2,3-Dihydrofurans are subunits of a range of biologically active compounds (e.g., aflatoxin B1 and clerodin);1 furthermore, they serve as extremely useful synthetic intermediates, since they can be transformed with good stereoselectivity into an array of highly functionalized tetrahydrofurans.2 Although many strategies for the synthesis of 2,3-dihydrofurans have been described, virtually no catalytic asymmetric processes have been developed.3
In 1967, Spencer reported that CuSO4 catalyzes the [4+1] cycloaddition of β-methoxy-α,β-unsaturated ketones with ethyl diazoacetate, leading to furans upon elimination of methanol from the presumed 2,3-dihydrofuran intermediate.4 Since this pioneering work, there have been several other studies of copper-catalyzed reactions of enones with diazo compounds, but none of these investigations has explored the possibility of accessing 2,3-dihydrofurans with control of relative or absolute stereochemistry.5
We recently decided to address this challenge, and in this report we describe our progress to date. Specifically, we have established that, through the use of planar-chiral bipyridine ligand bpy*,6 copper-catalyzed [4+1] cycloadditions of α,β-unsaturated ketones with diazoacetates can produce highly substituted 2,3-dihydrofurans in good yield, dr, and ee (eq 1).7,8
 |
(1) |
In initial studies, we explored cycloadditions of enones with diazo compounds in the presence of a variety of chiral ligands that have proved useful in other copper-catalyzed processes. Unfortunately, for the reaction of chalcone with t-butyl diazoacetate, a bis(oxazoline),9 a semicorrin,10 and a bis(azaferrocene)11 were not effective (Table 1, entries 1-3). On the other hand, a planar-chiral 2,2′-bipyridine (bpy*)12 provided promising yield, dr, and ee (entry 4).
Table 1.
Copper-catalyzed asymmetric [4+1] cycloadditions: Survey of ligands
 | ||||
|---|---|---|---|---|
| entry | ligand | yield (%)a | dr | ee (%) |
| 1 | bis(oxazoline) 1 | 12 | >20:1 | -20b |
| 2 | semicorrin 2 | <2 | - | - |
| 3 | bis(azaferrocene) 3 | 6 | >20:1 | 34 |
| 4 | (-)-bpy* | 45 | >20:1 | 60 |
| 5 | no ligand | 10 | >20:1 | - |
| 6 | no CuOTf, no ligand | <2 | - | - |
All data are the average of two runs.
Isolated yield of the trans diastereomer.
The opposite enantiomer is produced.
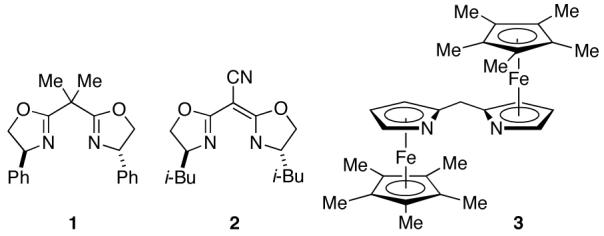
In order to improve upon our preliminary lead (Table 1, entry 4), we investigated the dependence of these Cu/bpy*-catalyzed asymmetric [4+1] cycloadditions on the steric demand of the diazoester (Table 2). Use of a small alkyl or aryl group led to lower ee (entries 2 and 3 versus entry 1). On the other hand, hindered aryl esters furnished higher enantioselectivities (entries 4-6), with the 2,6-diisopropylphenyl ester providing the best combination of yield, dr, and ee (entry 5).
Table 2.
Copper-catalyzed asymmetric [4+1] cycloadditions: Impact of the structure of the diazoester
 | ||||
|---|---|---|---|---|
| entry | R | yield (%)a | dr | ee (%) |
| 1 | t-Bu | 45 | >20:1 | 60 |
| 2 | Et | 43 | >20:1 | 37 |
| 3 | Ph | 44 | >20:1 | 37 |
| 4 | 2,6-dimethylphenyl | 63 | 7:1 | 83 |
| 5 | 2,6-diisopropylphenyl | 79 | 13:1 | 85 |
| 6 | 2,6-di-t-butyl-4-methylphenyl | 47 | 16:1 | 85 |
All data are the average of two runs.
Isolated yield of the trans diastereomer.
We have examined the scope of this copper-catalyzed asymmetric synthesis of 2,3-dihydrofurans (Table 3). The enantiomeric excesses are highest when the enone substituents are unsaturated. Thus, regardless of whether R or R1 is an electron-poor or an electron-rich aromatic group, good ee is typically observed (entries 2-6). Furthermore, the reaction proceeds with useful enantioselectivity when a heteroaromatic substituent is present (entries 7 and 8). Finally, an enone that bears an alkenyl group undergoes cycloaddition with high efficiency (entry 9).
Table 3.
Copper-catalyzed asymmetric [4+1] cycloadditions: Scope
 | |||||
|---|---|---|---|---|---|
| entry | R | R1 | yield (%)a | dr | ee (%) |
| 1 | Ph | Ph | 79 | 13:1 | 85 |
| 2 | 4-(F3C)C6H4 | Ph | 59 | 19:1 | 76 |
| 3 | 4-ClC6H4 | Ph | 77 | 19:1 | 88 |
| 4b | 4-(MeO)C6H4 | Ph | 84 | 19:1 | 92 |
| 5 | Ph | 4-ClC6H4 | 81 | >20:1 | 88 |
| 6 | Ph | 4-(MeO)C6H4 | 84 | 9:1 | 93 |
| 7 | N-Boc-2-pyrrolyl | Ph | 68 | >20:1 | 93 |
| 8 | Ph | 3-furyl | 63 | 6:1 | 87 |
| 9 | Ph | CH=CHPh | 76 | 7:1 | 93 |
| 10 | Ph | n-Bu | 92 | >20:1 | 78 |
| 11 | n-Hex | Ph | 69 | 13:1 | 75 |
| 12 | n-Hex | Me | 80 | >20:1 | 71 |
All data are the average of two runs.
Isolated yield of the trans diastereomer.
The product was hydrolyzed and then acetylated, prior to isolation.
This Cu/bpy*-catalyzed method for the synthesis of 2,3-dihydrofurans may also be applied to alkyl-substituted enones, although such cycloadditions proceed with more modest enantiomeric excess than those that bear only unsaturated groups. Nevertheless, the desired dihydrofurans are generally produced in good yield and with excellent diastereoselectivity (Table 3, entries 10-12).13
The 2,3-dihydrofuran products can be converted into a variety of other useful families of compounds without an erosion in dr or ee. Thus, a primary alcohol can be generated via treatment of the cycloaddition adduct with LiAlH4 (eq 2). Furthermore, hydrolysis and then acetylation affords an acyclic ester that bears an α and a β stereocenter (eq 3).
 |
(2) |
 |
(3) |
Deoxy-C-nucleosides are of interest in medicinal chemistry as mimics of naturally occurring nucleosides.14 We have established that our Cu/bpy*-catalyzed [4+1] cycloaddition can be applied to the expeditious catalytic asymmetric synthesis of this class of compounds (Figure 1). Cycloaddition of an α-diazoacetate to the illustrated vinylogous ester furnishes a 2,3-dihydrofuran, which is not isolated due to its sensitivity. Hydrogenation of the olefin and then reduction of the ester affords the desired tetrahydrofuran in good yield and diastereoselectivity (77% yield for three steps; >20:1 dr). Deprotection of the trimethylsilylethyl group then provides the deoxy-C-nucleoside (93% ee).15,16
Figure 1.

Catalytic asymmetric synthesis of deoxy-C-nucleosides.
In conclusion, we have described the first examples of diastereo- and enantioselective copper-catalyzed [4+1] cycloadditions of enones with diazo compounds. This new method furnishes synthetically useful, highly substituted 2,3-dihydrofuran derivatives with good efficiency and stereoselection. Additional studies of asymmetric copper-catalyzed reactions of diazo compounds are underway.
Supplementary Material
Acknowledgment
We thank Michael M.-C. Lo for preliminary studies. Support has been provided by the NIH (National Institute of General Medical Sciences: R01-GM66960), Merck Research Laboratories, and Novartis. Funding for the MIT Department of Chemistry Instrumentation Facility has been furnished in part by NIH IS10RR13886 and NSF DBI-9729592.
References
- (1).For a review and leading references, see: Kilroy TG, O’Sullivan TP, Guiry PJ. Eur. J. Org. Chem. 2005:4929–4949.
- (2).(a) For leading references to the asymmetric synthesis of tetrahydrofurans, see: Hou X-L, Yang Z, Yeung K-S, Wong HNC. Prog. Heterocycl. Chem. 2005;17:142–171. Elliott MC. J. Chem. Soc., Perkin Trans. 1. 2002:2301–2323. Faul MM, Huff BE. Chem. Rev. 2000;100:2407–2473. doi: 10.1021/cr940210s.
- (3).(a) For other catalytic asymmetric methods for the synthesis of 2,3-dihydrofurans from achiral precursors that proceed with good enantioselectivity, see: Evans DA, Sweeney ZK, Rovis T, Tedrow JS. J. Am. Chem. Soc. 2001;123:12095–12096. doi: 10.1021/ja011983i. Mueller P, Bernardinelli G, Allenbach YF, Ferri M, Grass S. Synlett. 2005:1397–1400. two examples, which differ in a silyl group. Ishitani H, Achiwa K. Heterocycles. 1997;46:153–156. one example.
- (4).Storm DL, Spencer TA. Tetrahedron Lett. 1967;8:1865–1867. Spencer TA, Villarica RM, Storm DL, Weaver TD, Friary RJ, Posler J, Shafer PR. J. Am. Chem. Soc. 1967;89:5497–5499. (c) See also: Murayama ST, Spencer TA. Tetrahedron Lett. 1969;10:4479–4482.
- (5).Anac O, Daut A. Liebigs Ann. Recueil. 1997:1249–1254. Anac O, Ozdemir AD, Sezer O. Helv. Chim. Acta. 2003;86:290–298. Anac O, Guengor FS, Kahveci C, Cansever MS. Helv. Chim. Acta. 2004;87:408–415. (d) See also: Paulissen R, Hayez E, Hubert AJ, Teyssie P. Tetrahedron Lett. 1974;15:607–608.
- (6).For the initial report of the synthesis of this ligand, see: Rios R, Liang J, Lo MM-C, Fu GC. Chem. Commun. 2000:377–378.
- (7).(a) For leading references to the chemistry of carbonyl ylides, see: Clark JS, editor. Nitrogen, Oxygen and Sulfur Ylide Chemistry. Oxford; New York: 2002. McMills MC, Wright D. Chem. Heterocycl. Compd. 2002;59:253–314. Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. Wiley; New York: 1998. Padwa A. Helv. Chim. Acta. 2005;88:1357–1374.
- (8).For leading references to catalytic asymmetric reactions of ylides formed from diazo compounds, see: Davies HML. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Springer; New York: 2004. pp. 83–94. Hodgson DM, Pierard FYTM, Stupple PA. Chem. Soc. Rev. 2001;30:50–61.
- (9).For a review of applications of bis(oxazoline)s in asymmetric catalysis, see: Desimoni G, Faita G, Jorgensen KA. Chem. Rev. 2006;106:3561–3651. doi: 10.1021/cr0505324.
- (10).For leading references, see: Pfaltz A. Synlett. 1999:835–842.
- (11).For leading references to previous applications, see: Maier TC, Fu GC. J. Am. Chem. Soc. 2006;128:4594–4595. doi: 10.1021/ja0607739.
- (12).(a) For reviews of chiral 2,2′-bipyridine ligands, see: Malkov AV, Kocovsky P. Curr. Org. Chem. 2003;7:1737–1757. Fletcher NC. J. Chem. Soc., Perkin Trans. 1. 2002:1831–1842.
- (13).Under our standard conditions, α,β-unsaturated esters are not suitable substrates.
- (14).(a) For some leading references, see: Kool ET. Acc. Chem. Res. 2002;35:936–943. doi: 10.1021/ar000183u. Loakes D. Nucleic Acids Res. 2001;29:2437–2447. doi: 10.1093/nar/29.12.2437. Watanabe KA. In: Chemistry of Nucleosides and Nucleotides. Townsend LB, editor. Vol. 3. Plenum; New York: 1994. pp. 421–535.
- (15).To the best of our knowledge, this is the first catalytic asymmetric synthesis of this deoxy-C-nucleoside (and the first synthesis of the “unnatural” enantiomer).
- (16).(a) For studies of this deoxy-C-nucleoside, see: Initial work: Millican TA, Mock GA, Chauncey MA, Patel TP, Eaton MAW, Gunning J, Cutbush SD, Neidle S, Mann J. Nucleic Acids Res. 1984;12:7435–7453. doi: 10.1093/nar/12.19.7435. Matsuda S, Romesberg FE. J. Am. Chem. Soc. 2004;126:14419–14427. doi: 10.1021/ja047291m. Mathis G, Hunziker J. Angew. Chem. Int. Ed. 2002;41:3203–3205. doi: 10.1002/1521-3773(20020902)41:17<3203::AID-ANIE3203>3.0.CO;2-K. Guckian KM, Schweitzer BA, Ren RX-F, Sheils CJ, Tahmassebi DC, Kool ET. J. Am. Chem. Soc. 2000;122:2213–2222. doi: 10.1021/ja9934854.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


