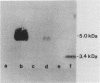
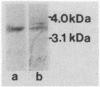
Abstract
The lipooligosaccharides (LOS) of strains of Haemophilus ducreyi, Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica contain epitopes that are antigenically and structurally similar to carbohydrates present in human glycosphingolipids. LOS from strains of Haemophilus influenzae and H. influenzae biogroup aegyptius were tested for the binding of monoclonal antibodies (MAbs) that bind to human glycosphingolipids possessing Gal beta 1-4GlcNAc (MAb 3F11) and Gal alpha 1-4Gal beta 1-4Glc (MAb anti-Pk). In solid-phase radioimmunoassays, the LOS of 18 of 19 H. influenzae type b (Hib), 8 of 19 nontypeable H. influenzae, and 10 of 20 H. influenzae biogroup aegyptius strains bound MAb anti-Pk. The LOS of 13 of 19 Hib, 10 of 16 nontypeable H. influenzae, and 2 of 18 H. influenzae biogroup aegyptius strains bound MAb 3F11. Neuraminidase treatment of the strains increased the binding of MAb 3F11 by more than twofold in 47% of the H. influenzae strains, suggesting that sialic acid occluded the LOS structure recognized by MAb 3F11. The material released from neuraminidase-treated Hib LOS was confirmed to be sialic acid by high-performance anion-exchange chromatography. A recombinant plasmid containing genes involved in Hib LOS biosynthesis directed the expression (assembly) of the 3F11 epitope in Escherichia coli. These studies demonstrate that H. influenzae and H. influenzae biogroup aegyptius express at least two LOS epitopes that are similar to those present in human glycosphingolipids. Sialic acid was present on the LOS of some H. influenzae strains and prevented the binding of MAb 3F11 to its epitope. The oligosaccharide portion of sialylated LOS may also resemble sialylated oligosaccharides present in human glycosphingolipids (gangliosides).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu Kwaik Y., McLaughlin R. E., Apicella M. A., Spinola S. M. Analysis of Haemophilus influenzae type b lipooligosaccharide-synthesis genes that assemble or expose a 2-keto-3-deoxyoctulosonic acid epitope. Mol Microbiol. 1991 Oct;5(10):2475–2480. doi: 10.1111/j.1365-2958.1991.tb02092.x. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Mandrell R. E., Shero M., Wilson M. E., Griffiss J. M., Brooks G. F., Lammel C., Breen J. F., Rice P. A. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis. 1990 Aug;162(2):506–512. doi: 10.1093/infdis/162.2.506. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Shero M., Jarvis G. A., Griffiss J. M., Mandrell R. E., Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987 Aug;55(8):1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnari A. A., Spinola S. M., Lesse A. J., Kwaik Y. A., Mandrell R. E., Apicella M. A. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990 May;8(5):353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Frosch M., Weisgerber C., Meyer T. F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John C. M., Griffiss J. M., Apicella M. A., Mandrell R. E., Gibson B. W. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J Biol Chem. 1991 Oct 15;266(29):19303–19311. [PubMed] [Google Scholar]
- Kuratana M., Inzana T. J., Anderson P. Sources of low-molecular-weight host factors that phenotypically increase the resistance of Haemophilus influenzae type b to bacteriolysis: non-identity with a factor active in gonococci. Microb Pathog. 1989 Jul;7(1):73–77. doi: 10.1016/0882-4010(89)90113-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Kim J. J., John C. M., Gibson B. W., Sugai J. V., Apicella M. A., Griffiss J. M., Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991 May;173(9):2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J Immunol. 1982 Nov;129(5):2172–2178. [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons N. J., Patel P. V., Tan E. L., Andrade J. R., Nairn C. A., Goldner M., Cole J. A., Smith H. Cytidine 5'-monophospho-N-acetyl neuraminic acid and a low molecular weight factor from human blood cells induce lipopolysaccharide alteration in gonococci when conferring resistance to killing by human serum. Microb Pathog. 1988 Oct;5(4):303–309. doi: 10.1016/0882-4010(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Spinola S. M., Kwaik Y. A., Lesse A. J., Campagnari A. A., Apicella M. A. Cloning and expression in Escherichia coli of a Haemophilus influenzae type b lipooligosaccharide synthesis gene(s) that encodes a 2-keto-3-deoxyoctulosonic acid epitope. Infect Immun. 1990 Jun;58(6):1558–1564. doi: 10.1128/iai.58.6.1558-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Weiser J. N., Lindberg A. A., Moxon E. R. Antigenic similarities in lipopolysaccharides of Haemophilus and Neisseria and expression of a digalactoside structure also present on human cells. Microb Pathog. 1990 Dec;9(6):441–450. doi: 10.1016/0882-4010(90)90062-u. [DOI] [PubMed] [Google Scholar]
- Yamasaki R., Bacon B. E., Nasholds W., Schneider H., Griffiss J. M. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods. Biochemistry. 1991 Oct 29;30(43):10566–10575. doi: 10.1021/bi00107a028. [DOI] [PubMed] [Google Scholar]
- Yamasaki R., Nasholds W., Schneider H., Apicella M. A. Epitope expression and partial structural characterization of F62 lipooligosaccharide (LOS) of Neisseria gonorrhoeae: IgM monoclonal antibodies (3F11 and 1-1-M) recognize non-reducing termini of the LOS components. Mol Immunol. 1991 Nov;28(11):1233–1242. doi: 10.1016/0161-5890(91)90010-h. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]