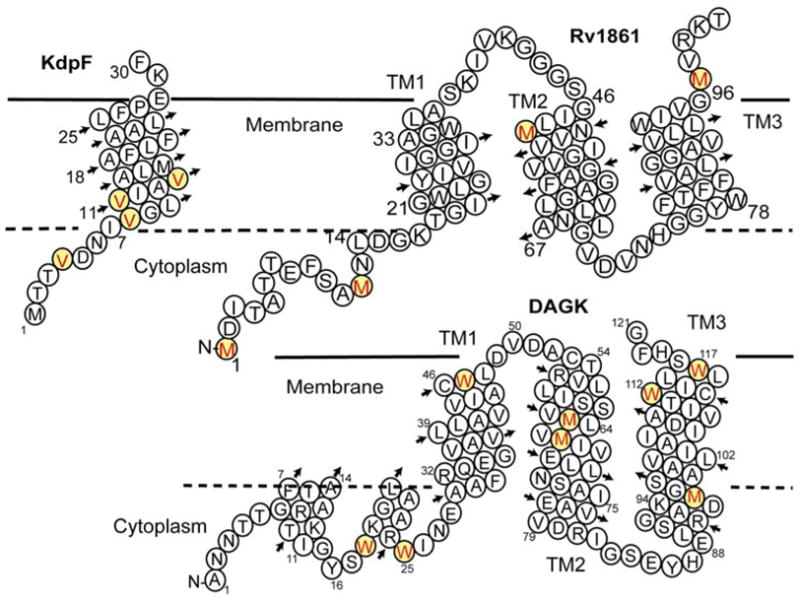
Secondary structure prediction for (A) KdpF and (B) Rv1861 both from Mtb, and (C) DAGK from E. coli5. This latter protein is a functional mutant engineered to be a particularly stable trimer6. Predictions for KdpF and Rv1861 were performed using TMHMM7. The Val, Met and Trp residues specifically labeled for PISEMA spectra are highlighted.
Fig. 2 shows the PISEMA spectra of KdpF, Rv1861, and DAGK in liquid crystalline lipid bilayer environments. Uniform helical structures generate patterns of resonances, known as PISA (Polar Index Slant Angles) wheels with 3.6 resonances per turn. Because it has been difficult to predict such wheels from crystallographic data in the Protein Data Bank due to the relatively low resolution of the membrane protein structures, the high sensitivity of the wheels to local structural variations and potential tensor variation (see supplemental materials), it has been questioned in the literature13 whether PISA wheels would be observed in membrane proteins. Here PISA wheels are clearly observed for two (KdpF and Rv1861) of the three proteins. We have previously argued that due to the scarcity of strong inter-helical interactions and the low dielectric of the membrane interstices that membrane proteins would have very regular helical TM structure10. Indeed, the uniformity of helical structures and the unusually short intra-helical hydrogen bonds have been documented10, 14, meaning that, in general, the helical structure is more uniform15,16 than observed in water soluble proteins where approximately 30% of all helical backbone carbonyls accept more than one hydrogen bond. The third protein, DAGK has TM helices displaying small tilt angles such that the PISA wheel, which disappears at a 0° tilt angle, is unresolved in the uniformly 15N labeled sample.
Figure 2.
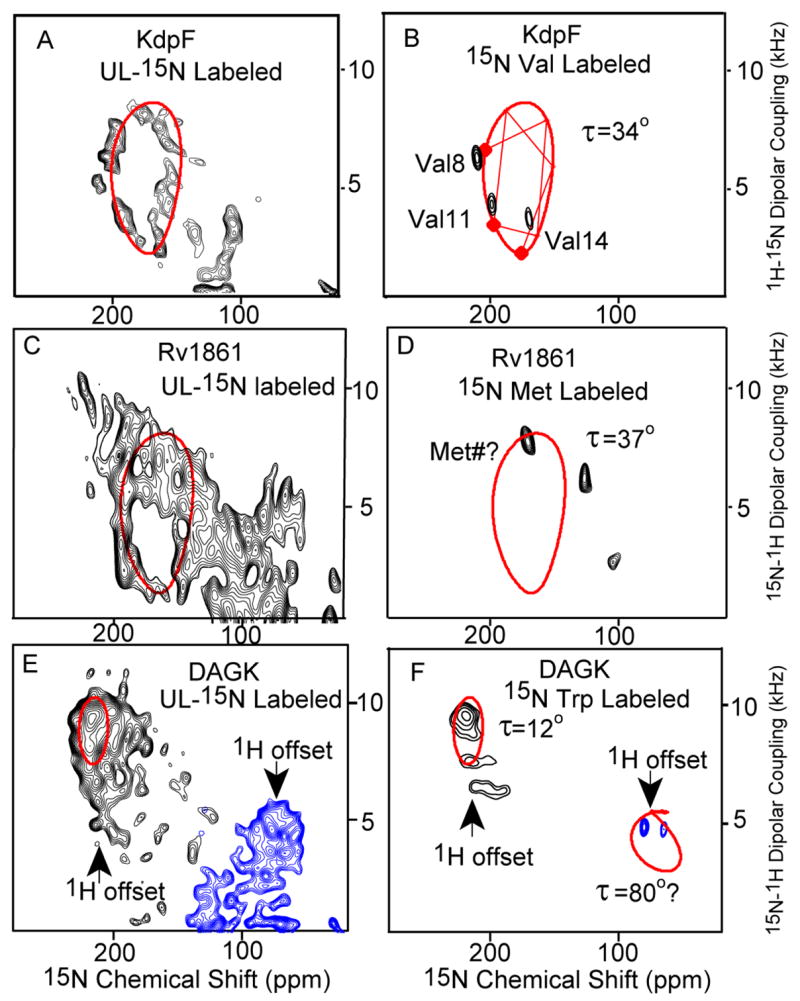
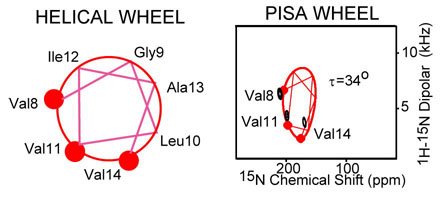
PISEMA spectra of KdpF (A&B), Rv1861 (C&D) and DAGK (E & F) of uniformly 15N-labeled (A,C&E) and amino acid specific labeled protein (B,D&F) expressed in E. coli and reconstituted into a mixture of lipids – dimyristoylphosphatidylcholine (DMPC) and phosphatidyl-glycerol (DMPG) in a 4:1 molar ratio. The samples were aligned between glass slides (see supplemental materials). Spectra were obtained with NHMFL low-E probes at 600 MHz except for the 15N UL KdpF spectrum which was obtained in the UWB 900 MHz magnet. The low-electric field feature was essential for this spectroscopy. 0.8 ms cross polarization contact time, an acquisition time of 4 ms during with SPINAL9 decoupling was applied, and a recycle delay of 6 s were used. To avoid the limitations of the 1H bandwidth the spectra of DAGK were obtained in two halves with different offset frequencies. Spectra acquisition time varies from 6 hours to 3 days. The PISA wheels were calculated using motionally averaged dipolar (ν|| = 10.375 kHz) and chemical shift tensors (σ11 = 57; σ22 = 81; σ33 = 228 ppm)10,11.
For KdpF the three valine residues in the TM helix are in a sequential pattern of i to i+3 to i+6. Based on a 100° inter-residue spacing about the helical axis the connectivity lines between the residues can be drawn on the wheel as in Fig. 2B resulting in the assignment of these resonances to residues Val8, Val11 and Val14. Therefore, not only can the tilt of this helix be characterized as 34° ± 3°, but the rotational orientation of the helix is also fixed by this residue specific assignment of the resonances. A fourth Val residue in the N-terminus may be highly dynamic generating a poorly cross-polarized signal and an 15N-1H dipolar coupling near 0 kHz.
Rv1861 has three putative TM helices and the PISEMA resonance intensity is distributed over most of the potential spectral area, and yet a characteristic wheel pattern is observed indicating a tilt of 37° ± 3°. Since the intensity pattern around the wheel is so strong we anticipate that two of the helices have this same tilt angle. While only three of the four Met resonances are observed, Met1 is likely to be highly dynamic, if it is not cleaved in the expressed protein. Of the remaining three Met residues Met49 is likely to be part of TM2 and Met97 to be part of TM3.
The helical tilt angles for the three TM helices of DAGK are smaller than those for KdpF and Rv1861. Unlike Rv1861 the intensity distribution is at the extremes of the dipolar and chemical shift ranges. All five of the Trp resonances are observed as shown in Fig. 2F. As predicted from the secondary structure characterization12 three of the resonances are in the TM region of the spectrum and two in the amphipathic surface bound region (70–80 ppm). TM3 has two Trp residues and a Met residue and TM2 has two additional Met residues. Shown in Fig. 3 are these resonances and theoretical PISA wheels reflecting a 9° and 15° helical tilt angle. The three observed resonances in TM3, Met96, Trp112, and Trp117 would be separated on the PISA wheel by 140° and 160°, respectively. For a regular helical structure these resonances would not be in the same half of the wheel and therefore the helical tilt is likely to be less than 15° and greater than 9° because of the separation of the Trp resonances or 12 ± 3°.
Figure 3.
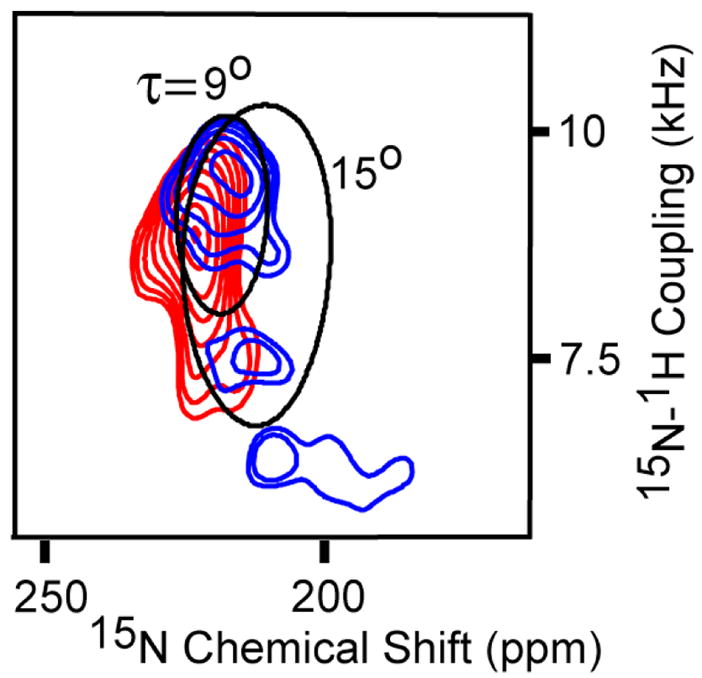
TM region of the PISEMA spectra of 15N-Trp (blue) and 15N-Met (red) labeled DAGK. Theoretical 9° and 15° PISA wheels are superimposed on spectra.
Proteins ranging in molecular weight from a 3.5 kDa monomer to an 82 kDa octamer have been uniformly aligned between glass slides demonstrating the feasibility of preparing full length membrane protein samples for solid state NMR structural characterization. Initial PISEMA spectra show that helical structures in membrane proteins can have a very regular structure resulting in resonance patterns known as PISA wheels.
Supplementary Material
Sample preparation information and additional spectra. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
This work was supported by GM-67476 from NIH and MCB-0235774 from NSF. The authors are grateful to C. Sanders for providing the DAGK plasmid and a protocol for DAGK sample purification. The experiments were largely conducted at the National High Field Magnetic Laboratory, supported by cooperative Agreement (DMR-0084173) and the State of Florida.
References
- 1.(a) De Angelis AA, Howell SC, Nevzorov AA, Opella SJ. J Am Chem Soc. 2006;128:12256–12267. doi: 10.1021/ja063640w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Traaseth NJ, Buffy JJ, Zamoon J, Veglia G. Biochemistry. 2006;45:13827–13834. doi: 10.1021/bi0607610. [DOI] [PubMed] [Google Scholar]; (c) Tian C, Gao FP, Pinto LH, Lamb R, Cross TA. Protein Sci. 2003;12:2597–2605. doi: 10.1110/ps.03168503. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Opella SJ, Marassi FM, Gesell JJ, Valente AP, Kim Y, Oblatt-Montal M, Montal M. Nat Struct Biol. 1999;6:374–379. doi: 10.1038/7610. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nishimura K, Kim S, Zhang L, Cross TA. Biochemistry. 2002;41:13170–13177. doi: 10.1021/bi0262799. [DOI] [PubMed] [Google Scholar]; (f) Kamihira M, Vosegaard T, Mason AJ, Straus SK, Nielsen NC, Watts A. J Struct Biol. 2005;149:7–16. doi: 10.1016/j.jsb.2004.10.002. [DOI] [PubMed] [Google Scholar]; (g) Ketchem R, Roux B, Cross TA. Structure. 1997;5:1655–1669. doi: 10.1016/s0969-2126(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 2.Hu J, Asbury TM, Achuthan S, Li C, Bertram R, Quine JR, Fu R, Cross TA. Biophys J. 2006;126 doi: 10.1529/biophysj.106.090183. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gor’kov PL, Chekmenev EY, Li C, Cotten M, Buffy JJ, Traaseth NJ, Veglia G, Brey WW. J Magn Reson. 2007;185:77–93. doi: 10.1016/j.jmr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wang J, Denny J, Tian C, Kim S, Mo Y, Kovacs F, Song Z, Nishimura K, Gan Z, Fu R, Quine JR, Cross TA. J Magn Reson. 2000;144:162–167. doi: 10.1006/jmre.2000.2037. [DOI] [PubMed] [Google Scholar]; (b) Marassi FM, Opella SJ. J Magn Reson. 2000;144:150–155. doi: 10.1006/jmre.2000.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Opella SJ, Nevzorov A, Mesleb MF, Marassi FM. Biochem Cell Biol. 2002;80:597–604. doi: 10.1139/o02-154. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Quine JR, Cross TA, Chapman MS, Bertram R. Bull Math Biol. 2004;66:1705–1730. doi: 10.1016/j.bulm.2004.03.006. [DOI] [PubMed] [Google Scholar]; (e) Ramamoorthy A, Wei YF, Lee DK. Ann Rep NMR Spectrosc. 2004;52:1–52. [Google Scholar]
- 5.Lightner VA, Bell RM, Modrich P. J Biol Chem. 1983;258:10856–10861. [PubMed] [Google Scholar]
- 6.Zhou Y, Bowie JU. J Biol Chem. 2000;275:6975–6979. doi: 10.1074/jbc.275.10.6975. [DOI] [PubMed] [Google Scholar]
- 7.Melen K, Krogh A, von Heijne G. J Mol Biol. 2003;327:735–744. doi: 10.1016/s0022-2836(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 8.Gassel M, Mollenkamp T, Puppe W, Altendorf K. J Biol Chem. 1999;274:37901–37907. doi: 10.1074/jbc.274.53.37901. [DOI] [PubMed] [Google Scholar]
- 9.Fung BM, Khitrin AK, Ermolaev K. J Magn Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Cross TA. Biophys J. 2002;83:2084–2095. doi: 10.1016/S0006-3495(02)73969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemical shifts are referenced to liquid ammonia at 0 ppm via a saturated solution of 15NH4NO3 at 26 ppm. Previous work from this lab referenced 15NH4NO3 at 0 ppm.
- 12.Oxenoid K, Kim HJ, Jacob J, Sönnichsen FD, Sanders CR. J Am Chem Soc. 2004;126:5048–5049. doi: 10.1021/ja049916m. [DOI] [PubMed] [Google Scholar]
- 13.Straus SK, Scott WR, Watts A. J Biomol NMR. 2003;26:283–295. doi: 10.1023/a:1024098123386. [DOI] [PubMed] [Google Scholar]
- 14.Olivella M, Deupi X, Govaerts C, Pardo L. Biophys J. 2002;82:3207–3213. doi: 10.1016/S0006-3495(02)75663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Kim S, Kovacs F, Cross TA. Protein Sci. 2001;10:2241–2250. doi: 10.1110/ps.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luecke H, Richter HT, Lanyi JK. Science. 1998;280:1934–1937. doi: 10.1126/science.280.5371.1934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample preparation information and additional spectra. This material is available free of charge via the internet at http://pubs.acs.org.